Introduction
Total joint arthroplasty is a safe and effective
method that dramatically improves quality of life and restores the
function of the patient with arthritis of the hip and knee
(1-4).
Although its general success is beyond dispute, postoperative
complications still accrue, including prosthetic joint infection
(PJI), which is an important cause of implant failure and revision
arthroplasty should be carried out in most of the cases (5-9).
The financial cost is estimated at U.S. $96,166 per patient
requiring revision arthroplasty for infection, which is 4.8 times
the cost of a primary arthroplasty (10,11). Due
to its insidious onset, early and accurate diagnosis is crucial.
Late diagnosis is known to decrease the chance of saving the
prosthesis and the joint function, leading to more bone destruction
and difficulty in revision surgery (12). Since currently there is no diagnostic
gold standard for the identification of PJI, diagnosis is currently
based on clinical signs, laboratory and microbiological tests,
histopathology and imaging studies (10,13-17).
However, aseptic prosthetic loosing may present with similar
symptoms as PJI and similar imaging features, which often leads to
incorrect diagnosis (18). So,
diagnosis of PJI remains a clinical challenge (19).
Laboratory tests including C reactive protein,
erythrocyte sedimentation rate, white blood cells count and gram
staining are currently recommended but are not specific,
particularly for the early stage of infection (20,21). In
the past two decades, new molecular techniques have been applied to
PJIs to increase the diagnostic yield including utilizing
polymerase chain reaction (PCR) (22). Bacterial ribosomal RNA (rRNA) PCR is
reported to be a rapid and more sensitive tool for microbiological
diagnosis in most studies, while other studies yielded
controversial results (20,23). Previous meta-analysis studies have
few references and comparatively little evidence (24). In the present study, new contents
were added on the basis of previous studies, including the new
studies in the past five years, patients and subgroup analysis.
Furthermore, the inclusion criteria were reformulated (16S rRNA was
selected as the target gene for diagnosis of PJI), and studies
using other genes were excluded. Therefore, the purpose of the
present study was to perform a meta-analysis to establish the
overall diagnostic accuracy of 16S rRNA PCR assays for diagnosing
PJI.
Materials and methods
Search strategy
A systemic search of the English medical literature
of using 16S rRNA PCR in diagnosis of PJI published between January
1980 and December 2018 was performed. The data collection and
reporting was in line with the Preferred Reporting Items for
Meta-Analyses (PRISMA) Statement (25). Databases including PubMed (www.ncbi.nlm.nih.gov/pubmed), Web of Science
(www.webofknowledge.com), Cochrane
Central Register (www.cochranelibrary.com), EMBASE (www.embase.com) and Wiley Online Library of Controlled
Trials (onlinelibrary.wiley.com) were used. The search
strategy was based on the combination of the terms: i) ‘PCR’ or
‘polymerase chain reaction’, ‘reverse transcription-PCR’,
‘real-time PCR’; and ii) ‘prosthesis infection’ or ‘prosthetic
joint infection’ or ‘septic loosening’. Searches were limited to
human subjects. Moreover, these searches were supplemented with
manual searches of references within the interested published
articles to identify additional studies. When necessary, the
authors were contacted for more information. Initially, there were
no restrictions as to the form of publication in order to achieve a
highly sensitive search. However, conference abstracts were
excluded due to the limited data presented.
Inclusion criteria and exclusion
criteria
Only studies meeting the following criteria were
included in this meta-analysis: i) Written in English language; ii)
16S rRNA was the targeted gene in the diagnosis of PJI; iii)
studies with definite clinical diagnosis of PJI: A sinus tract
connected to the prosthesis, purulent fluid visible in the synovial
fluid or surgical incision, microbiological cultures positive from
at least two samples around the prosthesis and acute inflammation
in the histopathological periprosthetic tissue sections; iv)
sensitivity and specificity were provided or can be calculated; v)
>10 patients or samples were included in the study.
Studies that fell under the following conditions
were excluded from the present study: i) Studies presenting
non-original data, conference abstracts, editorials, reviews,
guidelines and studies conducted in animals were excluded; ii)
studies with no definite clinical diagnosis of PJI and sensitivity
and specificity cannot be determined; iii) PCR assay with other
target genes in diagnosis of PJI.
Data extraction and quality
assessment
Two authors (YZ and SFS) independently reviewed the
titles and abstracts of the relevant articles in light of the
inclusion criteria. When an article's abstract fulfilled the
criteria, the full text was reviewed. Any disagreement in the
selection was resolved by a third author. The main elements
extracted included the authors' names, area, study design, clinical
sample, study years, sex, blinded status, age, sex and number of
patients. In order to evaluate the diagnostic performance, a 2x2
table including true-positive, false-positive, false-negative and
true-negative were used for identifying PJIs. These were derived
from the data provided in the studies. The quality assessment was
conducted on all of the included studies. The quality of the
included studies was assessed using the diagnostic accuracy study
quality tool (QUADAS-2) by Review Manager 5.3 (The Nordic Cochrane
Centre, The Cochrane Collaboration).
Statistical analysis
Recommended standard methods for diagnostic
meta-analysis were used (26,27).
Review Manager 5.3 (The Nordic Cochrane Centre, The Cochrane
Collaboration) was used for the quality evaluation and Meta-disc
software was used for statistical analysis (version 1.4). Various
indexes were calculated including sensitivity, specificity,
positive likelihood ratio (PLR), negative likelihood ratio (NLR)
and diagnostic odds ratio (DOR) with corresponding 95% confidence
intervals (CIs). A summary of receiver operating characteristic
(SROC) curves was obtained to assess the overall performance of the
tests by Meta-Disc 1.4. The area under the curve (AUC) displays the
trade-off between sensitivity and specificity. An AUC of 1.0
indicates perfect discriminatory ability to distinguish cases from
non-cases. The SROC and AUC range between 0 and 1, with higher
values indicating a better test performance (28,29).
Statistical heterogeneity was determined by chi-square test and I².
Meta-regression and subgroup analyses were performed to assess
potential heterogeneity and Deeks' funnel plot was used to evaluate
the publication bias analyzed with Meta-Disc 1.4 and Stata 12.0
(StataCorp LLC). All statistical tests were two-sided, P<0.05
was considered to indicate a statistically significant
difference.
Results
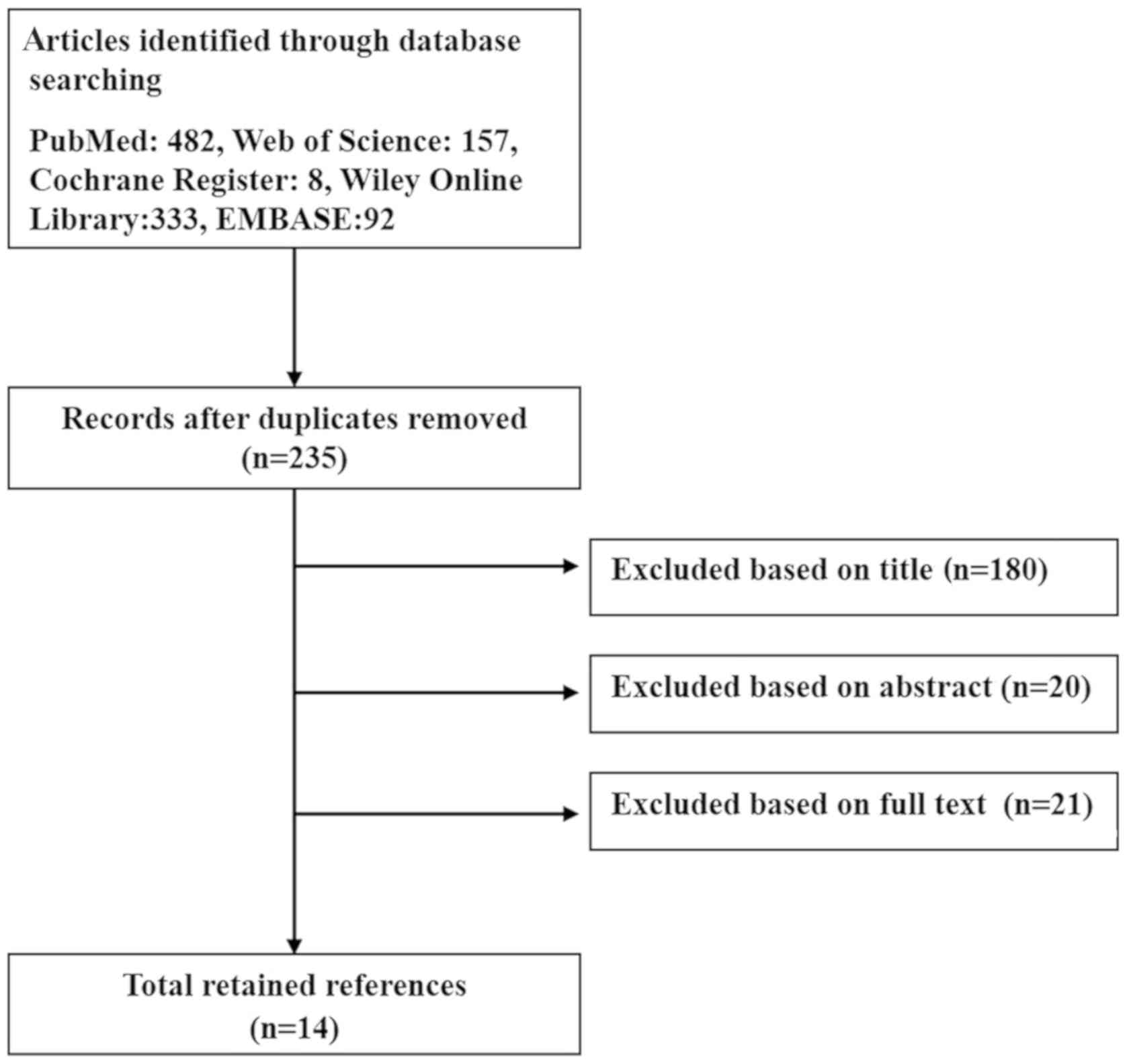
Following independent reviews of the title, abstract
and the full text, a total of 14 English language publications
(Fig. 1) of studies on 16S rRNA
expression for the diagnosis of PJI were included in the present
meta-analysis, based on the aforementioned inclusion and exclusion
criteria. A publication by Rak et al (30) used sonication on fluid and tissue
samples for the diagnosis of PJI. A total of 15 studies included in
the 14 publications enrolled 2,070 patients with an age range of
23-96 years. Table I presents
baseline characteristics of these studies including clinical
characteristics of the patients. Table
II shows true-positive, false-positive, false-negative and
true-negative of the 15 studies. A graphical summary of the
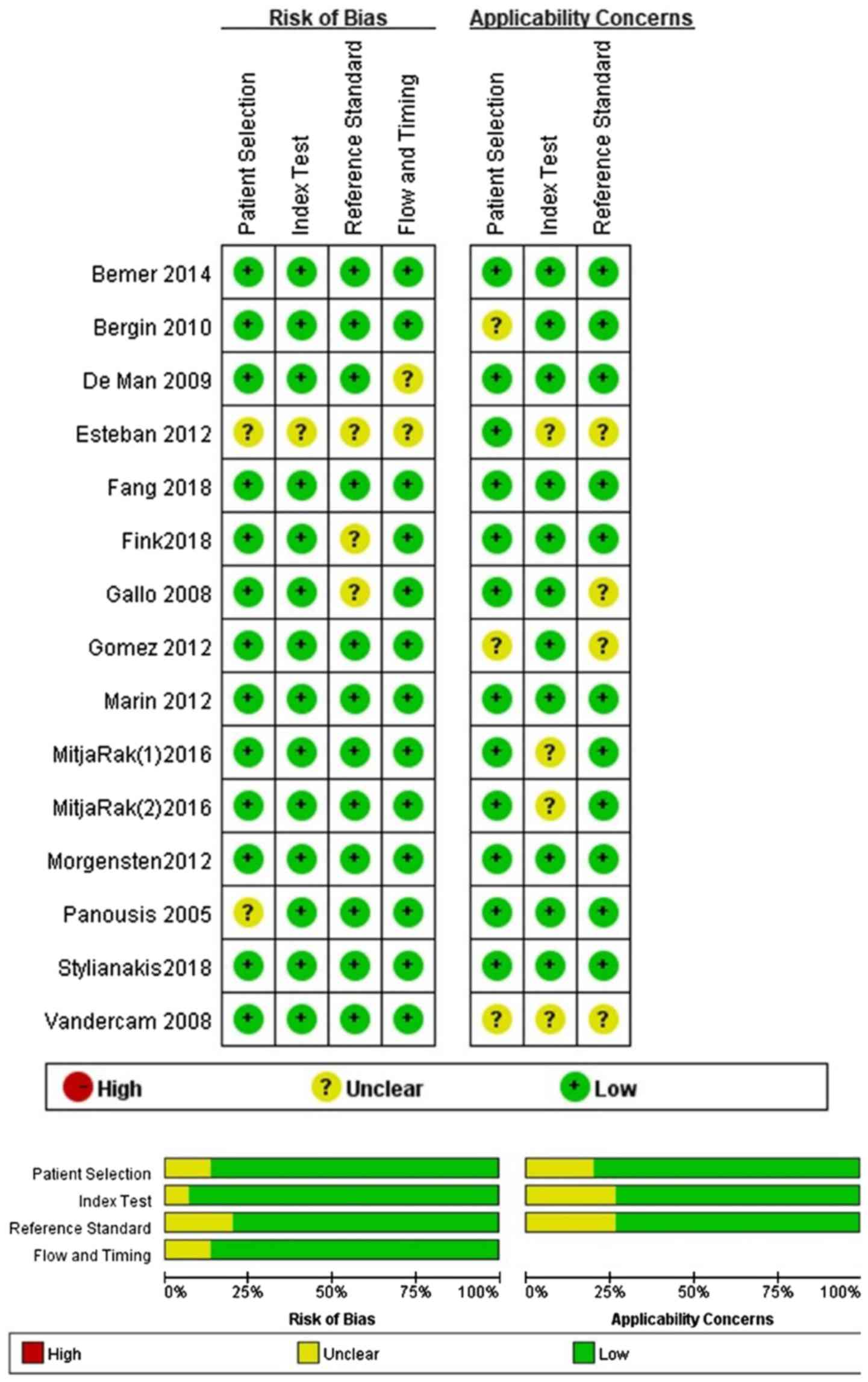
methodological assessment based on QUADAS-2 quality assessment for
the included studies is illustrated and all the included studies
demonstrated a relatively low risk of bias and applicability
concern (Fig. 2). Significant
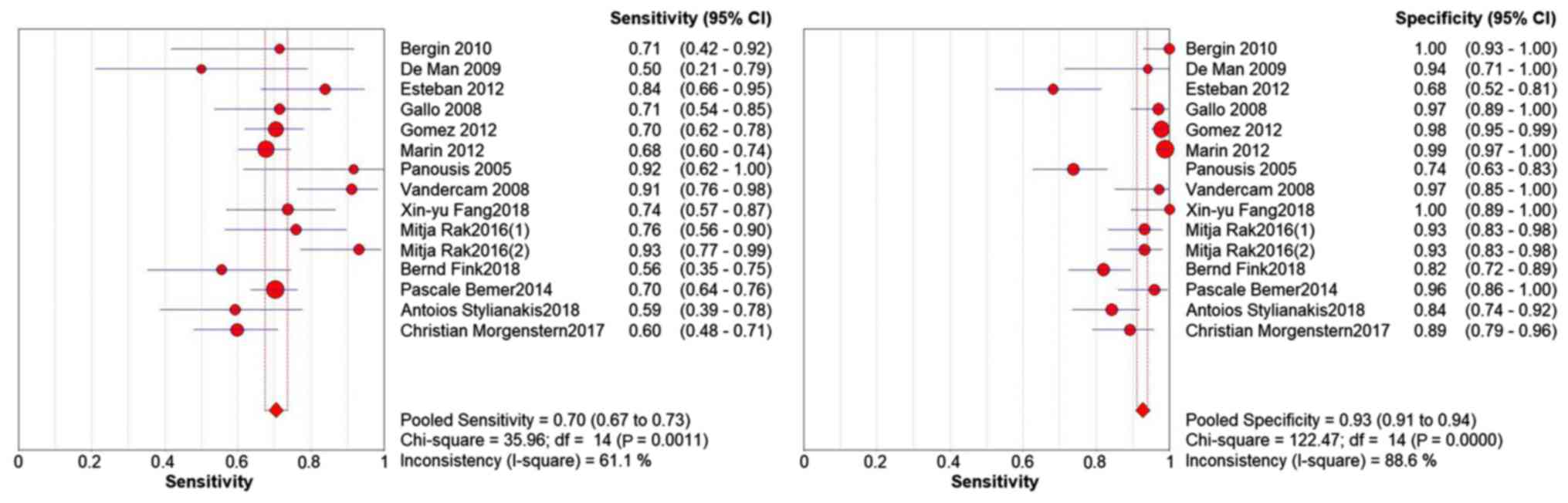
heterogeneity among studies was detected by sensitivity (I²=61.1%),
specificity (I²=88.6%, Figs. 3 and
4). This indicates significant
heterogeneity between studies. The Spearman correlation coefficient
was 0.082 (P=0.771), which indicated that the heterogeneity was not
caused by threshold effects between the included studies.
 | Table IDetails of the 15 studies included in
the present meta-analysis. |
Table I
Details of the 15 studies included in
the present meta-analysis.
| | | | | | | | | | Number of patients
(samples) |
|---|
| Study, author and
year (Refs) | Country | Data
collection | Study design | Biological
sample | Center | Blinded status | Age, years | Sex (m/f) | Septic | Aseptic |
|---|
| Bergin
2010(48) | USA | NR | Prospective | Joint fluid | Single-center | NR | NR | NR | 14 | 50 |
| De Man
2009(49) | Switzerland | 2001-2005 | Retrospective | Joint fluid and
periprosthetic tissue | Single-center | NR | 53-80 | 13/13 | 12 | 17 |
| Esteban
2012(35) | Spain | 2004-2009 | Retrospective | Sonicate fluid | Multi-center | NR | 23-96 | NR | 31 | 44 |
| Gallo 2008(34) | Czech Republic | 2003-2005 | Prospective | Joint fluid | Single-center | NR | 35-80 | 72/42 | 35 | 66 |
| Gomez 2012(36) | USA | 2006-2011 | Retrospective | Sonicate fluid | Single-center | Yes | 24-92 | 183/183 | 135 | 231 |
| Marin 2012(37) | Spain | 2004-2007 | Prospective | Joint fluid | Single-center | Yes | 33-92 | 86/36 | 176 | 321 |
| Panousis
2005(23) | UK | NR | Prospective | Joint fluid | Single-center | NR | 24-85 | 35/56 | 12 | 80 |
| Vandercam
2008(50) | Belgium | NR | Prospective | Intraoperative
tissue samples | Single-center | Yes | 41-82 | 21/20 | 34 | 35 |
| Fang 2018(38) | China | 2014-2016 | Prospective | Joint fluid | Single-center | Yes | 47-78 | 20/51 | 38 | 33 |
| Rak-PT
2016(30) | Slovenia | 2011-2016 | Prospective | Periprosthetic
tissue | Single-center | Yes | 29-88 | 21/46 | 29 | 58 |
| Rak-SF
2016(30) | Slovenia | 2011-2016 | Prospective | Sonicate fluid | Single-center | Yes | 29-88 | 21/46 | 29 | 58 |
| Fink 2018(45) | Germany | 2016-2017 | Prospective | Joint fluid | Single-center | Yes | 41-91 | 55/61 | 27 | 89 |
| Bemer 2014(51) | France | 2010-2012 | Prospective | Periprosthetic
tissue | Multi-center | Yes | 63-79 | 127/137 | 215 | 49 |
| Stylianakis
2018(52) | USA | 2011-2015 | Prospective | Joint fluid | Single-center | Yes | 49-90 | 32/82 | 27 | 70 |
| Morgenstern
2018(53) | Germany | 2014-2015 | Prospective | Joint fluid | Single-center | Yes | 32-92 | 54/88 | 77 | 65 |
 | Table IIData showing true-positives,
false-positives, false-negatives and true-negatives, the
sensitivities, specificities and the DOR of the 15 studies. |
Table II
Data showing true-positives,
false-positives, false-negatives and true-negatives, the
sensitivities, specificities and the DOR of the 15 studies.
| Study, author and
year (Refs) | Tp | Fp | Fn | Tn | Sensitivity | Specificity | DOR |
|---|
| Bergin
2010(48) | 10 | 0 | 4 | 50 | 0.71 | 1 | 235.67 |
| De Man
2009(49) | 6 | 1 | 6 | 16 | 0.50 | 0.94 | 16 |
| Esteban
2012(35) | 26 | 14 | 5 | 30 | 0.84 | 0.68 | 11.14 |
| Gallo 2008(34) | 25 | 2 | 10 | 64 | 0.71 | 0.97 | 80 |
| Gomez 2012(36) | 95 | 5 | 40 | 226 | 0.7 | 0.98 | 107.35 |
| Marin 2012(37) | 119 | 4 | 57 | 317 | 0.68 | 0.99 | 165.45 |
| Panousis
2005(23) | 11 | 21 | 1 | 59 | 0.92 | 0.74 | 30.9 |
| Vandercam
2008(50) | 31 | 1 | 3 | 34 | 0.91 | 0.97 | 351.33 |
| Fang 2018(38) | 28 | 0 | 10 | 33 | 0.74 | 1 | 30.90 |
| Rak-PT
2016(30) | 22 | 4 | 7 | 54 | 0.76 | 0.93 | 42.43 |
| Rak-SF
2016(30) | 27 | 4 | 2 | 54 | 0.93 | 0.93 | 182.25 |
| Fink 2018(45) | 15 | 16 | 12 | 73 | 0.55 | 0.82 | 5.70 |
| Bemer 2014(51) | 151 | 2 | 64 | 47 | 0.7 | 0.96 | 55.45 |
| Stylianakis
2018(52) | 16 | 11 | 11 | 59 | 0.59 | 0.84 | 7.80 |
| Morgenstern
2018(53) | 46 | 7 | 31 | 58 | 0.6 | 0.89 | 12.29 |
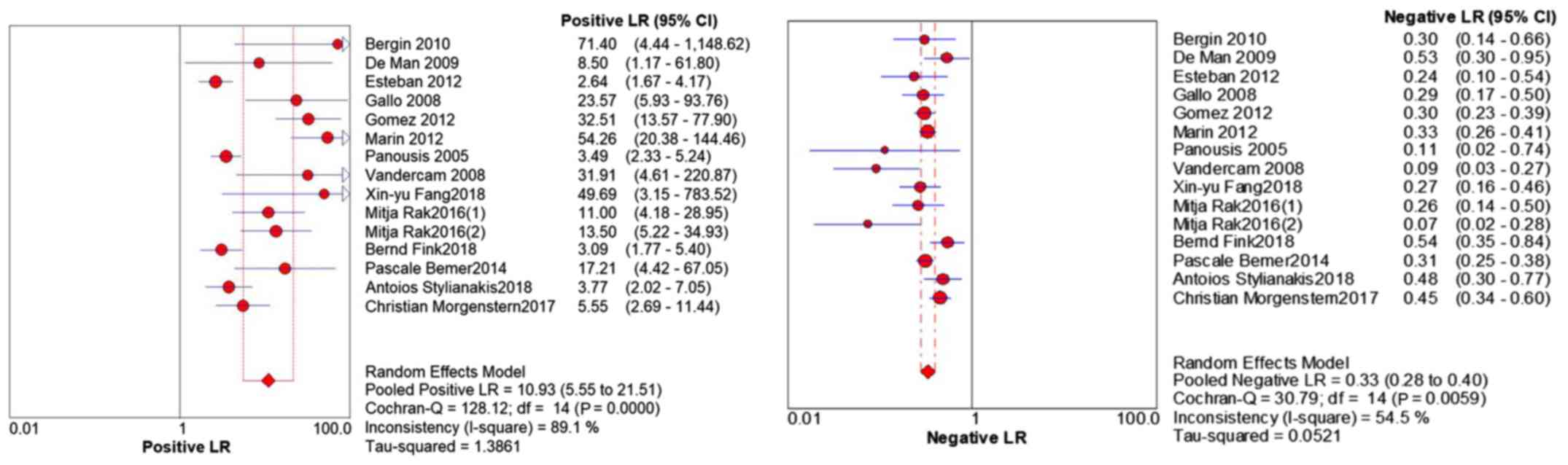
The pooled analysis revealed: Sensitivity, 0.70 (95%
CI 0.67-0.73); specificity, 0.93 (95% CI 0.91-0.94); PLR, 10.93
(95% CI 5.55-21.51); NLR, 0.33 (95% CI 0.28-0.40); and DOR, 41.77
(95% CI 19.90-87.68) (Figs. 3 and
4; Table III). The corresponding SROC
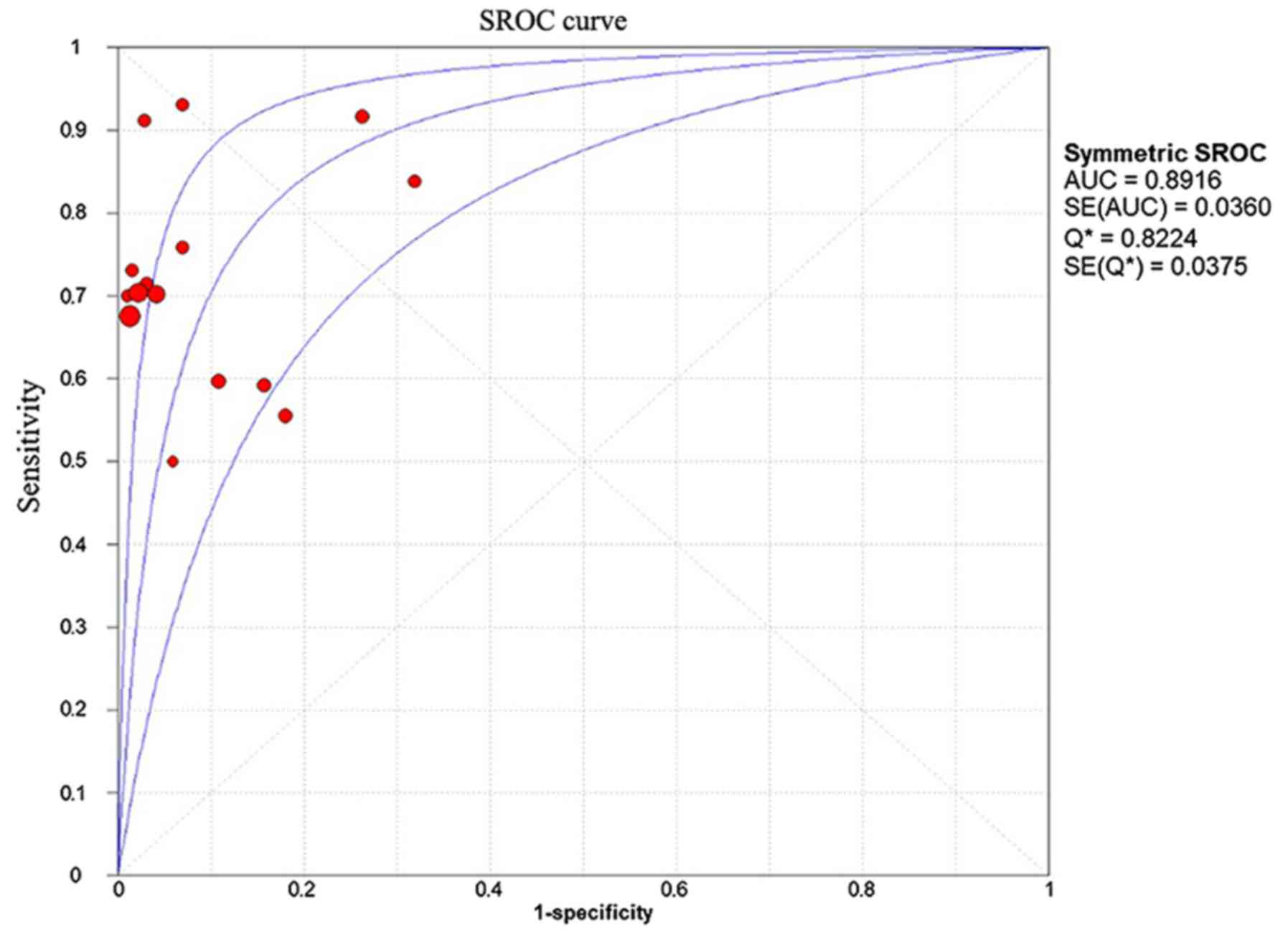
(Fig. 5) shows an AUC of 0.89, and
the pooled diagnostic accuracy is 0.82 with a standard error of
0.037, which indicates high overall accuracy of 16S rRNA PCR for
PJI.
 | Table IIISubgroup analyses. |
Table III
Subgroup analyses.
| A, Clinical
sample |
|---|
| Subgroup
analyses | Studies, n | Sensitivity (95%
CI) | Specificity (95%
CI) | PLR (95% CI) | NLR (95% CI) | DOR (95% CI) | AUC (SE) |
|---|
| Overall
studies | 15 | 0.70
(0.67-0.73) | 0.93
(0.91-0.94) | 10.93
(5.55-21.51) | 0.33
(0.28-0.40) | 41.77
(19.90-87.68) | 0.89 (0.0375) |
| Joint fluid | 8 | 0.67
(0.62-0.71) | 0.92
(0.90-0.94) | 10.09
(3.83-26.61) | 0.38
(0.31-0.64) | 32.89
(10.73-100.76) | 0.8128
(0.0794) |
| Sonicate fluid | 3 | 0.76
(0.69-0.82) | 0.93
(0.90-0.96) | 10.25
(1.45-72.45) | 0.22
(0.11-0.43) | 56.78
(10.44-308.89) | 0.9310
(0.0505) |
| Periprosthetic
tissue | 3 | 0.73
(0.68-0.78) | 0.95
(0.90-0.98) | 14.56
(7.02-30.23) | 0.22
(0.11-0.44) | 68.15
(24.43-190.08) | 0.9860
(0.0304) |
| B, Study
design |
| Subgroup
analyses | Studies, n | Sensitivity (95%
CI) | Specificity (95%
CI) | PLR (95% CI) | NLR (95% CI) | DOR (95% CI) | AUC (SE) |
| Prospective | 12 | 0.70
(0.67-0.74) | 0.93
(0.91-0.94) | 11.55
(5.47-24.39) | 0.33
(0.26-0.40) | 46.64
(19.44-111.87) | 0.9044
(0.0437) |
| Retrospective | 3 | 0.71
(0.64-0.78) | 0.93
(0.90-0.96) | 8.89
(0.86-91.97) | 0.34
(0.23-0.51) | 29.32
(5.36-160.51) | 0.8766
(0.0359) |
| C, Center |
| Subgroup
analyses | Studies, n | Sensitivity (95%
CI) | Specificity (95%
CI) | PLR (95% CI) | NLR (95% CI) | DOR (95% CI) | AUC (SE) |
| Single-center | 13 | 0.70
(0.66-0.73) | 0.94
(0.92-0.95) | 12.21
(5.76-25.87) | 0.33
(0.27-0.41) | 46.73
(20.11-108.61) | 0.9032
(0.0436) |
| Multi-center | 2 | 0.72
(0.66-0.77) | 0.83
(0.74-0.90) | 6.38
(0.44-92.65) | 0.31
(0.25-0.38) | 23.68
(4.56-120.92) | NA |
| D, Blinded
status |
| Subgroup
analyses | Studies, n | Sensitivity (95%
CI) | Specificity (95%
CI) | PLR (95% CI) | NLR (95% CI) | DOR (95% CI) | AUC (SE) |
| NR | 5 | 0.75
(0.66-0.83) | 0.85
(0.80-0.89) | 6.62
(2.62-16.75) | 0.32
(0.22-0.48) | 30.81
(10.81-87.81) | 0.8894
(0.0293) |
| Yes | 10 | 0.70
(0.67-0.73) | 0.95
(0.93-0.96) | 12.79
(5.74-28.48) | 0.33
(0.27-0.46) | 46.55
(17.88-121.20) | 0.8899
(0.0695) |
| E, Area |
| Subgroup
analyses | Studies, n | Sensitivity (95%
CI) | Specificity (95%
CI) | PLR (95% CI) | NLR (95% CI) | DOR (95% CI) | AUC (SE) |
| USA and Asia | 4 | 0.70
(0.63-0.76) | 0.96
(0.93-0.98) | 20.90
(3.12-139.86) | 0.33
(0.26-0.42) | 60.50
(9.19-398.40) | 0.5076
(0.1565) |
| Europe | 11 | 0.71
(0.67-0.74) | 0.91
(0.89-0.93) | 9.32
(4.46-19.50) | 0.32
(0.25-0.41) | 38.36
(16.47-89.32) | 0.9072
(0.0344) |
Due to the heterogeneity caused by the non-threshold
effects between the included studies, a subgroup analysis was
conducted to explore the possible sources of heterogeneity.
Subgroup analysis was conducted according to the clinical sample,
study design, center, blinded status and country (Table III). In the subgroup of the
clinical sample, both sonicated fluids and periprosthetic tissues
showed higher sensitivity (0.76, 95% CI 0.69-0.82; and 0.73, 95% CI
0.68-0.78, respectively) and specificity (0.93, 95% CI 0.90-0.96;
0.95, 95% CI 0.90-0.98) compared with joint fluid (sensitivity,
0.67, 95% CI 0.62-0.71; specificity, 0.92, 95%; CI 0.90-0.94). For
studies using the blind method, analysis showed a higher
specificity and lower sensitivity compared with the studies using
the non-blind method (Table III).
The analysis results of the remaining subgroups showed no
significant difference in diagnostic value. The results are shown
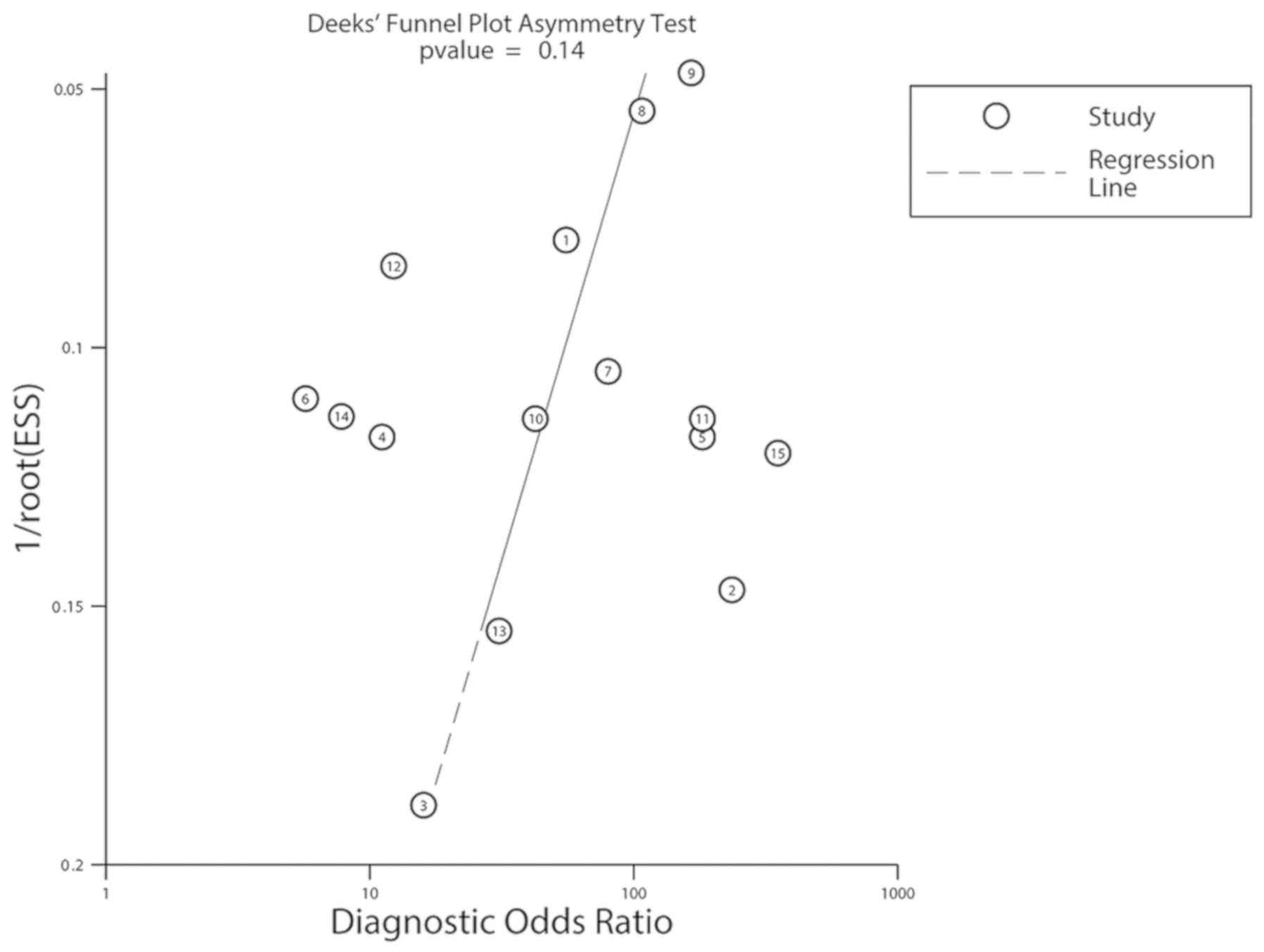
in Table III. Deeks' funnel chart
analysis revealed that there were no notable publication biases in
the included studies (P=0.14; Fig.
6). Studies 2 and 10 had the highest observed specificity,
which was indicative of a high test threshold and the highest
DOR.
Discussion
The diagnosis of PJI represents a notable clinical
challenge. In recent years, several guidelines have been released
for the correct diagnostic approach to this disease (31-38).
In the latest guidelines released in 2011, the authors claimed that
the diagnosis of PJI is established when one of the following
criteria have been fulfilled (32):
i) Sinus tract communicating with the prosthesis; ii) a
microorganism isolated by culture from at least two separate tissue
or fluid samples of affected prosthetic joint; iii) four of the
following six criteria exist: iiia) Elevated serum erythrocyte
sedimentation rate and elevated serum C-reactive protein
concentration; iiib) elevated synovial leukocyte count; iiic)
elevated synovial neutrophil percentage; iiid) presence of
purulence in the affected joint; iiie) isolation of a microorganism
in one culture of periprosthetic tissue or fluid; and iiif) more
than five neutrophils per high-power field observed from histologic
analysis of periprosthetic tissue at x400 magnification. Although
molecular methods are not included in these criteria and PCR assay
is not extensively tested in the routine of a clinical practice,
PCR can meet the demanding expectations of the orthopedic community
because it is helpful in making an early diagnosis of infection
(39-42).
After an extensive evaluation of the literature,
fifteen papers were identified on the usefulness of 16S rRNA in
diagnosing PJIs. These 15 studies involved 862 PJIs and 1,208
aseptic prostheses. The analysis of these studies shows the
sensitivities of 0.70 (range, 0.50-0.93). The false positive PCR
results may be due to contamination in the surgery process by skin,
vials used for collection of samples or by the presence of 16S rRNA
from nonviable bacteria present in sterilized medical devices
(23,30,43).
Contaminants can also be introduced during the PCR reaction by
reagents and equipment (44,45). The heterogeneity found by the present
meta-analysis was likely due to clinical sample, study design,
center, blinded status and the country used for PCR diagnosis. In
the clinical sample subgroup, joint fluid samples had a lower
sensitivity and specificity compared with all the other groups.
Sonicated fluid samples had a higher sensitivity (0.76/0.73) and
lower specificity (0.93/0.95) compared with the periprosthetic
tissue subgroup. A comparison among the different studies showed
that significantly heterogeneous specificity values were found in
spite of a limited range of pooled specificity value (0.91-0.94).
In other subgroups, the sensitivity and specificity were not
significantly different. The DOR is the ratio of the odds of a
positive test result in patients with the disease relative to the
patients without disease; which is a single indicator of test
accuracy that combines the data from sensitivity and specificity
into a single number. A higher value DOR indicates a better
discriminatory test performance. In the present meta-analysis, the
present study has found that the mean DOR was 41.77 and this value
was 68.15 in periprosthetic tissue sample, which indicated that
there was a high level of overall accuracy. But in joint fluid
sample, this value was 32.89 which was lower than the mean DOR. In
the present study, PLR and NLR were also used for the measurement
of diagnostic accuracy. A PLR value of 10.93 suggested that
patients with PJIs have a 10-fold greater chance of having a
positive 16S rRNA PCR test compared with the controls. NLR is found
to be 0.33 in the present meta-analysis, which indicates that if
the 16S rRNA PCR result is negative for an individual, the
probability of this individual having PJI is 33%, which is not low
enough to rule out PJI. The SROC approach shows a good overview of
the pooled results from several studies. The SROC curve and its AUC
demonstrate the tradeoff between sensitivity and specificity. The
data demonstrated that the AUC is 0.90. The meta-analysis data
demonstrated that both the AUC of sonicated fluid samples and
periprosthetic tissue samples (0.93 and 0.98, respectively) were
higher than that of total analysis, which indicated a higher level
of accuracy. Recently, PCR techniques have shown better value in
the diagnosis of PJI (46). PCR
theoretically has higher sensitivity and faster test time and is
not affected by antibiotics, compared with microbiological
cultures. However, the method of sample selection during PCR
analysis may affect the capability of diagnosing PJI. Most studies
have shown that the sonication of fluid samples can improve the
accuracy of PJI diagnosis (45,47). The
present results also show that the diagnostic value of ultrasound
fluid is significantly higher than that of joint fluid.
PCR is a rapid diagnostic test in the diagnosis of
PJI. It is particularly useful in patients who have received
antibiotic therapy (19). Bacterial
16S rRNA PCR is a broad-range PCR test, it is the most frequently
used molecular diagnostic method in PJI (48-53).
There are also other target genes reported in the PCR diagnosis of
PJI (54,55). The Mayo clinic's Patel team used
metagenomic next-generation sequencing (mNGS) based on Illumina
HiSeq 2500 instruments to test the joint fluid and sonicated fluid
of patients of revision arthroplasty. They found that mNGS is a
powerful tool to identify a wide range of PJI pathogens, including
difficult to detect pathogens in culture-negative infections
(56). Frank et al (57) used PCR to target the expression of
the Staphylococcus aureus icaA gene and the result showed
that the presence of icaA in a coagulase-negative staphylococcal
isolate associated with an arthroplasty is not a useful diagnostic
indicator of pathogenicity. Birmingham et al (58) used reverse transcription-quantitative
PCR to detect mRNA encoding for the bacterial genes groEL or femC,
and the result showed minimized false-positive detection of
nonviable bacteria. Multiplex PCR uses specific primers for a
number of microorganisms and allows the detection of multiple
pathogens with the one assay. However, the greatest limitation to
multiplex PCR is that some organisms are not included in the
commercially available kits (59).
In addition, both 16S rRNA and other target genes can be used as a
valuable method for the diagnosis of PJI. However, microbiological
cultures of the sample are also very important, as the antibiotic
sensitivity test can be beneficial to the treatment of
patients.
This meta-analysis has several limitations. Although
a broad search strategy was adopted by two independent reviewers at
all stages of the review process, there were only 15 publications
included, and the small overall number of patients resulted in wide
CIs and may have influenced the outcome. Therefore, it is still
difficult to make a definitive conclusion about the accuracy of
diagnosis of PJI. Further studies on a large scale may be needed to
confirm the diagnostic value of 16S rRNA PCR in PJI. Secondly,
there is no accepted gold standard, which is a common barrier to
all studies for diagnostic accuracy in the detection of PJI. To
date, most studies that have examined PJI have relied on diagnosis
through clinical manifestations and laboratory tests. There were
considerable heterogeneities of the selected studies. Therefore,
subgroup analysis was performed to obtain more accurate estimates,
and consequently, the findings of this meta-analysis should be
interpreted with caution. Finally, during the statistical analysis
the Meta-disc software has some advantages over Stata and RevMen in
exploration of heterogeneity (for instance, it was able to
calculate Chi-square and I-squared) and conduct meta-regression
analysis, however as the program also had some inherent statistical
shortcomings, the present study also used some functions of the
Stata software, such as publication bias.
In conclusion, the present meta-analysis suggests a
potential role for 16S rRNA PCR in the diagnosis of PJI. The
heterogeneity of the studies published until now means that more
studies are necessary in order to assess the true accuracy of 16S
rRNA PCR in the diagnosis of PJI. The results of PCR assays should
be interpreted in parallel with clinical findings and the results
of microbiological and other laboratory tests.
Acknowledgements
The authors thank Dr Rui-Qi Li from Beijing
Chao-Yang Hospital affiliated to Capital Medical University for
valuable technical assistance and support.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ, XYC and SFS conceptualized and designed this
study. YZ provided the study materials. YZ, WC and SF collected and
assembled the data. YZ and QCZ analyzed and processed the data. All
authors wrote the manuscript and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jones CA, Beaupre LA, Johnston DW and
Suarez-Almazor ME: Total joint arthroplasties: Current concepts of
patient outcomes after surgery. Rheum Dis Clin North Am. 33:71–86.
2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lavernia CJ, Guzman JF and Gachupin-Garcia
A: Cost effectiveness and quality of life in knee arthroplasty.
Clin Orthop Relat Res. 134–139. 1997.PubMed/NCBI
|
|
3
|
Pivec R, Johnson AJ, Mears SC and Mont MA:
Hip arthroplasty. Lancet. 380:1768–1777. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Van Manen MD, Nace J and Mont MA:
Management of primary knee osteoarthritis and indications for total
knee arthroplasty for general practitioners. J Am Osteopath Assoc.
112:709–715. 2012.PubMed/NCBI
|
|
5
|
Clohisy JC, Calvert G, Tull F, McDonald D
and Maloney WJ: Reasons for revision hip surgery: A retrospective
review. Clin Orthop Relat Res. 188–192. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sharkey PF, Hozack WJ, Rothman RH, Shastri
S and Jacoby SM: Insall Award paper. Why are total knee
arthroplasties failing today? Clin Orthop Relat Res. 7–13.
2002.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Munjal S, Phillips MJ and Krackow KA:
Revision total knee arthroplasty: Planning, controversies, and
management-infection. Instr Course Lect. 50:367–377.
2001.PubMed/NCBI
|
|
8
|
Mortazavi SM, Molligan J, Austin MS,
Purtill JJ, Hozack WJ and Parvizi J: Failure following revision
total knee arthroplasty: Infection is the major cause. Int Orthop.
35:1157–1164. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Shi S and Zhang X: Interaction of
staphylococcus aureus with osteoblasts (Review). Exp Ther
Med. 3:367–370. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Peel TN, Buising KL and Choong PF:
Prosthetic joint infection: Challenges of diagnosis and treatment.
ANZ J Surg. 81:32–39. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bozic KJ and Ries MD: The impact of
infection after total hip arthroplasty on hospital and surgeon
resource utilization. J Bone Joint Surg Am. 87:1746–1751.
2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kuo FC, Lu YD, Wu CT, You HL, Lee GB and
Lee MS: Comparison of molecular diagnosis with serum markers and
synovial fluid analysis in patients with prosthetic joint
infection. Bone Joint J. 100B:1345–1351. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Berbari EF, Hanssen AD, Duffy MC,
Steckelberg JM, Ilstrup DM, Harmsen WS and Osmon DR: Risk factors
for prosthetic joint infection: Case-control study. Clin Infect
Dis. 27:1247–1254. 1998.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Corvec S, Portillo ME, Pasticci BM, Borens
O and Trampuz A: Epidemiology and new developments in the diagnosis
of prosthetic joint infection. Int J Artif Organs. 35:923–934.
2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cobo J and Del Pozo JL: Prosthetic joint
infection: Diagnosis and management. Expert Rev Anti Infect Ther.
9:787–802. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Matthews PC, Berendt AR, McNally MA and
Byren I: Diagnosis and management of prosthetic joint infection.
BMJ. 338(b1773)2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tsukayama DT, Goldberg VM and Kyle R:
Diagnosis and management of infection after total knee
arthroplasty. J Bone Joint Surg Am. 85-A(Suppl 1):S75–S80.
2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Shaw JD, Miller S, Plourde A, Shaw DL,
Wustrack R and Hansen EN: Methylene blue-guided debridement as an
intraoperative adjunct for the surgical treatment of periprosthetic
joint infection. J Arthroplasty. 32:3718–3723. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Peel TN, Buising KL and Choong PF:
Diagnosis and management of prosthetic joint infection. Curr Opin
Infect Dis. 25:670–676. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cataldo MA, Petrosillo N, Cipriani M,
Cauda R and Tacconelli E: Prosthetic joint infection: Recent
developments in diagnosis and management. J Infect. 61:443–448.
2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ouyang Z, Zhai Z, Qin AN, Li H, Liu X, Qu
X and Dai K: Limitations of Gram staining for the diagnosis of
infections following total hip or knee arthroplasty. Exp Ther Med.
9:1857–1864. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Patel R, Osmon DR and Hanssen AD: The
diagnosis of prosthetic joint infection: Current techniques and
emerging technologies. Clin Orthop Relat Res. 55–58.
2005.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Panousis K, Grigoris P, Butcher I, Rana B,
Reilly JH and Hamblen DL: Poor predictive value of broad-range PCR
for the detection of arthroplasty infection in 92 cases. Acta
Orthop. 76:341–346. 2005.PubMed/NCBI
|
|
24
|
Qu X, Zhai Z, Li H, Li H, Liu X, Zhu Z,
Wang Y, Liu G and Dai K: PCR-based diagnosis of prosthetic joint
infection. J Clin Microbiol. 51:2742–2746. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Moher D, Shamseer L, Clarke M, Ghersi D,
Liberati A, Petticrew M, Shekelle P and Stewart LA: PRISMA-P Group.
Preferred reporting items for systematic review and meta-analysis
protocols (PRISMA-P) 2015 statement. Syst Rev. 4(1)2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Devillé WL, Buntinx F, Bouter LM, Montori
VM, de Vet HC, van der Windt DA and Bezemer PD: Conducting
systematic reviews of diagnostic studies: Didactic guidelines. BMC
Med Res Methodol. 2(9)2002.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang J and Leeflang M: Recommended
software/packages for meta-analysis of diagnostic accuracy. J Lab
Precision Med. 4:2019.
|
|
28
|
Walter SD: Properties of the summary
receiver operating characteristic (SROC) curve for diagnostic test
data. Stat Med. 21:1237–1256. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Jones CM and Athanasiou T: Summary
receiver operating characteristic curve analysis techniques in the
evaluation of diagnostic tests. Ann Thorac Surg. 79:16–20.
2005.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rak M, KavčIč M, Trebše R and CőR A:
Detection of bacteria with molecular methods in prosthetic joint
infection: Sonication fluid better than periprosthetic tissue. Acta
Orthop. 87:339–345. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Spangehl MJ, Masri BA, O'Connell JX and
Duncan CP: Prospective analysis of preoperative and intraoperative
investigations for the diagnosis of infection at the sites of two
hundred and two revision total hip arthroplasties. J Bone Joint
Surg Am. 81:672–683. 1999.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Parvizi J, Zmistowski B, Berbari EF, Bauer
TW, Springer BD, Della Valle CJ, Garvin KL, Mont MA, Wongworawat MD
and Zalavras CG: New definition for periprosthetic joint infection:
From the workgroup of the musculoskeletal infection society. Clin
Orthop Relat Res. 469:2992–2994. 2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Atkins BL, Athanasou N, Deeks JJ, Crook
DW, Simpson H, Peto TE, McLardy-Smith P and Berendt AR: Prospective
evaluation of criteria for microbiological diagnosis of
prosthetic-joint infection at revision arthroplasty. The OSIRIS
collaborative study group. J Clin Microbiol. 36:2932–2939.
1998.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Gallo J, Kolar M, Dendis M, Loveckova Y,
Sauer P, Zapletalova J and Koukalova D: Culture and PCR analysis of
joint fluid in the diagnosis of prosthetic joint infection. New
Microbiol. 31:97–104. 2008.PubMed/NCBI
|
|
35
|
Esteban J, Alonso-Rodriguez N, del-Prado
G, Ortiz-Pérez A, Molina-Manso D, Cordero-Ampuero J, Sandoval E,
Fernández-Roblas R and Gómez-Barrena E: PCR-hybridization after
sonication improves diagnosis of implant-related infection. Acta
Orthop. 83:299–304. 2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Gomez E, Cazanave C, Cunningham SA,
Greenwood-Quaintance KE, Steckelberg JM, Uhl JR, Hanssen AD, Karau
MJ, Schmidt SM, Osmon DR, et al: Prosthetic joint infection
diagnosis using broad-range PCR of biofilms dislodged from knee and
hip arthroplasty surfaces using sonication. J Clin Microbiol.
50:3501–3508. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Marin M, Garcia-Lechuz JM, Alonso P,
Villanueva M, Alcalá L, Gimeno M, Cercenado E, Sánchez-Somolinos M,
Radice C and Bouza E: Role of universal 16S rRNA gene PCR and
sequencing in diagnosis of prosthetic joint infection. J Clin
Microbiol. 50:583–589. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Fang XY, Li WB, Zhang CF, Huang ZD, Zeng
HY, Dong Z and Zhang WM: Detecting the presence of bacterial DNA
and RNA by polymerase chain reaction to diagnose suspected
periprosthetic joint infection after antibiotic therapy. Orthop
Surg. 10:40–46. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
39
|
Trampuz A, Osmon DR, Hanssen AD,
Steckelberg JM and Patel R: Molecular and antibiofilm approaches to
prosthetic joint infection. Clin Orthop Relat Res. 69–88.
2003.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Arciola CR, Collamati S, Donati E and
Montanaro L: A rapid PCR method for the detection of
slime-producing strains of staphylococcus epidermidis and S-aureus
in periprosthesis infections. Diagn Mol Pathol. 10:130–137.
2001.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tarkin IS, Henry TJ, Fey PI, Iwen PC,
Hinrichs SH and Garvin KL: PCR rapidly detects
methicillin-resistant staphylococci periprosthetic infection. Clin
Orthop Relat Res. 89–94. 2003.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gallo J, Kolar M, Koukalova D, Sauer P,
Loveckova Y and Zapletalova J: P1407. Cultivation versus PCR
analysis of joint fluid samples in prosthetic joint infection. Int
J Antimicrobial Agents. 29:S391–S392. 2007.
|
|
43
|
Clarke MT, Roberts CP, Lee PT, Gray J,
Keene GS and Rushton N: Polymerase chain reaction can detect
bacterial DNA in aseptically loose total hip arthroplasties. Clin
Orthop Relat Res. 132–137. 2004.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Millar BC, Xu J and Moore JE: Risk
assessment models and contamination management: Implications for
broad-range ribosomal DNA PCR as a diagnostic tool in medical
bacteriology. J Clin Microbiol. 40:1575–1580. 2002.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Fink B, Steurer M, Hofäecker S, Schäfer P,
Sandow D, Schuster P and Oremek D: Preoperative PCR analysis of
synovial fluid has limited value for the diagnosis of
periprosthetic joint infections of total knee arthroplasties. Arch
Orthop Trauma Surg. 138:871–878. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Mariaux S, Tafin UF and Borens O:
Diagnosis of persistent infection in prosthetic two-stage exchange:
PCR analysis of sonication fluid from bone cement spacers. J Bone
Jt Infect. 2:218–223. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Sebastian S, Malhotra R, Sreenivas V,
Kapil A, Chaudhry R and Dhawan B: Utility of 16S rRNA PCR in the
synovial fluid for the diagnosis of prosthetic joint infection. Ann
Lab Med. 38:610–612. 2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Bergin PF, Doppelt JD, Hamilton WG, Mirick
GE, Jones AE, Sritulanondha S, Helm JM and Tuan RS: Detection of
periprosthetic infections with use of ribosomal RNA-based
polymerase chain reaction. J Bone Joint Surg Am. 92:654–663.
2010.PubMed/NCBI View Article : Google Scholar
|
|
49
|
De Man FHR, Graber P, Lüem M, Zimmerli W,
Ochsner PE and Sendi P: Broad-range PCR in selected episodes of
prosthetic joint infection. Infection. 37:292–294. 2009.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Vandercam B, Jeumont S, Cornu O, Yombi JC,
Lecouvet F, Lefèvre P, Irenge LM and Gala JL: Amplification-based
DNA analysis in the diagnosis of prosthetic joint infection. J Mol
Diagn. 10:537–543. 2008.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Bemer P, Plouzeau C, Tande D, Léger J,
Giraudeau B, Valentin AS, Jolivet-Gougeon A, Vincent P, Corvec S,
Gibaud S, et al: Evaluation of 16S rRNA gene PCR sensitivity and
specificity for diagnosis of prosthetic joint infection: A
prospective multicenter cross-sectional study. J Clin Microbiol.
52:3583–3589. 2014.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Stylianakis A, Schinas G, Thomaidis PC,
Papaparaskevas J, Ziogas DC, Gamaletsou MN, Daikos GL, Pneumaticos
S and Sipsas NV: Combination of conventional culture, vial culture,
and broad-range PCR of sonication fluid for the diagnosis of
prosthetic joint infection. Diagn Microbiol Infect Dis. 92:13–18.
2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Morgenstern C, Cabric S, Perka C, Trampuz
A and Renz N: Synovial fluid multiplex PCR is superior to culture
for detection of low-virulent pathogens causing periprosthetic
joint infection. Diagn Microbiol Infect Dis. 90:115–119.
2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Arciola CR, Campoccia D, Baldassarri L,
Donati ME, Pirini V, Gamberini S and Montanaro L: Detection of
biofilm formation in Staphylococcus epidermidis from implant
infections. Comparison of a PCR-method that recognizes the presence
of ica genes with two classic phenotypic methods. J Biomed Mater
Res Part A. 76:425–430. 2006.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Montanaro L, Arciola CR, Borsetti E,
Brigotti M and Baldassarri L: A polymerase chain reaction (PCR)
method for the identification of collagen adhesin gene (CNA) in
staphylo coccus-induced prosthesis infections. New Microbiol.
21:359–363. 1998.PubMed/NCBI
|
|
56
|
Miao Q, Ma Y, Wang Q, Pan J, Zhang Y, Jin
W, Yao Y, Su Y, Huang Y, Wang M, et al: Microbiological diagnostic
performance of metagenomic next-generation sequencing when applied
to clinical practice. Clin Infect Dis. 67(Suppl 2):S231–S240.
2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Frank KL, Hanssen AD and Patel R: icaA is
not a useful diagnostic marker for prosthetic joint infection. J
Clin Microbiol. 42:4846–4849. 2004.
|
|
58
|
Birmingham P, Helm JM, Manner PA and Tuan
RS: Simulated joint infection assessment by rapid detection of live
bacteria with real-time reverse transcription polymerase chain
reaction. J Bone Joint Surg Am. 90:602–608. 2008.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Portillo ME, Salvadó M, Sorli L, Alier A,
Martínez S, Trampuz A, Gómez J, Puig L and Horcajada JP: Multiplex
PCR of sonication fluid accurately differentiates between
prosthetic joint infection and aseptic failure. J Infect.
65:541–548. 2012.PubMed/NCBI View Article : Google Scholar
|