Introduction
Gestational diabetes mellitus (GDM), as one of the
most frequent complications in pregnancy, is also one of the common
threats to the life and safety of mothers and infants (1). It can easily lead to premature
delivery, malformation and even miscarriage and dystocia of
pregnant women (2). In recent years,
the incidence of GDM has increased, which brings a heavy burden on
families and society (3). However,
except some studies (4,5) indicating that insulin resistance during
pregnancy may trigger GDM, its pathogenesis remains poorly
understood. The complex pathogenesis of GDM results in a lack of
effective GDM screening markers during pregnancy, which is
unfavorable for the diagnosis of GDM (6).
As a kind of non-coding micro-RNA, the
post-transcriptional level of miRNA is closely related to the
pathophysiological processes of many diseases (7). Studies (8,9) in
recent years have shown that miRNA is one of the essential
biological regulators in insulin resistance, and many miRNAs have
been proved to be effective diagnostic markers for GDM. For
example, a study (10) pointed out
that miR-330-3p was upregulated in GDM and was related to the
patient's insulin resistance. Other studies have further explored
the role of miRNA in GDM. Some scholars (11) found that miR-494 protected the
pancreatic β-cell function of GDM by targeting PTEN. Among them,
miR-409-5p has been proven to play a role as an oncogene in a
variety of tumors. For example, a study (12) found that miR-409-5p was up-regulated
in breast cancer, and inhibiting its expression can significantly
suppress the growth of breast cancer cells. While recent evidence
(13) revealed that miR-409-5p is
associated with the occurrence of diabetes. However, the
relationship between miR-409-5p and GDM has not been studied
yet.
Therefore in this study, we explored the expression
of miR-409-5p in GDM and its relationship with insulin resistance,
so as to provide more possibilities for the clinical diagnosis and
mechanism research of GDM.
Patients and methods
General information
A total of 149 pregnant women, including 76 patients
with GDM (GDM group) and 73 normal pregnant women (control group),
with an average age of 27.02±3.17 years, who underwent antenatal
examination in the hospital from April 2015 to January 2017 were
selected as the study participants. Inclusion criteria: The GDM
group included patients who were diagnosed and met the diagnostic
criteria of GDM (14). Glucose
screening is currently recommended at 24-28 weeks of gestation
(15), so patients diagnosed during
their pregnancy were included. Exclusion criteria: Patients with
diabetes before pregnancy; patients with other complications during
pregnancy; patients with multiple pregnancies; patients with
malignant tumors; patients with severe infectious diseases;
patients who had used glucocorticoids; patients with severe hepatic
and renal dysfunction; patients who refused to participate in the
study. The patients and their families agreed to participate in the
study and signed an informed consent form. This study was approved
by the Ethics Committee of Taizhou First People's Hospital
(Taizhou, China) (TZFPHPH201503A), and conformed to the Declaration
of Helsinki.
Detection of miR-409-5p expression by
RT-PCR
Fasting venous blood samples were taken from all the
patients, centrifuged at 4˚C for 10 min at 1,500 x g, and the
obtained serum (3 ml) was processed for detection. The total RNA in
serum was extracted with TRIzol reagent (Thermo Fisher Scientific,
Inc.), and its purity and concentration were detected by
ultraviolet spectrophotometer. Then 5 µg of the total RNA was taken
for reverse transcription of cDNA according to the instructions of
the reverse transcription kit (TransGen Biotech). The amplification
system of miR-409-5p (PCR kit, TransGen Biotech) was as follows:
cDNA: 1 µl, upstream and downstream primers (concentrations: 10
µmol/l): 0.4 µl each, 2X TransTaq® Tip Green qPCR
SuperMix: 10 µl, Passive Reference Dye (50X): 0.4 µl, and finally
ddH2O was added to a total volume of 20 µl.
Amplification conditions: PCR reaction conditions: pre-denaturation
at 94˚C for 45 sec, denaturation at 94˚C for 10 sec, annealing at
60˚C for 45 sec, totaling 40 cycles. Three replicate wells were set
for each sample and the experiment was performed three times. With
U6 as the internal reference, 2-∆∆ct was employed to
analyze the data. Primer sequences: miR-409-5p: F, 5'-AGGTTACCC
GAGCAACTTTG-3', R, 5'-GTGTCGTGGAGTCGGCAA-3'; U6: F,
5'-GCTTCGGCAGCACATATACTAAAAAT-3', R,
5'-CGCTTCACGAATTTGCGTGTCAT-3'.
Detection of other biochemical
indicators
The plasma triacylglycerol (TG), total cholesterol
(TC), low-density lipoprotein cholesterol (LDL-C), and high-density
lipoprotein cholesterol (HDL-C) were detected by automatic standard
routine enzymatic methods (Abbott Aeroset 2000, Abbott Corp.). The
level of glycosylated hemoglobin (HbAlc) was determined by an
automatic biochemical analyzer. Fasting plasma glucose (FPG) was
measured by glucose oxidase method, and fasting insulin (FINS) was
tested by radioimmunoassay. The homeostatic model assessment (HOMA)
was calculated by the formulate of HOMA-insulin resistance
(HOMA-IR) index (16) = (FBG x
FINS)/22.5.
Statistical methods
The experimental data in this study was
statistically analyzed using SPSS 19.0. The counting data were
verified by the chi-square test, while the measurement data were
expressed as mean ± standard deviation. The inter-group comparison
was performed by the independent t-test. GraphPad Prism 6 software
was adopted to draw the required illustrations of this experiment,
and Pearson was employed for correlation analysis. Receiver
operating characteristic (ROC) curve was used to analyze the
diagnostic value of miR-409-5p in GDM. P<0.05 indicates a
statistically significant difference.
Results
Patient information
There were no significant differences between the
two groups in terms of age, body mass index (BMI) or gravidity
(P>0.05), but there were differences in TG, TC, and HDL-C
(P<0.001) (Table I).
 | Table IPatient information. |
Table I
Patient information.
| Factors | GDM group n=76 | Control group
n=73 | t/χ2 | P-value |
|---|
| Age, years | | | 0.001 | 0.970 |
|
≤27 | 45 (59.21) | 43 (58.90) | | |
|
>27 | 31 (40.79) | 30 (41.10) | | |
| Pre-pregnancy BMI,
kg/m2 | | | 0.003 | 0.954 |
|
≤23 | 42 (55.26) | 40 (54.79) | | |
|
>23 | 34 (44.74) | 33 (45.21) | | |
| Educational
level | | | 0.001 | 0.975 |
|
Below junior
high school | 21 (27.63) | 20 (27.40) | | |
|
Junior high
school or above | 55 (72.37) | 53 (72.60) | | |
| Place of
residence | | | 0.016 | 0.901 |
|
Rural | 32 (42.11) | 30 (41.10) | | |
|
Urban | 44 (57.89) | 43 (58.90) | | |
| Gravidity, times | | | 0.002 | 0.961 |
|
≤1 | 57 (75.00) | 55 (75.34) | | |
|
>1 | 19 (25.00) | 18 (24.66) | | |
| Gestational week of
delivery | 38.21±2.01 | 38.45±2.08 | 0.716 | 0.475 |
| TC (mmol/l) | 5.93±0.47 | 5.45±0.41 | 6.632 | <0.001 |
| TG (mmol/l) | 2.81±0.54 | 1.97±0.32 | 11.49 | <0.001 |
| LDL-C (mmol/l) | 2.74±0.48 | 2.63±0.43 | 1.471 | 0.143 |
| HDL-C (mmol/l) | 1.29±0.26 | 1.67±0.37 | 7.277 | <0.001 |
Expression and diagnostic value of
miR-409-5p in the two groups
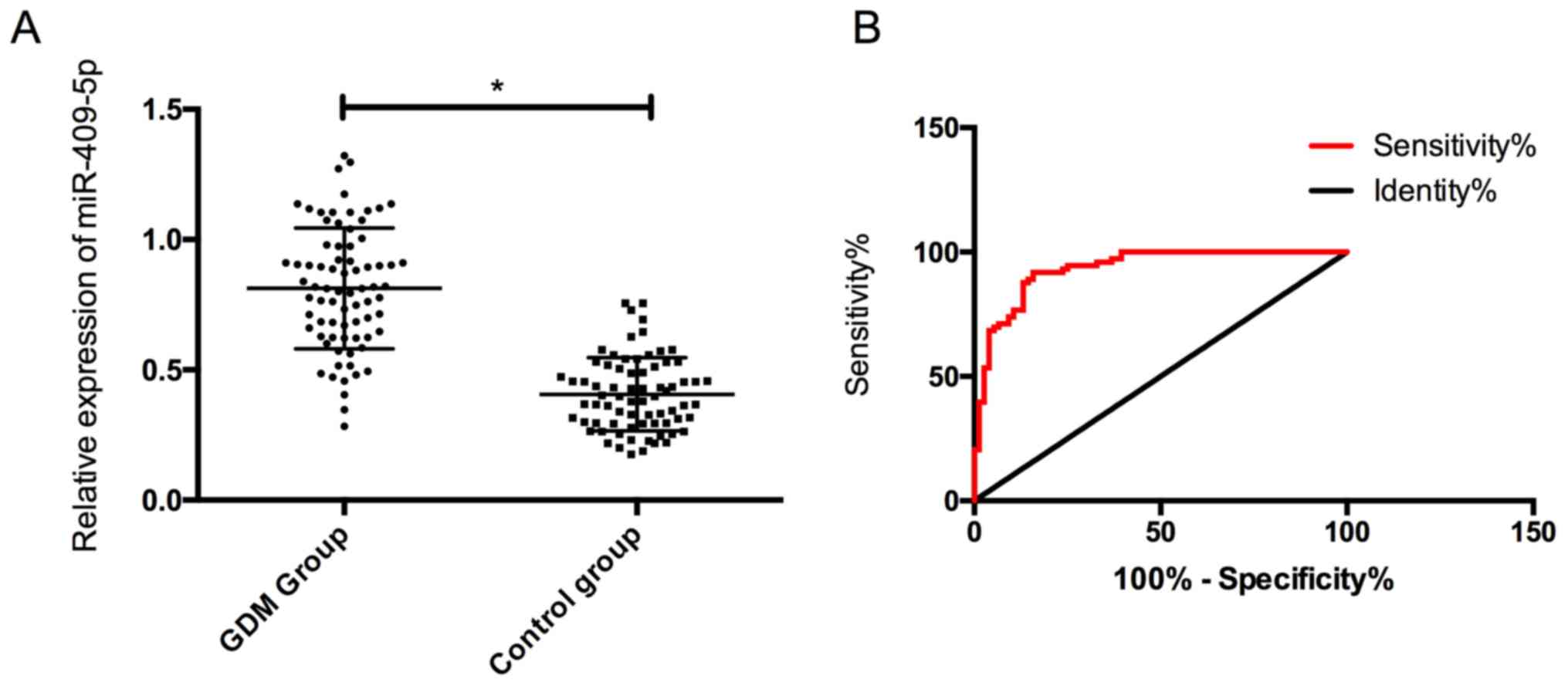
The expression of serum miR-409-5p in the GDM group
was (0.78±0.24), which was noticeably higher than (0.42±0.14) in
the control group, and the difference was statistically significant
(P<0.05). In addition, it was found that the the ROC curve of
miR-409-5p in the diagnosis of GDM was 0.933, indicating a high
diagnostic value (Fig. 1).
Comparison of other biochemical
indicators between the two groups
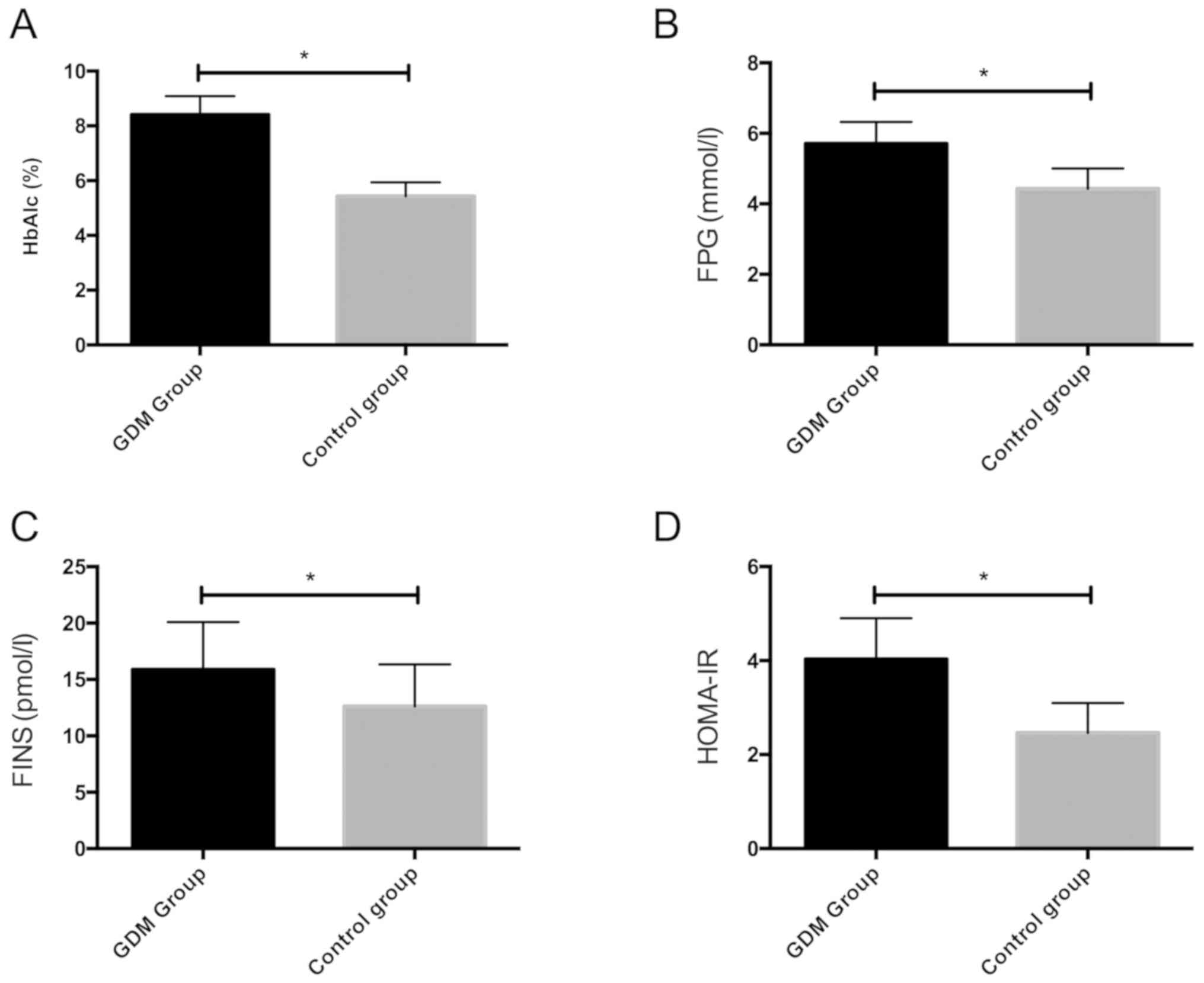
We detected HbAlc, FPG and FINS of the two groups of
pregnant women, and calculated HOMA-IR. The results showed that
HbAlc, FPG, FINS and HOMA-IR in the GDM group were remarkably
higher than those in the control group, with statistically
significant differences (P<0.05) (Fig. 2).
Correlation of serum miR-409-5p with
HbAlc, FPG, FINS and HOMA-IR in pregnant women with GDM
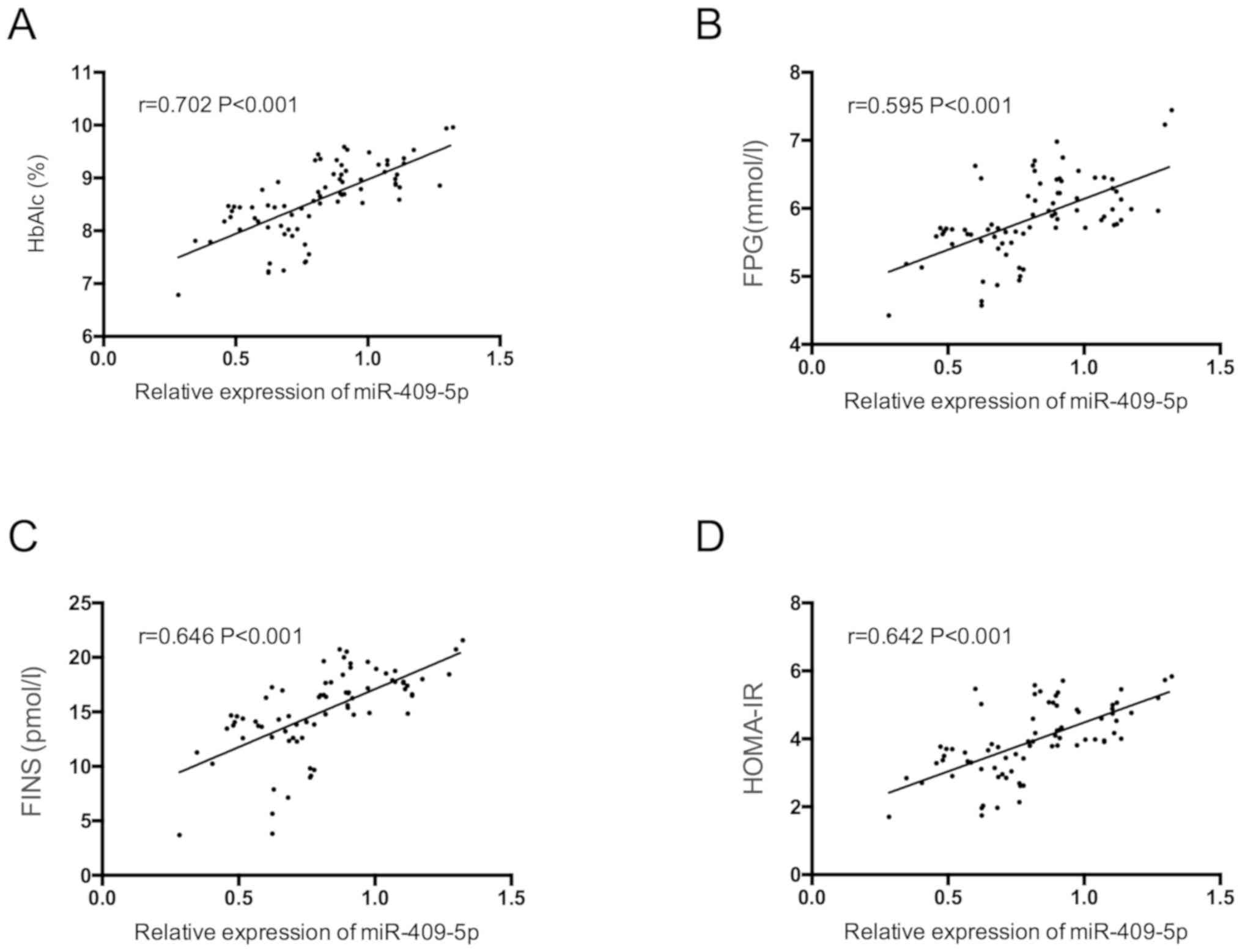
miR-409-5p was positively correlated with HbAlc,
FPG, FINS and HOMA-IR in pregnant women with GDM (P<0.05)
(Fig. 3).
Expression of miR-409-5p in patients
with different insulin resistance
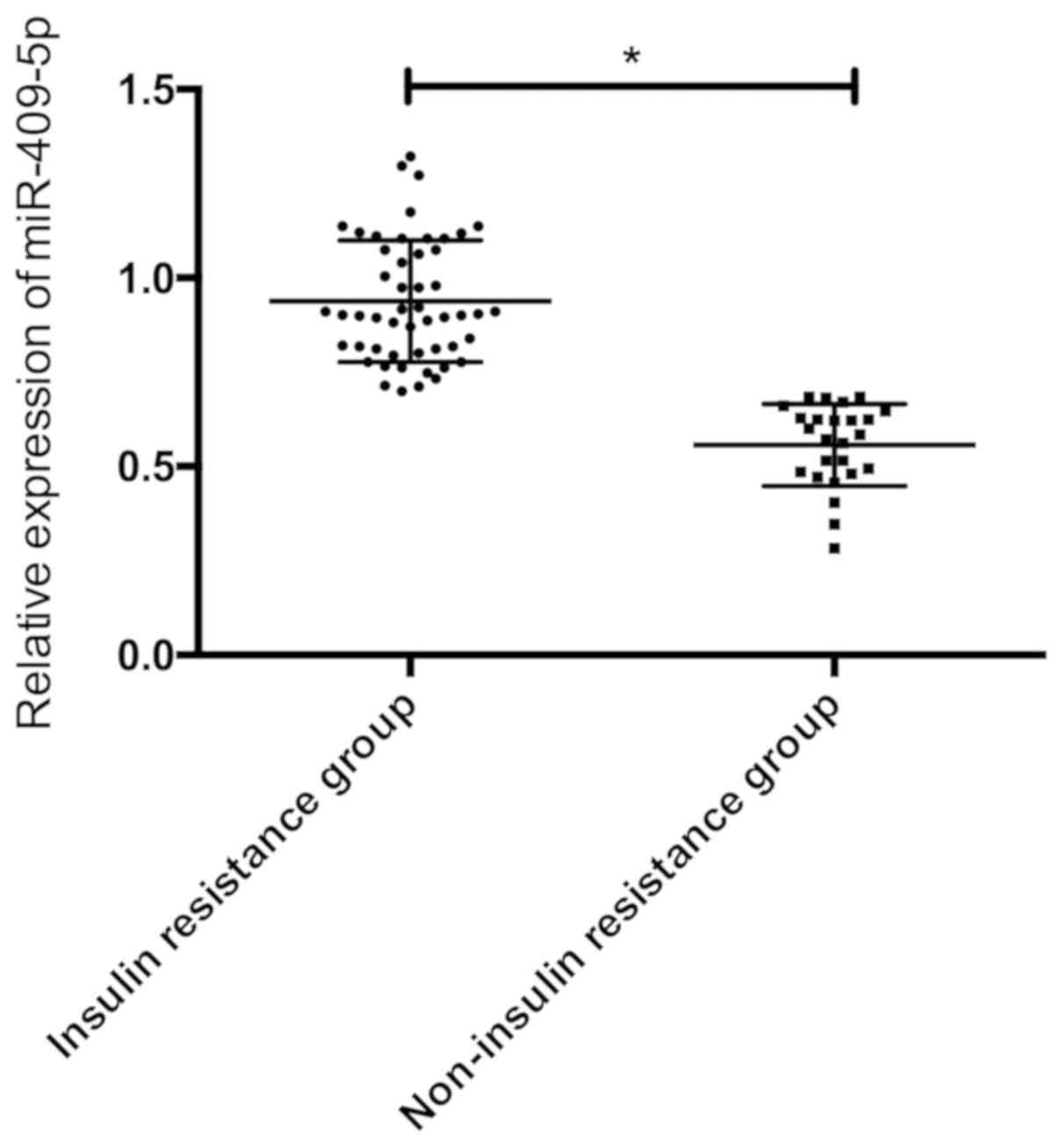
According to the insulin resistance index, the GDM
patients were divided into 51 cases of insulin resistance group
(HOMA-IR ≥1.66) and 25 cases of non-insulin resistance group
(HOMA-IR <1.66) to compare the serum miR-409-5p expression in
the two groups. The results showed that serum miR-409-5p level in
the insulin resistance group was dramatically higher than that in
the non-insulin resistance group, and the difference was
statistically significant (P<0.05) (Fig. 4).
Multivariate analysis of the
occurrence of GDM
Based on the above results, we found that the
occurrence of GDM was associated with serum miR-409-5p, HbAlc, FPG,
FINS, and HOMA-IR. These factors were listed as independent
variables and assigned values, and took GDM as the dependent
variable for multivariate analysis by logistic regression analysis
(Table II). The results revealed
that the increase of miR-409-5p, FINS and HOMA-IR were all
independent risk factors for GDM.
 | Table IIMultivariate analysis. |
Table II
Multivariate analysis.
| Factors | β | S.E | Wald | OR | 95% CI | P-value |
|---|
| miR-409-5p | 0.343 | 0.157 | 5.442 | 1.465 | 1.008-1.871 | <0.05 |
| HbAlc | 0.113 | 0.508 | 0.294 | 1.061 | 0.742-1.674 | 0.153 |
| FPG | 0.228 | 0.384 | 0.341 | 1.249 | 0.903-1.714 | 0.129 |
| FINS | 0.421 | 0.128 | 6.237 | 1.745 | 1.166-2.354 | <0.05 |
| HOMA-IR | 0.509 | 0.086 | 8.626 | 2.432 | 1.505-3.774 | <0.05 |
Discussion
GDM is considered to be a severe glucose intolerance
during pregnancy. In recent years, increasing number of women have
been diagnosed with GDM, which has become a common public health
problem (17). For many patients,
untimely diagnosis and treatment will inevitably bring severe
adverse effects on the mother and fetus (18). As one of the mechanisms of GDM,
insulin resistance has been reported in previous research (19) indicating that many pregnant women
with GDM have more severe insulin resistance than non-pregnant
women, the possible mechanism of action, however, remains
unknown.
It is well-established that miRNAs play a vital part
in the occurrence and progression of many diseases, including
diabetes (20,21). For example, research (22) identified that the serum miR-132
expression in patients with GDM was decreased, and it may be used
as a biomarker for the diagnosis of GDM. In this study, we found
that the expression of miR-409-5p was up-regulated in the serum of
GDM patients, and ROC analysis exhibited that it had a high
diagnostic value for GDM. Previous studies on miR-409-5p mainly
focused on its role in tumors. For example, a study (23) revealed that miR-409 inhibited the
invasion and metastasis of tumor cells by directly targeting the
radixin in gastric cancer. Another study (24) showed that miR-409 inhibited the
development of non-small cell lung cancer (NSCLC) by directly
targeting SPIN1. In our study, for the first time it was found that
miR-409-5p was up-regulated in GDM, which provides certain ideas
and directions for follow-up research.
To further analyze the relationship between
miR-409-5p and GDM patients, we tested other relevant biochemical
indicators of GDM and calculated HOMA-IR. The results demonstrated
that compared with normal pregnant women, the HbAlc, FPG, FINS and
HOMA-IR were significantly increased in GDM patients, and
correlation analysis revealed a positive correlation between
miR-409-5p and serum HbAlc, FPG, FINS and HOMA-IR. As is know,
insulin resistance is an important index for evaluating islet cell
function in diabetes mellitus (25),
our study found there was a positive correlation between miR-409-5p
and insulin resistance index in patients with GDM. Subsequently, we
compared the expression of miR-409-5p in patients with different
insulin resistance, and noted that the serum miR-409-5p level in
patients with insulin resistance was significantly higher than that
without, which validated the previous idea on the one hand, and on
the other hand, it suggested that miR-409-5p may affect the
function of islet cells, thus affecting the pathogenesis of GDM.
However, more basic experiments and clinical studies are needed to
confirm this hypothesis. Many studies have explored the effect of
miRNA on islet function. For example, evidence (26) found that miR-375 was highly expressed
in islet cells, which could inhibit glucose-induced insulin
secretion. Another study (27)
conducted animal experiments and also pointed out that miR-29 was
highly expressed in diabetic rats and could induce insulin
resistance. Finally, we analyzed the causes of GDM, and the results
showed that the increase of miR-409-5p, FINS, and HOMA-IR were all
independent risk factors for GDM. Studies in the past (28,29)
indicated that insulin resistance may be one of the causes of GDM,
but the possible effect of miR-409-5p in GDM has not been studied
yet. The present study is the first that found miR-409-5p may be an
independent risk factor for the occurrence of GDM, which we
speculate may be related to the association between miR-409-5p and
insulin resistance, but more basic experiments are yet to be
performed for confirmation.
In conclusion, miR-409-5p is highly expressed in the
serum of GDM patients, and it is positively correlated with insulin
resistance index of GDM patients, which may be a potential target
for clinical diagnosis and treatment of GMD. However, this study
also has deficiencies. Relevant basic experiments in vivo
and in vitro, which are absent in the present study, are
needed to support our conclusions. As there are relatively few
studies on miR-409-5p in GDM, our conclusions need to be further
confirmed.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CT conceived the study and drafted the manuscript.
LW collected the general information and analyzed the data. HT and
LG detected miR-409-5p expression using RT-PCR. SZ and XC were
responsible for the detection of the biochemical indicators. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Taizhou First People's Hospital (Taizhou, China) (TZFPHPH201503A),
and conformed to the Declaration of Helsinki. Patients who
participated in this research signed an informed consent and had
complete clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ranheim T, Haugen F, Staff AC, Braekke K,
Harsem NK and Drevon CA: Adiponectin is reduced in gestational
diabetes mellitus in normal weight women. Acta Obstet Gynecol
Scand. 83:341–347. 2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
O'Sullivan EP, Avalos G, O'Reilly MW,
Dennedy MC, Gaffney G and Dunne F: Atlantic DIP collaborators:
Erratum to. Atlantic Diabetes in Pregnancy (DIP): the prevalence
and outcomes of gestational diabetes mellitus using new diagnostic
criteria. Diabetologia. 59:873. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lee KH, Han YJ, Chung JH, Kim MY, Ryu HM,
Kim JH, Kwak DW, Kim SH, Yang S and Kim M: Treatment of gestational
diabetes diagnosed by the IADPSG criteria decreases excessive fetal
growth. Obstet Gynecol Sci. 63:19–26. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Li J, Leng J, Li W, Zhang C, Feng L, Wang
P, Chan JCN, Hu G, Yu Z and Yang X: Roles of insulin resistance and
beta cell dysfunction in macrosomia among Chinese women with
gestational diabetes mellitus. Prim Care Diabetes. 12:565–573.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shaat N, Ignell C, Katsarou A and Berntorp
K: Glucose homeostasis, beta cell function, and insulin resistance
in relation to vitamin D status after gestational diabetes
mellitus. Acta Obstet Gynecol Scand. 96:821–827. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bartolo S, Vambergue A and Deruelle P:
Screening for gestational diabetes: Still many unsolved issues. J
Gynecol Obstet Biol Reprod (Paris). 45:105–111. 2016.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
7
|
Pheiffer C, Dias S, Rheeder P and Adam S:
Decreased expression of circulating miR-20a-5p in South African
women with gestational diabetes mellitus. Mol Diagn Ther.
22:345–352. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Feng J, Xing W and Xie L: Regulation of
microorganisms in diabetes. Int J Mol Sci. 17(1729)2016.
|
|
9
|
Tang XW and Qin QX: miR-335-5p induces
insulin resistance and pancreatic islet β-cell secretion in
gestational diabetes mellitus mice through VASH1-mediated TGF-β
signaling pathway. J Cell Physiol. 234:6654–6666. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sebastiani G, Guarino E, Grieco GE,
Formichi C, Delli Poggi C, Ceccarelli E and Dotta F: Circulating
microRNA (miRNA) expression profiling in plasma of patients with
pestational diabetes mellitus reveals upregulation of miRNA
miR-330-3p. Front Endocrinol. 8(345)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
He Y, Bai J, Liu P, Dong J, Tang Y, Zhou
J, Han P, Xing J, Chen Y and Yu X: miR-494 protects pancreatic
β-cell function by targeting PTEN in gestational diabetes mellitus.
EXCLI J. 16:1297–1307. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yu H, Xing H, Han W, Wang Y, Qi T, Song C,
Xu Z, Li H and Huang Y: MicroRNA-409-5p is upregulated in breast
cancer and its downregulation inhibits cancer development through
downstream target of RSU1. Tumour Biol.
39(1010428317701647)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Massaro JD, Polli CD, Costa E Silva M,
Alves CC, Passos GA, Sakamoto-Hojo ET, Rodrigues de Holanda Miranda
W, Bispo Cezar NJ, Rassi DM, Crispim F, et al: Post-transcriptional
markers associated with clinical complications in Type 1 and Type 2
diabetes mellitus. Mol Cell Endocrinol. 490:1–14. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Metzger BE, Gabbe SG, Persson B, Buchanan
TA, Catalano PA, Damm P, Dyer AR, Leiva A, Hod M, Kitzmiler JL, et
al: International Association of Diabetes and Pregnancy Study
Groups Consensus Panel: International association of diabetes and
pregnancy study groups recommendations on the diagnosis and
classification of hyperglycemia in pregnancy. Diabetes Care.
33:676–682. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Demir E, Ozkan H, Seckin KD, Sahtiyancı B,
Demir B, Tabak O, Kumbasar A and Uzun H: Plasma zonulin levels as a
non-invasive biomarker of intestinal permeability in women with
gestational diabetes mellitus. Biomolecules: Jan 11, 2019 (Epub
ahead of print). doi: 10.3390/biom9010024.
|
|
16
|
Van Winden K, Montoro M, Korst LM and
Ouzounian JG: A homeostatic model assessment of insulin resistance
(HOMA-IR) relates to gestational diabetes, glycemic control [1K].
Obstet Gynecol. 129(112S)2017.
|
|
17
|
Wei YM, Yan J and Yang HX: Identification
of severe gestational diabetes mellitus after new criteria used in
China. J Perinatol. 36:90–94. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang X, Yang T, Miao J, Liu H, Wu K, Guo
J, Chen J and Li T: Correlation between maternal and fetal insulin
resistance in pregnant women with gestational diabetes mellitus.
Clin Lab. 64:945–953. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ebert T, Hindricks J, Kralisch S, Lossner
U, Jessnitzer B, Richter J, Blüher M, Stumvoll M and Fasshauer M:
Serum levels of fractalkine are associated with markers of insulin
resistance in gestational diabetes. Diabet Med. 31:1014–1017.
2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wander PL, Boyko EJ, Hevner K, Parikh VJ,
Tadesse MG, Sorensen TK, Williams MA and Enquobahrie DA:
Circulating early- and mid-pregnancy microRNAs and risk of
gestational diabetes. Diabetes Res Clin Pract. 132:1–9.
2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li L, Wang S, Li H, Wan J, Zhou Q, Zhou Y
and Zhang C: microRNA-96 protects pancreatic β-cell function by
targeting PAK1 in gestational diabetes mellitus. Biofactors.
44:539–547. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhou X, Xiang C and Zheng X: miR-132
serves as a diagnostic biomarker in gestational diabetes mellitus
and its regulatory effect on trophoblast cell viability. Diagn
Pathol. 14(119)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zheng B, Liang L, Huang S, Zha R, Liu L,
Jia D, Tian Q, Wang Q, Wang C, Long Z, et al: MicroRNA-409
suppresses tumour cell invasion and metastasis by directly
targeting radixin in gastric cancers. Oncogene. 31:4509–4516.
2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Song Q, Ji Q, Xiao J, Li F, Wang L, Chen
Y, Xu Y and Jiao S: miR-409 inhibits human non-small-cell lung
cancer progression by directly targeting SPIN1. Mol Ther Nucleic
Acids. 13:154–163. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Retnakaran R, Ye C, Connelly PW, Hanley
AJ, Sermer M and Zinman B: Serum apoA1 (Apolipoprotein A-1),
insulin resistance, and the risk of gestational diabetes mellitus
in human pregnancy - brief report. Arterioscler Thromb Vasc Biol.
39:2192–2197. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kraus M, Greither T, Wenzel C,
Bräuer-Hartmann D, Wabitsch M and Behre HM: Inhibition of
adipogenic differentiation of human SGBS preadipocytes by
androgen-regulated microRNA miR-375. Mol Cell Endocrinol.
414:177–185. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Dooley J, Garcia-Perez JE, Sreenivasan J,
Schlenner SM, Vangoitsenhoven R, Papadopoulou AS, Tian L,
Schonefeldt S, Serneels L, Deroose C, et al: The microRNA-29 family
dictates the balance between homeostatic and pathological glucose
handling in diabetes and obesity. Diabetes. 65:53–61.
2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Andersson-Hall U, Gustavsson C, Pedersen
A, Malmodin D, Joelsson L and Holmäng A: Higher concentrations of
BCAAs and 3-HIB are associated with insulin resistance in the
transition from gestational diabetes to type 2 diabetes. J Diabetes
Res. 2018(4207067)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li J-Y, Wu G-M, Hou Z and Cao YM:
Expression of C1q/TNF-related protein-3 (CTRP3) in serum of
patients with gestational diabetes mellitus and its relationship
with insulin resistance. Eur Rev Med Pharmacol Sci. 21:5702–5710.
2017.PubMed/NCBI View Article : Google Scholar
|