Introduction
Allergic rhinitis (AR) is the allergic inflammation
of immune cells in the nasal mucosa and is characterized by
clinical symptoms and complaints including itching, allergic
conjunctivitis, rhinorrhea, nasal congestion and disturbed
olfaction (1). AR affects ~10% of
the population worldwide and an increase in the frequency of this
disease has been reported (2). AR is
closely associated with other inflammatory diseases, including
asthma, chronic rhinosinusitis, allergic conjunctivitis and otitis
media with effusion and adenoid hypertrophy, which may affect the
quality of life of patients with AR (3,4).
The imbalance of type 2 T helper (Th2) cells, a
subset of CD4+ T cells, has an essential role in the
pathogenesis and immunological characteristics of AR (5). A previous study reported that
immunoglobulin (Ig)E produced by Th2 cells binds to IgE receptors
on the surface of mast cells to sensitize these cells, resulting in
the release of histamine and leukotrienes, which induce an AR
attack (6). Interleukin (IL)-4 and
IL-13 secreted by activated Th2 cells are recognized as key
mediators of the pathogenesis of AR, leading to IgE isotype switch
(7). Furthermore, IL-17a and
interferon (IFN)-γ are inflammatory cytokines produced by Th2 cells
that may induce the expression of other cytokines and mucosal
proteins. IL-17a and IFN-γ are involved in the development and
regulation of AR (8,9). Shirasaki et al (10) reported that Th2 cells were abundant
in the nasal mucosa during AR and low Th2 cell counts relieved
AR-associated symptoms, suggesting that Th2 cells may be a
potential target for the treatment of AR (10).
IL-37 is a unique member of the IL-1 family with
broad anti-inflammatory roles during autoimmune diseases, including
systemic lupus erythematosus, psoriasis and asthma (11-13).
Several studies have reported that IL-37 alleviates allergic
inflammation during AR (14-16).
In animal models, administration of recombinant human IL-37 may
attenuate the local allergic symptoms of AR and decrease the
expression of cytokines produced by Th2 and Th17 cells in the nasal
mucosa (15). IL-37 has also been
demonstrated to regulate aberrant T-cell immune responses in
patients with AR (16).
Wang et al (14) reported that phosphorylation of STAT
proteins, together with IL-37 signaling and exogenous IL-37
treatment, is able to attenuate allergic symptoms (nasal rubbing
and sneezing) by decreasing STAT6 or STAT3 expression levels in
CD4+ T-cell subsets (14). The increased expression of IL-1R8 in
CD4+ T cells may be associated with the potential role
of IL-37 during AR-mediated anti-inflammation (16). Further studies have focused on the
role of IL-37 in limiting innate inflammation primarily by
inhibiting the production of proinflammatory cytokines by
macrophages or epithelial cells (17,18).
However, the underlying anti-inflammatory mechanism of IL-37, as
well as its ability to alleviate the allergic symptoms of AR, have
yet to be determined.
C-C motif cytokine ligand (CCL)11 is critical for
attracting eosinophils during allergic asthma in mice (19,20).
IL-4/IL-13 signaling may induce CCL11 secretion in Th2 cells
(21), and interruption of IL-4 and
IL-13 synthesis significantly decreases CCL11 production, which
further reduces pulmonary eosinophilia in several asthma models
(22,23). In a previous animal study, IL-37 has
been reported to inhibit the production of IL-4/IL-13 but induce
the production of CCL11, thereby stimulating anti-inflammatory
mechanisms in allergic asthma (24).
However, the exact mechanisms by which IL-37 inhibits IL-4/IL-13
and induces CCL11 to exert its anti-inflammatory effects in AR have
yet to be elucidated.
On the basis of the aforementioned studies, it was
hypothesized that IL-37 alleviates the allergic symptoms of AR via
CCL11 signaling in Th2 cells. To further understand the role of
IL-37 in CCL11 signaling, a mouse model of AR was established by
treating animals with ovalbumin (OVA). The allergic symptoms were
investigated and the serum and nasal lavage fluid levels of IgE,
IgG1, IgG2a, IFN-γ, IL-4, IL-13, IL-17a and CCL11, as well as
histamine and substance P, were determined by ELISA. In addition,
the number of eosinophils was determined by histopathological
observation.
Materials and methods
Animals
A total of 40 male BALB/c mice (age, 6-8 weeks;
weight, 20-23 g) were purchased from Beijing SPF Vital Laboratory
Animal Technology Company. The mice were housed in a limited access
rodent facility with 8 rats per polycarbonate cage in a room with a
12 h light, 12 h dark cycle (lights on from 7:00 a.m. to 7:00
p.m.), with a relative humidity maintained at 55±15%, and a
constant temperature (24±2˚C). The experiments were performed in
accordance with the National Institutes of Health Guidelines for
the Care and Use of Laboratory Animals and approved by the Animal
Care and Use Committee of Beijing Jishuitan Hospital (Beijing,
China).
Experimental procedure
To induce sensitization, mice were administered with
an intraperitoneal injection of 1 mg/ml OVA (Grade V;
Sigma-Aldrich; Merck KGaA) and 20 mg/ml aluminum hydroxide
(Sigma-Aldrich; Merck KGaA) in sterile saline (0.1 ml/mouse) on
days 1, 3, 5, 7, 9, 11 and 13. To induce AR, mice were subsequently
intranasally administered 60 mg/ml OVA in sterile saline (20
µl/mouse) from day 20 to day 30(14). The control group (n=10) did not
receive any treatment.
Experimental design and
pharmacological treatment
The experimental design is presented in Fig. 1. OVA-exposed mice were randomly
assigned to one of the following three groups (n=10 per group): The
OVA group received no treatment; the OVA + Sal group received 1 µg
saline by intraperitoneal injection prior to each OVA challenge and
for 5 days between day 26 and day 30; and the OVA + IL-37 group
received 1 µg recombinant human IL-37 (dissolved in 50 µl sterile
PBS, R&D Systems, Inc.) by intraperitoneal injection prior to
each OVA challenge and for 5 days between day 26 and day 30
according to a previous protocol (14).
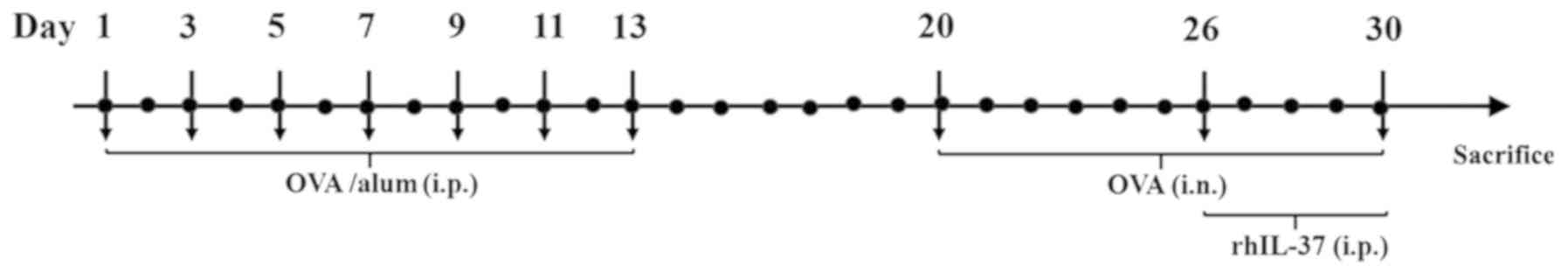 | Figure 1Experimental design of the mouse
model of AR. To induce sensitization, mice were administered OVA
and alum i.p. on days 1, 3, 5, 7, 9, 11 and 13. To induce AR, the
mice were challenged i.n. with OVA from day 20 to 30 and were
subsequently administered rhIL-37 i.p. for 5 consecutive days from
day 26 to 30. AR, allergic rhinitis; OVA, ovalbumin; alum, aluminum
hydroxide; i.p., intraperitoneally; i.n., intranasally; rhIL-37,
recombinant human interleukin 37. |
Evaluation of allergic symptoms
Nasal symptoms were evaluated by counting the
frequency of nasal rubbing and sneezing (25). The frequency of nasal rubbing and
sneezing within a 15-min period after the final allergen challenge
was recorded by two observers blinded to the experimental
conditions.
Collection of serum and nasal lavage
fluid and ELISA
At 24 h after the last administration of OVA, the
mice were anesthetized with isoflurane. Cardiac blood samples were
harvested from each mouse in EDTA-coated tubes, maintained on ice,
centrifuged at 4,000 x g for 10 min at 4˚C and subsequently, serum
was isolated. ELISA was performed to determine the following: Total
IgE (Mouse IgE ELISA kit; cat. no. SEKM-0095; Beijing Solarbio
Science & Technology Co., Ltd.), IgG1 (Mouse IgG1 ELISA kit;
cat. no. SEKM-0097; Beijing Solarbio Science & Technology Co.,
Ltd.), IgG2a (Mouse IgG2a ELISA kit; cat. no. SEKM-0099; Beijing
Solarbio Science & Technology Co., Ltd.), IFN-γ (Mouse IFN-γ
ELISA kit; cat. no. PI508; Beyotime Institute of Biotechnology),
IL-4 (Mouse IL-4 ELISA kit; cat. no. PI612; Beyotime Institute of
Biotechnology), IL-13 (Mouse IL-13 PicoKine ELISA kit; cat. no.
EK0425; Boster Biological Technology), IL-17a (Mouse IL-17a ELISA
kit; cat. no. PI545; Beyotime Institute of Biotechnology) and CCL11
(Mouse CCL11 PicoKine ELISA kit; cat. no. EK0330; Boster Biological
Technology). Concentrations were determined by ELISA using specific
rabbit polyclonal antibodies (Abcam) according to the
manufacturer's protocol.
After the collection of blood, the mice were
sacrificed with an overdose of isoflurane. When the cardiac
activity and respiration ceased 15 min later, nasal lavage fluid
was collected using an 18-gauge catheter. In brief, the trachea was
partly resected, a catheter was inserted from the trachea into the
nasopharynx and the nasal passages were gently perfused with 1 ml
saline. The nasal lavage fluid was centrifuged at 10,000 x g for 10
min at 4˚C. The concentrations of IFN-γ, IL-4, IL-13, IL-17a and
CCL11 were determined by ELISA using the aforementioned kits. In
addition, histamine (Histamine ELISA kit; cat. no. ab213975; Abcam)
and substance P (Substance P ELISA kit; cat. no. ab133029; Abcam)
were measured by ELISA.
Histopathologic observation
After 24 h of the last challenge on day 30, a
portion of the nasal mucosa was excised and fixed in 10% formalin
for 3 days at room temperature. The tissues were cut into 4-µm
sections and stained for 1 min at room temperature using a
haematoxylin-eosin staining kit (Beyotime Institute of
Biotechnology) to assess the extent of inflammatory cell
infiltration. A total of 5 random fields of vision in the nasal
mucosal sections were examined using an Olympus FluoView 1200
confocal microscope (Olympus Corp.) and photomicrographs
(magnification, x400) of representative nasal mucosa areas were
acquired. The thickness of the nasal mucosa and the number of
eosinophils were calculated using Image J software (Version 1.5.1;
National Institutes of Health)
Statistical analysis
Values are expressed as the mean ± standard
deviation. Statistical analyses were performed using SPSS software
(version 23.0; IBM Corp.) and GraphPad Prism software (version 7.0;
GraphPad Software, Inc.). One-way analysis of variance followed by
Bonferroni's correction was used for multiple comparisons among
four groups. The correlation between two variables was assessed by
Pearson correlation analysis. P<0.05 was considered to indicate
a statistically significant difference.
Results
Allergic symptoms
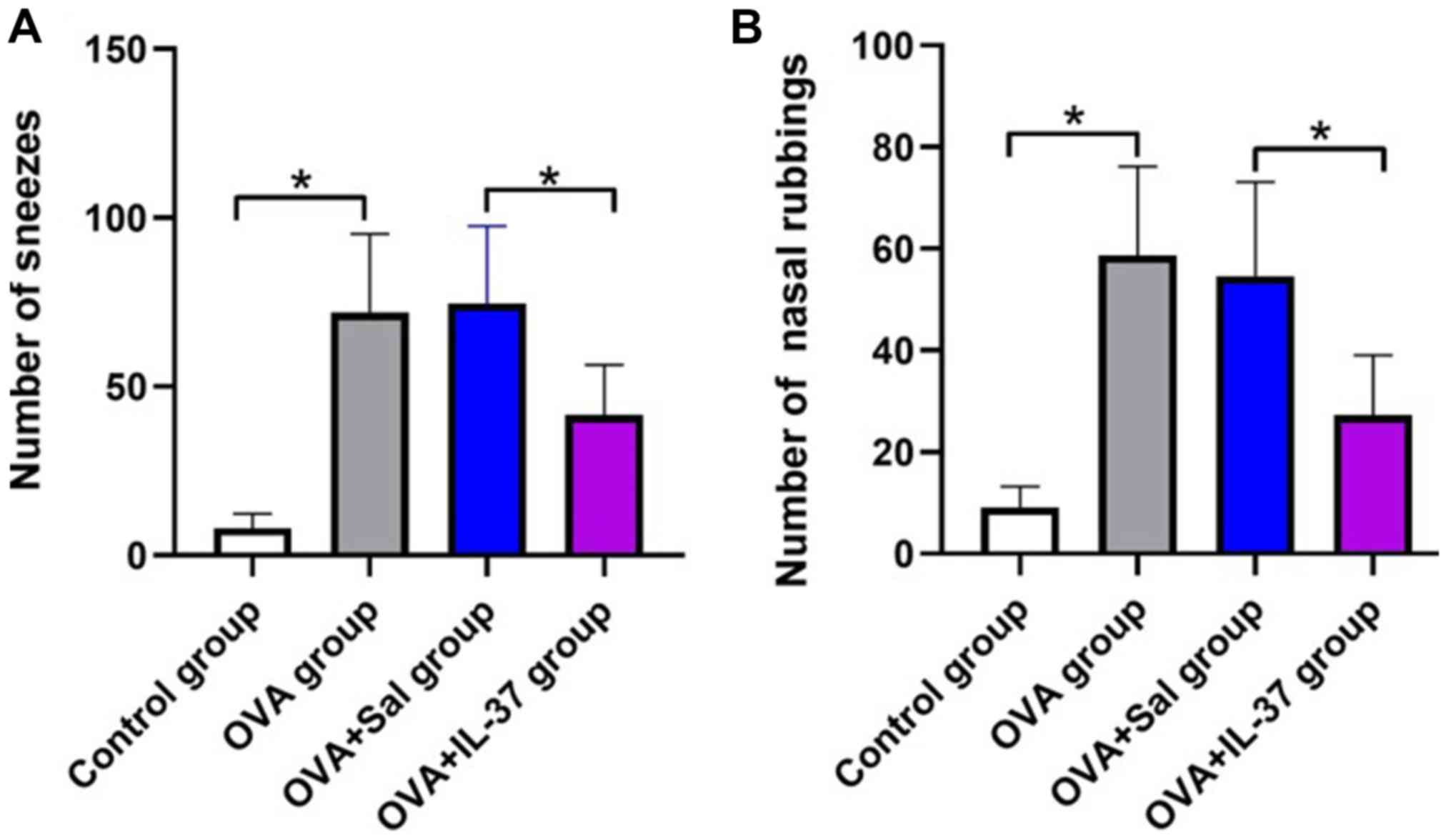
The effects of IL-37 administration on the allergic
symptoms of the mice with OVA-induced AR are presented in Fig. 2. The frequency of nasal rubbing
(P<0.001) and sneezing (P<0.05) in the OVA group was
significantly increased compared with that in the control group
(Fig. 2A and B). After IL-37 administration, the
frequency of nasal rubbing (P<0.001) and sneezing (P<0.05) in
the OVA + IL-37 group was significantly decreased compared with
that in the OVA + Sal group (Fig. 2A
and B).
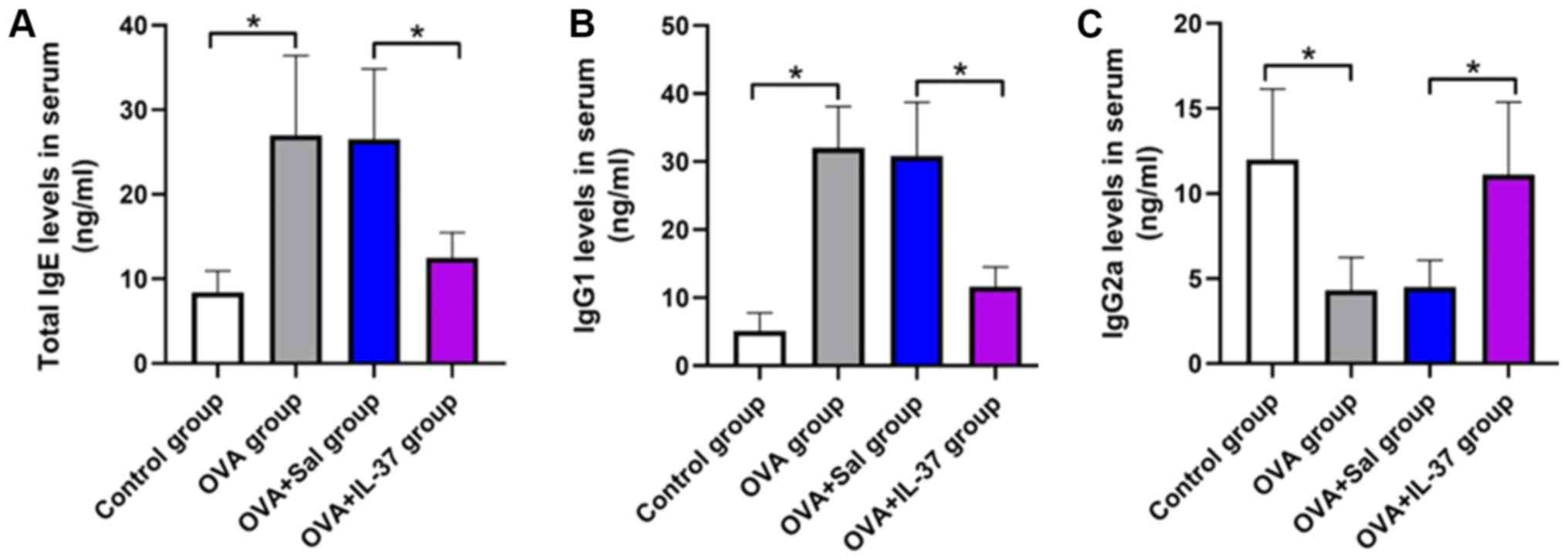
Serum IgE, IgG1 and IgG2a levels
Serum IgE, IgG1 and IgG2a levels in mice were
measured by ELISA and the results are presented in Fig. 3. IgE levels in the serum were
significantly higher in the OVA group compared with those in the
control group (P<0.001; Fig. 3A).
Treatment with IL-37 significantly reduced the IgE levels in the
serum of OVA-treated mice (P<0.001; Fig. 3A). IgG1 levels in the OVA group were
significantly increased compared with those in the control group.
However, IgG1 serum levels were decreased in the OVA + IL-37 group
compared with the OVA group (P<0.001; Fig. 3B). Furthermore, IgG2a serum levels
were significantly decreased in the OVA group compared with those
in the control group, but this effect was reversed by IL-37
treatment (P<0.001; Fig. 3C).
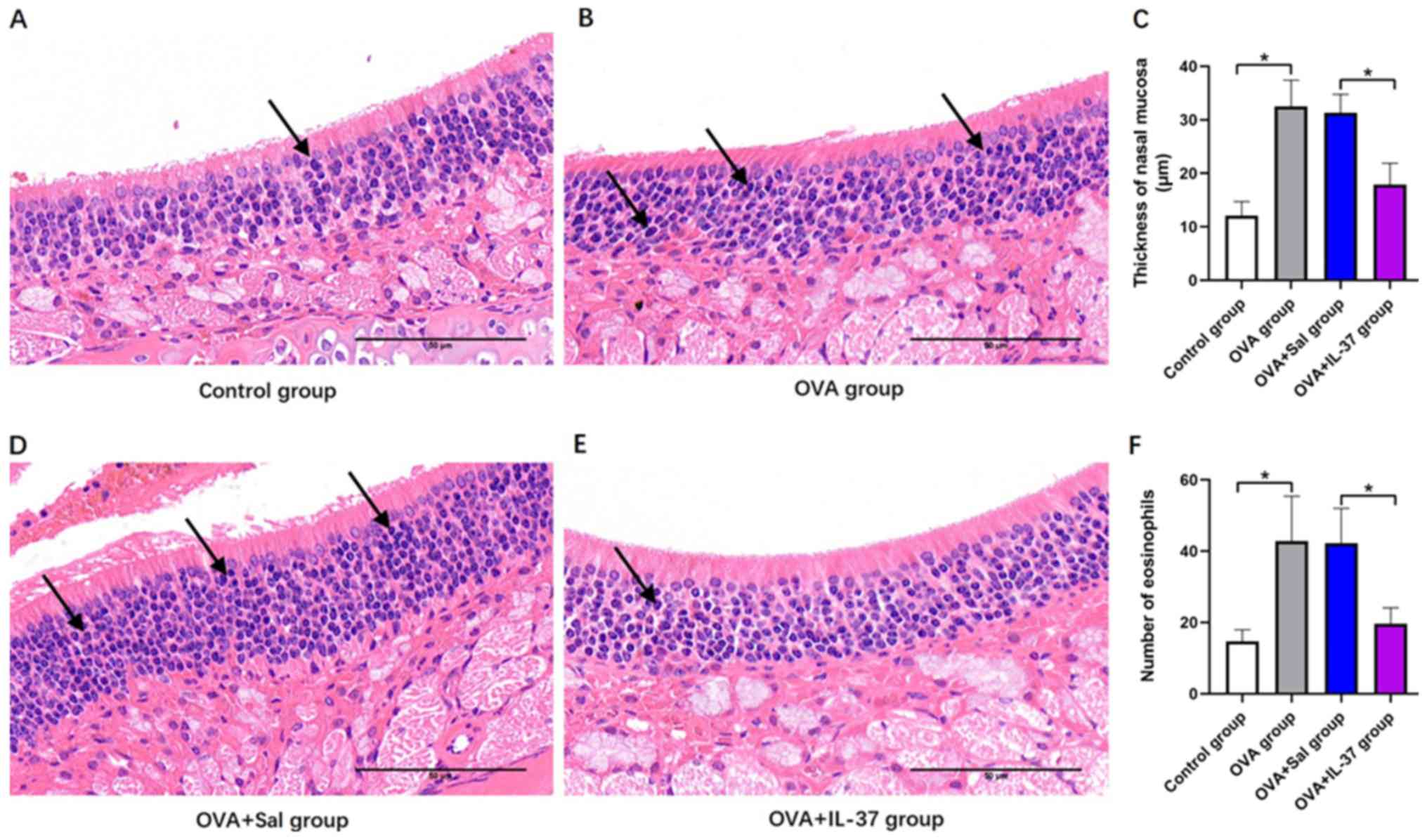
Thickness of the nasal mucosa and
number of eosinophils
The thickness of the nasal mucosa and number of
eosinophils were histologically determined (Fig. 4). Representative histological images
for the different groups are provided in Fig. 4A, B,
D and E. The thickness of the nasal mucosa was
significantly increased in the OVA group compared with that in the
control group (P<0.001; Fig. 4C).
Following IL-37 treatment, a significant decrease in the thickness
of the nasal mucosa of OVA-treated mice was observed (P<0.001;
Fig. 4C). The eosinophil count in
the nasal mucosa was significantly increased in the OVA group
compared with that in the control group (P<0.001; Fig. 4F), and similarly, following IL-37
treatment, OVA-treated mice displayed a significant decrease in the
eosinophil count (P<0.001; Fig.
4F).
Nasal lavage fluid cytokine or
chemical mediator levels
As presented in Fig.
5, the levels of cytokines and chemical mediators, including
IL-4 (Fig. 5B), IL-13 (Fig. 5C), IL-17a (Fig. 5D), CCL11 (Fig. 5E), histamine (Fig. 5F) and substance P (Fig. 5G), were significantly increased in
the nasal lavage fluid of the OVA group compared with those in the
control group. However, IFN-γ levels were decreased in the nasal
lavage fluid of the OVA group compared with those in the control
group (P<0.01; Fig. 5A). After
IL-37 treatment, the effects of OVA on the levels of the
abovementioned cytokines and chemical mediators in the nasal lavage
fluid were significantly inhibited or even reversed (P<0.001;
Fig. 5A-F). A positive correlation
between the levels of CCL11 and IL-4 (r2=0.6765;
P<0.05; Fig. 5I), IL-13
(r2=0.8650; P<0.05; Fig.
5J), IL-17a (r2=0.4562; P<0.05; Fig. 5K), histamine (r2=0.8439;
P<0.05; Fig. 5L) and substance P
(r2=0.8406; P<0.05; Fig.
5M) was identified. Furthermore, a negative correlation between
the levels of CCL11 and IFN-γ was obtained (r2=0.6641;
P<0.05; Fig. 5H).
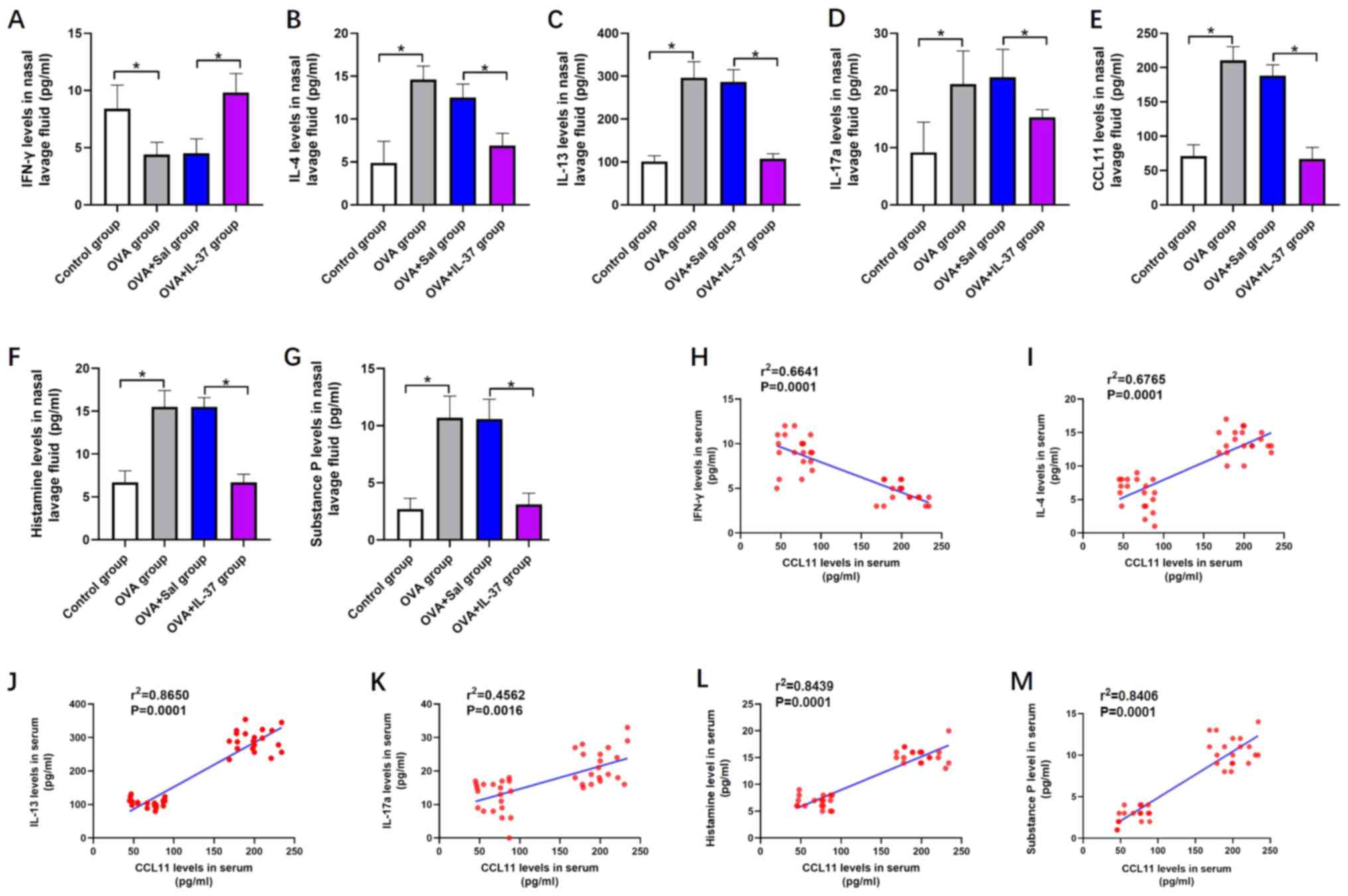 | Figure 5Systemic administration of IL-37
reverses OVA-induced effects on the levels of cytokines or chemical
mediators in the nasal lavage fluid in a mouse model of allergic
rhinitis, and the correlation between CCL11 and IFN-γ, IL-4, IL-13,
IL-17a, histamine and substance P. Nasal lavage fluid level of (A)
IFN-γ, (B) IL-4, (C) IL-13, (D) IL-17a, (E) CCL11, (F) histamine
and (G) substance P. The correlation between nasal lavage fluid
levels of CCL11 and (H) IFN-γ, (I) IL-4, (J) IL-13, (K) IL-17a, (L)
Histamine and (M) Substance. P Values are expressed as the mean ±
standard deviation (n=40). *P<0.05, as indicated. IL,
interleukin; OVA, ovalbumin; CCL11, C-C motif cytokine ligand 11;
IFN-γ, interferon-γ; Sal, saline. |
Serum cytokine levels
The serum cytokine levels are presented in Fig. 6. The serum levels of IL-4 (Fig. 6B), IL-13 (Fig. 6C), IL-17a (Fig. 6D) and CCL11 (Fig. 6E) were significantly increased, while
the levels of IFN-γ were significantly decreased (Fig. 6A) in the OVA group compared with
those in the control group (P<0.001). Following IL-37 treatment,
the effect of OVA on the serum levels of each cytokine was
significantly inhibited or reversed (P<0.001; Fig. 6A-E). A positive correlation between
the serum levels of CCL11 and IL-4 (r2=0.6817;
P<0.05; Fig. 6G), IL-13
(r2=0.6955; P<0.05; Fig.
6H) and IL-17a (r2=0.2384; P<0.05; Fig. 6I) was identified. By contrast, a
negative correlation between the serum levels of CCL11 and IFN-γ
(r2=0.4294; P<0.05; Fig.
6F) was obtained.
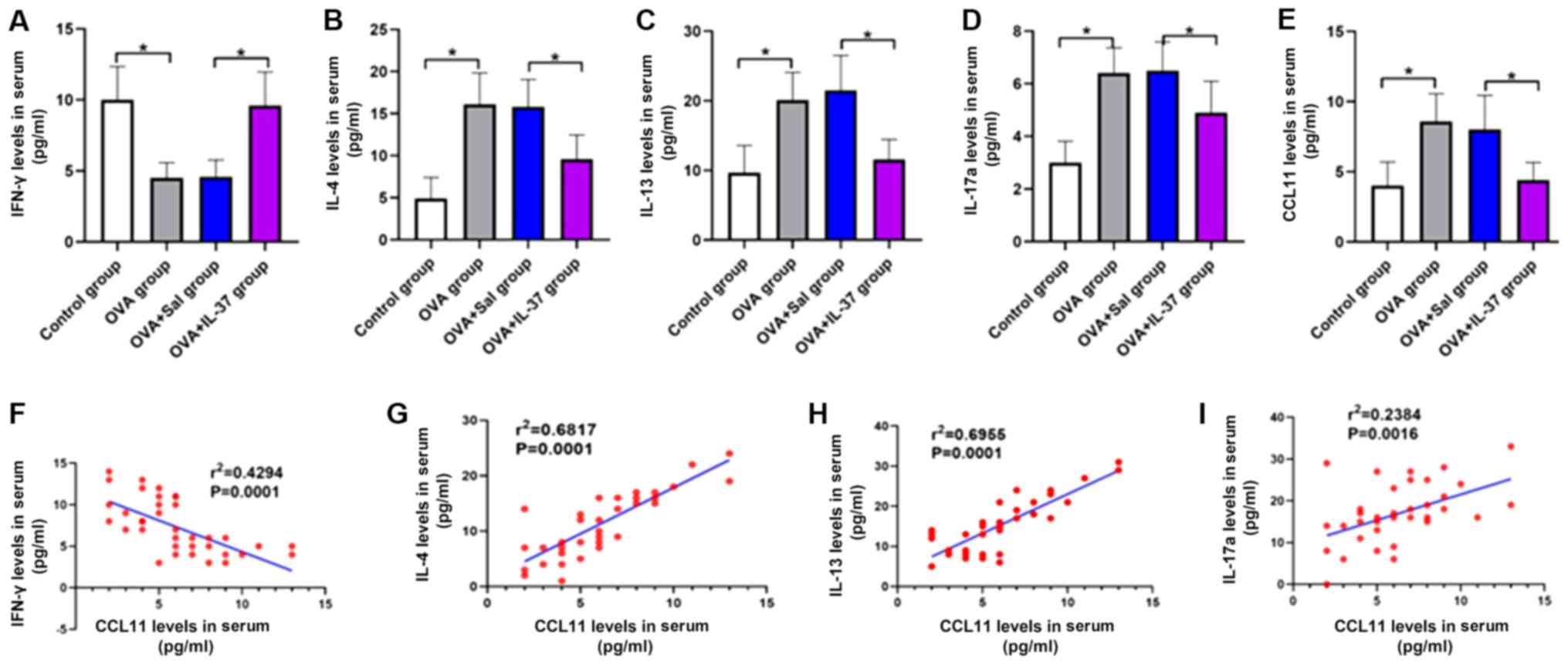 | Figure 6Systemic administration of IL-37
reverses the OVA-induced effects on serum levels of IFN-γ, IL-4,
IL-13, IL-17a and CCL11 in a mouse model of allergic rhinitis, and
the correlation between serum levels of CCL11 and IFN-γ, IL-4,
IL-13 and IL-17a. Serum levels of (A) IFN-γ, (B) IL-4, (C) IL-13,
(D) IL-17a and (E) CCL11. The correlation between serum levels of
CCL11 and (F) IFN-γ, (G) IL-4, (H) IL-13 and (I) IL-17a. Values are
expressed as the mean ± standard deviation (n=40).
*P<0.05, as indicated. IL, interleukin; OVA,
ovalbumin; IFN-γ, interferon-γ; CCL11, C-C motif cytokine ligand
11; Sal, saline. |
Discussion
In the present study, the protective effect and
ability of IL-37 to attenuate the allergic inflammatory response by
decreasing the production of CCL11 in a mouse model of OVA-induced
AR was investigated. Allergic symptoms, including nasal mucosal
eosinophilia, increased thickness of the nasal mucosa, as well as
altered serum IgE, IgG1 and Ig G2a levels, were reduced or reversed
in AR mice treated with IL-37. Furthermore, IL-37 decreased the
levels of cytokines, including IL-4, IL-13, IL-17a and CCL11, in
the serum and nasal lavage fluid of mice with OVA-induced AR.
However, the level of IFN-γ in the serum and nasal lavage fluid of
mice could be increased by IL-37 treatment. In addition,
OVA-induced increases in the levels of histamine and substance P
were reversed by IL-37 administration. CCL11 levels displayed a
positive correlation with the levels of IL-4, IL-13, IL-17a,
histamine and substance P. CCL11 displayed a negative correlation
with the expression of IFN-γ, which suggested that IL-37 may
attenuate the allergic inflammatory response by decreasing CCL11
expression in an OVA-induced mouse model of AR.
Immunoglobulins have major roles in mediating
allergic and inflammatory reactions. The expression of several
immunoglobulin antibodies, including IgE, IgG1 and IgG2a, has been
reported to be involved in B-cell immune responses controlled by
cytokines produced by Th cells, such as TNF-α, IL-4, IL-5, IL-10,
IL-12 and IL-13 (1,26). In particular, allergens may activate
the binding of IgE to the high-affinity IgE receptor FcεRI on the
surface of eosinophils. Subsequently, chemical mediators are
released into the surrounding tissues, causing allergic symptoms
(2,27). In the present study, the altered
serum profiles of IgE, IgG1 and Ig G2a in OVA-treated mice were
reversed by IL-37 treatment, suggesting that IL-37 may downregulate
immune responses in CD4+ T cells. Following IL-37
treatment, the OVA group displayed significantly reduced allergic
symptoms (nasal rubbing and sneezing). The results suggested that
systemic administration of IL-37 decreased the hallmarks of
experimental AR by decreasing the production of IgE, IgG1 and
IgG2a.
The infiltration of eosinophils into the mucosa and
the thickness of the nasal mucosa are important pathological
features of AR. Limited chronic inflammation and a priming effect
are stimulated by eosinophil infiltration into the nasal mucosa,
both of which may intensify nasal hyperreactivity (28). In the present study,
histopathological observations indicated a high degree of
eosinophil infiltration in the interstitium of the nasal mucosa and
an increase in the thickness of the nasal mucosa in the mouse model
of AR. Furthermore, the degree of eosinophil infiltration and the
thickness of the nasal mucosa were reduced by IL-37, which is
consistent with the results of a study performed by Shahsavan et
al (29). These results
indicated that IL-37 may attenuate the allergic inflammatory
response by decreasing eosinophil infiltration in the interstitium
of the nasal mucosa.
IL-4 and IL-13 are immunoregulatory cytokines
secreted predominantly by activated Th2 cells and serve as key
mediators of the allergic response, including the induction of
isotype switching to IgE and the promotion of eosinophil migration
across the endothelium of Th2 lymphocytes (30,31).
Increasing evidence suggests that Th2 cytokines, including IL-4 and
IL-13, which are downregulated by T cells, are elevated in patients
with AR, inducing the itch response via signaling in sensory
neurons (32,33). IL-4 and IL-13 are secreted primarily
by eosinophils and basophils, and they bind to the IgE receptor
with high affinity to initiate IgE-dependent inflammatory reactions
and IgE production by B cells (34).
Furthermore, IL-4 may induce polyamine synthesis, which exerts
anti-inflammatory effects (35). In
the present study, IL-37 decreased the IL-4 and IL-13 levels in the
serum and nasal lavage fluid, indicating that IL-37 attenuated the
allergic inflammatory response by downregulating IL-4 and IL-13
expression. Huang et al (36)
reported that IL-37 is able to downregulate IL-4/IL-13 expression
by inhibiting STAT6 activation and STAT3 phosphorylation. The
results also suggested a potential underlying mechanism of IL-37,
indicating that IL-37 reversed the serum profile of IgE and
eosinophil infiltration in the mucosa.
IL-17 is involved in airway hyperreactivity and
mucus hypersecretion in the upper airway during AR (37). After OVA administration, the IL-17
level was significantly increased, which is consistent with the
results of a previous study (1). By
contrast, IL-37 administration reversed the OVA-induced increases
in the serum and nasal lavage fluid levels of IL-17, indicating
that IL-37 may reverse allergic symptoms (nasal rubbing and
sneezing). IFN-γ is the principal Th1 effector cytokine, which
affects Th1/Th2 differentiation, triggers macrophage production and
inhibits Th2-cell proliferation (38). Zhu et al (39) reported that reductions in IFN-γ
release in in vitro co-culture with IL-37 may partly rely on the
suppressed transcriptional activity of NF-κB (39). The present study suggested that the
concentration of IFN-γ was increased in the OVA + IL-37 group
compared with that in the OVA group, further indicating that IL-37
shifted the Th2 immune response to allergens to a Th1 immune
response.
Although the levels of IL-4, IL-13 and IL-17 were
significantly reduced by IL-37, similar to the results reported in
previous studies (14,16,24,39), the
mechanism of IL-37 in the regulation of cytokines has yet to be
determined. CCL11 is critical for the attraction of eosinophils
during allergic asthma. Therefore, the present study investigated
the serum and nasal lavage fluid levels of CCL11. CCL11 levels were
significantly increased in the OVA group compared with those in the
control group and were reduced following treatment with IL-37. Of
note, CCL11 displayed a positive correlation with various
cytokines, including IL-4, IL-13 and IL-17. According to the
literature, the inhibitory effect of IL-37 on IL-4/IL-13 is
mediated by a reduction in CCL11 production in house dust
mite-induced allergic asthma (24).
Therefore, IL-37-mediated alterations to the levels of CCL11 may
have a role in the regulation of cytokines, including IFN-γ, IL-4,
IL-13 and IL-17.
Despite the important role of IL-37 in the
regulation of CCL11-mediated OVA-induced AR, IL-37 has previously
been reported to inhibit STAT6 activation and STAT3 phosphorylation
to alleviate pulmonary eosinophilia and airway remodeling (36). The possible mechanisms by which IL-37
regulates CCL11 and CCL11 subsequently regulates serum cytokine
levels were not identified in the present study and further
investigation is thus required. In addition, the IL-2 receptor has
been implicated in immune responses and may be a potential
mechanism by which IL-37 relieves allergic inflammation (40). Collectively, the results of the
present study along with those of previous studies suggest that
further investigation is required to identify the underlying
mechanisms of IL-37 during AR.
The present study reported novel results on the
effect of IL-37 in a mouse model of OVA-induced AR. The results
suggested that IL-37 reversed allergic symptoms and the allergic
inflammatory response by downregulating serum cytokine levels and
inhibiting CCL11 expression.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL conceived the idea and designed the study. HL, YS
and SQ performed the experiments. HL and YS analyzed the data and
performed the statistical analysis. HL wrote the manuscript. All
authors have reviewed the manuscript and read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Care
and Use Committee of Beijing Jishuitan Hospital (Beijing,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fan Y, Piao CH, Hyeon E, Jung SY, Eom JE,
Shin HS, Song CH and Chai OH: Gallic acid alleviates nasal
inflammation via activation of Th1 and inhibition of Th2 and Th17
in a mouse model of allergic rhinitis. Int Immunopharmacol.
70:512–519. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Skoner DP: Allergic rhinitis: Definition,
epidemiology, pathophysiology, detection, and diagnosis. J Allergy
Clin Immunol. 108 (Suppl):S2–S8. 2001.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Meltzer EO, Blaiss MS, Derebery MJ, Mahr
TA, Gordon BR, Sheth KK, Simmons AL, Wingertzahn MA and Boyle JM:
Burden of allergic rhinitis: Results from the Pediatric Allergies
in America survey. J Allergy Clin Immunol. 124 (Suppl):S43–S70.
2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sritipsukho P, Satdhabudha A and
Nanthapisal S: Effect of allergic rhinitis and asthma on the
quality of life in young Thai adolescents. Asian Pac J Allergy
Immunol. 33:222–226. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pilette C, Jacobson MR, Ratajczak C, Detry
B, Banfield G, VanSnick J, Durham SR and Nouri-Aria KT: Aberrant
dendritic cell function conditions Th2-cell polarization in
allergic rhinitis. Allergy. 68:312–321. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ciprandi G, Marseglia GL, Castagnoli R,
Valsecchi C, Tagliacarne C, Caimmi S and Licari A: From IgE to
clinical trials of allergic rhinitis. Expert Rev Clin Immunol.
11:1321–1333. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Akdis M and Akdis CA: Mechanisms of
allergen-specific immunotherapy: Multiple suppressor factors at
work in immune tolerance to allergens. J Allergy Clin Immunol.
133:621–631. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang M, Zhang W, Shang J, Yang J, Zhang L
and Bachert C: Immunomodulatory effects of IL-23 and IL-17 in a
mouse model of allergic rhinitis. Clin Exp Allergy. 43:956–966.
2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Moon IJ, Hong SL, Kim DY, Lee CH, Rhee CS
and Min YG: Blocking interleukin-17 attenuates enhanced
inflammation by staphylococcal enterotoxin B in murine allergic
rhinitis model. Acta Otolaryngol. 132 (Suppl 1):S6–S12.
2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shirasaki H, Kanaizumi E, Seki N and Himi
T: Correlation of Local FOXP3-Expressing T Cells and Th1-Th2
Balance in Perennial Allergic Nasal Mucosa. Int J Otolaryngol.
2011(259867)2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Boraschi D, Lucchesi D, Hainzl S, Leitner
M, Maier E, Mangelberger D, Oostingh GJ, Pfaller T, Pixner C,
Posselt G, et al: IL-37: A new anti-inflammatory cytokine of the
IL-1 family. Eur Cytokine Netw. 22:127–147. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ye L, Ji L, Wen Z, Zhou Y, Hu D, Li Y, Yu
T, Chen B, Zhang J, Ding L, et al: IL-37 inhibits the production of
inflammatory cytokines in peripheral blood mononuclear cells of
patients with systemic lupus erythematosus: Its correlation with
disease activity. J Transl Med. 12(69)2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Teng X, Hu Z, Wei X, Wang Z, Guan T, Liu
N, Liu X, Ye N, Deng G, Luo C, et al: IL-37 ameliorates the
inflammatory process in psoriasis by suppressing proinflammatory
cytokine production. J Immunol. 192:1815–1823. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang J, Shen Y, Li C, Liu C, Wang ZH, Li
YS, Ke X and Hu GH: IL-37 attenuates allergic process via
STAT6/STAT3 pathways in murine allergic rhinitis. Int
Immunopharmacol. 69:27–33. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kim DH, Kim SW, Kim SW and Kang JM:
Interleukin-37 relieves allergic inflammation in a house dust mite
allergic rhinitis murine model. Iran J Allergy Asthma Immunol.
16:404–417. 2017.PubMed/NCBI
|
|
16
|
Li C, Shen Y, Wang J, Ma ZX, Ke X, Wang
ZH, Hong SL and Hu GH: Increased expression of IL-1R8 and a
possible immunomodulatory role of its ligand IL-37 in allergic
rhinitis patients. Int Immunopharmacol. 60:152–159. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nold MF, Nold-Petry CA, Zepp JA, Palmer
BE, Bufler P and Dinarello CA: IL-37 is a fundamental inhibitor of
innate immunity. Nat Immunol. 11:1014–1022. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Nold-Petry CA, Lo CY, Rudloff I, Elgass
KD, Li S, Gantier MP, Lotz-Havla AS, Gersting SW, Cho SX, Lao JC,
et al: IL-37 requires the receptors IL-18Rα and IL-1R8 (SIGIRR) to
carry out its multifaceted anti-inflammatory program upon innate
signal transduction. Nat Immunol. 16:354–365. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Kim J, Merry AC, Nemzek JA, Bolgos GL,
Siddiqui J and Remick DG: Eotaxin represents the principal
eosinophil chemoattractant in a novel murine asthma model induced
by house dust containing cockroach allergens. J Immunol.
167:2808–2815. 2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Pope SM, Zimmermann N, Stringer KF, Karow
ML and Rothenberg ME: The eotaxin chemokines and CCR3 are
fundamental regulators of allergen-induced pulmonary eosinophilia.
J Immunol. 175:5341–5350. 2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kuperman DA and Schleimer RP:
Interleukin-4, interleukin-13, signal transducer and activator of
transcription factor 6, and allergic asthma. Curr Mol Med.
8:384–392. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hosokawa Y, Hosokawa I, Shindo S, Ozaki K
and Matsuo T: IL-4 Modulates CCL11 and CCL20 Productions from
IL-1β-Stimulated Human Periodontal Ligament Cells. Cell Physiol
Biochem. 38:153–159. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Miyagawa Y, Murakami A and Ebihara N: The
proteolytic effect of mast cell tryptase to
eotaxin-1/CCL11·eotaxin-2/CCL24 and eotaxin-3/CCL26 produced by
conjunctival fibroblasts. Jpn J Ophthalmol. 63:215–220.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lv J, Xiong Y, Li W, Cui X, Cheng X, Leng
Q and He R: IL-37 inhibits IL-4/IL-13-induced CCL11 production and
lung eosinophilia in murine allergic asthma. Allergy. 73:1642–1652.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bui TT, Piao CH, Song CH and Chai OH:
Skullcapflavone II attenuates ovalbumin-induced allergic rhinitis
through the blocking of Th2 cytokine production and mast cell
histamine release. Int Immunopharmacol. 52:77–84. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhao N, Liu Y, Liang H and Jiang X: Bone
marrow-derived mesenchymal stem cells reduce immune reaction in a
mouse model of allergic rhinitis. Am J Transl Res. 8:5628–5636.
2016.PubMed/NCBI
|
|
27
|
Humbles AA, Lloyd CM, McMillan SJ, Friend
DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH, et
al: A critical role for eosinophils in allergic airways remodeling.
Science. 305:1776–1779. 2004.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Canonica GW and Compalati E: Minimal
persistent inflammation in allergic rhinitis: Implications for
current treatment strategies. Clin Exp Immunol. 158:260–271.
2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Shahsavan S, Pirayesh A, Samani OZ,
Shirzad H, Zamani MA, Amani S, Kazemi SM, Moghni M, Deris F, Bageri
N, et al: The relationship between IL-17A and IL-22 expression and
clinical severity in patients with moderate/severe persistent
allergic rhinitis. Am J Otolaryngol. 40:173–178. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rosenberg HF, Phipps S and Foster PS:
Eosinophil trafficking in allergy and asthma. J Allergy Clin
Immunol. 119:1303–1310; quiz 1311-1312. 2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wills-Karp M, Luyimbazi J, Xu X, Schofield
B, Neben TY, Karp CL and Donaldson DD: Interleukin-13: Central
mediator of allergic asthma. Science. 282:2258–2261.
1998.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhong H, Fan XL, Fang SB, Lin YD, Wen W
and Fu QL: Human pluripotent stem cell-derived mesenchymal stem
cells prevent chronic allergic airway inflammation via
TGF-β1-Smad2/Smad3 signaling pathway in mice. Mol Immunol.
109:51–57. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Norris GT and Kipnis J: Immune cells and
CNS physiology: Microglia and beyond. J Exp Med. 216:60–70.
2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Rubtsov YP, Rasmussen JP, Chi EY, Fontenot
J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR Jr,
et al: Regulatory T cell-derived interleukin-10 limits inflammation
at environmental interfaces. Immunity. 28:546–558. 2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Stockwell BR and Jiang X: A Physiological
Function for Ferroptosis in Tumor Suppression by the Immune System.
Cell Metab. 30:14–15. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Huang N, Liu K, Liu J, Gao X, Zeng Z,
Zhang Y and Chen J: Interleukin-37 alleviates airway inflammation
and remodeling in asthma via inhibiting the activation of NF-κB and
STAT3 signalings. Int Immunopharmacol. 55:198–204. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Xuekun H, Qintai Y, Yulian C and Gehua Z:
Correlation of gammadelta-T-cells, Th17 cells and IL-17 in
peripheral blood of patients with allergic rhinitis. Asian Pac J
Allergy Immunol. 32:235–239. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kidd P: Th1/Th2 balance: The hypothesis,
its limitations, and implications for health and disease. Altern
Med Rev. 8:223–246. 2003.PubMed/NCBI
|
|
39
|
Zhu J, Dong J, Ji L, Jiang P, Leung TF,
Liu D, Ng LG, Tsang MS, Jiao D, Lam CW, et al: Anti-allergic
inflammatory activity of interleukin-37 is mediated by novel
signaling cascades in human eosinophils. Front Immunol.
9(1445)2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Campbell TM and Bryceson YT: IL2RB
maintains immune harmony. J Exp Med. 216:1231–1233. 2019.PubMed/NCBI View Article : Google Scholar
|