Introduction
Heat stroke (HS) is a severe and life-threatening
disease, characterized by excessive hyperthermia and nervous system
symptoms such as confusion, seizures or loss of consciousness
(1). HS can be divided into two
types: Labor HS (caused by high-intensity physical work) and
non-labor HS (caused by a high-temperature environment) (1). With increased temperature caused by
global warming, the number of reported HS cases has increased
within the last two decades (1).
According to its pathophysiological characteristics, HS is a type
of high fever-associated systemic inflammatory response, which can
lead to multiple organ dysfunction syndrome (MODS) and the
appearance of encephalopathy, especially central nervous system
injury (2).
The small intestines can prevent bacterial invasion
and participate in the process of water balance and solute
transport within the body (3). In
the initiation stage of HS inflammation, high fever is conducive to
endotoxin leakage from the intestinal mucosa to the systemic
circulation (4). Heat stimulation
and high-intensity physical activity can damage intestinal mucosal
cells, resulting in tissue hypoxia, ATP depletion, acidosis and
oxidative stress and intestinal mucosal barrier dysfunction
(4,5). Previous animal and cell studies have
demonstrated that heat stress can induce tissue hyperthermia and
decrease intestinal blood flow, which may subsequently lead to
tight junction disorders and pro-inflammatory cytokine release, or
further induce the systemic inflammatory response (6,7). Under
these circumstances, the inflammatory reaction can cause vascular
endothelial damage, which can aggravate the damage to the
intestinal mucosal barrier (8).
Mesenchymal stem cells (MSCs) serve a number of
roles, including enhancing angiogenesis and neurogenesis by
secreting nutrient factors, regulating the immune system and
exhibiting anti-inflammatory effects (5). MSCs may exhibit immunomodulatory
effects, which are based on the mechanisms cell contact and
paracrine signaling, and involve the release of soluble
inflammatory factors (6). In
particular, MSCs have been indicated to stimulate immune regulatory
factors, including interleukin (IL)-6, IL-10, prostaglandins,
transforming growth factor-β (TGF-β) and nitric oxide (7). Furthermore, regulatory T cell
production can be promoted and their inhibitory effect increased by
MSCs, which can lead to the inhibition of intestinal inflammation
by inhibiting macrophage production and regulating macrophage
phenotype via prostaglandin E2 (PGE2) (8). Furthermore, aside from their potent
anti-inflammatory effects, MSCs have been demonstrated to
accelerate tissue repair, mainly through promoting epithelial
formation, granulation tissue formation and angiogenesis (9). It has been hypothesized that the early
onset of HS is due to intestinal ischemia, which results in
intestinal mucosal barrier function damage and leads to the
induction of the intestinal flora and the systemic inflammatory
response (10). Furthermore, studies
have reported that MSCs improved intestinal mucosal barrier
function by repairing tight junctions and increasing the number of
regenerating crypts and zonula occludens 1 protein (11-13).
Previous studies investigating the use of stem cells
for the treatment of diabetes (14),
stroke (15) and brain or spinal
cord injury (16) have made rapid
progress. Previous studies have focused on the beneficial effects
of stem cells on HS brain injury (17,18);
however, a small number of studies have focused on intestinal
injury during the initiation of inflammation (19). The current study aimed to investigate
whether MSCs serve a protective role in intestinal injury and
systemic inflammation that is caused by heat stroke. The present
study aimed to identify effective methods and references that can
be used in the treatment of HS and the improvement of patient
prognosis. In the current study, a rat model of HS was established.
The study group received MSCs injection intravenously following the
successful formation of the model, while the control group was
injected with 0.3 ml normal saline. By observing the survival rate,
biochemical indicators and cytokine expression levels of the two
groups and examining the pathological condition of intestinal
mucosa at different time points, the results demonstrated whether
MSCs serve a protective role in intestinal injury and systemic
inflammation caused by heat stroke.
Materials and methods
Animals
A total of 90 mature (age, 8 weeks; weight, 180-220
g) and 20 immature (age, 4 weeks; weight, 60-80 g) male
Sprague-Dawley rats were provided by the Experimental Animal Center
of Chinese PLA General Hospital. The current research complied with
the statement of relevant ethical standards (the Animal Research:
Reporting of in vivo Experiments reporting guidelines)
(20) and was approved by the Ethics
Committee of the Chinese PLA General Hospital (approval no.
2017-X13-10). All animals were conducted in accordance with the
National Institutes of Health Guidelines for Animal Care and Use
(21). The animals were kept in
cages at room temperature of 22-25˚C, air humidity of 40%, air
pressure of 101.325 kPa and 12 h light/dark cycles. All animals had
free access to common rat feed and water. At the end of the
experiment, rats were euthanized by neck dislocation following
anesthesia.
Adipose-derived MSCs isolation,
culture and identification
As described previously (22,23),
MSCs derived from fat were isolated and purified from immature
rats. A total of 3 rats were euthanized by neck dislocation at
different time points (at days 1, 7, 14 and 28). Adipose tissues
were separated from the groin and then sliced and digested with
0.05% trypsin and 0.1% collagen I. The digestive solution was
filtered and centrifuged in 2,000 x g. Cells were washed twice with
PBS and cultured at 37̊C in humidified air with 5% CO2
in low-glucose DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.),
80 units/ml penicillin and 0.2 mg/ml streptomycin. MSCs were
identified as described previously (22,23).
Immunophenotypes of MSCs were stained at room temperature for 15
min with Allophycocyanin-conjugated cluster of differentiation (CD)
90 (1:20; cat. no. 561409; BD Biosciences), fluorescein
isothiocyanate (FITC)-conjugated CD44 (1:20; cat. no. 550974; BD
Biosciences), R-phycoerythrin-conjugated CD54 (1:20; cat. no.
554970; BD Biosciences), FITC-conjugated CD45 (1:20; cat. no.
554877; BD Biosciences), FITC-conjugated CD34 (1:20; cat. no.
11-0341-82; eBioscience; Thermo Fisher Scientific, Inc.) and
FITC-conjugated CD11b (1:20; cat. no. 554982; BD Biosciences) and
analyzed using a BD Accuri C6 flow cytometry software system
(version 1.0.264.21; BD Biosciences). Additionally, adipocyte
differentiation of MSCs was detected by Oil Red O staining
(Sigma-Aldrich; Merck KGaA) at room temperature for 1 h, and
osteoblast differentiation was detected by Alizarin Red staining
(Sigma-Aldrich; Merck KGaA) at room temperature for 30 min. The
cells were observed using an inverted light microscope
(magnification, x40; Olympus BX53; Olympus Corporation). Freshly
harvested early passage MSCs were used in all subsequent
experiments.
HS injury rat model induction and
treatment
The HS damage model was established as described
previously (24,25). A total of 5 mature rats at a time
were kept at 40˚C and 40% humidity. The rectal temperature of rats
was continuously measured. When rectal temperatures reached 42˚C,
the general condition including hind limb weakness and lethargy of
the rats was observed. All rats were restored to room temperature
and were given a peritoneal injection of 5 ml saline. The HS injury
model rats were divided into control (HS) and study (HS + MSCs)
groups (n=40 per group). After the model was established
successfully, 2x106 MSCs suspended in 0.3 ml of saline
were injected into the HS+MSCs group and 0.3 ml of saline were
injected into the HS group via the tail vein. Rats in the HS and HS
+ MSCs groups were further divided into early (day 1), intermediate
(days 7 and 14) and late (day 28) groups (n=10 per group). Rats in
the normal control group (n=10) were not exposed to the high
temperature and injected with 0.3 ml of saline. All rats were
observed for 28 days following HS injury with or without MSCs
infusion to estimate survival rate.
Determination of the effects of
infused MSCs on cytokines and biochemical markers in rats with HS
injury
To detect the level of inflammatory factors [IL-1β,
IL-6, IL-10 and tumor necrosis factor-α (TNF-α)] and chemokines
[eotaxin and regulated upon activation normal T cell expressed and
secreted (Rantes)], blood (n=5 per group) and intestinal tissues
(n=5 per group) were collected on day 1 (early), days 7 and 14
(intermediate) and day 28 (late) following MSCs infusion and
following the rats being euthanized. The blood samples were
centrifuged at 2,000 x g for 10 min at 4˚C and the supernatant was
harvested. Brain samples were homogenized in a 10-fold volume of
cold PBS. The homogenate was centrifuged at 12,000 x g for 15 min
at 4˚C and the supernatant was kept at -80˚C until subsequent
measurement. Procarta Plex™ Analyst software (version 1.0;
eBioscience; Thermo Fisher Scientific, Inc.) was used to determine
the concentration of inflammatory factors and chemokines in blood
and tissue lysates. For biochemical determination, blood samples
were collected on day 1 (early), days 7 and 14 (intermediate), and
day 28 (late) following MSCs or saline infusion for every group.
Blood samples were centrifuged at 2,000 x g for 10 min at 4˚C and
the supernatant was collected for determination. The ALT (alanine
aminotransferase), AST (aspartate aminotransferase), ALP (alkaline
phosphatase), LDH (lactate dehydrogenase), CREA (creatinine) and UA
(uric acid) were determined using an automated biochemical analyzer
(7170-A; Hitachi, Ltd.).
Histological examinations
Intestinal tissue specimens were prepared by
perfusion fixation with 4% paraformaldehyde (Wuhan Servicebio
Technology Co., Ltd) at room temperature on days 1, 7, 14 and 28
following MSCs or saline infusion (n=3 in each group), until the
liver turned white, the samples were collected and eluted three
times using PBS. Intestinal tissues were dissected and immersed in
4% paraformaldehyde (Wuhan Servicebio Technology Co., Ltd.) for 12
h initially and then immersed in 30% sucrose solution for 24 h at
room temperature. The tissues were implanted in a cooled embedding
medium (optimal cutting temperature solution; Sakura Finetek USA,
Inc.). Following immediate freezing, tissues were cut using a
frozen section machine (Leica Microsystems) into 7 µm thick slices
for dyeing. The sections were stained with hematoxylin and eosin,
as in previous studies (26).
Stained sections were visualized and scanned using a Pannoramic
MIDI CaseViewer 2.0 System (3DHISTECH Ltd.). According to Chiu
et al (27), the degree of
injury to the intestinal tissues was evaluated, with each degree of
injury ranging from 0 to 5 points.
Statistical analysis
SPSS software (version 23.0; IBM Corp.) was used to
the analyze data. All experiments were conducted independently in
triplicate. Data is expressed as mean ± standard deviation. A
Kolmogorov Smirnov test was used to assess whether the data was
normally distributed, and a Levene test was used to analyze the
homogeneity of variance. ANOVA and Tukey's test was used for
continuous variables subject following data tests of normality and
equivariance. Kaplan-Meier method was used for survival analysis of
rats in each group. Otherwise, nonparametric statistical analysis
(Mann-Whitney U test) was conducted for Chiu's score of intestinal
tissue. P<0.05 was considered to indicate a statistically
significant difference.
Results
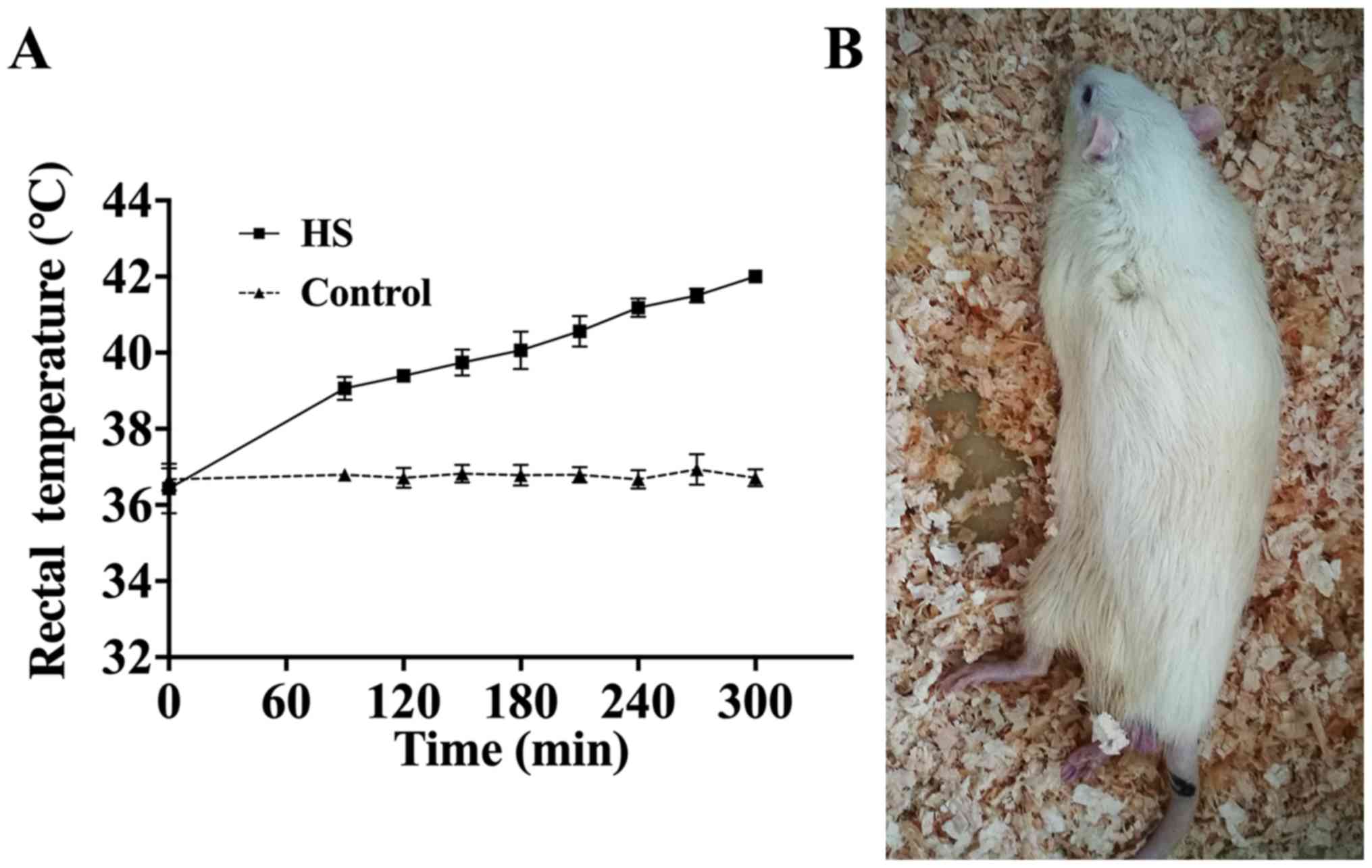
Establishment of the rat heat stroke
model
As reported by previous studies (22,23),
modeling was considered to be complete once the rats exhibited
symptoms of heat stress (including hind limb weakness and lethargy)
and when rectal temperatures reached 42˚C. Real time rectal
temperature changes in rats were monitored to identify a raised
temperature of 42̊C (Fig. 1A) and 80
rat models of heat stroke were obtained. The normal state of the
rats (body temperature 42̊C) after modeling is presented in
Fig. 1B.
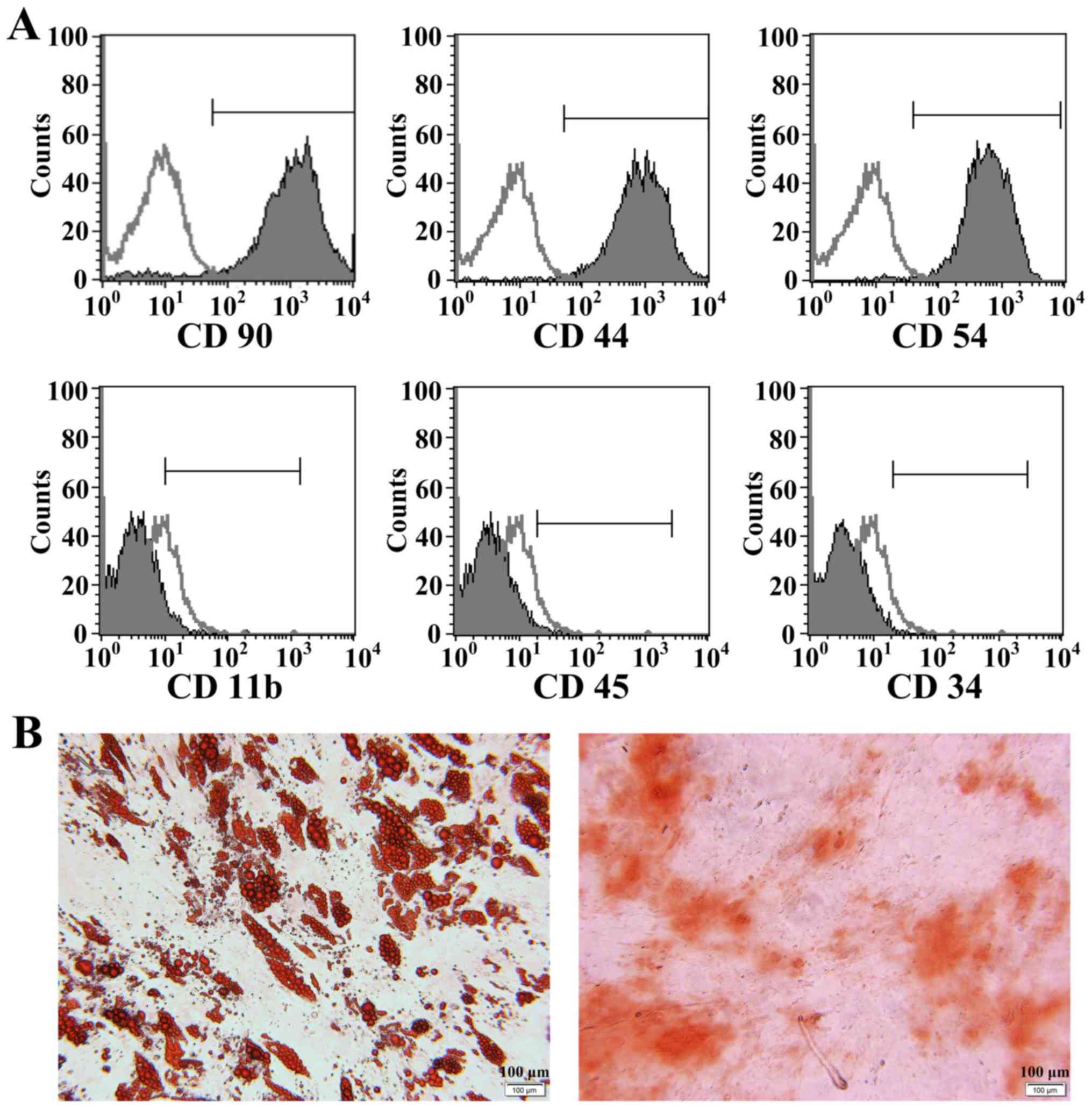
Identification of MSCs
MSCs injected into the rats were identified by flow
cytometry and differentiation ability. Following immunophenotyping,
these MSCs were positive for CD90, CD44 and CD54, and negative for
CD11b, CD45 and CD34 (Fig. 2A). Oil
Red O and Alizarin Red staining demonstrated that the cells
exhibited adipogenic and osteogenic differentiation (Fig. 2B).
Effects of MSCs on survival rate and
organ function
Rats underwent necessary rescue measures
simultaneously in the study (HS + MSCs) and control (HS) groups.
The results demonstrated in the study group, the time required for
rats to lower to normal body temperature was shorter (Fig. 3A) and mortality was significantly
reduced (Fig. 3B) compared with the
control group. Survival analysis indicated that the 28-day survival
rate of the study group was significantly higher compared with the
control group (Fig. 3C). At 24 h
post-infusion of MSCs, the levels of biochemical indicators alanine
aminotransferase (ALT), aspartate aminotransferase (AST), alkaline
phosphatase (ALP), lactate dehydrogenase (LDH), creatinine (CREA)
and uric acid (UA) in each group were detected. Compared with the
control group, the level of all biochemical indicators in the study
group were significantly improved, and the level of biochemical
indicators in the normal group was lower compared with the other
two groups (Fig. 3D-I).
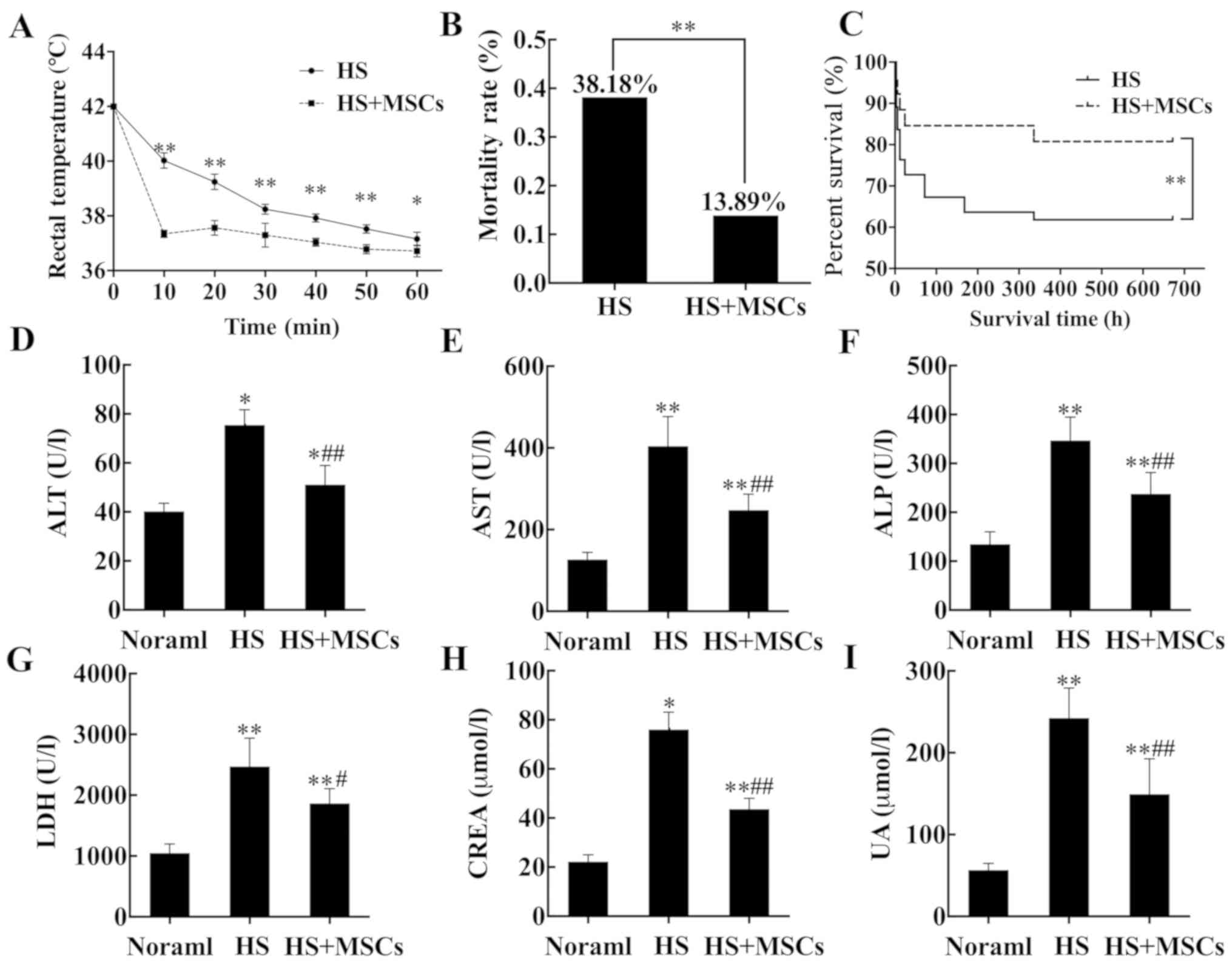 | Figure 3Cooling process, survival analysis
and detection of organ markers. (A) Following the establishment of
the model, rats were subjected to the temperature changes of rats
were monitored and recorded. (B) Mortality rates were compared
between the HS and HS + MSCs groups. (C) Survival analysis was
performed for 28 days. Serum markers reflecting organ functions
were measured, including (D) ALT, (E) AST, (F) ALP, (G) LDH, (H)
CREA and (I) UA (n=5) and the results were analyzed.
*P<0.05 and **P<0.01 vs. normal group.
#P<0.05 and ##P<0.01 vs. control group.
HS, heat stroke; MSCs, mesenchymal stem cells; ALT, alanine
aminotransferase; AST, glutamic oxalate aminotransferase; ALP,
alkaline phosphatase; LDH, lactate dehydrogenase; CREA, creatinine;
UA, uric acid. |
Effects of MSCs on inflammatory
factors and chemokines in blood
The levels on proinflammatory cytokines IL-1β, IL-6
and TNF-α were significantly decreased compared with the control
group (Fig. 4A, B and D) and
the level of pro-inflammatory factors in the study group was
obviously lower compared with the control group. TNF-α levels were
significantly decreased in the study group compared with the
control group (Fig. 4D).
Furthermore, the level of IL-10 in the study group was
significantly increased in a time-dependent manner compared with
the control group, approaching normal levels at day 28 (Fig. 4C). The blood level of Rantes in the
study group was lower at day 1 compared with the control group,
then increased gradually. By day 7 the level of Rantes was
significantly higher compared with the control group (Fig. 4E). Accordingly, the level of eotaxin
in the study group peaked on day 1, then gradually decreased on the
day 14, and increased gradually on day 28 (Fig. 4F). However, the overall level of
eotaxin remained markedly higher compared with the control group at
all subsequent time-points.
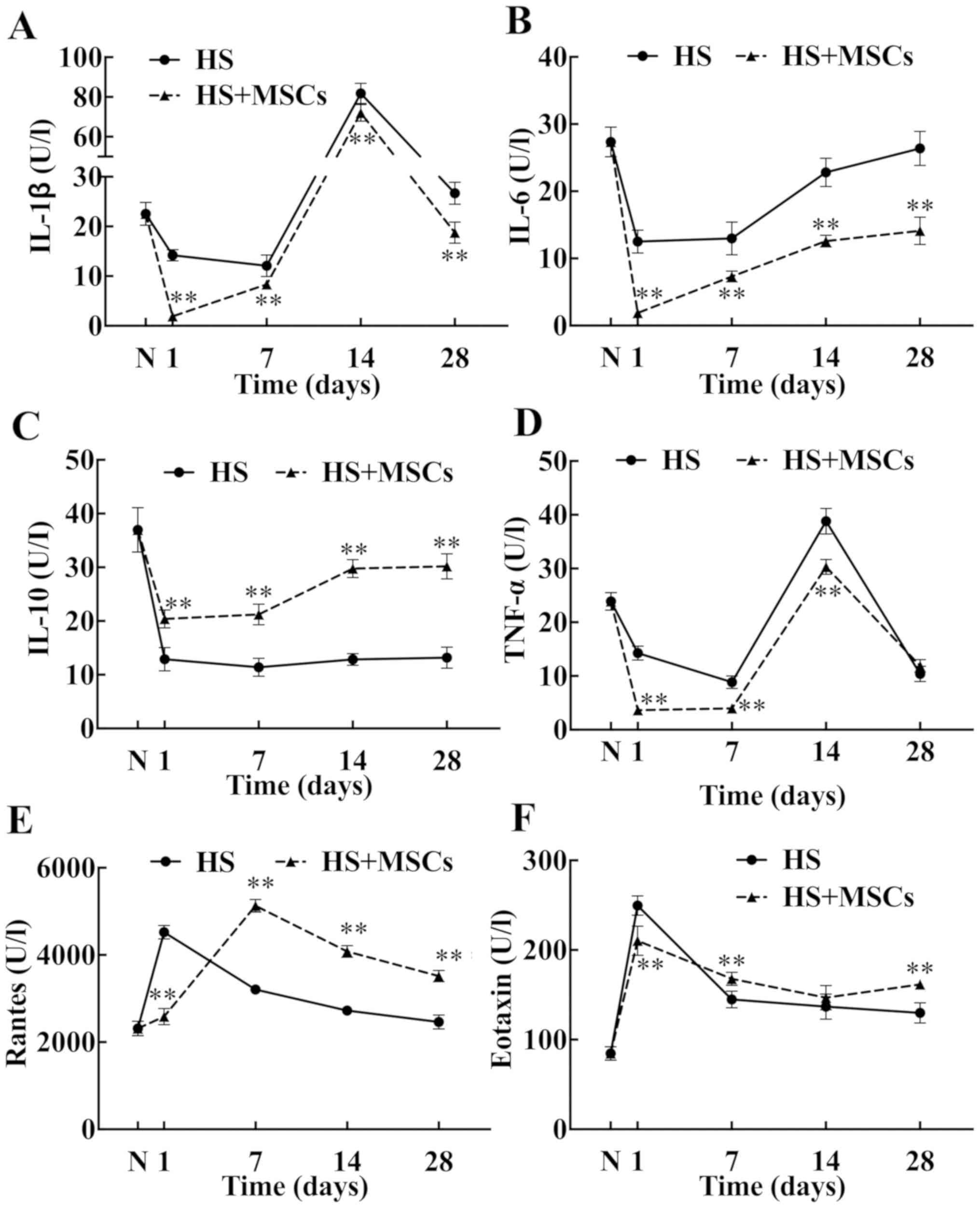 | Figure 4Inflammatory cytokines and chemokines
in blood. The levels of inflammatory cytokines and chemokines in
serum were measured on days 1, 7, 14 and 28 and plotted into a
broken-line graph. The proinflammatory cytokines tested included
(A) IL-1β, (B) IL-6 and (D) TNF-α (n=5), and the anti-inflammatory
cytokine tested was (C) IL-10 (n=5). The chemokines detected were
(E) Rantes (n=5) and (F) eotaxin (n=5). These factors reflected the
level of systemic inflammatory response. **P<0.01 vs.
control group. IL, interleukin; TNF-α, tumor necrosis factor-α;
Rantes, regulated upon activation normal T cell expressed and
secreted; HS, heat stroke; MSCs, mesenchymal stem cells; N, normal
group. |
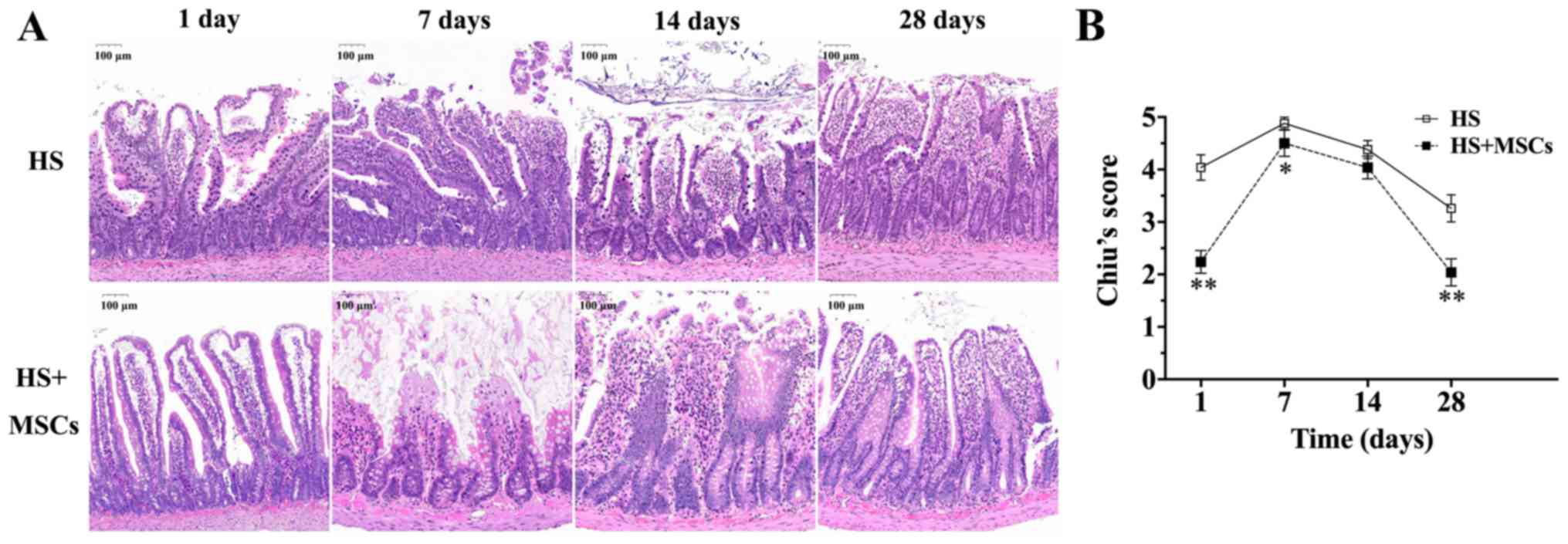
Intestinal histology
The pathological changes in the intestines of rats
in each group are presented in Fig.
5. Following the successful establishment of the control group,
numerous epithelial layers were separated from the lamina propria
on both sides of the small intestinal villi and parts of the top of
the villi were damaged. Additionally, there was an accumulation of
inflammatory cells beneath the endothelium in the control group
(Fig. 5A). According to Chiu's
scores, intestinal injury gradually worsened from day 1 to day 7
(Fig. 5B). The highest score was
4.88±0.12 at day 7 in the control group, after which pathological
injury gradually decreased. At day 28, Chiu's score was 3.26±0.26.
The trend of variation of intestinal pathological scores in the
study group were similar compared with the control group. However,
pathological damage in the study group was significantly improved
compared with the control group.
Effects of MSCs on inflammatory
factors and chemokines in intestinal tissues
The level of IL-1β in the study group was
significantly lower compared with the control group at the early
stage (day 1), but the level of IL-1β in the control group began to
decrease after day 1, and was significantly lower than that in the
study group on day 7, until day 28 (Fig.
6A). The levels of pro-inflammatory factors, including IL-6 and
TNF-α, in the intestinal tissues of the study group were
significantly lower compared with the control group (Fig. 6B and D). The trend of variation of IL-1β between
groups was similar (Fig. 6A);
however, the highest level of IL-1β in the study group was not
similar to that in the control group and reverted to a normal level
earlier than the control group. The difference in IL-6 and TNF-α
levels between groups was evident at the early stage; however, the
overall levels of both were lower in the study group compared with
the control group (Fig. 6B and
D). Furthermore, compared with the
control group, the levels of anti-inflammatory factor IL-10 in the
study group increased significantly at the early stage (day 1 and
day 7; Fig. 6C). Chemokines,
including Rantes and eotaxin, were detected in intestinal tissues
(Fig. 6E and F). The level of Rantes in the early stage
(day 1) in the study group was significantly higher compared with
the control group, but on the day 7 and 14, the levels of Rantes in
the study group were significantly lower compared with the control
group. Furthermore, while the trend of variation of eotaxin was
similar between groups, eotaxin levels in the study group was
markedly lower compared with the control group from day 1. The
results therefore indicated that the levels of Rantes and eotaxin
in the intestinal tissue were higher compared with the peripheral
blood.
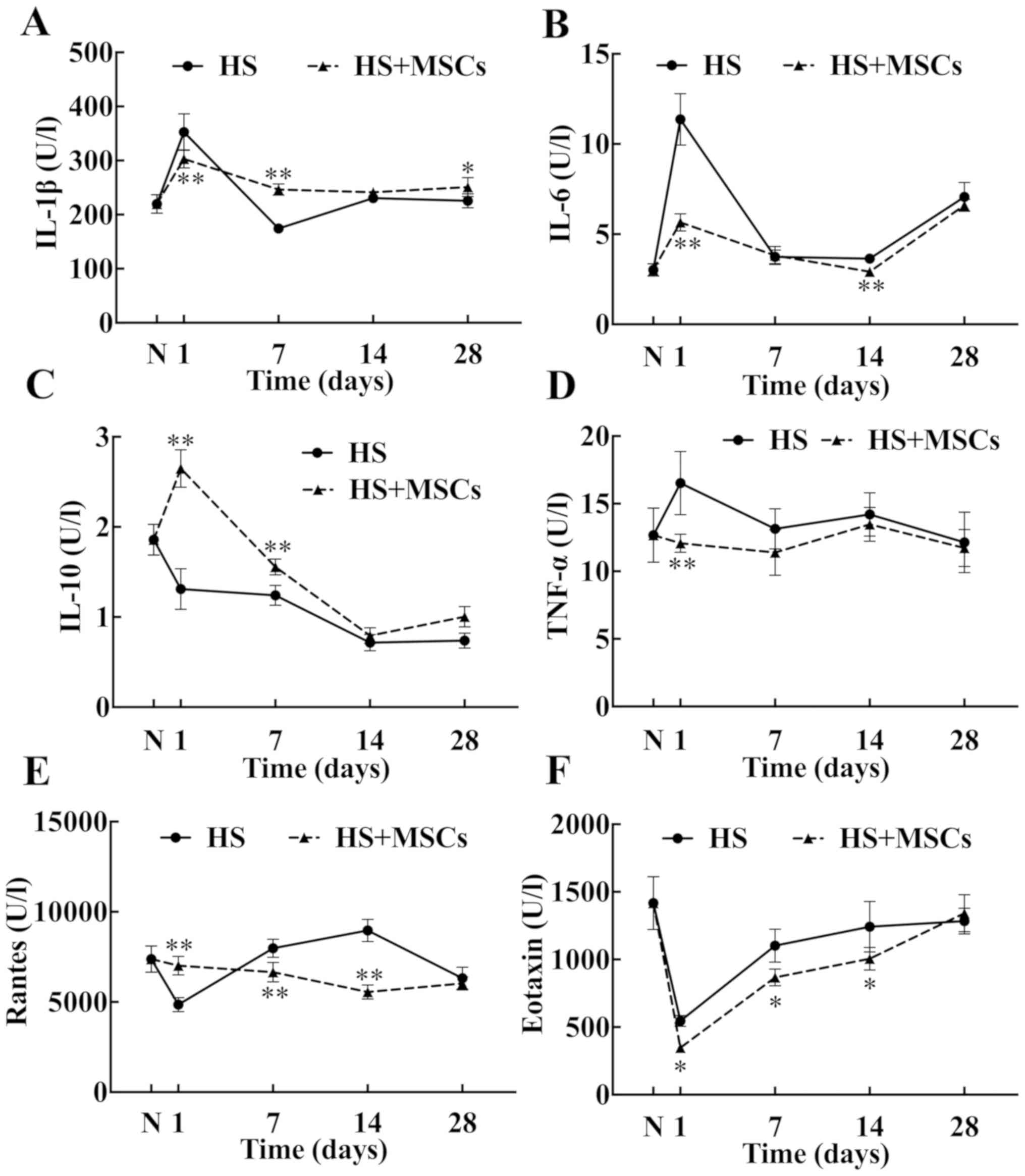 | Figure 6Inflammatory cytokines and chemokines
in the intestinal tissues. The levels of inflammatory cytokines and
chemokines in the intestinal tissues were measured on days 1, 7, 14
and 28 and plotted into a broken-line graph. The proinflammatory
cytokines tested were (A) IL-1β, (B) IL-6 and (D) TNF-α (n=5), and
anti-inflammatory cytokine tested was (C) IL-10 (n=5). The
chemokines detected were (E) Rantes (n=5) and (F) eotaxin (n=5).
These factors reflected the level of inflammatory response in
intestinal tissues. *P<0.05 and
**P<0.01 vs. control group. IL, interleukin; TNF-α,
tumor necrosis factor-α; Rantes, regulated upon activation normal T
cell expressed and secreted; HS, heat stroke; MSCs, mesenchymal
stem cells; N, normal group. |
Discussion
At present, it is hypothesized that the intestine is
the organ that initiates the systemic inflammatory response to heat
stroke (10). High heat stimulation
and intense exercise can lead to intestinal ischemia, intestinal
mucosal cells damage and intestinal mucosal barrier dysfunction
(28,29). In addition to tissue hypoperfusion
that is caused by hypotension, coagulation dysfunction also
increases intestinal permeability, especially in tight junctions
within the intestinal mucosal epithelium (28). Normal intestinal flora promotes
digestion and absorption, protects liver and cardiovascular
function (30), and improves iron
metabolism (31). However, the
destruction of the intestinal mucosal barrier causes intestinal
flora and endotoxins to enter into the circulation (4), resulting in a systemic inflammatory
response and MODS.
Hyperthermia can cause a decrease in intestinal
blood flow, damage to intestinal mucosa and loss of tight junction
integrity (10). Lambert et
al (32) observed that the
intestinal permeability of fluorescein isothiocyanate-glucose
increased in anesthetized rats with core temperatures of 42.5˚C,
and the pathological changes of the small intestine were clearer
than those in the colon. A study also demonstrated that
transmission electron microscopy revealed that intestinal
epithelial cells were damaged, microvilli were lost, tight
junctions were opened and mitochondria were swollen and vacuolated
(32). Similarly, the current study
demonstrated that following successful heat stroke modeling,
numerous epithelial layers were separated from the lamina propria
on both sides of the small intestinal villi and part of the tops of
the villi were damaged, accompanied by accumulation of inflammatory
cells beneath the endothelium. The results also revealed that the
damage to intestinal mucosa was the most serious in the heat shock
group at day 7 in the control group, with a Chiu's score of
4.88±0.12. The disruption of intestinal mucosal barrier function
leads to immense endotoxin entry into the circulation, which
increases the production and release of pro-inflammatory factors,
including IL-6, IL-1β and TNF-α, activates endothelial cells,
stimulates the release of endothelial activating factors and
induces local or systemic inflammation (33). The results of the current study
demonstrated that the levels of IL-1β, IL-6 and TNF-α in intestinal
tissues of the rats in the control group increased to varying
degrees at day 1 following successful modeling.
MSCs have numerous functions, including regulating
immune function and repairing tissue regeneration (6,9). MSCs
used in the current study were positive for CD90, CD44 and CD54,
negative for CD11b, CD45 and CD34. Oil red O and alizarin red
staining showed that the cells had adipogenic and osteogenic
differentiation. A previous study has demonstrated that human
umbilical cord blood stem cells improved the prognosis of heat
stroke by reducing circulatory shock, improving brain injury and
regulating inflammatory response (34). Similarly, a previous study has
demonstrated that the application of human umbilical cord blood
stem cells increased IL-10 levels and reduce TNF-α levels in the
serum of mice with HS (18).
Furthermore, a previous study has demonstrated that by using
peripheral circulation measurement, MSCs improved the
anti-inflammatory effect in sepsis to a certain degree, improve
function of organs, including the lungs and kidneys, reduce the
protein expression of inflammatory biomarkers and ultimately
reduced the mortality of sepsis rats (35). Additionally, Weil et al
(36) revealed that MSCs treatment
prominently reduced the level of endotoxin-induced left ventricular
myocardial inflammation biomarkers. In the current study, mortality
statistics and survival analysis revealed that MSCs significantly
improve the survival rate of HS rats. HS can cause multiple organ
failure (2). The levels of certain
organ markers were examined and the results reported that the
levels of ALT, AST, ALP, LDH, CERA and UA in MSCs-treated rats
decreased significantly, indicating that MSCs exhibited an
effective protective effect on the liver and kidneys.
As the largest direct barrier between the
environment and the host environment, gastrointestinal mucosa
serves a key role in the regulation of immune system and the
acquisition of tolerance against dietary antigens and the
intestinal microbiota (37).
Intestinal mucosal barriers allow nutrient uptake and immune
surveillance, while limiting the transport of potentially harmful
antigens and microorganisms (37).
Intestinal integrity and immune homeostasis can be maintained by
the dynamic regulation of intestinal mucosal structure and
molecular interactions (37). The
main pathophysiological mechanism of sepsis and HS is the
recruitment of immune cells to produce an overwhelming immune
response (2). Pathogen-associated
molecular patterns, such as lipopolysaccharides, peptidoglycan and
bacterial DNA, as well as damage-related molecular patterns
(DAMPs), including mitochondrial DNA, high-mobility histone B1 and
serum amyloid A, are upregulated by the recruitment of neutrophils
and macrophages (38). As a first
line of defense, cells migrate to intestinal tissue and produce
proinflammatory cytokines, which are clinically typical of local
and systemic inflammation (39,40).
Additionally, apoptosis and necrosis damage mucosal epithelium and
lead to a cycle of DAMP release, resulting in increased
inflammation and an imbalance of mucosal homeostasis (41). Therefore, normal intestinal function
is crucial, and the destruction of the intestinal mucosal barrier
causes bacterial translocation, leading to serious systemic
inflammatory response (37).
Histological staining was performed on the intestinal tissues of
rats and evaluated using Chiu's score. The results revealed that
the condition of intestinal mucosa improved in the early stage (day
1 following model establishment), which may be related to the
self-repair function of intestinal mucosa; however, the rapid
release of inflammatory factors aggravated mucosal damage.
Furthermore, the intestinal scores of HS rats treated with MSCs on
day 1, 7, 14 and 28 were significantly lower than that of the
control group. Therefore, MSCs was indicated to significantly
improve intestinal mucosal damage and protect intestinal barrier
function.
Inflammatory factors and chemokines in intestinal
tissue and peripheral blood were detected in the study and control
groups. The results of intestinal tissue demonstrated that the
levels of pro-inflammatory factors in the intestine were elevated
due to local inflammation. However, in the early stages, the levels
of pro-inflammatory factors in the study group were significantly
lower compared with the control group, indicating that MSCs
inhibited local inflammation. Levels of pro-inflammatory factors in
peripheral blood were decreased, which may be associated with the
inflammation of pro-inflammatory factors in tissues. However,
levels of pro-inflammatory factors were decreased following MSCs
treatment compared with the control group, indicating that MSCs
also inhibited systemic inflammation. The level of
anti-inflammatory factors IL-10 in the intestinal tissues differed
from levels in peripheral blood. In intestinal tissues, IL-10
levels decreased in the control group, compared with the study
group, indicating that MSCs promoted the local release of
anti-inflammatory factors. Furthermore, IL-10 levels in the
peripheral blood of both groups decreased; however, levels in the
study group were significantly higher compared with the control
group, indicating that MSCs promoted the systemic release of
anti-inflammatory factors. This implied that MSCs may serve an
anti-inflammatory role by increasing the level of anti-inflammatory
factors. Additionally, the levels of two chemokines, Rantes and
eotaxin, in intestinal tissue and peripheral blood were measured,
were detected and the results indicated that their levels in
intestinal tissues were significantly higher compared with levels
in the peripheral blood, indicating that chemokines may serve a
more significant role in local tissues. In intestinal tissues, the
level of eotaxin in the study group was significantly lower
compared with the control group on the days 1, 7 and 14. An
increase in eotaxin levels in the later stage was associated with
its main role in the acute phase of inflammation. The level of
Rantes in the study group decreased, which may be associated with
the role of Rantes in the middle and late stages of inflammation.
Therefore, it may be concluded that MSC treatment significantly
reduced chemokine levels, thereby inhibiting local tissue
inflammation.
The current study demonstrated that MSCs have a
positive protective effect on intestinal damage caused by HS.
Combined with the results of previous studies, the present study
hypothesized that MSCs may play a role in intestinal protection
through the following aspects: As macrophages are key cells in a
variety of intestinal inflammatory diseases, MSCs secrete
macrophage-regulating molecules, including indoleamine
2,3-dioxygenase, TGF-β and PGE2, which further stimulate
macrophages to produce IL-10 by stimulating the production of
PGE2(42). Toll-like receptors
(TLRs) are activated by intestinal-derived bacterial products and
immune cells when the body is damaged (43). The immunomodulatory effects of MSCs
activate TLRs and regulate the metallothionein 1-matrix
metallopeptidase/janus kinase/signal transduced and activator of
transcription 3 signaling pathway induced by TLR-2/6 targeting
neovascularization (44). TLR4
activates the endotoxin-induced innate immune system and the NF-κB
pathway, further stimulating the expression of pro-inflammatory
factors (45). MSCs reduce the level
of TLR4, leading to the reduction of pro-inflammatory factors and
alleviating intestinal inflammatory response (46). A previous study has demonstrated that
MSCs inhibited the proliferation and activation of cluster of
differentiation (CD)4+ T cells and the differentiation
of CD4+ T cells into Th1 and Th17 cells (47). These inhibitory effects are
associated with the increased secretion of CD4+CD25 + forkhead box
(Fox) p3 + regulatory T cells (Treg) and IL-10(47). A previous study on acute kidney
injury reported that MSCs therapy effectively reduced the
expression of IL-17-related chemokines in serum and kidney tissues,
as well as the infiltration of renal neutrophils (48). This is consistent with the results of
the intestinal tissues assessed in the current study. Furthermore,
the previous study demonstrated that MSCs restored the balance
between Th17 cells and CD4 + CD25 + Foxp3 + Treg cells in
intestinal tissues (48). Therefore,
the current study hypothesized that MSCs affected the
differentiation and expression of T cells, thereby regulating local
and systemic immune function. Additionally, previous studies have
revealed that MSCs may activate the Wingless-related integration
site/β-catenin signaling pathway, which is required for G-protein
coupled receptor (LGR) 5+ cell proliferation through
secretory factors (12),
significantly stimulate intestinal epithelial cell proliferation,
increase the number of LGR5+ intestinal stem cells and
increase intestinal angiogenesis, thus serving a protective role in
intestinal mucosa and promoting injury repair (49).
The current study proposed the use of MSCs for the
treatment of HS and reported that MSCs effectively alleviated the
inflammatory response of the whole body and local tissue, and
protected the intestinal mucosal barrier. The present study
demonstrated the anti-inflammatory and protective effects of MSCs
on organs and provided new theories and methods for the treatment
of HS, which are required to improve the prognosis of patients with
HS. However, the signaling pathways involved in the regulation of
the inflammatory response and the protection of organ function via
MSCs in HS was not elucidated and subsequent experiments are
required. Additionally, currently, there are also no uniform
criteria for assessing the timing or dose of MSCs treatment and
further studies will need to be conducted to determine these.
In conclusion, the current study demonstrated that
MSCs reduced the levels of pro-inflammatory factors, regulated
immune status and alleviated intestinal injury in rats with HS.
Therefore, MSCs improved the systemic inflammatory response and
tissue damage caused by HS and ultimately reduced the mortality of
rats with HS.
Acknowledgements
Not applicable.
Funding
HK received a grant from the National Natural
Science Foundation of China (grant no. 81671966), the Beijing
Municipal Natural Science Foundation (grant no. 7182155) and the
Application Research and Achievement Extension of Clinical
Characteristics in Chinese Capital Foundation (grant no.
Z171100001017160).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HK designed the current study, raised funds and
revised the manuscript. LW conducted experiments, data collection
and analysis and wrote the manuscript. ZD performed certain
experiment and provided guidance for data analysis. RY, YanZ and MY
performed certain data collection and analysis. YL, YuZ and JH
conducted certain experiments. FZ designed the study and supervised
the research. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The current research complied with the statement of
relevant ethical standards (the Animal Research: Reporting of in
vivo Experiments reporting guidelines) (20) and was approved by the Ethics
Committee of the Chinese PLA General Hospital (approval no.
2017-X13-10).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Alan N, Peiris SJ and Rabiya N: Heat
Stroke. JAMA. 318(2503)2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Epstein Y and Yanovich R: Heatstroke. N
Engl J Med. 380:2449–2459. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Haussner F, Chakraborty S, Halbgebauer R
and Huber-Lang M: Challenge to the intestinal mucosa during sepsis.
Front Immunol. 10(891)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lim CL and Mackinnon LT: The roles of
exercise-induced immune system disturbances in the pathology of
heat stroke the dual pathway model of heat stroke. Sports Med.
36:39–64. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kalladka D and Muir KW: Brain repair: Cell
therapy in stroke. Stem Cells Cloning. 7:31–44. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mishra VK, Shih HH, Parveen F, Lenzen D,
Ito E, Chan TF and Ke LY: Identifying the therapeutic significance
of mesenchymal stem cells. Cells. 9(1145)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang Y, Chen X, Cao W and Shi Y:
Plasticity of mesenchymal stem cells in immunomodulation:
Pathological and therapeutic implications. Nat Immunol.
15:1009–1016. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Park HJ, Kim J, Saima FT, Rhee KJ, Hwang
S, Kim MY, Baik SK, Eom YW and Kim HS: Adipose-derived stem cells
ameliorate colitis by suppression of inflammasome formation and
regulation of M1-macrophage population through prostaglandin E2.
Biochem Biophys Res Commun. 498:988–995. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fu X, Liu G, Halim A, Ju Y, Luo Q and Song
AG: Mesenchymal stem cell migration and tissue repair. Cells.
8(784)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Pires W, Veneroso CE, Wanner SP, Pacheco
DAS, Vaz GC, Amorim FT, Tonoli C, Soares DD and Coimbra CC:
Association between exercise-induced hyperthermia and intestinal
permeability: A systematic review. Sports Med. 47:1389–1403.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang W, Shen ZY, Song HL, Yang Y, Wu BJ,
Fu NN and Liu T: Protective effect of bone marrow mesenchymal stem
cells in intestinal barrier permeability after heterotopic
intestinal transplantation. World J Gastroenterol. 20:7442–7451.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gong W, Guo M, Han Z, Wang Y, Yang P, Xu
C, Wang Q, Du L, Li Q, Zhao H, et al: Mesenchymal stem cells
stimulate intestinal stem cells to repair radiation-induced
intestinal injury. Cell Death Dis. 7(e2387)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Te Winkel J, John QE, Hosfield BD, Drucker
NA, Das A, Olson KR and Markel TA: Mesenchymal stem cells promote
mesenteric vasodilation through hydrogen sulfide and endothelial
nitric oxide. Am J Physiol Gastrointest Liver Physiol.
317:G441–G446. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Davey GC, Patil SB, O'Loughlin A and
O'Brien T: Mesenchymal stem cell-based treatment for microvascular
and secondary complications of diabetes mellitus. Front Endocrinol
(Lausanne). 5(86)2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tobin MK, Bonds JA, Minshall RD,
Pelligrino DA, Testai FD and Lazarov O: Neurogenesis and
inflammation after ischemic stroke: What is known and where we go
from here. J Cereb Blood Flow Metab. 34:1573–1584. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Penha EM, Meira CS, Guimaraes ET, Mendonça
MV, Gravely FA, Pinheiro CM, Pinheiro TM, Barrouin-Melo SM,
Ribeiro-Dos-Santos R and Soares MB: Use of autologous mesenchymal
stem cells derived from bone marrow for the treatment of naturally
injured spinal cord in dogs. Stem Cells Int.
2014(437521)2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hsuan YC, Lin CH, Chang CP and Lin MT:
Mesenchymal stem cell-based treatments for stroke, neural trauma,
and heat stroke. Brain Behav. 6(e00526)2016.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Tseng L, Chen S, Lin M and Lin Y:
Umbilical cord blood-derived stem cells improve heat tolerance and
hypothalamic damage in heat stressed mice. Biomed Res Int.
2014(685683)2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang XM, Zhang YJ, Wang W, Wei YQ and
Deng HX: Mesenchymal stem cells to treat Crohn's disease with
fistula. Hum Gene Ther. 28:534–540. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kilkenny C, Browne WJ, Cuthill IC, Emerson
M and Altman DG: Improving bioscience research reporting: The
ARRIVE guidelines for reporting animal research. PLoS Biol.
8(e1000412)2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
NIH (National Institutes of Health U.S.A).
Guide for the Care and Use of Laboratory Animals. The National
Academies Press, Washington, D.C, pp246, 2011.
|
|
22
|
Si Y, Zhao Y, Hao H, Liu J, Guo Y, Mu Y,
Shen J, Cheng Y, Fu X and Han W: Infusion of mesenchymal stem cells
ameliorates hyperglycemia in type 2 diabetic rats identification of
a novel role in improving insulin sensitivity. Dibaetes.
61:1616–1625. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Deng Z, Xu H, Zhang J, Yang C, Jin L, Liu
J, Song H, Chen G, Han W and Si Y: Infusion of adiposederived
mesenchymal stem cells inhibits skeletal muscle mitsugumin 53
elevation and thereby alleviates insulin resistance in type 2
diabetic rats. Mol Med Rep. 17:8466–8474. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ji J, Gu Z, Li H, Su L and Liu Z:
Cryptdin-2 predicts intestinal injury during heatstroke in mice.
Int J Mol Med. 41:137–146. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Phillips NA, Welc SS, Wallet SM, King MA
and Clanton TL: Protection of intestinal injury during heat stroke
in mice by interleukin-6 pretreatment. J Physiol. 593:739–753.
2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Deng ZH Jr, Yan GT, Wang LH, Zhang JY, Xue
H and Zhang K: Leptin relieves intestinal ischemia/reperfusion
injury by promoting ERK1/2 phosphorylation and the NO signaling
pathway. J Trauma Acute Care Surg. 72:143–149. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chiu CJ, McArdle AH, Brown R, Scott HJ and
Gurd FN: Intestinal mucosal lesion in low-flow States. I. A
morphological, hemodynamic, and metabolic reappraisal. Arch Surg.
101:478–483. 1970.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Dokladny K, Moseley PL and Ma TY:
Physiologically relevant increase in temperature causes an increase
in intestinal epithelial tight junction permeability. Am J Physiol
Gastrointest Liver Physiol. 290:G204–G212. 2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Pals KL, Chang RT, Ryan AJ and Gisolfi CV:
Effect of running intensity on intestinal permeability. J Appl
Physiol. 82:571–576. 1997.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Skrypnik K, Bogdański P, Łoniewski I,
Reguła J and Suliburska J: Effect of probiotic supplementation on
liver function and lipid status in rats. Acta Sci Pol Technol
Aliment. 17:185–192. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Skrypnik K, Bogdanski P, Schmidt M and
Suliburska J: The effect of multispecies probiotic supplementation
on iron status in rats. Biol Trace Elem Res. 192:234–243.
2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lambert GP, Gisolfi CV, Berg DJ, Moseley
PL, Oberley LW and Kregel KC: Selected Contribution:
Hyperthermia-induced intestinal permeability and the role of
oxidative and nitrosative stress J Appl. Physiol. 92:1750–1761.
2002.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yan YE, Zhao YQ, Wang H and Fan M:
Pathophysiological factors underlying heatstroke. Med Hypotheses.
67:609–617. 2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu WS, Chen CT, Foo NH, Huang HR, Wang
JJ, Chen SH and Chen TJ: Human umbilical cord blood cells protect
against hypothalamic apoptosis and systemic inflammation response
during heatstroke in rats. Pediatr Neonatol. 50:208–216.
2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chang C, Leu S, Sung HC, Zhen YY, Cho CL,
Chen A, Tsai TH, Chung SY, Chai HT, Sun CK, et al: Impact of
apoptotic adipose-derived mesenchymal stem cells on attenuating
organ damage and reducing mortality in Rat sepsis syndrome induced
by cecal puncture and ligation. J Transl Med.
10(244)2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Weil BR, Manukyan MC, Herrmann JL, Wang Y,
Abarbanell AM, Poynter JA and Meldrum DR: Mesenchymal stem cells
attenuate myocardial functional depression and reduce systemic and
myocardial inflammation during endotoxemia. Surgery. 148:444–452.
2010.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Romero ES, Cotoner CA, Camacho CP, Bedmar
MC and Vicario M: The intestinal barrier function and its
involvement in digestive disease. Rev Esp Enferm Dig. 107:686–696.
2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Bianchi ME: DAMPs, PAMPs and alarmins all
we need to know about danger. J Leukoc Biol. 81:1–5.
2007.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Nascimento LO, Massari P and Wetzler LM:
The role of TLR2 in infection and immunity. Front Immunol.
3(79)2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Schnoor M, Garcia Ponce A, Vadillo E,
Pelayo R, Rossaint J and Zarbock A: Actin dynamics in the
regulation of endothelial barrier functions and neutrophil
recruitment during endotoxemia and sepsis. Cell Mol Life Sci.
74:1985–1997. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Jiang LY, Zhang M, Zhou TE, Yang ZF, Wen
LQ and Chang JX: Changes of the immunological barrier of
intestinalmucosa in rats with sepsis. World J Emerg Med. 1:138–143.
2010.PubMed/NCBI
|
|
42
|
Németh K, Leelahavanichkul A, Yuen PS,
Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller
BH, Brown JM, et al: Bone marrow stromal cells attenuate sepsis via
prostaglandin E(2)-dependent reprogramming of host macrophages to
increase their interleukin-10 production. Nat Med. 15:42–49.
2009.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Yiu JH, Dorweiler B and Woo CW:
Interaction between gut microbiota and toll-like receptor: From
immunity to metabolism. J Mol Med (Berl). 95:13–20. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zgheib A, Pelletier-Bonnier E, Levros LCJ
and Annabi B: Selective JAK/STAT3 signalling regulates
transcription of colony stimulating factor-2 and -3 in
Concanavalin-A-activated mesenchymal stromal cells. Cytokine.
63:187–193. 2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Poltorak A, He X, Smirnova I, Liu MY, Van
Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al:
Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations
in Tlr4 gene. Science. 282:2085–2088. 1998.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Niu GC, Liu L, Zheng L, Zhang H, Shih DQ
and Zhang X: Mesenchymal stem cell transplantation improves chronic
colitis-associated complications through inhibiting the activity of
toll-like receptor-4 in mice. BMC Gastroenterol.
18(127)2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Crawford PL, Kurte M, Bravo-Alegría J,
Contreras R, Nova-Lamperti E, Tejedor G, Noël D, Jorgensen C,
Figueroa F, Djouad F and Carrión F: Mesenchymal stem cells generate
a CD4+CD25+Foxp3+ regulatory T cell population during the
differentiation process of Th1 and Th17 cells. Stem Cell Res Ther.
4(65)2013.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Luo CJ, Zhang FJ, Zhang L, Geng YQ, Li QG,
Hong Q, Fu B, Zhu F, Cui SY, Feng Z, et al: Mesenchymal stem cells
ameliorate sepsis-associated acute kidney injury in mice. Shock.
41:123–129. 2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Soontararak S, Chow L, Johnson V, Coy J,
Wheat W, Regan D and Dow S: Mesenchymal stem cells (MSC) derived
from induced pluripotent stem cells (iPSC) equivalent to
adipose-derived MSC in promoting intestinal healing and microbiome
normalization in mouse inflammatory bowel disease model. Stem Cells
Transl Med. 7:456–467. 2018.PubMed/NCBI View Article : Google Scholar
|