Introduction
Coronary artery disease (CAD) is a frequent
cardiovascular disease that is mainly caused by coronary
atherosclerosis. It may result in arterial blood vessel stenosis,
blockage or spasm, further leading to myocardial ischemia and
hypoxia and/or a series of lesions (1-3).
CAD may also cause further conditions, including angina pectoris,
arrhythmia, heart failure or sudden death (4,5).
Therefore, early diagnosis and treatment of CAD are of high
clinical significance. In recent years, as medicine, science and
technologies continue to progress and imaging technologies develop
rapidly, the spatial and temporal resolution of CT scanning, which
is widely used in the clinical diagnosis of CAD, have been markedly
improved (6-8).
Adipose tissue, which is now considered as an
endocrine organ, secretes various fat factors to affect the
metabolism and certain pathological processes, including
inflammation, oxidative stress and apoptosis (9,10). Among
adipocytokines, visfatin may be involved in the development of
obesity-associated diseases, including diabetes and cardiovascular
disease. Adiponectin is considered as an important anti-atherogenic
and anti-diabetic protein and also as an anti-inflammatory protein
(9). In recent years, the
association between epicardial adipose tissue (EAT) and
atherosclerosis has attracted increasing attention (11,12). EAT
is a specialized energy storage organ located between the
myocardium and epicardium, which serve to provide energy (13-15).
Previous studies have reported that EAT potentially causes local
inflammation and may be seen as a marker for the risk of
cardiovascular disease (16).
Studies have indicated that EAT is an important risk factor for
atherosclerotic diseases and metabolic syndrome (17,18). The
correlation between EAT and CAD has been previously reported;
however, it is controversial whether EAT may be regarded as a
marker to predict the occurrence and development of CAD (19-21).
Therefore, in the present study, the epicardial fat volume (EFV)
was measured by CT scanning to explore the correlation between the
EFV and coronary artery lesions and left ventricular function, so
as to provide a theoretical basis for the diagnosis of clinical
CAD.
Patients and methods
Patient population
The present study was approved by the Medical Ethics
Committee of The Affiliated Changzhou No. 2 People's Hospital of
Nanjing Medical University (Changzhou, China) and all patients
volunteered to participate in the present study and provided
written informed consent. A total of 61 patients with known or
suspected CAD who received cardiac CT scanning and coronary
angiography at Changzhou No. 2 People's Hospital, the Affiliated
Hospital of Nanjing Medical University (Changzhou, China) between
December 2017 and October 2018 were enrolled. The inclusion
criterion was as follows: One or more major coronary arteries with
a diameter stenosis of ≥50%. Patients with iodine contrast agent
allergy, arrhythmias and hepatic and/or renal insufficiency were
excluded. Those subjects who previously had a coronary artery
operation or coronary bypass surgery, or those with respiratory
diseases who cannot hold their breath for >10 sec were also
excluded. The treatment of these patients mainly included
administration of anti-hypertension drugs (amlodipine tablets,
furosemide tables, spironolactone), statin drugs (pravastatin
tablets, atorvastatin calcium tablets, rosuvastatin calcium
tablets), anti-platelet agglutination drugs (aspirin enteric-coated
tablet, clopidogrel hydrogen sulphate tablets) and nitrates
(nitroglycerin tablet).
Data collection
Prior to CT scanning, the age, gender, family
history of CAD, history of myocardial infarction, as well as
smoking and drinking status of all participants were recorded. The
body height and weight of the participants were measured to
calculate the body mass index (BMI) and body surface area (BSA) as
follows: BMI (kg/m2) = body weight (kg)/height
(m)2, BSA (m2) = 0.006 x height (cm) + 0.013
x body weight (kg)-0.153. A catheter-tipped micromanometer
(SPC-454D, Millar Instrument Co.) and a polygraph system (RMC-2000;
Nihon Kohden, Inc.), were used to measure and record the pulse
pressure, systolic blood pressure (SBP) and diastolic blood
pressure (DBP). Hypertension was defined as SBP ≥140 mmHg and/or
DBP ≥90 mmHg or use of anti-hypertensive drugs. An automatic
biochemistry analyzer (7060; Hitachi) was used to determine the
levels of low-density lipoprotein-cholesterol (LDL-C), high-density
lipoprotein-cholesterol (HDL-C), total cholesterol (TC) and fasting
blood glucose (FBG). Dyslipidemia was defined as TC >200 mg/dl,
LDL-C ≥130 mg/dl and HDL-C <40 mg/dl for males or HDL-C <50
mg/dl for females or those who used lipid-lowering medications.
Diabetes mellitus was defined as FBG ≥126 mg/dl or treatment with
hypoglycemic drugs.
Instruments and scanning methods
The CT examination equipment used in the present
study was a 64-slice spiral CT scanner (SOMATOM Definition; Siemens
AG) and the scanning parameters were as follows: Tube voltage, 120
kV; tube current, 90-160 mA; thread pitch, 0.2; slice thickness,
0.60-1.25 mm; reconstruction thickness and reconstruction interval,
5 mm. As the contrast media, Ultravist (370 mg I/ml; Schering AG)
and Omnipaque (350 mgI/ml; GE Healthcare Ltd) at a volume of 60-80
ml (scaled to body weight) was injected at 4.5-5.0 ml/sec. After
the injection, 40 ml normal saline was injected at the same flow
rate.
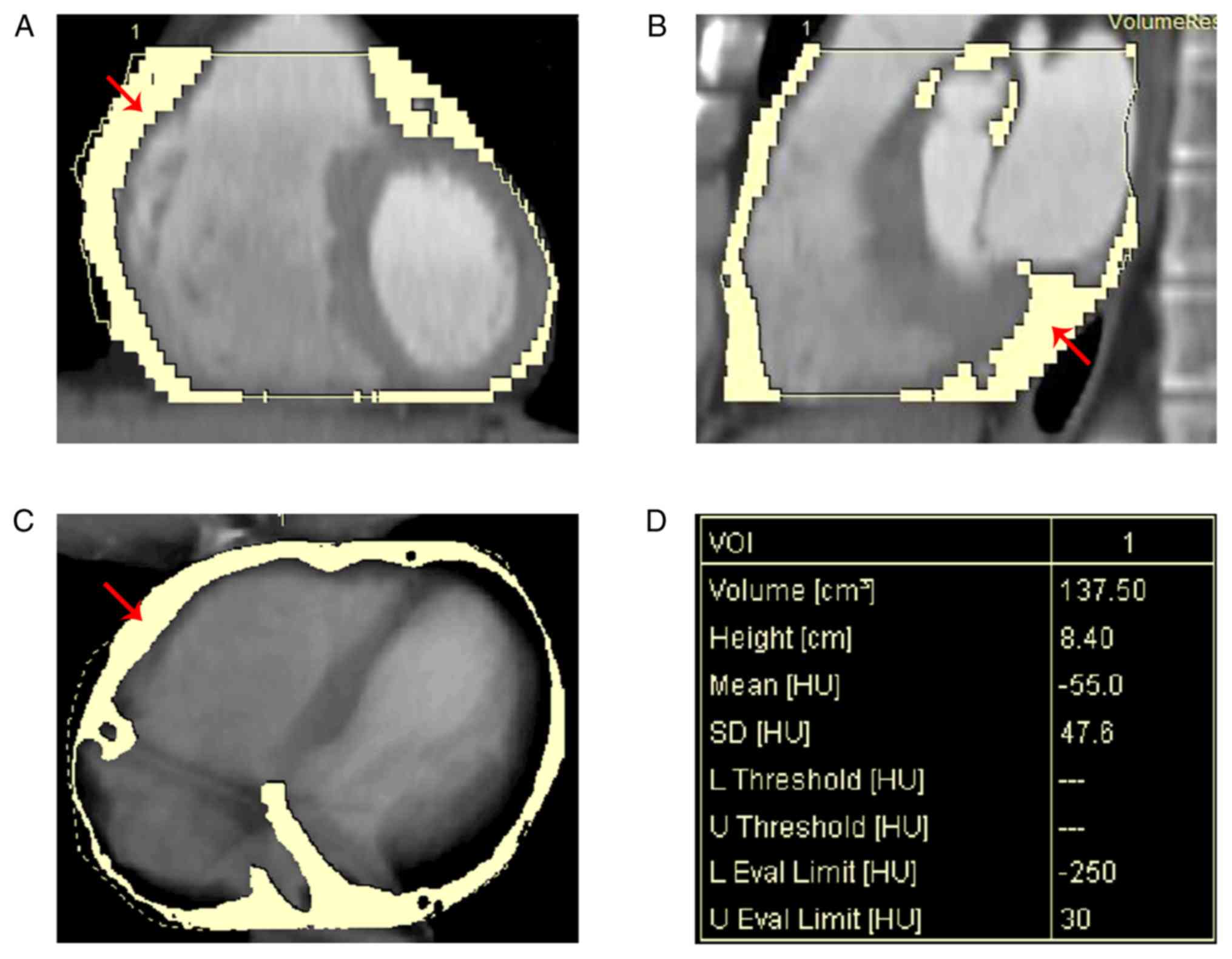
Measurement of EFV
After the scanning, the EFV data were analyzed using
the Volume Viewer program (version 1.2.0.0) of the semi-automatic
off-line workstation (Simens AG). The measurements were performed
by two experienced radiologists who were blinded regarding the
clinical condition of the patients (presence or absence of CAD).
The pericardium was manually traced from the bifurcation of the
pulmonary trunk to the last section containing any images of the
heart to obtain a region of interest (ROI). The Hounsfield units
(HU) threshold of -190 to -30 HU was used to isolate the fat
content inside the ROI. The threshold value was visually adjusted
by the radiologists to ensure that all adipose tissue in the
pericardium was included (Fig.
1).
Assessment of the severity of CAD
Coronary angiography was performed on the patients
by 64-slice multislice spiral CT. The degree of coronary artery
stenosis was evaluated according to the coronary artery image
evaluation criteria of the American Heart Association (22). The severity of CAD was based on the
evaluation criteria of the Gensini scoring system (23) and analyzed by two experienced
radiologists; a consensus was reached through discussion in the
event of any disagreement. The degree of coronary artery stenosis
was divided into groups of 25, 50, 75, 90, 99 and 100%, with
corresponding scores of 1, 2, 4, 8, 16 and 32, respectively. The
coronary arteries in different segments were multiplied by the
corresponding coefficients as follows: Score x5 for left major
disease; score x2.5 for left anterior descending (LAD) proximal
segment; score x1.5 for LAD middle segment; score x1 for LAD distal
segment; score x1 for first diagonal branch; score x0.5 for second
diagonal branch; score x2.5 for left circumflex artery (LCX)
proximal segment, score x1 for LCX distal segment and posterior
descending branch; score x0.5 for posterior lateral artery; score
x1 for right coronary artery proximal, middle and distal segment
and posterior descending branch. The final total integral was the
sum of the integral of lesion branch, with a higher score
indicating a more serious lesion degree.
Detection of left ventricular
function
The participants underwent a complete transthoracic
echocardiographic examination using the GE Vingmed System V (GE
Healthcare) with a 3.5-MHz phased-array probe. Three consecutive
cycles were averaged for each parameter and analyzed by two
cardiologists blinded to the data of the participants. Left
ventricular end diastolic diameter (LVEDD), interventricular septal
thickness (IVS), left ventricular posterior wall thickness (LVPW),
left ventricular mass (LVM) and LVM index (LVMI) were measured and
recorded according to the recommendations of the American Society
of Echocardiography (24).
Statistical analysis
The statistical analysis was performed using SPSS
version 20.0 (IBM Corp.). Kolmogorov-Smirnov test was used to
assess normality of data distribution. The enumeration data were
expressed as n (%) and non-normally distributed data were expressed
as the median and the 25 and 75th percentile quartiles (Q1 and Q3).
The degrees of correlation between variables were determined by
performing a Pearson's correlation analysis. Kruskal-Wallis
analysis of variance was used to compare differences among the
groups. P-values were 2-sided and a value of P<0.05 was
considered to indicate statistical significance.
Results
Clinical characteristics of the
participants
The clinical characteristics of the participants are
provided in Table I. A total of 61
patients with suspected CAD, including 29 (52.5%) females and 32
(47.5%) males, with a median age of 63 years (Q1 and Q3: 55 and 73
years), were selected as the research subjects in this study. The
patients had a median BMI of 23.37 kg/m2 (Q1 and Q3:
21.8 and 26.3 kg/m2), a median BSA of 1.65 m2
(Q1 and Q3: 1.6 and 1.8 m2), a median pulse of 75
beats/min (Q1 and Q3: 69 and 88 beats/min), a median SBP of 130
mmHg (Q1 and Q3: 120 and 146 mmHg) and a median DBP of 76 mmHg (Q1
and Q3: 70 and 88 mmHg). Among the 61 participants, 28 (45.9%) had
hypertension, 13 (21.3%) had diabetes mellitus, 7 (11.5%) had
dyslipidemia and 2 (3.3%) had a history of myocardial infarction.
Regarding the smoking and drinking status of the participants, 11
(18.0%) were current smokers and 6 (9.8%) were former smokers,
while 44 (72.1%) had never smoked; furthermore, 5 (8.2%) were
drinkers and 56 (91.8%) were non-drinkers. One participant (1.6%)
had a family history of CAD and the remaining 60 participants
(98.4%) did not have a family history of CAD. Among the 61
subjects, 43 patients (70.5%) received medications and 18 (29.5%)
did not take any medications.
 | Table IBasic data of the included subjects
(n=61). |
Table I
Basic data of the included subjects
(n=61).
| Characteristic | Value |
|---|
| Age (years) | 63 (55, 73) |
| Sex |
|
Male | 32 (52.5) |
|
Female | 29 (47.5) |
| BMI
(kg/m2) | 23.37 (21.8,
26.3) |
| BSA
(m2) | 1.65 (1.6,1.8) |
| Pulse
(beats/min) | 75 (69, 88) |
| SBP (mmHg) | 130 (120,146) |
| DBP (mmHg) | 76 (70, 88) |
| Coronary risk
factor |
|
Hypertension | 28 (45.9) |
|
Diabetes
mellitus | 13 (21.3) |
|
Dyslipidemia | 7 (11.5) |
|
History of
myocardial infarction | 2 (3.3) |
| Smoking status |
|
Current
smoker | 11 (18.0) |
|
Former
smoker | 6 (9.8) |
|
Never
smoker | 44 (72.1) |
| Drinking
status |
|
Yes | 5 (8.2) |
|
No | 56 (91.8) |
| Family history of
CAD |
|
Yes | 1 (1.6) |
|
No | 60 (98.4) |
| Intake of
medications |
|
Yes | 43 (70.5) |
|
No | 18 (29.5) |
Association between EFV and risk
factors for CAD
The distribution of EFV among the participants is
provided in Fig. 2A and Table II; the median EFV among all
participants was 86.41 cm3 (Q1 and Q3: 66.66 and 112.23
cm3). The median Gensini score among all participants
was 4 (Q1 and Q3: 0 and 9). Table
II provides the median EFV for subgroups based on the risk
factors associated with CAD. In addition, the association between
EFV and risk factors associated with CAD was evaluated, and it was
revealed that hypertension, diabetes mellitus, dyslipidemia,
history of myocardial infarction and smoking had no significant
effect on the EFV (P>0.05).
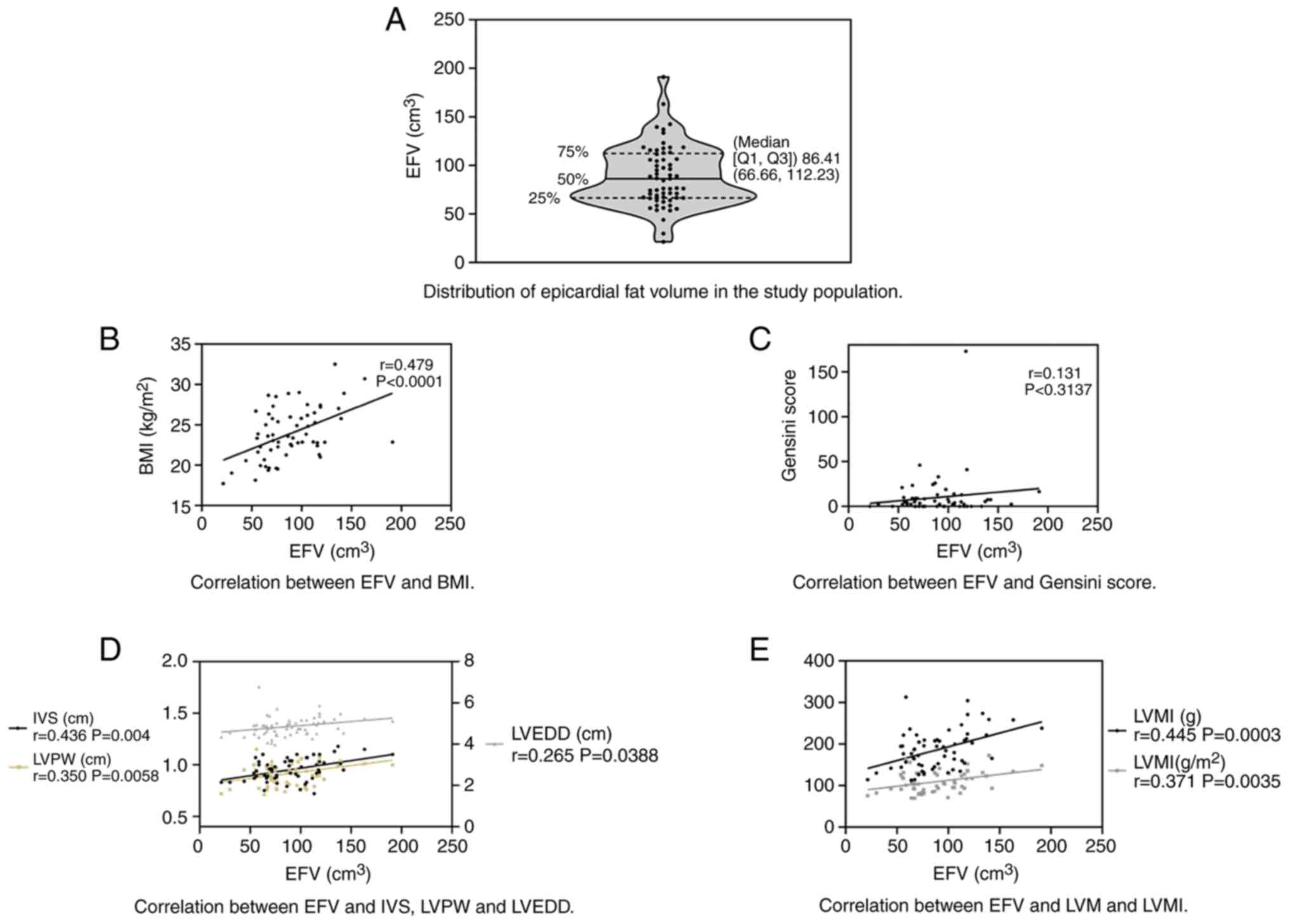 | Figure 2Association between EFV and left
ventricular function analyzed by Pearson correlation analysis. (A)
Violin plot indicating the distribution of EFV among the study
participants; the median EFV was 86.84 cm3 (Q1 and Q3,
66.66 and 112.23 cm3, respectively). (B) Correlation
between EFV and BMI (r=0.479, P<0.0001). (C) Correlation between
EFV and Gensini score (r=0.131, P=0.3137). (D) Correlation between
EFV and IVS (r=0.436, P=0.004), LVPW (r=0.350, P=0.0058) and LVEDD
(r=0.265, P=0.0388). (E) Correlation between EFV and LVM (r=0.445,
P=0.0003) and LVMI (r=0.371, P=0.0035). EFV, epicardial fat volume;
BMI, body mass index; IVS, interventricular septal thickness; LVPW,
left ventricular posterior wall thickness; LVEDD, left ventricular
end diastolic diameter; LVM, left ventricular mass; LVMI, LVM
index; Q1, first quartile; Q3, third quartile. |
 | Table IIAssociation between EFV and risk
factors of CAD. |
Table II
Association between EFV and risk
factors of CAD.
| Item/subgroup | n | EFV | P-value |
|---|
| All patients | 61 | 86.41 (66.66,
112.23) | |
| Hypertension | | | 0.57 |
|
Yes | 28 | 80.68 (70.92,
114.67) | |
|
No | 33 | 90.12 (58.69,
109.67) | |
| Diabetes
mellitus | | | 0.42 |
|
Yes | 13 | 89.06 (68.69,
127.41) | |
|
No | 48 | 85.52 (66.34,
106.28) | |
| Dyslipidemia | | | 0.96 |
|
Yes | 7 | 76.73
(71.23,89.06) | |
|
No | 54 | 86.61 (65.68,
113.00) | |
| History of
myocardial infarction | | | 0.08 |
|
Yes | 2 | 60.15 | |
|
No | 59 | 86.81 (67.20,
112.88) | |
| Smoking | | | 0.79 |
|
Current
smoker | 11 | 71.41 (66.22,
112.88) | |
|
Former
smoker | 6 | 104.37 (57.85,
122.54) | |
|
Never
smoker | 44 | 86.61
(66.83,105.74) | |
Correlation analysis
As provided in Fig.
2B-E, the Pearson's correlation analysis indicated that the EFV
was significantly positively correlated with the BMI (r=0.479,
P<0.0001), IVS (r=0.436, P=0.004), LVPW (r=0.350, P=0.0058),
LVEDD (r=0.265, P=0.0388), LVM (r=0.445, P=0.0003) and LVMI
(r=0.371, P=0.0035). However, there was no significant correlation
between the EFV and the Gensini score (r=0.131, P=0.31375).
Discussion
CAD is a common disease seriously affecting human
health and the risk factors include smoking, drinking, obesity,
hypertension, dyslipidemia, diabetes mellitus, family history of
CAD, history of myocardial infarction and/or abdominal fat
accumulation (25-27).
In addition to these factors, EAT has been reported to be involved
in the pathogenesis of CAD (28). As
an endocrine organ, EAT may directly affect the vascular wall and
promote the progression of atherosclerosis by releasing
inflammatory cytokines and mediators (29,30). At
present, the volume of epicardial fat may be determined by a
variety of imaging techniques, including echocardiography, CT and
cardiovascular magnetic resonance (31,32). Due
to its non-invasiveness, reliable results and capacity to provide
abundant information, CT imaging technology is widely used in the
detection of CAD and EAT (33,34).
In the present study, CT scanning and standard
methods were used to evaluate the degree of coronary artery
lesions. It should be noted that in the present study, the
association between EFV and clinical outcomes was not assessed,
though it was attempted to analyze the association between the EFV
and the severity of CAD. To date, the association between EAT and
the severity of CAD has been under debate (35). In most studies, the EFV was proved to
be significantly correlated with the Gensini score, suggesting that
the EFV is closely associated with CAD (36,37).
Conversely, no correlation between the EFV and Gensini score was
detected in the present study, which may be explained by the fact
that only one patient in the study had a Gensini score of >60
(lack of critical patients). On the other hand, in the study by
Tanami et al (38), a cohort
of 320 patients with suspected CAD who underwent 320-detector row
CT angiography exhibited a lack of association between the EFV and
the presence and severity of CAD, which was similar to the present
results. In addition, there are also certain differences between
the present study and that of Tanami et al (38): In the present study, the correlation
between the EFV and Gensini score was determined, while the latter
study explored the correlation between EFV and the coronary
calcification score.
It has been reported that the EFV was elevated with
the increase of risk factors for CAD and was significantly
associated with hypertension, diabetes, age and hyperlipidemia
(39). Hell et al (40) determined the volume and density of
EAT in CAD patients by using non-contrast CT, indicating that
hypertension was the only risk factor affecting the volume and
density of EAT. However, it has also been revealed that EAT is
independent of traditional risk factors and contributes to fatal
and non-fatal coronary events regardless of cardiovascular risk
factors (41,42). Hachiya et al (43) analyzed 134 patients who underwent
multi-detector CT to assess them for CAD and observed that the
increase in EFV was associated with augmented central aortic
pressure and left ventricular diastolic dysfunction, but not with
age, gender, hypertension, dyslipidemia, diabetes mellitus or prior
myocardial infarction. In the present study, the risk factors for
CAD, including hypertension, diabetes mellitus, dyslipidemia,
history of myocardial infarction and smoking had no effect on the
EFV. These conflicting results may be due to limitations of the
measurement methods.
Overweight and obesity are important risk factors
for CAD and the relevant evaluation indexes include BMI, waist
circumference, hip circumference and percentage of body/visceral
fat (44-46).
EAT is a type of visceral fat with metabolic activity and is a
source of multiple fat factors (47). Previous studies have indicated a
direct association of EAT with the BMI and waist circumference
(48,49). In the present study, the EFV was
positively correlated with the BMI, suggesting that with the
increase of the EFV, the BMI and the risk of CAD were also
increased. In addition, EFV was positively correlated with IVS,
LVPW, LVEDD, LVM and LVMI. In the analysis of the association
between EVF and left cardiac function, the indicators of left
cardiac function adopted in the present study were different from
those used in other studies (50,51).
Left ventricular dysfunction significantly increases the rate of
mortality in CAD patients (52).
Multiple studies have confirmed the correlation between EAT and
LVM, left atrial size and left ventricular diastolic dysfunction
(53-55),
and this may be explained by the fact that the secretion of
pro-inflammatory cytokines, free fatty acids and atherogenic
cytokines by EAT may lead to arteriosclerosis (56). Vural et al (57) reported that patients with left
ventricular diastolic dysfunction had a significantly increased
EFV. Nerlekar et al (58)
indicated that an increased EFV was associated with diastolic
dysfunction. In addition, EAT is directly associated with the
myocardium, may be subjected to local compressive forces and may
affect the morphology and function of the heart by paracrine and
mechanical interactions (57,59). EAT
induces inflammation by secreting a variety of adipokines and
inflammatory factors, and persistent inflammation may lead to
collagen deposition, thereby causing impaired left ventricular
relaxation function and finally affecting the diastolic function of
the left ventricle (58). Previous
studies have reported that epicardial fat may increase the
accumulation of myocardial triglycerides, which may cause apoptosis
of myocardial cells, increase oxidative stress and damage of
cardiac function (60,61). In a state of cardiovascular disease,
the EAT dilates, becoming hypoxic and dysfunctional and recruiting
phagocytic cells, which may lead to increasing detrimental
adipocytokines and eventually impaired cardiac function (61). In view of this, the EFV is correlated
with the BMI and left ventricular function, which has certain
clinical significance for the early diagnosis of diseases linked to
obesity and left ventricular function.
The present study has certain limitations. For
instance, the number of patients included in the study was small
and patients with severe coronary artery lesions were not included
in the study, as only 1 out of the 61 patients with suspected CAD
had a Gensini score of >60. In the present study, the EFV was
associated with left heart function, whereas poor left heart
function is not a direct diagnostic basis for CAD, as coronary
angiography remains the diagnostic gold standard for CAD (62). In addition, a previous study has
indicated that abdominal adipose tissue was an important index for
cardiovascular disease (63).
Detection of abdominal adipose tissue may be more effective in fat
evaluation and this will be our future research direction.
In conclusion, in the present study, the EFV
determined using CT scanning was not associated with CAD based on
the Gensini scores, but was positively correlated with the BMI,
IVS, LVPW, LVEDD, LVM and LVMI. The association between EFV and BMI
and left ventricular function still has certain clinical guiding
significance. Further studies are required to determine the
association between the EFV and coronary heart disease, and it may
be suggested that the cohort size should be expanded or the
evaluation indicators (e.g. the elasticity of ascending aorta) may
be increased to enhance the reliability. In addition, the guiding
significance of the EFV should be verified by examining the
association between the EFV and heart diseases other than CAD. The
association between the EFV and left ventricular diastolic function
should be further explored and whether the location of epicardial
fat affects left ventricular function also remains to be
investigated.
Acknowledgements
Not applicable.
Funding
This work was supported by the Jiangsu Provincial
Commission of Health and Family Planning Surface Project (grant no.
H201658).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
Substantial contributions to conception and design:
RHY; data acquisition, data analysis and interpretation: XQT, TW,
HFS, XW, XQW and CJP; drafting the article or critical revision for
important intellectual content: RHY; final approval of the version
to be published: All authors; agreement to be accountable for all
aspects of the work to ensure that questions regarding the accuracy
or integrity of the work are appropriately investigated and
resolved: All authors.
Ethics approval and consent to
participate
All procedures involving human participants were in
accordance with the ethical standards of the institutional and/or
national research committee and with the 1964 Helsinki declaration
and its later amendments or comparable ethical standards. The
present study was approved by the Medical Ethics Committee of The
Affiliated Changzhou No. 2 People's Hospital of Nanjing Medical
University (Changzhou, China) and all patients volunteered to
participate in the present study and provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Potz BA, Parulkar AB, Abid RM, Sodha NR
and Sellke FW: Novel molecular targets for coronary angiogenesis
and ischemic heart disease. Coron Artery Dis. 28:605–613.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Marateb HR and Goudarzi S: A noninvasive
method for coronary artery diseases diagnosis using a
clinically-interpretable fuzzy rule-based system. J Res Med Sci.
20:214–223. 2015.PubMed/NCBI
|
|
3
|
Olie RH, van der Meijden PEJ and Ten Cate
H: The coagulation system in atherothrombosis: Implications for new
therapeutic strategies. Res Pract Thromb Haemost. 2:188–198.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Goyal V, Jassal DS and Dhalla NS:
Pathophysiology and prevention of sudden cardiac death. Can J
Physiol Pharmacol. 94:237–244. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zulkifli Amin H, Zulkifli Amin L and
Zulkifli Amin F: Paramount importance of angiotensin receptor
neprilysin inhibitor in heart failure management. Acta Med Iran.
54:823–824. 2016.PubMed/NCBI
|
|
6
|
Han D, Lee JH, Hartaigh BO and Min JK:
Role of computed tomography screening for detection of coronary
artery disease. Clin Imaging. 40:307–310. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dalager MG, Bøttcher M, Thygesen J,
Andersen G and Bøtker HE: Different plaque composition and
progression in patients with stable and unstable coronary syndromes
evaluated by cardiac CT. Biomed Res Int.
2015(401357)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Saba L, Anzidei M, Piga M, Ciolina F,
Mannelli L, Catalano C, Suri JS and Raz E: Multi-modal CT scanning
in the evaluation of cerebrovascular disease patients. Cardiovasc
Diagn Ther. 4:245–262. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Matsuzawa Y: The metabolic syndrome and
adipocytokines. Expert Rev Clin Immunol. 3:39–46. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Vidal H: Obesity and inflammation: The
adipocytokines. Ann Endocrinol (Paris). 64:S40–S44. 2003.PubMed/NCBI(In French).
|
|
11
|
Vacca M, Di Eusanio M, Cariello M,
Graziano G, D'Amore S, Petridis FD, D'orazio A, Salvatore L,
Tamburro A, Folesani G, et al: Integrative miRNA and whole-genome
analyses of epicardial adipose tissue in patients with coronary
atherosclerosis. Cardiovasc Res. 109:228–239. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fitzgibbons TP and Czech MP: Epicardial
and perivascular adipose tissues and their influence on
cardiovascular disease: Basic mechanisms and clinical associations.
J Am Heart Assoc. 3(e000582)2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yang TT, Fish AF, Kong WM, Gao X, Huang J,
Feng JT, Zhu JY, Chen T and Lou QQ: Correlates of pericardial
adipose tissue volume using multidetector CT scanning in cardiac
patients in China. Int J Cardiol. 244:285–289. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nagy E, Jermendy AL, Merkely B and
Maurovich-Horvat P: Clinical importance of epicardial adipose
tissue. Arch Med Sci. 13:864–874. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kitagawa T, Yamamoto H, Sentani K,
Takahashi S, Tsushima H, Senoo A, Yasui W, Sueda T and Kihara Y:
The relationship between inflammation and neoangiogenesis of
epicardial adipose tissue and coronary atherosclerosis based on
computed tomography analysis. Atherosclerosis. 243:293–299.
2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bertaso AG, Bertol D, Duncan BB and Foppa
M: Epicardial fat: Definition, measurements and systematic review
of main outcomes. Arq Bras Cardiol. 101:e18–e28. 2013.PubMed/NCBI View Article : Google Scholar : (In English,
Portuguese).
|
|
17
|
Fatma E, Bunyamin K, Savas S, Mehmet U,
Selma Y, Ismail B, Sabri C, Gulzade O, Ibrahim D and Mehmet Y:
Epicardial fat thickness in patients with rheumatoid arthritis. Afr
Health Sci. 15:489–495. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kocyigit I, Gungor O, Unal A, Yasan M,
Orscelik O, Tunca O, Eroglu E, Sipahioglu MH, Tokgoz B, Ozdogru I,
et al: A low serum free triiodothyronine level is associated with
epicardial adipose tissue in peritoneal dialysis patients. J
Atheroscler Thromb. 21:1066–1074. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hartiala O, Magnussen CG, Bucci M,
Kajander S, Knuuti J, Ukkonen H, Saraste A, Rinta-Kiikka I,
Kainulainen S, Kähönen M, et al: Coronary heart disease risk
factors, coronary artery calcification and epicardial fat volume in
the Young Finns Study. Eur Heart J Cardiovasc Imaging.
16:1256–1263. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wu J, Zhang X, Li X and Yang L: Adipose
tissue volume differences around the heart between subjects without
coronary atherosclerosis and coronary heart disease patients. Acta
Cardiol. 71:291–298. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sironi AM, Petz R, De Marchi D, Buzzigoli
E, Ciociaro D, Positano V, Lombardi M, Ferrannini E and Gastaldelli
A: Impact of increased visceral and cardiac fat on cardiometabolic
risk and disease. Diabet Med. 29:622–627. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Taylor AJ, Cerqueira M, Hodgson JM, Mark
D, Min J, O'Gara P and Rubin GD: American College of Cardiology
Foundation Appropriate Use Criteria Task Force; Society of
Cardiovascular Computed Tomography; American College of Radiology
et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010
appropriate use criteria for cardiac computed tomography. A report
of the American College of Cardiology Foundation appropriate use
criteria task force, the society of cardiovascular computed
tomography, the American College of Radiology, the American Heart
Association, the American Society of Echocardiography, the American
Society of Nuclear Cardiology, the North American Society for
cardiovascular imaging, the society for cardiovascular angiography
and interventions, and the society for cardiovascular magnetic
resonance. J Am Coll Cardiol. 56:1864–1894. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gensini GG: A more meaningful scoring
system for determining the severity of coronary heart disease. Am J
Cardiol. 51(606)1983.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Schiller NB, Shah PM, Crawford M, DeMaria
A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D,
Schnittger I, et al: Recommendations for quantitation of the left
ventricle by two-dimensional echocardiography American Society of
Echocardiography Committee on standards, subcommittee on
quantitation of two-dimensional echocardiograms. J Am Soc
Echocardiogr. 2:358–367. 1989.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang G, Li Y, Peng Y, Tang J and Li H:
Association of polymorphisms in MALAT1 with risk of coronary
atherosclerotic heart disease in a Chinese population. Lipids
Health Dis. 17(75)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hajar R: Diabetes as ʻcoronary artery
disease risk equivalentʼ: A historical perspective. Heart Views.
18:34–37. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Florido R, Zhao D, Ndumele CE, Lutsey PL,
McEvoy JW, Windham BG, Pankow JS, Guallar E and Michos ED: Physical
activity, parental history of premature coronary heart disease, and
incident atherosclerotic cardiovascular disease in the
atherosclerosis risk in communities (ARIC) study. J Am Heart Assoc.
5(e003505)2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Aydin AM, Kayali A, Poyraz AK and Aydin K:
The relationship between coronary artery disease and pericoronary
epicardial adipose tissue thickness. J Int Med Res. 43:17–25.
2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Topaloglu O, Sayki Arslan M, Turak O,
Ginis Z, Sahin M, Cebeci M, Ucan B, Cakir E, Karbek B, Ozbek M, et
al: Three noninvasive methods in the evaluation of subclinical
cardiovascular disease in patients with acromegaly: Epicardial fat
thickness, aortic stiffness and serum cell adhesion molecules. Clin
Endocrinol (Oxf). 80:726–734. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tanaka K and Sata M: Roles of perivascular
adipose tissue in the pathogenesis of atherosclerosis. Front
Physiol. 9(3)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Izgi C: Epicardial adipose tissue: Just a
predictor or a local player for coronary atherosclerosis? Anatol J
Cardiol. 15:360–362. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Douglass E, Greif S and Frishman WH:
Epicardial fat: Pathophysiology and clinical significance. Cardiol
Rev. 25:230–235. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yuce G, Türkvatan A and Yener Ö: Can
aortic atherosclerosis or epicardial adipose tissue volume be used
as a marker for predicting coronary artery disease? J Cardiol.
65:143–149. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Romijn MA, Danad I, Bakkum MJ, Stuijfzand
WJ, Tulevski II, Somsen GA, Lammertsma AA, van Kuijk C, van de Ven
PM, Min JK, et al: Incremental diagnostic value of epicardial
adipose tissue for the detection of functionally relevant coronary
artery disease. Atherosclerosis. 242:161–166. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kim SH, Chung JH, Kwon BJ, Song SW and
Choi WS: The associations of epicardial adipose tissue with
coronary artery disease and coronary atherosclerosis. Int Heart J.
55:197–203. 2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ghaderi F, Eshraghi A, Shamloo AS and
Mousavi S: Assosiation of epicardial and pericardial fat thickness
with coronary artery disease. Electron Physician. 8:2982–2989.
2016.PubMed/NCBI View
Article : Google Scholar
|
|
37
|
Yang C, Li L, Zha Y and Peng Z:
Correlation between epicardial adipose tissue and severity of
coronary artery stenosis evaluated by 64-MDCT. Clin Imaging.
40:477–480. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Tanami Y, Jinzaki M, Kishi S, Matheson M,
Vavere AL, Rochitte CE, Dewey M, Chen MY, Clouse ME, Cox C, et al:
Lack of association between epicardial fat volume and extent of
coronary artery calcification, severity of coronary artery disease,
or presence of myocardial perfusion abnormalities in a diverse,
symptomatic patient population: Results from the CORE320
multicenter study. Circ Cardiovasc Imaging.
8(e002676)2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Bo X, Ma L, Fan J, Jiang Z, Zhou Y, Zhang
L and Li W: Epicardial fat volume is correlated with coronary
lesion and its severity. Int J Clin Exp Med. 8:4328–4334.
2015.PubMed/NCBI
|
|
40
|
Hell MM, Ding X, Rubeaux M, Slomka P,
Gransar H, Terzopoulos D, Hayes S, Marwan M, Achenbach S, Berman DS
and Dey D: Epicardial adipose tissue volume but not density is an
independent predictor for myocardial ischemia. J Cardiovasc Comput
Tomogr. 10:141–149. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Hajsadeghi F, Nabavi V, Bhandari A, Choi
A, Vincent H, Flores F, Budoff M and Ahmadi N: Increased epicardial
adipose tissue is associated with coronary artery disease and major
adverse cardiovascular events. Atherosclerosis. 237:486–489.
2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Mahabadi AA, Berg MH, Lehmann N, Kälsch H,
Bauer M, Kara K, Dragano N, Moebus S, Jöckel KH, Erbel R and
Möhlenkamp S: Association of epicardial fat with cardiovascular
risk factors and incident myocardial infarction in the general
population: The Heinz Nixdorf Recall Study. J Am Coll Cardiol.
61:1388–1395. 2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Hachiya K, Fukuta H, Wakami K, Goto T,
Tani T and Ohte N: Relation of epicardial fat to central aortic
pressure and left ventricular diastolic function in patients with
known or suspected coronary artery disease. Int J Cardiovasc
Imaging. 30:1393–1398. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Sinha SK, Thakur R, Jha MJ, Goel A, Kumar
V, Kumar A, Mishra V, Varma CM, Krishna V, Singh AK and Sachan M:
Epicardial adipose tissue thickness and its association with the
presence and severity of coronary artery disease in clinical
setting: A cross-sectional observational study. J Clin Med Res.
8:410–419. 2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Antonopoulos AS, Oikonomou EK, Antoniades
C and Tousoulis D: From the BMI paradox to the obesity paradox: The
obesity-mortality association in coronary heart disease. Obes Rev.
17:989–1000. 2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Sharma S, Batsis JA, Coutinho T, Somers
VK, Hodge DO, Carter RE, Sochor O, Kragelund C, Kanaya AM, Zeller
M, et al: Normal-weight central obesity and mortality risk in older
adults with coronary artery disease. Mayo Clin Proc. 91:343–351.
2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Sequeira DI, Ebert LC, Flach PM, Ruder TD,
Thali MJ and Ampanozi G: The correlation of epicardial adipose
tissue on postmortem CT with coronary artery stenosis as determined
by autopsy. Forensic Sci Med Pathol. 11:186–192. 2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Shaheen S, Hassan N, Awan M, Zehra N and
Manzoor A: Epicardial adipose tissue (EAT) thickness and its
association with BMI and waist circumference in healthy adults and
coronary artery disease (CAD) patients. Br J Med Med Res. 11:1–10.
2016.
|
|
49
|
Iacobellis G, Mohseni M, Bianco SD and
Banga PK: Liraglutide causes large and rapid epicardial fat
reduction. Obesity (Silver Spring). 25:311–316. 2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Fontes-Carvalho R, Fontes-Oliveira M,
Sampaio F, Mancio J, Bettencourt N, Teixeira M, Rocha Gonçalves F,
Gama V and Leite-Moreira A: Influence of epicardial and visceral
fat on left ventricular diastolic and systolic functions in
patients after myocardial infarction. Am J Cardiol. 114:1663–1669.
2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ng AC, Goo SY, Roche N, van der Geest RJ
and Wang WY: Epicardial adipose tissue volume and left ventricular
myocardial function using 3-dimensional speckle tracking
echocardiography. Can J Cardiol. 32:1485–1492. 2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Wang Y, Ma H, Hao X, Yang J, Chen Q, Lu L
and Zhang R: Low serum calcium is associated with left ventricular
systolic dysfunction in a Chinese population with coronary artery
disease. Sci Rep. 6(22283)2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Topuz M and Dogan A: The effect of
epicardial adipose tissue thickness on left ventricular diastolic
functions in patients with normal coronary arteries. Kardiol Pol.
75:196–203. 2017.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Kırış A, Kırış G, Turan OE, Öztürk M,
Şahin M, İlter A, Bektaş O, Kutlu M, Kaplan Ş and Gedikli Ö:
Relationship between epicardial fat tissue and left ventricular
synchronicity: An observational study. Anatol J Cardiol.
15:990–994. 2015.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Bakkum MJ, Danad I, Romijn MA, Stuijfzand
WJ, Leonora RM, Tulevski II, Somsen GA, Lammertsma AA, van Kuijk C,
van Rossum AC, et al: The impact of obesity on the relationship
between epicardial adipose tissue, left ventricular mass and
coronary microvascular function. Eur J Nucl Med Mol Imaging.
42:1562–1573. 2015.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Doğan M, Turak O, Akyel A, Grboviç E,
Mendi MA, Oksüz F, Doğan A, Cimen T, Bilgin M, Sunman H, et al:
Increased epicardial adipose tissue thickness is linked to aortic
stiffness in patients with primary hypertension. Blood Press.
23:222–227. 2014.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Vural M, Talu A, Sahin D, Elalmis OU,
Durmaz HA, Uyanık S and Dolek BA: Evaluation of the relationship
between epicardial fat volume and left ventricular diastolic
dysfunction. Jpn J Radiol. 32:331–339. 2014.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Nerlekar N, Muthalaly RG, Wong N, Thakur
U, Wong DTL, Brown AJ and Marwick TH: Association of volumetric
epicardial adipose tissue quantification and cardiac structure and
function. J Am Heart Assoc. 7(e009975)2018.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Fernandes-Cardoso A, Santos-Furtado M,
Grindler J, Ferreira LA, Andrade JL and Santo MA: Epicardial fat
thickness correlates with P-wave duration, left atrial size and
decreased left ventricular systolic function in morbid obesity.
Nutr Metab Cardiovasc Dis. 27:731–738. 2017.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Kankaanpää M, Lehto HR, Pärkkä JP, Komu M,
Viljanen A, Ferrannini E, Knuuti J, Nuutila P, Parkkola R and Iozzo
P: Myocardial triglyceride content and epicardial fat mass in human
obesity: Relationship to left ventricular function and serum free
fatty acid levels. J Clin Endocrinol Metab. 91:4689–4695.
2006.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Lin HH, Lee JK, Yang CY, Lien YC, Huang JW
and Wu CK: Accumulation of epicardial fat rather than visceral fat
is an independent risk factor for left ventricular diastolic
dysfunction in patients undergoing peritoneal dialysis. Cardiovasc
Diabetol. 12(127)2013.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Peng-Hui Jian L-FX and Tie-Jun Zhang:
Study on ultrasound evaluation of carotid atherosclerosis and its
predicting value for coronary heart disease. 22 12: 4, 2016.
|
|
63
|
Neeland IJ, Ayers CR, Rohatgi AK, Turer
AT, Berry JD, Das SR, Vega GL, Khera A, McGuire DK, Grundy SM and
de Lemos JA: Associations of visceral and abdominal subcutaneous
adipose tissue with markers of cardiac and metabolic risk in obese
adults. Obesity (Silver Spring). 21:E439–E447. 2013.PubMed/NCBI View Article : Google Scholar
|