Introduction
Insomnia is a subjective experience characterized by
difficulty in falling asleep and/or staying asleep, which results
in poor sleep quality and/or quantity (1). The sleep characteristics of the elderly
are different from those of young people (2). In China, the prevalence of insomnia is
17% and the percentage of the people whose sleep time is <6 h is
23% (3), with increasing prevalence
of insomnia among the elderly (4).
The prevalence of elderly insomnia in China is 47.2% (5) and 57% in America (6). Insomnia severely affects the physical
health and life quality of the elderly (7-11)
and increases social and economic burden (6).
The main clinical characteristic of ageing insomnia
is difficulty initiating sleep (12). Ageing individuals spend more time in
the lighter stages of sleep than in deep sleep in non-rapid eye
movement sleep (NREM), as demonstrated by electroencephalography
(13). The main treatments of ageing
insomnia are psychological/behavioral therapies, pharmacological
treatment or a combination of both (6). Pharmacological treatments are initially
recommended when non-pharmacological options do not attain
satisfying sleep (14). Currently,
the commonly used ageing animal models include: D-galactose
(D-gal)-induced subacute (15),
β-amyloid induced (16,17), thymic senescence (18), rapid (19) and natural (20) ageing models. The ageing model induced
by D-gal has been widely used (21,22) and
has been evaluated in behavioral, biochemical and neurochemical
aspects (23). The modeling methods
of animal models of insomnia mainly include chemical reagent
stimulation using intraperitoneal injection of
para-chlorophenylalanine (PCPA) (24), the horizontal platform environmental
deprivation method (25), the stress
stimulation sleep deprivation method (26), the gentle stimulation deprivation
method and the forced exercise deprivation method (27). Rat and mouse models can be used;
however, rat models are optimal due to model stability in the
establishment of the ageing model induced by D-gal (28). Therefore, the current study
established rat models.
Inflammatory factors are closely related to sleep
and ageing (29,30). Sleep has a regulatory effect on
immune function, with pro-inflammatory cytokines reaching their
peak levels during early night sleep and anti-inflammatory
cytokines reaching their peak levels during daytime waking hours
(31). Inflammatory cytokines
interleukin (IL)-1 and tumor necrosis factor (TNF)-α participate
the regulation of physiological sleep in the central nervous system
(32). Inflammatory cytokines are
also involved in oxidative stress, and inflammation is associated
with aging (33). IL-1β, IL-6 and
TNF-α are the key inflammatory cytokines involved in oxidative
stress. They are closely related to reactive oxygen species (ROS)
and promote the activation of NF-κB, as well as the expression of
proinflammatory factor-induced nitric oxide synthase and
cyclooxygenase-2(34). Sleep is
closely related to neurotransmitters (35). Glutamate, an important excitatory
amino acid transmitter in brain tissue, can stimulate the activity
of neurons (36). Gamma-aminobutyric
acid (GABA), an important inhibitory neurotransmitter in the
central nervous system, can inhibit neuronal excitability and exert
sedative and hypnotic effects (37).
Glutamate and GABA play an important role in maintaining the
balance of nerve cell inhibition and excitation function (38).
In the current study, the ageing insomnia rat model
was induced by continuous subcutaneous injection of D-gal and
intraperitoneal injection of PCPA. The levels of senility and sleep
characteristics of ageing insomnia rat model were evaluated,
including memory function, sleep duration, inflammation factors and
neurotransmitters. The results of the current study may provide
experimental evidence for further research on ageing insomnia.
Materials and methods
Reagents
D-gal (purity, 99%) was purchased from Beijing
Solarbio Science and Technology Co., Ltd. (cat no. 1013G051). PCPA
and pentobarbital were purchased from Sigma-Aldrich, Merck KGaA
(cat no. SHBJ7057). IL-1β (cat no. JL20884), IL-6 (cat no.
JL20896), TNF-α (cat no. JL29364), 5-hydroxytryptamine (5-HT; cat
no. JL13043), glutamate (cat no. JL13664) and gamma-aminobutyric
acid (GABA; cat no. JL47835) kits were purchased from Shanghai
JiangLai Biotech. Co. Ltd. TRIzol® reagent was obtained
from Invitrogen (Invitrogen; Thermo Fisher Scientific, Inc.).
RevertAid First Strand cDNA Synthesis kit was purchased from Thermo
Fisher Scientific, Inc. TB Green Premix Ex Taq II (Tli RNase Plus)
was purchased from Takara Bio, Inc.
Animals
A total of 40 male Sprague Dawley rats (weight,
200±20 g; age, 2 months) were obtained from the Xinjiang Medical
University Animal experiment center [approval protocol no. SYXK (X)
2018-003]. The rats were housed in a specific pathogen-free
environment with room temperature of 25±2˚C, 12-h light/dark cycles
and free access to water and food. Their health and behavior were
monitored daily. The primary humane endpoints used to determine
when animals should be euthanized were reduced heart and
respiration rate. ‘Guidelines for euthanasia of experimental
animals’ was followed to minimize suffering and distress of the
animals (39).
Experimental procedures were conducted in accordance
with the China Experimental Animals Administration Legislation and
were approved by the Ethics Committee of Xinjiang Medical
University.
Establishment of rat models
Ageing rat model induced by D-gal or by PCPA are two
classical rat models used in ageing and insomnia research,
respectively (21,23). D-gal and PCPA were used to establish
ageing insomnia rat model in the current study, which was a
composite rat model. A total of 40 rats were randomly divided into
4 groups: Controls, PCPA group (insomnia group), D-gal group
(ageing group) and PCPA+D-gal group (ageing insomnia group) with 10
rats in each group.
Controls were subcutaneously injected with normal
saline (120 mg/kg) for 42 days, then intraperitoneally injected
with normal saline (300 mg/kg) for 3 days. Rats in the PCPA group
were subcutaneously injected with normal saline (120 mg/kg) for 42
days, then intraperitoneally injected with PCPA (300 mg/kg) for 3
days. Rats in the D-gal group were subcutaneously injected with
D-gal (120 mg/kg) for 42 days and then intraperitoneally injected
with normal saline (300 mg/kg) for 3 days. Rats in the PCPA+D-gal
group were subcutaneously injected with D-gal (120 mg/kg) for 42
days and then intraperitoneally injected with PCPA (300 mg/kg) once
a day for 3 days. Acute and/or adverse reactions of D-gal and PCPA
were not observed during the experiment. Weight gain was measured
every week and for the final time on the day 46.
Morris water maze test
The spatial memory of the rats was assessed via the
Morris water maze test on day 46. The Morris Water Maze (Chengdu
Taimeng Co., Ltd.) consisted of a tank (radius, 120 cm; height, 50
cm) containing water at a height of 30 cm and a temperature of
~25˚C. The water maze was divided into 4 quadrants and the escape
platform (diameter, 12 cm) was placed at a fixed position in the
3rd quadrant, 2.5 cm under the water. The midpoint was
selected as the fixed entry point in each quadrant. The rats were
placed into the water with their backs to the wall of the pool and
detained on the platform for 10 sec as a sign of success in finding
the platform. A water maze device was used to record the time
required for the rats to find the platform from the entry point,
which was defined as escape latency. Rats stayed on the platform
for 10 sec and then the next quadrant experiment was conducted.
When the rats took >2 min to find the target platform, they were
directed to the platform, stayed there for 10 sec and escape
latency was recorded as 2 min. For spatial navigation training, the
experiment was conducted once a day at each water entry point in
each quadrant for a total of 4 times for 5 days. It is hypothesized
that the time of each rat crossing the original platform within 2
min reflects the spatial memory ability, and the platform was
removed only on the 6th day. Latency time to the platform and the
number of target crossings were recorded and processed by a
computer equipped with a TaiMeng Behavior Analysis System (Chengdu
Taimeng Co., Ltd.).
Measurement of pentobarbital-induced
sleeping behavior
Experiments were carried out on day 46.
Pentobarbital was diluted with 0.9% physiological saline and
intraperitoneally injected into the rats (35 mg/kg). The rats were
then placed into another cage. Sleep latency was recorded as time
elapsed between the pentobarbital injection and the time that rats
could maintain 60 sec without flipping. Sleep time was recorded as
the time between the elapse and the time that rats could not
continue to remain in the supine position within 30 sec.
Sample collection
Following behavioral tests, the rats were
anesthetized with 10% chloral hydrate (Chengdu Kelong Chemical Co.,
Ltd.) by intraperitoneal injection into the abdominal cavity with
300 mg/kg (40). No sign of
peritonitis was observed following the injection. No rats died
during the experiment and all 40 rats were anesthetized. Blood
samples (8-10 ml) were collected from the abdominal aorta. The rats
died of hemorrhagic shock following blood sample collection. Death
was determined by non-spontaneous breathing, lack of heartbeat and
cold limbs. Cardiac and respiratory arrest were observed for 3-5
min to confirm death. Body weight at the time of sacrifice (on day
46) are shown in Table SI. Brain
tissue was dissected on ice for further analysis. Experiments,
including anesthesia of rats, abdominal aortic blood collection,
brain tissue separation, plasma centrifugation (2,000 x g for 15
min at 4˚C), liquid nitrogen quick-freezing tissue, labeling and
preservation of tissue and body disposal lasted ~5 h.
ELISA
Neural serum levels of IL-1β, IL-6, TNF-α and
neurotransmitter levels of 5-HT, glutamate and in brain tissue were
detected using ELISA kits, according to the manufacturer's
protocol.
Reverse transcription quantitative
(RT-q) PCR
The hippocampi were isolated and collected to
examine the mRNA level of inflammatory cytokines and
neurotransmitter receptors. RNA extraction was conducted using
TRIzol® reagent. cDNA was synthesized using a RevertAid
First Strand cDNA Synthesis kit, according to the manufacturers'
instructions. RT-qPCR reactions were conducted by a CFX96 RT-qPCR
system (Bio-Rad Laboratories, Inc.) using TB Green™
Premix Ex Taq™ II (Tli RNase Plus). The thermocycling
conditions included pre-denaturation at 95˚C for 10 min,
denaturation at 95˚C for 10 sec, annealing for 10 sec (annealing
temperatures: 60˚C for nuclear factor κ-light-chain-enhancer of
activated B cells (NF-κB), 5-hydroxytryptamine 1A receptor
(5-HT1AR) and metabotropic glutamate receptor 2
(mGluR2); 62˚C for IL-6 and GABA receptor α1 subtype
(GABAARα1); 62.5˚C for TNF-α), extension at
72˚C for 10 sec, 40 cycles of extension at 95˚C for 15 sec and
extension at 60˚C for 1 min. Measurements were performed in
triplicate. The mRNA level of inflammatory cytokines and
neurotransmitters was normalized to reference GAPDH gene and
quantified as relative expression of mRNA using the
2-ΔΔCq method (41). The
primer sequences are listed in Table
I.
 | Table IForward and reverse primers used for
reverse transcription quantitative PCR analysis. |
Table I
Forward and reverse primers used for
reverse transcription quantitative PCR analysis.
| Primer | Forward | Reverse |
|---|
| IL-6 |
5'-AGGAGTGGCTAAGGACCAAGACC-3' |
5'-TGCCGAGTAGACCTCATAGTGACC-3' |
| TNF-α |
5'-CCAATGGCGTGGAGCTGAGAG-3' |
5'-TCTGGTAGGAGACGGCGATGC-3' |
| NF-κb |
5'-TGTGGTGGAGGACTTGCTGAG G-3' |
5'-AGTGCTGCCTTGCTGTTCTTGAG-3' |
|
5-HT1AR |
5'-AGGACCACGGCTACACCATCTAC-3' |
5'-CTGACAGTCTTGCGGATTCGGAAG-3' |
| mGluR2 | 5'-ATC
ACTGGTGGTATTGGCGGTTCC-3' |
5'-TGGCACTGGTAGAGGCGTAGC-3' |
|
GABAARα1 |
5'-TGAGCACACTGACTGGAAGAAGC-3' |
5'-TGGTCTCAGGCGATTGTCATAACC-3' |
| GAPDH |
5'-GACATGCCGCCTGGAGAAAC-3' |
5'-AGCCCAGGATGCCCTTTAGT-3' |
Statistical analysis
Each experiment was repeated three times. Data are
presented as the mean ± standard deviation or standard error. SPSS
software (version 21.0; IBM Corp.) was used for statistical
analysis. One-way ANOVA and Tukey's post-hoc test were performed to
compare differences between groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Insomnia rats and ageing insomnia rats
lost body weight rapidly on day 45 due to sleep deprivation induced
by PCPA injection
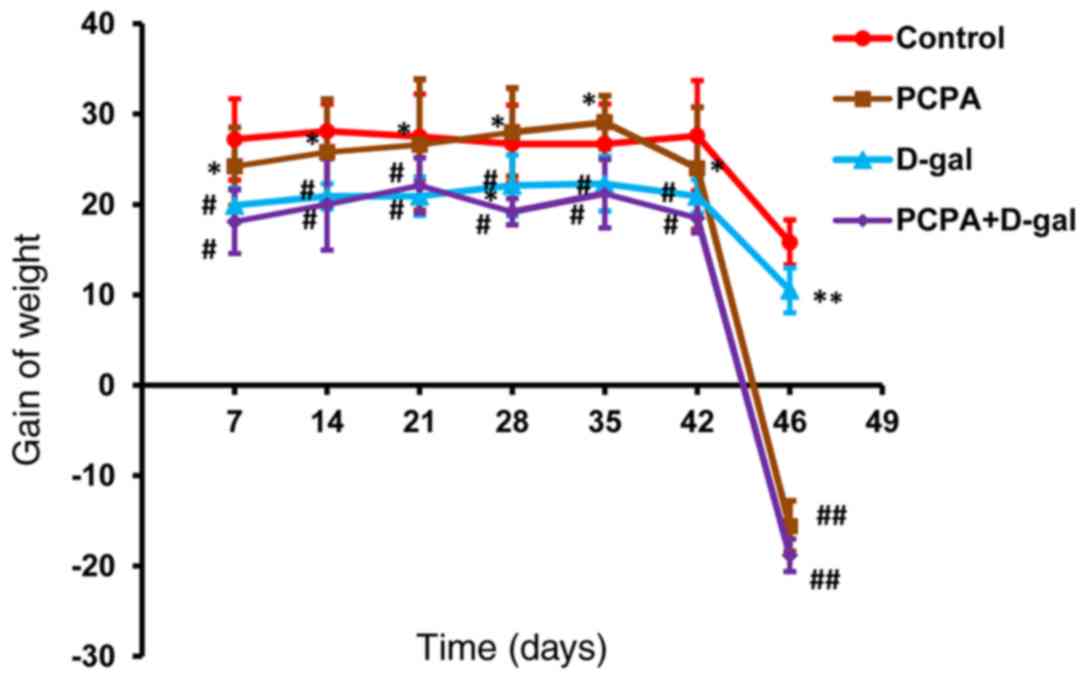
The body weight of the rats in different groups were
initially observed following model establishment. The results
demonstrated that rats in the D-gal and PCPA+D-gal groups appeared
to shed hair and move slower from the 5th week, while rats in the
PCPA and the PCPA+D-gal groups appeared to be excited and irascible
from day 43. Weight gain in the D-gal and PCPA+D groups was
significantly lower compared with controls on days 7-42 (Fig. 1). Furthermore, weight gain in the
PCPA group was significantly higher compared with PCPA+D group on
days 7-42. Weight gain in the PCPA and PCPA+D-gal groups was
significantly lower compared with controls on day 45. Moreover,
weight gain in the D-gal group was significantly higher compared
with the PCPA+D-gal group on day 45.
Spatial memory ability decreases in
ageing insomnia PCPA+D-gal rats
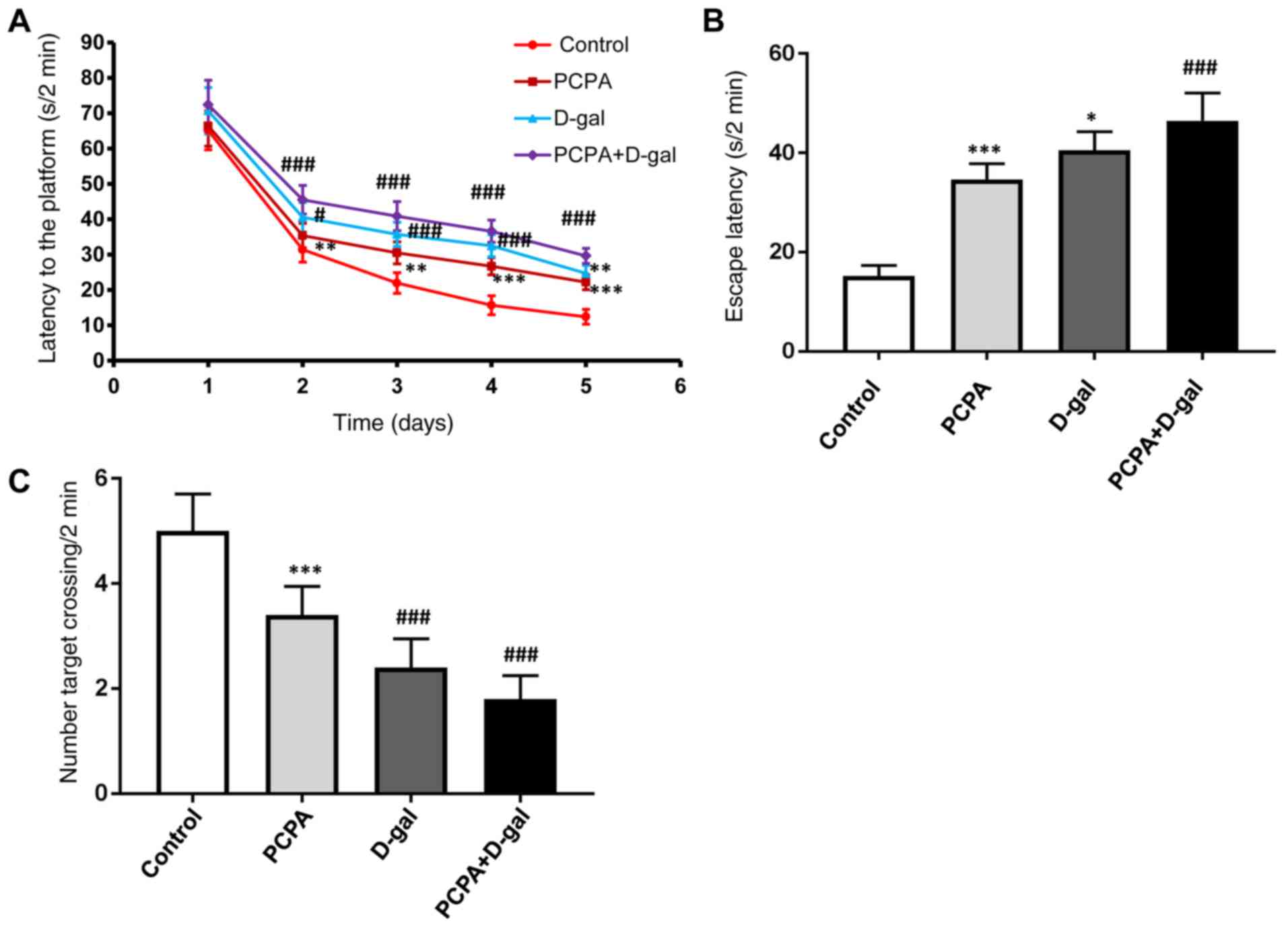
A Morris water maze was performed to assess the
spatial memory ability of the rats. Rats in the PCPA+D-gal groups
suffered significant impairment in spatial learning ability on
account of the longer latency time compared with controls from days
2-5 (Fig. 2A). Furthermore, escape
latency in the PCPA group was significantly shorter compared with
the PCPA+D-gal group from days 2-5 (Fig.
2A). No significant difference of escape latency was found
between the ageing D-gal and insomnia PCPA groups (Fig. 2A).
Subsequently, the spatial memory was evaluated on
the 6th day. The escape latency of rats in PCPA+D-gal groups was
longer compared with the control group (Fig. 2B). Furthermore, escape latency in the
D-gal and PCPA groups was shorter compared with the PCPA+D-gal
group (Fig. 2B).
Rats in D-gal and PCPA+D-gal groups exhibited lower
target crossing numbers compared with the control group (Fig. 2C). Additionally, there were
significantly more target crossings in the PCPA group compared with
the PCP+D-gal group (Fig. 2C). No
significant difference was found between the PCPA and D-gal groups
(Fig. 2C).
According to the results of Morris water maze tests,
the current study concluded that the spatial memory ability of the
PCPA+D-gal ageing insomnia rats significantly decreased compared
with the control group.
Ageing insomnia PCPA+D-gal rats
exhibit shorter sleep latency and sleep time
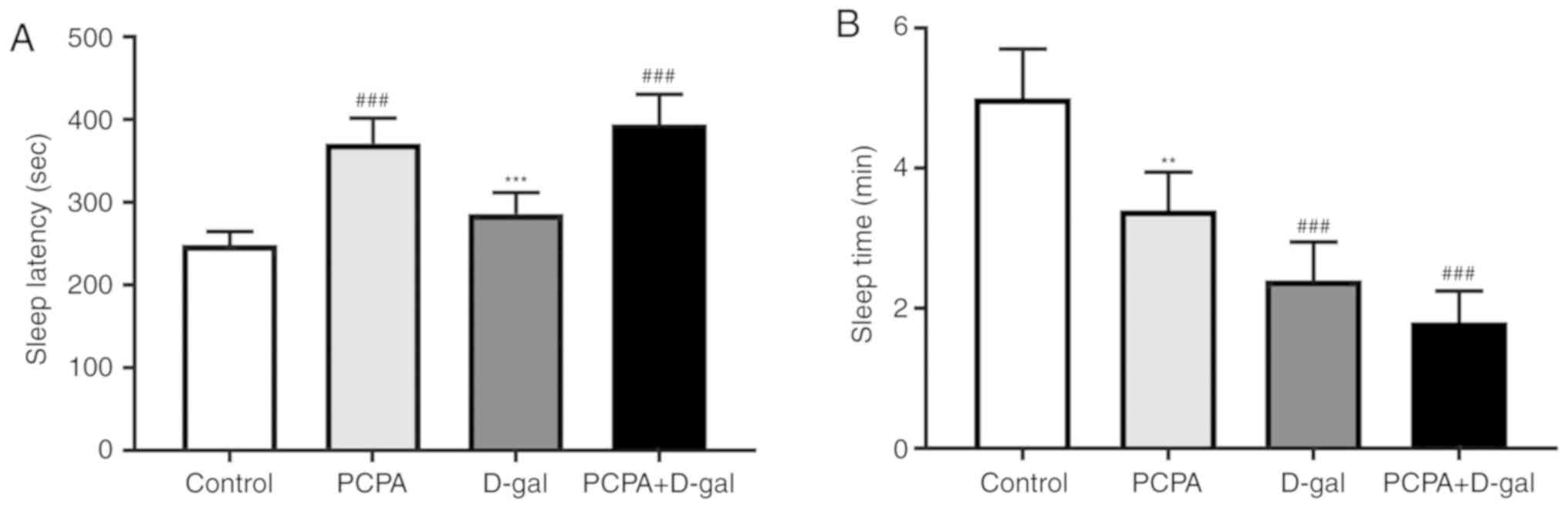
Sleep latency and sleep time through pentobarbital
injections was investigated. The results demonstrated that sleep
latency of PCPA and PCPA+D-gal rats was significantly longer
compared with the control group (Fig.
3A). Furthermore, D-gal rats exhibited shorter sleep latency
compared with the PCPA+D-gal group (Fig.
3A).
The sleep time of D-gal and PCPA+D-gal rats was
significantly shorter compared with the control group (Fig. 3B). Additionally, PCPA rats exhibited
longer sleep time compared with the PCPA+D-gal group (Fig. 3B). There was no significant
difference between the PCPA and D-gal groups (Fig. 3B).
Expression of pro-inflammatory
cytokines and neurotransmitter receptors at mRNA level are also
influenced by D-gal and PCPA injections
Hippocampal IL-6, TNF-α, NF-κB, 5-HT1AR,
mGluR2 and GABAARα1 mRNA expression was
determined using RT-qPCR to establish whether inflammatory
cytokines and neurotransmitter receptors were involved in cognitive
impairment and insomnia. The results demonstrated that IL-6, TNF-α
and NF-κB mRNA expression were upregulated in the hippocampus of
rats in the PCPA, D-gal and PCPA+D-gal groups compared with the
control group (Fig. 4A-C). However,
there was no significant difference in the IL-6, TNF-α and NF-κB
mRNA expression levels in the PCPA and D-gal groups compared with
the PCPA+D-gal group. Additionally, the PCPA and PCPA+D-gal groups
demonstrated downregulated 5-HT1AR mRNA expression
compared with the control group (Fig.
4D). The PCPA, D-gal and PCPA+D-gal groups demonstrated
downregulated GABAARα1 mRNA expression
compared with the control group (Fig.
4F). No significant difference was discovered between D-gal,
PCPA and PCPA+D-gal groups. Furthermore, the PCPA and PCPA+D-gal
groups exhibited upregulated mGluR2 mRNA expression compared with
the control group (Fig. 4E). The
D-gal group also demonstrated downregulated mGluR2 mRNA expression
compared with the PCPA+D-gal group. The results indicate that the
subcutaneous injection of D-gal, intraperitoneal injection of PCPA
and combined injection of D-gal and PCPA altered inflammatory
cytokine and neurotransmitter receptor mRNA expression in the rat
hippocampus.
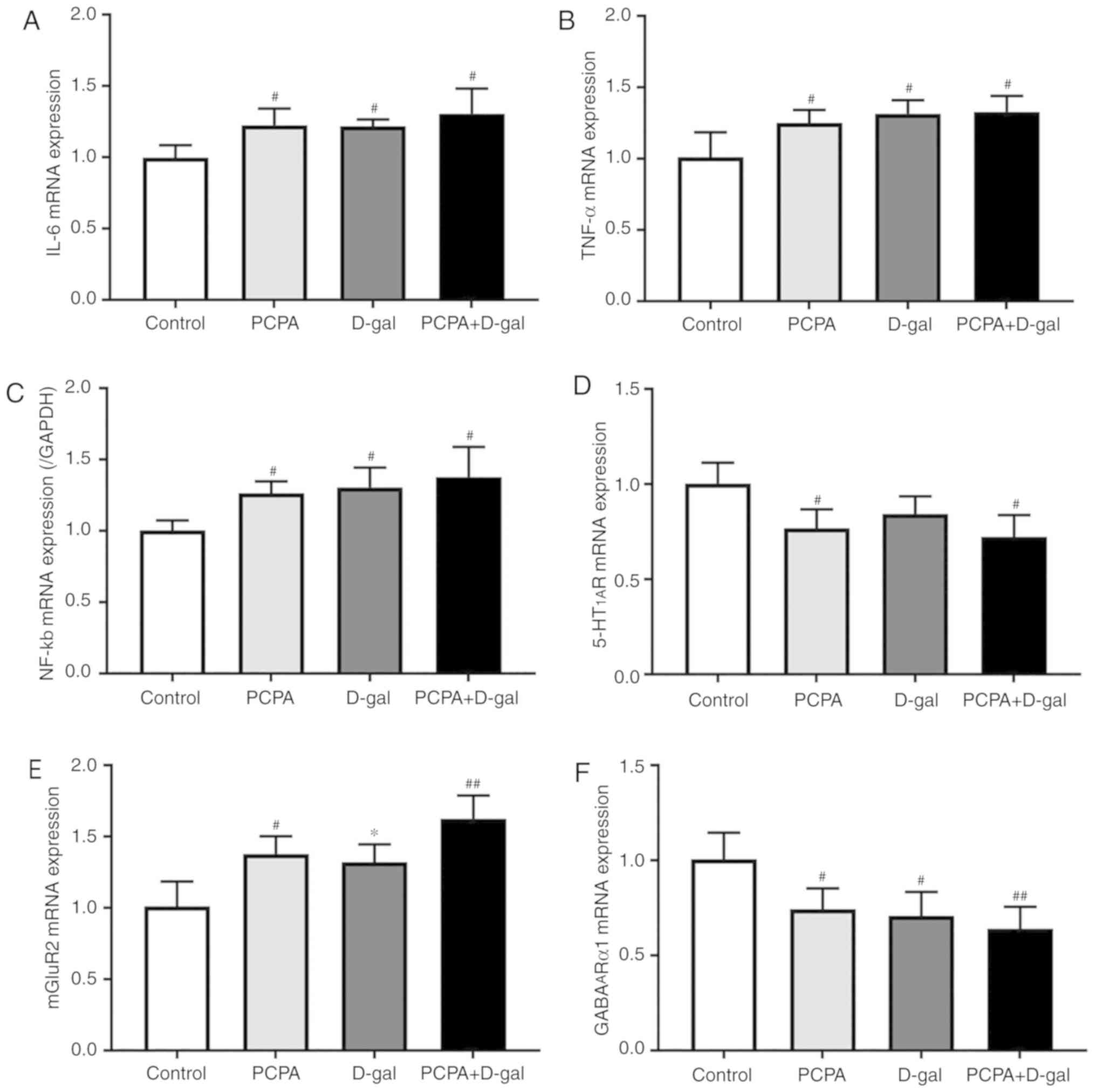 | Figure 4Gene expression of inflammatory
factors IL-6, TNF-α, NF-κb and neurotransmitter receptors of
5-HT1AR, mGluR2, Gamma-aminobutyric acid A receptor α1
subtype in the hippocampus were assayed using RT-PCR. mRNA
expression of (A) IL-6, (B) TNF-α, (C) NF-κb, (D)
5-HT1AR, (E) mGluR2 mRNA, and (F)
GABAARα1. Data are presented as mean ± SEM.
#P<0.05, ##P<0.01 vs. control group.
*P<0.05 vs. PCPA+D-gal group. IL-6, interleukin 6;
TNF-α, tumor necrosis factor α; NF-κb, nuclear factor
κ-light-chain-enhancer of activated B cells; 5-HT1AR,
5-hydroxytryptamine 1A receptor; mGluR2, metabotropic glutamate
receptor 2. |
Expression levels of pro-inflammatory
cytokines in neural serum and neurotransmitters in neural
tissue
Subsequently, ELISA assays were used to investigate
the expression of pro-inflammatory cytokines and neurotransmitters.
Serum expression of IL-1β, IL-6 and TNF-α in the PCPA+D-gal rats
were significantly increased compared with the control group
(Fig. 5A-C). Furthermore, the levels
of these cytokines in the PCPA and D-gal rats were significantly
decreased compared with the PCPA+D-gal group. No significant
difference of IL-1β, IL-6 and TNF-α was found between the PCPA and
D-gal groups. The results indicated that the subcutaneous injection
of D-gal, intraperitoneal injection of PCPA and combined injection
of D-gal and PCPA all induced significant chronic inflammation in
the rats.
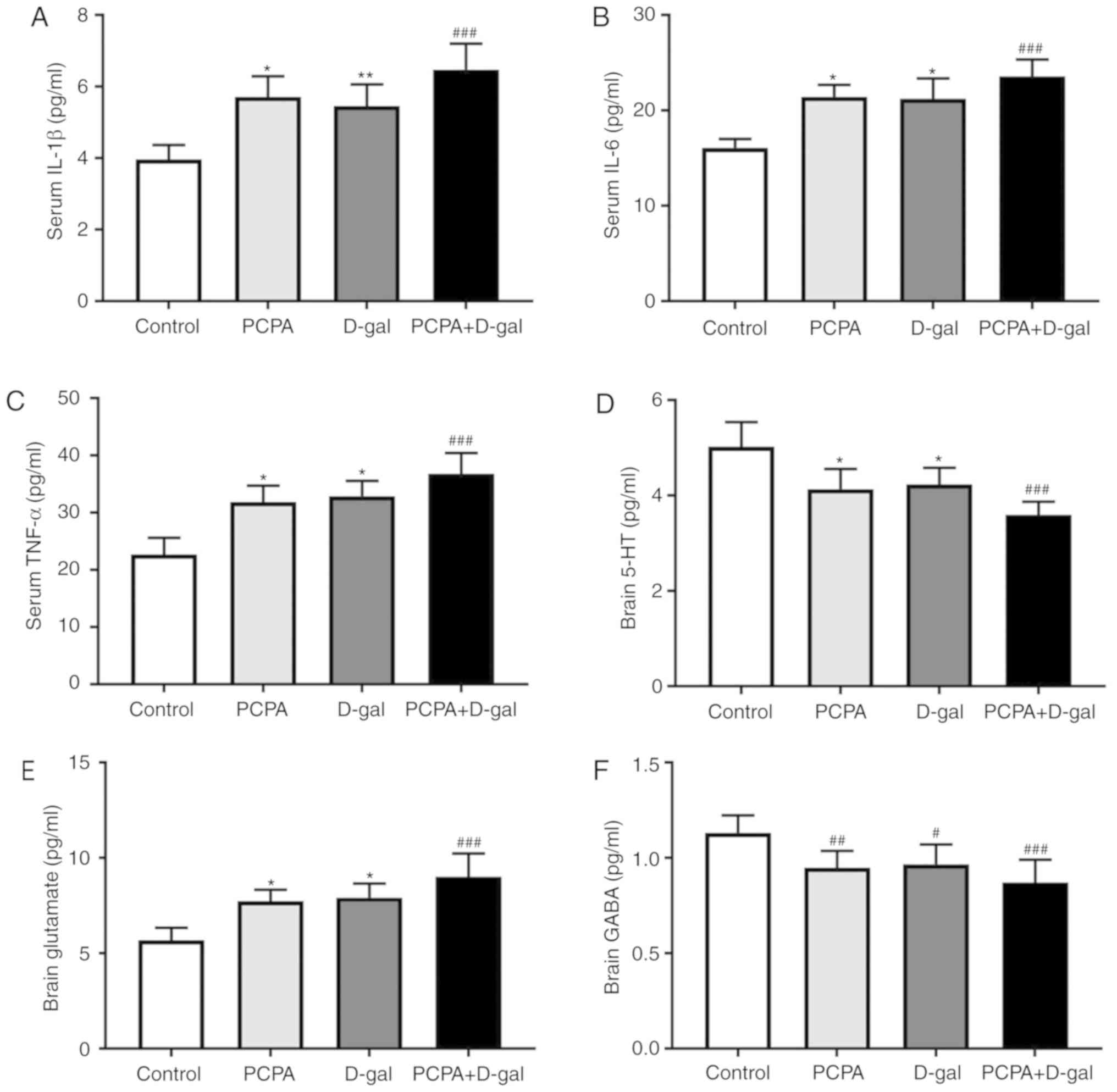 | Figure 5Protein expression of inflammatory
factors IL-1β, IL-6, TNF-α in serum and expression of 5-HT,
glutamate, GABA in neural tissue were assayed via ELISA. Serum (A)
IL-1β, (B) IL-6 (C), TNF-α and neural (D) 5-HT, (E) glutamate and
(F) GABA. Data are presented as mean ± SEM. #P<0.05,
##P<0.01 and ###P<0.001 vs. control
group. *P<0.05, **P<0.01 vs. PCPA+D-gal
group. IL, interleukin, TNF-α, tumor necrosis factor α; 5-HT,
5-hydroxytryptamine; GABA, gamma-aminobutyric acid; PCPA,
para-chlorophenylalanine; D-gal, D-galactose. |
Furthermore, neural levels of 5-HT, glutamate and
GABA were measured. The results demonstrated a significant decrease
in the levels of 5-HT in the PCPA+D-gal groups compared with the
control group (Fig. 5D). The levels
of 5-HT in brains of D-gal and PCPA rats were significantly higher
compared with the PCPA+D-gal group. The PCPA+D-gal groups exhibited
a significant increase in the levels of glutamate compared with the
control group (Fig. 5E).
Furthermore, neural glutamate levels in the D-gal and PCPA rats
were significantly decreased compared with the PCPA+D-gal group
(Fig. 5E). The PCPA and PCPA+D-gal
groups exhibited a significant decrease in GABA levels compared
with the control group (Fig. 5F). No
significant difference was found in the levels of 5-HT, glutamate
and GABA between the D-gal and PCPA groups. These results
demonstrated that the subcutaneous injection of D-gal,
intraperitoneal injection of PCPA and the combined injection of
D-gal and PCPA induced expression changes of the neurotransmitters
in brains of the rats.
Discussion
Oxidative damage induced by ROS serves a crucial
role in the pathophysiology of ageing (42,43).
High doses of D-gal induce ROS overexpression via the metabolism of
D-gal (44). The injection of D-gal
results in the increase of oxygen free radicals in the rat brain
(21). Additionally, ageing
alterations induced by D-gal are similar to natural ageing
processes (45). A previous study
demonstrated that the levels of neurotransmitters in the brain of
PCPA-induced insomnia rats were altered (23). Therefore, D-gal and PCPA were used in
this study to establish rat models of ageing and insomnia,
respectively. Furthermore, another previous study reported that
D-gal-induced ageing rats lose weight rapidly compared with the
control group (46). In the current
study, the results indicated that rats in both D-gal and PCPA+D-gal
groups lost weight rapidly compared with control rats.
Cognitive decline increases with age during natural
ageing (47,48). It has been reported that in the
Morris water maze, the latency time of D-gal rats was longer, the
number of target crossings was lower and that cognitive function
decreased (49). Sleep disorder is
closely related to cognition (50)
and patients with primary insomnia have been reported to suffer
from subjective memory impairment in virtual water mazes (51). Sleep duration is negatively
correlated with age in older adults (52) and chronic insomnia in the elderly is
likely to exacerbate cognitive impairment (53). In the current study, D-gal-induced
ageing rats, PCPA-induced the insomnia rats and D-gal- and
PCPA-induced ageing insomnia rats demonstrated cognitive decline in
the Morris water maze. Therefore, the ageing and insomnia model
exhibited decreased cognitive function.
Chronic inflammation is closely related to ageing
(54). Inflammatory factors, such as
IL-1β, IL-6 and TNF-α, serve roles in D-gal-induced oxidative
stress to simulate the ageing process (46). It has been reported that the plasma
concentrations of IL-1β, IL-6 and TNF-α in the D-gal and PCPA
induced ageing insomnia rats were significantly higher compared
with the control group (55).
Furthermore, inflammatory cytokines affect neurotransmitters
related to sleep, such as norepinephrine and 5-HT, which are
transmitted to the central nervous system (56,57).
IL-1β, IL-6 and TNF-α are the key inflammatory factors related to
sleep regulation (58), and IL-1β
increases non-rapid eye movement sleep in electroencephalography
(59). The I-κ/NF-κB signaling
pathway is associated with sleep regulation and the immune system
and hippocampus play a central role in insomnia (60). A previous study demonstrated that
plasma levels of IL-6 and TNF-α in PCPA-induced insomnia rats were
significantly higher compared with the control group (25). In the current study, the results
demonstrated that the serum content of IL-1β, IL-6 and TNF-α and
mRNA expression of IL-6, TNF-α and NF-κB in neural tissues in the
PCPA+D-gal group were higher compared with control rats.
Glutamate is an important excitatory amino acid
transmitter in brain tissue (36).
mGluR2 is distributed in both pre- and post-synaptic neurons,
inhibits adenylate cyclization and regulates ion channel receptors
by coupling with Gi/o, and negatively regulates neurotransmitter
release (61). mGluR2 is involved in
the physiology of sleep (62).
Additionally, GABA is a major inhibitory neurotransmitter in the
central nervous system (37). GABA
inhibits neuronal excitation in the nervous system, and GABA
receptors are widely used in the treatment of anxiety disorder,
insomnia, epilepsy (62). Glutamate
and GABA serve important roles in maintaining the stability of the
balance between the inhibitory and excitatory functions of nerve
cells (63). It has been
demonstrated the amount of GABA in the brain tissue is positively
associated with the changes in sleep-wake depth (64). Furthermore, GABA serves inhibitory
roles by binding to its receptor (65) and GABAARα1 has
a sedative effect (66). It has been
reported that the expression of glutamate in cerebral cortex,
hypothalamus and hippocampus of in PCPA-induced insomnia rats
increased, while the content of 5-HT and GABA decreased compared
with the control group (23). Woo
et al (67) found that the
expression of GABAARα1 protein was
upregulated in the hypothalamus of mice with improved sleep time.
In the current study, the results demonstrated that expression of
5-HT and GABA decreased while glutamate increased in the D-gal- and
PCPA-induced ageing insomnia rats compared with the control group.
This indicated that ageing insomnia may be associated with 5-HT,
glutamate and GABA neurotransmitters.
The present study established an ageing insomnia rat
model by injection of D-galactose and PCPA. Changes in memory
ability, inflammatory factors and neurotransmitters of model rats
were observed. Future studies should include more behavioral tests,
age-related redox marker detections (oxidative stress), apoptotic
proteins and body weight/5-HT measurements in order to verify the
results of the present study and elucidate ageing insomnia in the
rat model.
In conclusion, the current study established an
ageing insomnia rat model by an injection combination of D-gal with
PCPA, and evaluated the changes in cognitive behavior, sleep
duration, inflammation factors and neurotransmitters in PCPA+D-gal
ageing insomnia rats. This ageing insomnia rat model induced by
D-gal and PCPA may an provide experimental model for further
research on ageing insomnia.
Supplementary Material
The body weight of rats in each group
on day 45.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81560762 and
81960837), the Xinjiang Uygur Autonomous Region Healthy Young
Medical Science and Technology Talents Special Research Project
(grant no. WJWY-201919), General Project of the Natural Science
Foundation of Xinjiang Uygur Autonomous Region (grant no.
2020D01A32) and Xinjiang Uygur Autonomous Region 13th Five-Year Key
Discipline (grant no. 1005).
Availability of data and materials
The datasets used and/or analysed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XR, QW, XZ and ND designed the study; XZ supplied
the samples for this study; XR, GW, TL and DY analyzed the data; XZ
supervised the whole study; XR and XZ wrote he manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures were conducted in
accordance with China Experimental Animals Administration
Legislation and were approved by the Ethics Committee of Xinjiang
Medical University (Urumqi, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chinese guidelines for diagnosis and
treatment of insomnia in adults (2017 version). Chin J Neurol.
51:324–335. 2018.
|
|
2
|
Qaseemg A, Kansagara D, Forciea MA, Cooke
M and Denberg TD: Clinical Guidelines Committee of the American
College of Physicians. Management of Chronic insomnia disorder in
adults: A clinical practice guideline from the American college of
physicians. Ann Intern Med. 165:125–133. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Chen YP, Kartsonaki C, Clarke R, Guo Y, Yu
C, Bian Z, Jiang Q, Li S, Chen J, Li L, et al: Characteristics and
correlates of sleep duration, daytime napping, snoring and insomnia
symptoms among 0.5 million Chinese men and women. Sleep Med.
44:67–75. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang YM, Song M, Wang R, Shi L, He J, Fan
TT, Chen WH, Wang L, Yu LL, Gao YY, et al: Insomnia and
multimorbidity in the community elderly in China. J Clin Sleep Med.
13:591–597. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Liu Y, Dong YH, Li XY, et al:
Meta-analysis of the prevalence of sleep disorder among Chinese
elderly aged 60 years and over. Modern Prev Med, 2014.
|
|
6
|
Abad VC and Guilleminault C: Insomnia in
elderly patients: Recommendations for pharmacological management.
Drugs Aging. 35:791–817. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gulia KK and Kumar VM: Sleep disorders in
the elderly: A growing challenge. Psychogeriatrics. 18:155–165.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kim WH, Kim JH, Kim BS, Chang SM, Lee DW,
Cho MJ and Bae JN: The role of depression in the insomnia of people
with subjective memory impairment, mild cognitive impairment, and
dementia in a community sample of elderly individuals in South
Korea. Int Psychogeriatr. 29:653–661. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Leblanc MF, Desjardins S and Desgagne A:
Sleep cognitions associated with anxiety and depression in the
elderly. Clin Interv Aging. 10:575–582. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Nagai M, Hoshide S, Nishikawa M, Shimada K
and Kario K: Sleep duration and insomnia in the elderly:
Associations with blood pressure variability and carotid artery
remodeling. Am J Hypertens. 26:981–989. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhuang J, Zhan Y, Zhang F, Tang Z, Wang J,
Sun Y, Ding R, Hu D and Yu J: Self-reported insomnia and coronary
heart disease in the elderly. Clin Exp Hypertens. 38:51–55.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ahmed AE, Al-Jahdali H, Fatani A, Al-Rouqi
K, Al-Jahdali F, Al-Harbi A, Baharoon S, Ali YZ, Khan M and
Rumayyan A: The effects of age and gender on the prevalence of
insomnia in a sample of the Saudi population. Ethn Health.
22:285–294. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Moraes W, Piovezan R, Poyares D,
Bittencourt LR, Santos-Silva R and Tufik S: Effects of aging on
sleep structure throughout adulthood: A population-based study.
Sleep Med. 15:401–409. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sateia MJ, Buysse DJ, Krystal AD, Neubauer
DN and Heald JL: Clinical practice guideline for the pharmacologic
treatment of chronic insomnia in adults: An American academy of
sleep medicine clinical practice guideline. J Clin Sleep Med.
13:307–349. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Haider S, Liaquat L, Shahzad S, Sadir S,
Madiha S, Batool Z, Tabassum S, Saleem S, Naqvi F and Perveen T: A
high dose of short term exogenous D-galactose administration in
young male rats produces symptoms simulating the natural aging
process. Life Sci. 124:110–119. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Liu L, Zhao YH, Zeng CQ and Zeng Y:
Research progress in pharmacological effects of Uncaria Hook on
Alzheimer disease models. Yao Xue Xue Bao. 51:536–542.
2016.PubMed/NCBI(In Chinese).
|
|
17
|
Wang G, Chen L, Pan X, Chen J, Wang L,
Wang W, Cheng R, Wu F, Feng X, Yu Y, et al: The effect of
resveratrol on beta amyloid-induced memory impairment involves
inhibition of phosphodiesterase-4 related signaling. Oncotarget.
7:17380–17392. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Novoseletskaya AV, Kiseleva NM, Zimina IV,
Bystrova OV, Belova OV, Inozemtsev AN, Arion VY and Sergienko VI:
Thymus polypeptide preparation tactivin restores learning and
memory in thymectomied rats. Bull Exp Biol Med. 159:623–625.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sasaki K, Han J, Shimozono H, Villareal MO
and Isoda H: Caffeoylquinic acid-rich purple sweet potato extract,
with or without anthocyanin, imparts neuroprotection and
contributes to the improvement of spatial learning and memory of
SAMP8 mouse. J Agric Food Chem. 61:5037–5045. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Salazar C, Valdivia G, Ardiles ÁO, Ewer J
and Palacios AG: Genetic variants associated with neurodegenerative
Alzheimer disease in natural models. Biol Res.
49(14)2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chang YM, Tamilselvi S, Lin HJ, Tsai CC,
Lin YM, Day CH, Viswanadha VP, Chang HN, Kuo WW and Huang CY:
Alpinia oxyphylla Miq extract ameliorates cardiac fibrosis
associated with D-galactose induced aging in rats. Environ Toxicol.
34:172–178. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li G, Yu J, Zhang L, Wang Y, Wang C and
Chen Q: Onjisaponin B prevents cognitive impairment in a rat model
of D-galactose-induced aging. Biomed Pharmacother. 99:113–120.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhao FF, Zhou YZ, Gao L, Qin XM and Du GH:
Advances in the study of the rat model of aging induced by
D-galactose. Yao Xue Xue Bao. 52:347–354. 2017.PubMed/NCBI(In Chinese).
|
|
24
|
Bo A, Si L, Wang Y, Bao L and Yuan H:
Mechanism of Mongolian medical warm acupuncture in treating
insomnia by regulating miR-101a in rats with insomnia. Exp Ther
Med. 14:289–297. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hu Y, Wang YN, Zhang GQ, Dong XZ, Liu WW
and Liu P: Gan-Dan-Liang-Yi-Tang alleviates
p-chlorophenylalanine-induced insomnia through modification of the
serotonergic and immune system. Exp Ther Med. 12:3087–3092.
2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang B, Zhang Q and Ma YN: Effect of
chronic restraint stress on rats' sleep phase and intervention of
suanzaoren decoction. Inf Traditional Chin Med. 31:126–129.
2014.
|
|
27
|
Leenaars CH, Dematteis M, Joosten RN,
Eggels L, Sandberg H, Schirris M, Feenstra MG and Van Someren EJ: A
new automated method for rat sleep deprivation with minimal
confounding effects on corticosterone and locomotor activity. J
Neurosci Methods. 196:107–117. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhao FF, Zhou YZ and Gao L: Research
progress of d-galactose induced aging rat model. Chin J Pharm.
52:347–354. 2017.
|
|
29
|
Kang WS, Park HJ, Chuang JH and Kim JW:
REM sleep deprivation increases deprivation the expression of
interleukin genes in mice hypothalamus. Neuorosci Lett. 556:73–78.
2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li J, Zhang YC and Chen G: Effect of
ginkgo biloba extract EGb761 on hippocampal neuronal injury and
carbonyl stress of D-Gal-Induced aging rats. Evid Based Complement
Alternat Med. 2019(5165910)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ganz FD: Sleep and immune function. Crit
Care Nurse. 32:e19–e25. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Mullington JM, Simpson NS, Meier-Ewert HK
and Haack M: Sleep loss and inflammation. Best Pract Res Clin
Endocrinol Metab. 24:775–784. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sun L, Zhao Q, Xiao Y, Liu X, Li Y, Zhang
J, Pan J and Zhang Z: Trehalose targets Nrf2 signal to alleviate
d-galactose induced aging and improve behavioral ability. Biochem
Biophys Res Commun. 521:113–119. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hritcu L, Bagci E, Aydin E and Mihasan M:
Antiamnesic and antioxidants effects of ferulagoangulata, essential
oil against scopolamine-induced memory impairment in laboratory
rats. Neurochem Res. 40:1799–1809. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang W and Xu TL: Chloride homeostasis
differentially affects GABA(A)receptor-and glycine
receptor-mediated effects on spontaneous circuit activity in
hippocampal cell culture. Neurosci Lett. 406:11–16. 2006.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Shi YF and Yu YQ: The roles of glutamate
in sleep and wakefulness. Zhejiang Da Xue Xue Bao Yi Xue Ban.
42:583–590. 2013.PubMed/NCBI(In Chinese).
|
|
37
|
Jembrek MJ and Vlainic J: GABA receptors:
Pharmacological potential and pitfalls. Curr Pharm Des.
21:4943–4959. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
He B, Bi K, Jia Y, Wang J, Lv C, Liu R,
Zhao L, Xu H, Chen X and Li Q: Rapid analysis of neurotransmitters
in rat brain using ultra-fast liquid chromatography and tandem mass
spectrometry: Application to a comparative study in normal and
insomnic rats. J Mass Spectrom. 48:969–978. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Cicero L, Fazzotta S, Palumbo VD, Cassata
G and Lo Monte AI: Anesthesia protocols in laboratory animals used
for scientific purposes. Acta Biomed. 89:337–342. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zeng X, Zhang L, Sun L, Zhang D, Zhao H,
Jia J and Wang W: Recovery from rat sciatic nerve injury in
vivo through the use of differentiated MDSCs in vitro.
Exp Ther Med. 5:193–196. 2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Yang C, Du YK, Wang J, Luan P, Yang QL,
Huang WH and Yuan L: Transplanted Adipose-derived stem cells
ameliorate testicular dysfunction in a D-galactose-induced aging
rat model. J Cell Physiol. 230:2403–2414. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhen YZ, Lin YJ, Li KJ, Zhang GL, Zhao YF,
Wang MM, Wei JB, Wei J and Hu G: Effects of rhein lysinate on
D-galactose-induced aging mice. Exp Ther Med. 11:303–308.
2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Qu Z, Zhang J, Yang H, Huo L, Gao J, Chen
H and Gao W: Protective effect of tetrahydropalmatine against
d-galactose induced memory impairment in rat. Physiol Behav.
154:114–125. 2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Cardoso A, Magano S, Marrana F and Andrade
JP: D-Galactose high-dose administration failed to induce
accelerated aging changes in neurogenesis, anxiety, and spatial
memory on young male wistar rats. Rejuvenation Res. 18:497–507.
2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Chen P, Chen F and Zhou B: Antioxidative,
anti-inflammatory and anti-apoptotic effects of ellagic acid in
liver and brain of rats treated by D-galactose. Sci Rep.
8(1465)2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Bubbico G, Chiacchiaretta P, Parenti M, di
Marco M, Panara V, Sepede G, Ferretti A and Perrucci MG: Effects of
second language learning on the plastic aging brain: Functional
connectivity, cognitive decline, and reorganization. Front
Neurosci. 13(423)2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Koen JD and Rugg MD: Neural
dedifferentiation in the aging brain. Trends Cogn Sci. 23:547–559.
2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zhu J, Mu X, Zeng J, Xu C, Liu J, Zhang M,
Li C, Chen J, Li T and Wang Y: Ginsenoside Rg1 prevents cognitive
impairment and hippocampus senescence in a rat model of
D-galactose-induced aging. PLoS One. 9(e101291)2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Sindi S, Johansson L, Skoog J, Mattsson
AD, Sjöberg L, Wang HX, Fratiglioni L, Kulmala J, Soininen H,
Solomon A, et al: Sleep disturbances and later cognitive status: A
multi-centre study. Sleep Med. 52:26–33. 2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Kuhn M, Hertenstein E, Feige B, Landmann
N, Spiegelhalder K, Baglioni C, Hemmerling J, Durand D, Frase L,
Klöppel S, et al: Declarative virtual water maze learning and
emotional fear conditioning in primary insomnia. J Sleep Res.
27(e12693)2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Dzierzewski JM, Dautovich N and Ravyts S:
Sleep and cognition in older adults. Sleep Med Clin. 13:93–106.
2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Porter VR, Buxton WG and Avidan AY: Sleep,
cognition and dementia. Curr Psychiatry Rep. 17(97)2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Jurk D, Wilson C, Passos JF, Oakley F,
Correia-Melo C, Greaves L, Saretzki G, Fox C, Lawless C, Anderson
R, et al: Chronic inflammation induces telomere dysfunction and
accelerates ageing in mice. Nat Commun. 2(4172)2014.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Duan DD, Wang KX, Zhou YZ, Qin XM, Gao L
and Du GH: Baicalein exerts beneficial effects in
d-galactose-induced aging rats through attenuation of inflammation
and metabolic dysfunction. Rejuvenation Res. 20:506–516.
2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Ruiz FS, Andersen ML, Martins RC, Zager A,
Lopes JD and Tufik S: Immune alterations after selective rapid eye
movement or total sleep deprivation in healthy male volunteers.
Innate Immun. 18:44–54. 2012.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Zhao Q, Peng C, Wu X, Chen Y, Wang C and
You Z: Maternal sleep deprivation inhibits hippocampal neurogenesis
associated with inflammatory response in young offspring rats.
Neurobiol Dis. 68:57–65. 2014.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Hurtado-Alvarado G, Pavón L,
Castillo-García SA, Hernández ME, Domínguez-Salazar E,
Velázquez-Moctezuma J and Gómez-González B: Sleep loss as a factor
to induce cellular and molecular inflammatory variations. Clin Dev
Immunol. 2013(801341)2015.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Dantzer R: Cytokine-induced sickness
behavior: Where do we stand? Brain Behav Immun. 15:7–24.
2001.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Xiang B, Liu K, Yu M, Liang X, Huang C,
Zhang J, He W, Lei W, Chen J, Gu X and Gong K: Systematic genetic
analyses of GWAS data reveal an association between the immune
system and insomnia. Mol Genet Genomic Med.
7(e00742)2019.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Trabanco AA and Cid JM: mGluR2 positive
allosteric modulators: A patent review (2009-present). Expert Opin
Ther Pat. 23:629–647. 2013.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Wood CM, Wafford KA, McCarthy AP, Hewes N,
Shanks E, Lodge D and Robinson ESJ: Investigating the role of
mGluR2 versus mGluR3 in antipsychotic-like effects, sleep-wake
architecture and network oscillatory activity using novel Han
Wistar rats lacking mGluR2 expression. Neuropharmacology.
140:246–259. 2018.PubMed/NCBI View Article : Google Scholar
|
|
63
|
He B, Li Q, Jia Y, Zhao L, Xiao F, Lv C,
Xu H, Chen X and Bi K: A UFLC-MS/MS method for simultaneous
quantitation of spinosin, mangiferin and ferulic acid in rat
plasma: Application to a comparative pharmacokinetic study in
normal and insomnic rats. J Mass Spectrom. 47:1333–1340.
2012.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Weber F, Chung S, Beier KT, Xu M, Luo L
and Dan Y: Control of REM sleep by ventral medulla GABAergic
neurons. Nature. 526:435–438. 2015.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Hoffmann KM, Beltran L, Ziemba PM, Hatt H
and Gisselmann G: Potentiating effect of glabridin from Glycyrrhiza
glabra on GABAA receptors. Biochem Biophys Rep.
6:197–202. 2016.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Mckernan RM, Rosahl TW, Reynolds DS, Sur
C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, et
al: Sedative but not anxiolytic properties of benzodiazepines are
mediated by the GABA(A) receptor alpha1 subtype. Nat Neurosci.
3:587–592. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
67
|
Woo JH, Ha TW, Kang JS, Hong JT and Oh KW:
Potentiation of decursinol angelate on pentobarbital-induced
sleeping behaviors via the activation of GABAA-Ergic systems in
rodents. Korean J Physiol Pharmacol. 21:27–36. 2017.PubMed/NCBI View Article : Google Scholar
|