Introduction
Rheumatoid arthritis (RA) is a chronic systemic
inflammatory disease. Currently, RA is considered as an autoimmune
disease in clinical practice (1).
The main manifestations of RA are those of symmetrical, chronic and
progressive polyarthritis. As the development of the disease
continues, the articular cartilage and capsule will eventually be
destroyed (2). RA is a very common
disease with high incidence in clinic, which has been on the rise
due to the aging of population in recent years (3,4). RA can
be accompanied by various degrees of dysfunction in the early stage
and skeletal muscle atrophy in the late stage, which is one of the
major causes of labor loss and disability (5). At present, there is no specific
treatment for RA in clinical practice, and the main focus has been
on the comprehensive treatment of inflammation and sequelae
(6). For the treatment of RA,
clinical requirements include controlling inflammation of joints
and tissue, maintaining normal joint function and repairing the
damaged joints. Comprehensive treatment can achieve certain
efficacy for most patients in the early stage (7). However, there are still some patients
whose bone tissue is irreversibly damaged when the disease develops
through the middle and late stages. At this time, only artificial
joint replacement, arthroplasty and other operations can achieve
the therapeutic purpose, and in more serious cases, only amputation
can prevent further necrosis of the bone tissue (8). Therefore, the treatment of RA is
particularly important at the early stage of the disease. At
present, the diagnosis for RA is relatively complicated in clinical
practice. As RA has no special symptoms and the specificity of
imaging examination is low in clinical practice, complicated series
of examinations are usually required to confirm the occurrence of
RA (9), which are not conducive to
early screening of RA in clinical practice. Therefore, in order to
find an effective and convenient diagnostic method, researchers are
constantly exploring various markers of RA (10).
With in-depth research, the clinical application of
microRNA (miR) has gradually become a hot research topic in various
diseases. mRNA, as a non-coding short-chain RNA with a length of
about 22 nt, is mainly used to inhibit the translation and
transcription process of target genes by binding the untranslated
regions (UTRs) at the 3' end of target gene mRNA downstream,
achieving the effect of changing the expression of target genes
(11). miR-495 is located on
chromosome 14q32.31 and was recently discovered to be an extremely
important derivative of the miR family (12). Yang et al (13) proved that miR-495 activates NF-κB
signaling pathway to inhibit chondrocyte apoptosis in
osteoarthritis. miR-326 can inhibit the expression of NPB1, an
important gene of MAPK signaling pathway, by combining with its
3'UTR, promoting tumor cell apoptosis and inhibiting proliferation,
invasion and metastasis (14). Wang
et al (15) explored the role
of miR-326 in osteosarcoma and showed that miR-326 can regulate the
role of NOB1 as an oncogene in osteosarcoma. Both miR-495 and
miR-326 are closely related to bone tissue growth, metabolism or
bone tissue diseases. However, there is no study on the expression
and significance of miR-495 and miR-326 in RA. In the present
study, it was assumed that miR-495 and miR-326 may also participate
in the occurrence and development of RA, and this was confirmed
through experimental analysis, providing a new reference for the
clinical diagnosis and treatment of RA in the future.
Subjects and methods
General data
A total of 107 RA patients, admitted to the Yidu
Central Hospital of Weifang (Weifang, China) from February 2016 to
February 2019, and 112 healthy subjects, who underwent physical
examination during the same period, were selected as the research
subjects for prospective analysis. The RA patients served as the
study group, including 59 males and 48 females, 52-71 years of age,
with a mean age of 60.5±7.7 years. The control group consisted of
64 males and 48 females, 50-70 years of age, with a mean age of
59.2±8.3 years. The study was approved by the Ethics Committee of
Yidu Central Hospital of Weifang and signed written informed
consents were obtained from the patients and/or guardians.
Inclusion and exclusion criteria
Inclusion criteria
Patients were 30-75 years of age. The RA clinical
diagnostic criteria were as follows: i) Morning stiffness lasted
for 1 h (every day) with a course of at least 6 weeks; ii) there
were ≥3 arthroncus for at least 6 weeks; iii) swelling of wrist,
metacarpal finger and proximal knuckle lasted for at least 6 weeks;
iv) symmetrical joint swelling lasted for at least 6 weeks; v)
there were subcutaneous nodules; vi) changes were observed on the
hand X-ray scans; and vii) rheumatoid factor (RF) was positive
(titer >1:20). The patients met the above 7 diagnostic criteria
and were diagnosed with typical RA. If >4 criteria were
satisfied, the patient was diagnosed with RA. After diagnosis, the
patients received follow-up treatment in the Yidu Central Hospital
of Weifang. Additionally, in the study were included patients who
had not been treated with any antibiotics within 3 months before
admission and had a complete medical record. Signed written
informed consents were obtained from all patients or their
immediate family members.
Exclusion criteria
Patients with tumor, cardiovascular or
cerebrovascular diseases, other autoimmune or infectious diseases;
patients with liver or renal insufficiency due to organ failure;
patients with drug allergy; patients with physical disabilities who
had been bedridden for a long time and could not take care of
themselves; patients with low treatment compliance due to mental
disorders; transferred patients; pregnant women; patients with
history of bone tissue surgery.
Inclusion criteria for the control
group
Subjects who underwent physical examination in the
Yidu Central Hospital of Weifang, whose all test results were
normal, with no medical history, who agreed to cooperate and
participate in the study.
Research methods Treatment
methods
In strict accordance with RA clinical treatment
guidelines (16), non-steroidal
anti-inflammatory drugs were used as the first-line treatment
scheme, and slow acting anti-rheumatic drugs were used as
second-line drugs. A low dose (<10 mg/day) of glucocorticoids
was given to patients with rheumatoid vasculitis, severe RA or when
the treatment was ineffective. Immune purification and
hematopoietic stem cell transplantation were also carried out for
patients who did not respond to the aforementioned treatment. In
cases when the illness and serious joint dysfunction could not be
effectively controlled by the medical treatment, the patients were
treated by surgery (release of carpal tunnel syndrome, repair after
tendon tear, synovial resection, or even joint replacement for
severe cases), according to the patient's medical condition.
Detection methods
A total of 4 ml of fasting venous blood were
collected from all subjects before treatment (before any treatment
course) and after treatment (after all treatment courses were
completed), and the samples were placed at room temperature for 30
min. Next, centrifugation was carried out for 10 min (1,050 x g,
4˚C) to obtain the upper serum, which was stored in a refrigerator
at -80˚C for further analysis. The expression of miR-495 and
miR-326 in the serum was detected by RT-qPCR. Total RNA was
extracted from the collected serum using TRIzol™ LS
reagent (10296010; Invitrogen: Thermo Fisher Scientific, Inc.) and
the purity, concentration and integrity of total RNA was detected
by UV spectrophotometer and agarose gel electrophoresis.
PrimeScript™ RT reagent (RR036A; Takara Bio, Inc.) and
TB Green® Fast qPCR Mix (RR430A; Takara Bio, Inc.) were
used for the reverse transcription of total RNA, according to the
manufacturer's protocol. Next, PCR amplification was carried out.
The PCR reaction system was as follows: 1 µl of cDNA, 0.4 µl of
upstream and downstream primers, 10 µl of 2X TransTaq®
Tip Green qPCR SuperMix (AQ141-01; Beijing Transgen Biotech Co.,
Ltd.), 0.4 µl of Passive Reference Dye (50X) (75768 500 UL;
Shanghai Yanqi Biotechnology Co., Ltd.), and finally
ddH2O was added for a final volume of 20 µl. The PCR
reaction conditions were as follows: Pre-denaturation at 94˚C for
30 sec, denaturation at 94˚C for 5 sec, annealing at 60˚C for 30
sec, with a total of 40 cycles. Each sample was provided with 3
repeated wells, and the experiment was carried out 3 times. U6 was
used as internal reference and 2-ΔΔCq method was used
for data analysis (17). The primer
sequences of miR-495, miR-326 and U6 are presented in Table I.
 | Table IPrimer sequences of miR-495, miR-326
and U6. |
Table I
Primer sequences of miR-495, miR-326
and U6.
| Genes | Upstream | Downstream |
|---|
| miR-495 |
5'-TCCGATTCTTCACGTGGTAC-3' |
5'-GTGCAGGGTCCGAGGT-3' |
| miR-326 |
5'-GCAGCACGCTAGGTAGTTTCC-3' |
5'-TATCGTTGTTCTCCACTCCTTGAC-3' |
| U6 |
5'-TCCGATCGTGAAGCGTTC-3' |
5'-GTGCAGGGTCCGAGGT-3' |
Observation indicators Main
indicators
The expression of miR-495 and miR-326 in the
peripheral blood of the two groups of subjects, the diagnostic
value of miR-495 and miR-326 for RA, and the changes of miR-495 and
miR-326 expression in the study group before and after treatment
were observed.
Secondary indicators
The predictive value of miR-495 combined with
miR-326 for complications during RA treatment, the expression of
miR-495 and miR-326 in RA patients with different clinical
pathology, and the correlation between miR-495 and miR-326 with RF
were observed.
Statistical analysis
The experimental data were analyzed by SPSS 24.0
statistical software (Shanghai Yuchuang Network Technology Co.,
Ltd.) and all graphical results were visualized by GraphPad Prism 8
(Shenzhen SoftHead Software Technology Co., Ltd.). Counting data
were expressed in the form of rate, and chi-square test was used
for their comparison between groups. Measurement data were
expressed as the mean ± SD, and t-test was used for inter-group
comparisons, whereas one-way analysis of variance, with LSD post
hoc test, for multi-group comparisons. The diagnostic value of
miR-495 and miR-326 was determined by ROC curve analysis.
Multivariate Logistic analysis was used to calculate the
independent variable joint model and then the ROC curve was
analyzed. Pearson's correlation coefficient analysis was used for
the investigation of the correlation between miR-495 and miR-326
with RF. P<0.050 was considered to indicate a statistically
significant difference.
Results
General data comparison
Age, body mass index, white blood cells, red blood
cells, platelets, fasting blood glucose, systolic blood pressure,
diastolic blood pressure, sex, smoking history, drinking history,
exercise, residence, nationality and family history were compared
between the two groups and the differences were not statistically
significant (P>0.050) (Table
II).
 | Table IIGeneral data comparison between the
two groups of subjects [mean ± SD, n (%)]. |
Table II
General data comparison between the
two groups of subjects [mean ± SD, n (%)].
| Variables | Study group
(n=107) | Control group
(n=112) | t or
χ2 | P-value |
|---|
| Age (years) | 60.5±7.7 | 59.2±8.3 | 1.200 | 0.231 |
| BMI
(kg/cm2) | 23.52±2.63 | 23.36±3.05 | 0.415 | 0.679 |
| White blood cells
(x109/l) | 6.85±2.54 | 6.71±2.26 | 0.431 | 0.667 |
| Red blood cells
(x1012/l) | 4.87±1.36 | 4.61±1.56 | 1.312 | 0.191 |
| Platelets
(x109/l) | 258.62±54.21 | 249.62±46.26 | 1.324 | 0.187 |
| Fasting blood
glucose (mmol/l) | 18.51±3.45 | 17.89±3.85 | 1.253 | 0.212 |
| Systolic pressure
(mmHg) | 132.59±15.26 | 128.97±14.91 | 1.776 | 0.077 |
| Diastolic pressure
(mmHg) | 76.24±9.17 | 75.16±8.95 | 0.379 | 0.882 |
| Course of disease
(years) | 2.16±0.86 | | | |
| Sex | | | 0.089 | 0.765 |
|
Male | 59 (55.14) | 64 (57.14) | | |
|
Female | 48 (44.86) | 48 (42.86) | | |
| Smoking
history | | | 0.853 | 0.356 |
|
Yes | 63 (58.88) | 59 (52.68) | | |
|
No | 44 (41.12) | 53 (47.32) | | |
| Drinking
history | | | 0.344 | 0.558 |
|
Yes | 52 (48.60) | 50 (44.64) | | |
|
No | 55 (51.40) | 62 (55.36) | | |
| Exercise habit | | | 0.662 | 0.416 |
|
Yes | 32 (29.91) | 28 (25.00) | | |
|
No | 75 (70.09) | 84 (75.00) | | |
| Residence | | | 1.392 | 0.239 |
|
Urban | 62 (57.94) | 56 (50.00) | | |
|
Rural | 45 (42.06) | 56 (50.00) | | |
| Nationality | | | 1.474 | 0.225 |
|
Han | 102 (95.33) | 110 (98.21) | | |
|
Minorities | 5 (4.67) | 2 (1.79) | | |
| Family history | | | 2.307 | 0.129 |
|
Yes | 26 (24.30) | 18 (16.07) | | |
|
No | 81 (75.70) | 94 (83.93) | | |
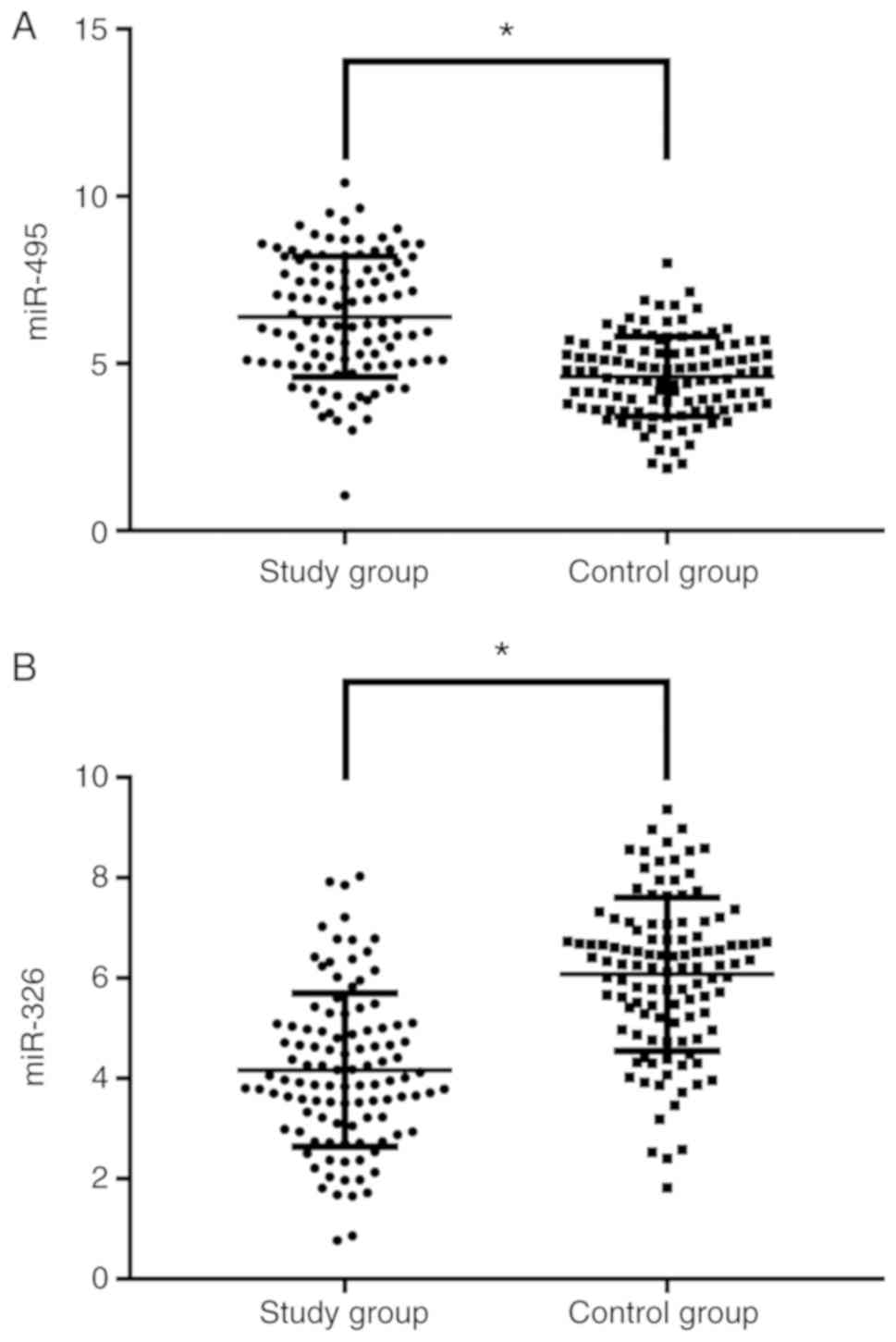
Comparison of miR-495 and miR-326
expression between the two groups
The expression of miR-495 in the study group was
significantly higher than that in the control group (P<0.001),
and miR-326 expression in the study group was significantly lower
than that in the control group (P<0.001) (Fig. 1).
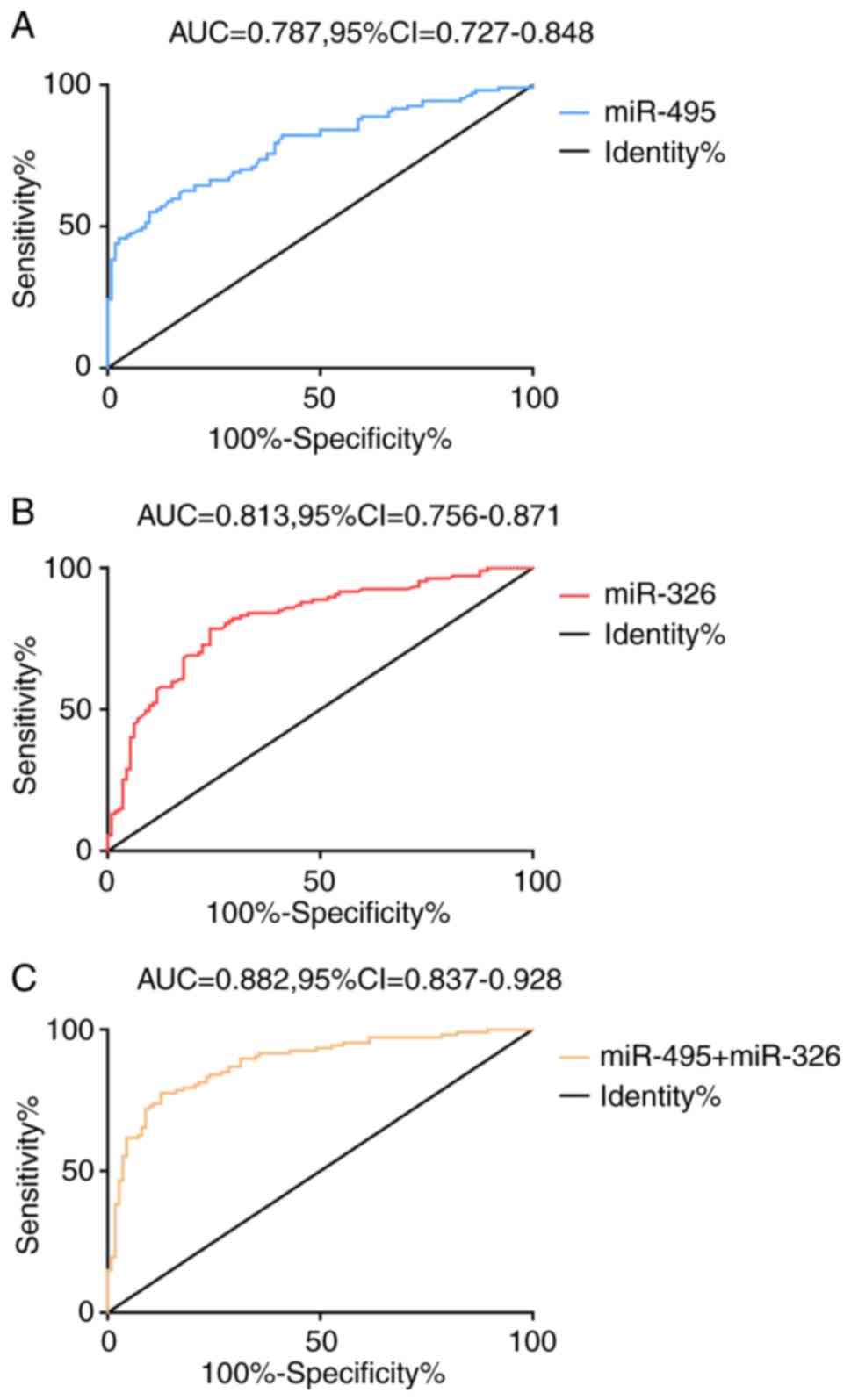
Diagnostic value of miR-495 and
miR-326 for RA
The results of the ROC curve analysis revealed that
when the cut-off value was 6.05, the diagnostic sensitivity and
specificity of miR-495 for RA were 55.14 and 90.18%, respectively.
When the cut-off value was 5.11, the diagnostic sensitivity and
specificity of miR-326 for RA were 78.50 and 75.89%, respectively.
miR-495 and miR-326 were taken as two independent variables, and
binary Logistic regression analysis was carried out to obtain the
Logistic regression model. Logt(P)=0.753 + (-0.739 x miR-495) +
(-0.313 x miR-326). When the cut-off value was 0.55, the
sensitivity and specificity of the model in diagnosing RA were
77.57 and 87.50%, respectively (Fig.
2 and Table III).
 | Table IIIDiagnostic value of miR-495 and
miR-326 for RA. |
Table III
Diagnostic value of miR-495 and
miR-326 for RA.
| Items | miR-495 | miR-326 | miR-495 +
miR-326 |
|---|
| Cut-off | 6.05 | 5.11 | 0.55 |
| Sensitivity
(%) | 55.14 | 78.50 | 77.57 |
| Specificity
(%) | 90.18 | 75.89 | 87.50 |
| AUC | 0.787 | 0.813 | 0.882 |
| Std. error | 0.031 | 0.029 | 0.023 |
| 95% CI | 0.727-0.848 | 0.756-0.871 | 0.837-0.928 |
| P-value | <0.001 | <0.001 | <0.001 |
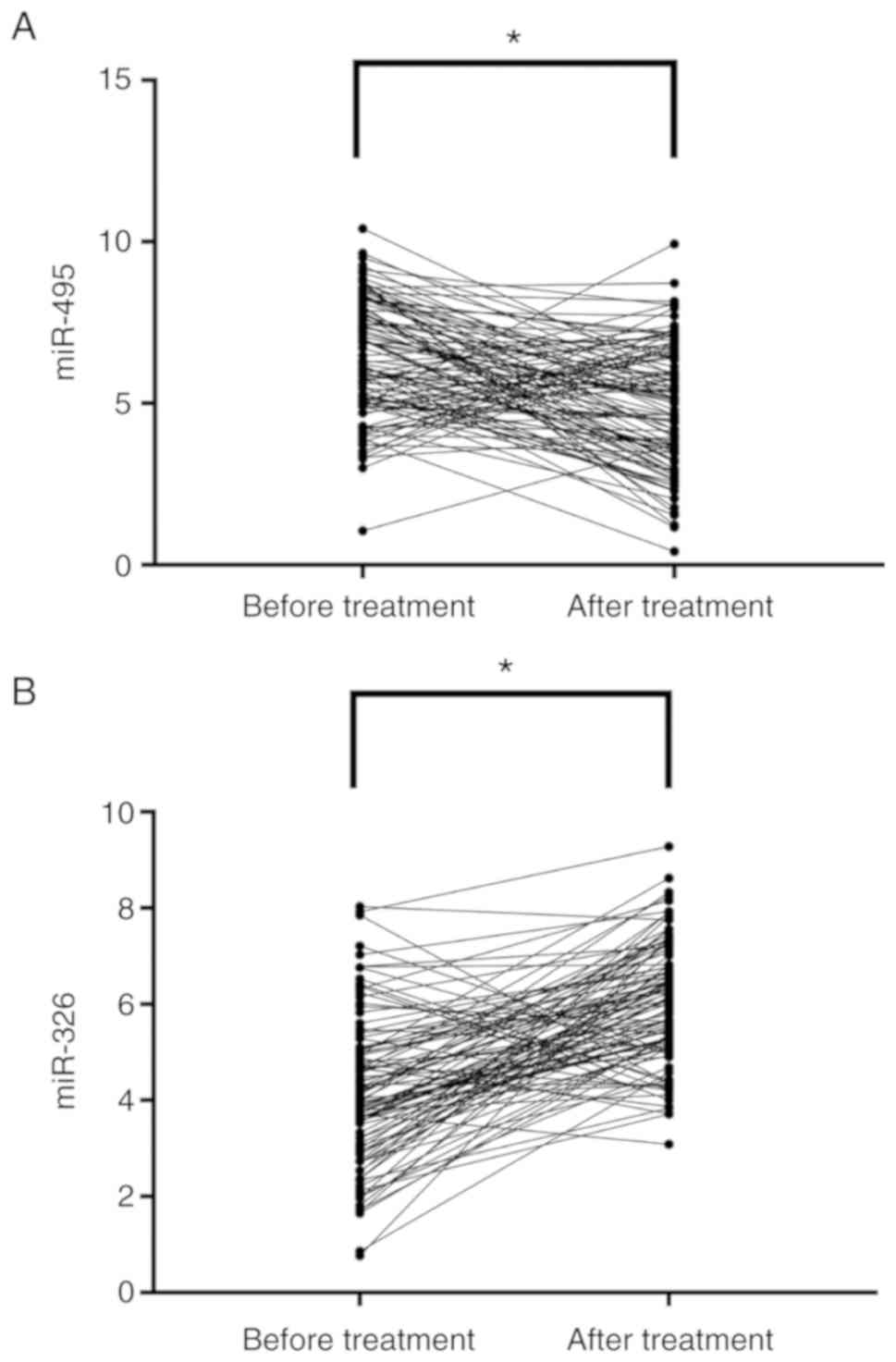
Changes of miR-495 and miR-326
expression in the study group after treatment
After treatment, miR-495 expression was
significantly decreased (P<0.001) compared to that before
treatment, whereas miR-326 expression was significantly increased
(P<0.001), in the study group (Fig.
3).
Expression difference of miR-495 and
miR-326 in RA patients with different clinical pathology
The expression of miR-495 had no significant
difference among patients with different functional activity
(P>0.050). However, miR-495 expression was closely related to
the course of the disease, the stage of pathological changes, the
disease progression and the clinical manifestations (P≤0.050).
miR-326 expression was closely related to the course of the
disease, the pathological activity stage, the disease progression,
the functional activity and the clinical manifestations
(P<0.050) (Table IV).
 | Table IVComparison of miR-495 and miR-326
expression in RA patients with different clinicopathology. |
Table IV
Comparison of miR-495 and miR-326
expression in RA patients with different clinicopathology.
| Variables | n | miR-495 | t or F | P-value | miR-326 | t or F | P-value |
|---|
| Course of disease,
years | | | 4.261 | <0.001 | | 4.188 | <0.001 |
|
<2.16 | 42 | 6.10±1.26 | | | 4.03±1.35 | | |
|
≥2.16 | 65 | 7.34±1.59 | | | 3.14±0.85 | | |
| Stage of
pathological changes | | | 5.321 | 0.007 | | 23.551 | <0.001 |
|
Acute active
prieod | 26 | 5.42±1.05 | | | 4.58±0.52 | | |
|
Subacute
active period | 30 | 5.86±1.26 | | | 3.87±0.62 | | |
|
Chronic
delay period | 16 | 6.38±1.58 | | | 3.05±1.08 | | |
|
Stationary
phase | 35 | 7.83±1.12 | | | 2.62±1.20 | | |
| Disease
progressiona | | | 9.323 | <0.001 | | 13.184 | <0.001 |
|
Stage I | 21 | 5.31±1.32 | | | 3.86±1.05 | | |
|
Stage
II | 29 | 6.03±1.52 | | | 2.68±1.26 | | |
|
Stage
III | 39 | 6.35±1.05 | | | 2.26±1.24 | | |
|
Stage
IV | 18 | 7.48±1.41 | | | 1.62±1.06 | | |
| Functional
activityb | | | 2.101 | 0.058 | | 3.179 | 0.027 |
|
Grade I | 9 | 5.84±1.52 | | | 4.05±1.52 | | |
|
Grade
II | 42 | 6.24±1.26 | | | 3.23±1.42 | | |
|
Grade
III | 42 | 6.30±1.48 | | | 2.86±1.26 | | |
|
Grade
IV | 14 | 7.21±1.84 | | | 2.52±0.58 | | |
| Clinical
manifestationsc | | | 7.082 | 0.001 | | 52.152 | <0.001 |
|
Grade 0 | 8 | 5.41±1.24 | | | 5.42±1.21 | | |
|
Grade I | 34 | 6.25±1.82 | | | 4.27±1.54 | | |
|
Grade
II | 65 | 7.05±1.16 | | | 2.52±0.54 | | |
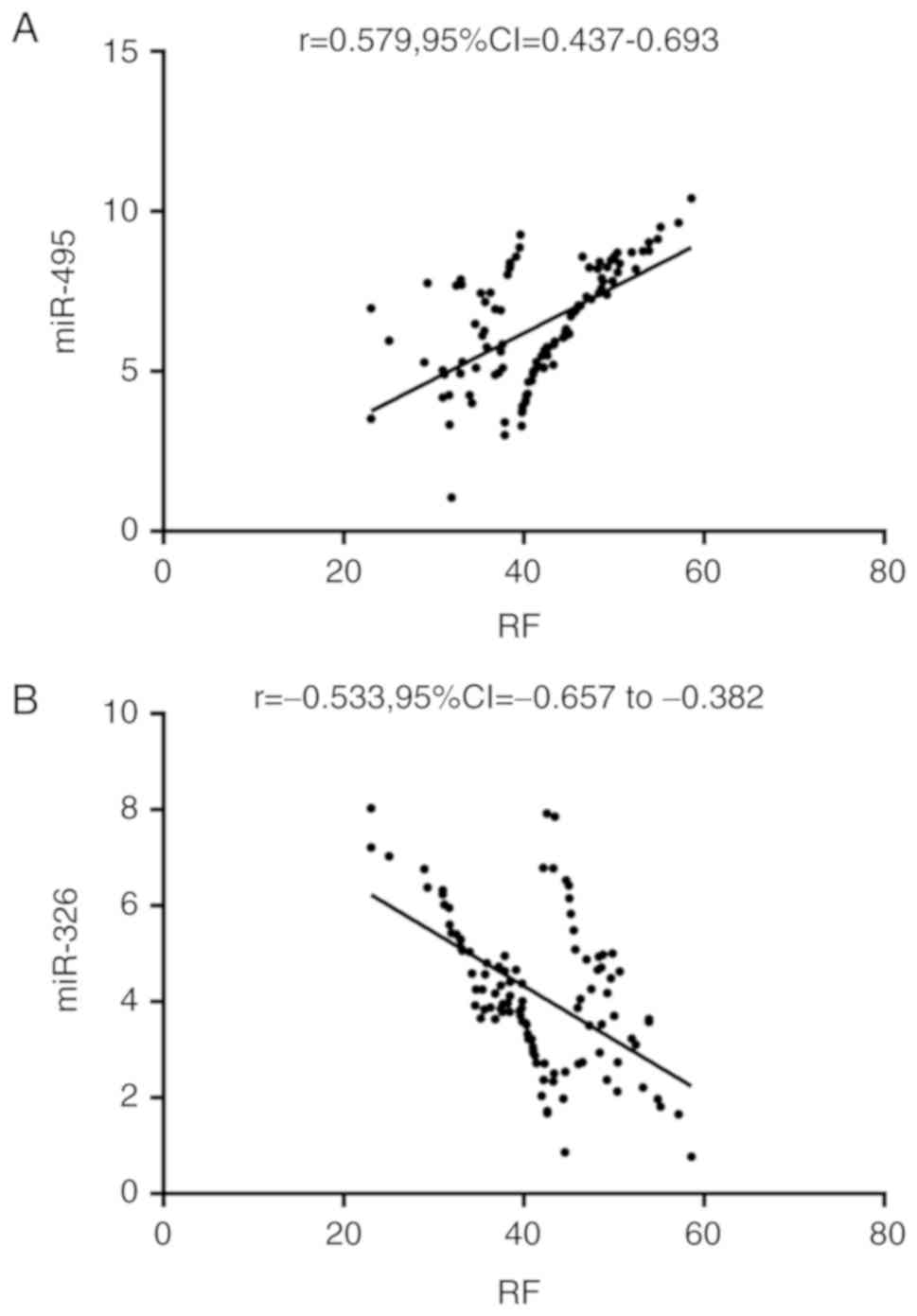
Correlation analysis of miR-495 and
miR-326 with RF
Pearson's correlation coefficient analysis showed
that miR-495 was positively correlated with RF (r=0.579,
P<0.001), whereas miR-326 was negatively correlated with RF
(r=-0.533, P<0.001) (Fig. 4).
Predictive value of miR-495 combined
with miR-326 for complications during treatment
In the study group, 21 patients had complications
during treatment, including 8 cases of synovitis, 1 case of
arteritis, 3 cases of chronic pleural effusion, 1 case of
myocarditis, 6 cases of anemia, and 2 cases of glomerulonephritis.
The incidence of complications was 19.63%. The patients with
complications were regarded as the poor group (n=21), and patients
without complications were regarded as the excellent group (n=86).
miR-495 and miR-326 were taken as two independent variables and
binary Logistic regression analysis was carried out. The Logistic
regression model obtained was: Logit(P)=2.638 + (-1.589 x miR-495)
+ (-16.232 x miR-326). When the cut-off value was 0.52, the
sensitivity and specificity of the model for predicting
complications during the RA treatment were 84.88 and 95.24%,
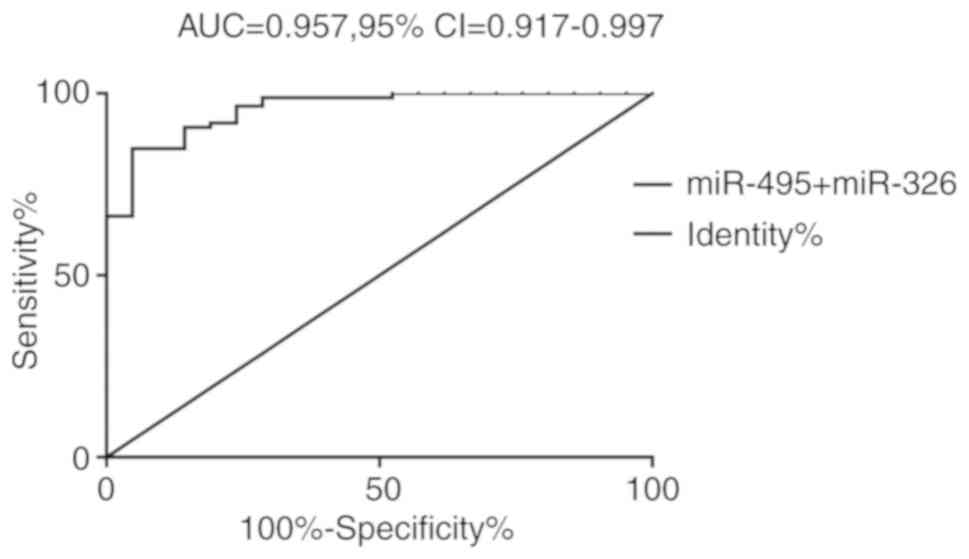
respectively (Fig. 5 and Table V).
 | Table VDiagnostic value of miR-495 combined
with miR-326 for complications during treatment. |
Table V
Diagnostic value of miR-495 combined
with miR-326 for complications during treatment.
| Items | miR-495 +
miR-326 |
|---|
| Cut-off | 0.52 |
| Sensitivity
(%) | 84.88 |
| Specificity
(%) | 95.24 |
| AUC | 0.957 |
| Std. error | 0.020 |
| 95% CI | 0.917-0.997 |
| P-value | <0.001 |
Discussion
The incidence of RA is on the rise year by year and
the clinical challenges are increasing (18). Although RA is not a deadly disease,
the complications caused by its development are extremely harmful,
such as infarction caused by artery invasion, myocarditis and
valvulitis caused by heart invasion, as well as drug-induced
gastrointestinal mucosal lesions, and spinal cord lesions in
patients with serious illness (19,20). At
present, the clinical diagnosis of RA is still based on the
diagnostic criteria developed by the American Rheumatology
Association in 1987. Thus, not only the RA examination is extremely
complicated, but also specific RA cases that may have appeared at
present due to the development of the disease cannot be diagnosed
based on these diagnostic criteria. Therefore, the exploration of
serum markers is particularly important for the clinical diagnosis
and treatment of RA.
The experimental results of the present study showed
that miR-495 expression was significantly increased in RA patients,
whereas miR-326 expression was significantly decreased, in
consistency with the results reported by Clark et al and
Kefas et al (21,22) on cardiomyopathy and glioma. This
suggests that miR-495 and miR-326 may be involved in the occurrence
and progression of RA. Further analysis of the relationship between
miR-495, miR-326 and the clinical pathology of RA patients showed
that miR-495 was closely related to the course of the disease, the
stage of pathological changes, the disease progression and the
clinical manifestations. In addition, miR-326 was closely related
to the course of the disease, the stage of pathological changes,
the disease progression, the functional activity and the clinical
manifestations (P<0.050). These results further confirm the
close relationship between miR-495, miR-326 and RA. According to a
previous report, miR-495 can inhibit cartilage differentiation
process in bone marrow mesenchymal stem cells (23) by targeting Sox9, and Tanaka (24) has suggested that Sox9 may be an
effective tool for future RA joint repair. Therefore, the mechanism
of miR-495 on RA may be through the regulation of Sox9 affecting
the integrity of cartilage tissue. However, Gao et al
(25) have suggested that Notch-1
plays an important role in the mediation of RA angiogenesis and
Zhang et al (26) have shown
that the expression of miR-495 is inhibited in retinal neurons.
Thus, miR-495 could be a potential therapeutic target for RA and
its therapeutic mechanism may be through the upregulation of
Notch-1. Mao et al (27) and
Formosa et al (28) have
shown that miR-495 is expressed at low levels in esophageal and
prostate cancer. The differences with the results of the present
study suggest that miR-495 may have specific expression and play
different roles in different tissues and cells, which needs further
investigation. In addition, miR-326 has been proven to bind 3'UTR
of helper T cell 17 differentiation inhibitor Ets-1 to inhibit
Ets-1 expression (29). A previous
study has shown that Ets-1 can be used as one of the markers of RA,
and can promote the progression of diseases in RA by supplying
blood to inflammatory tissue and recruiting immune ability and
inflammatory cells (30). Therefore,
the mechanism of miR-326 may be through the upregulation of Ets-1
expression to enhance inflammatory factors in RA patients. Another
study has also pointed out that miR-495 affects the occurrence of
mandatory spondylitis through the targeted inhibition of
DVL-2(31) and the study by Tomofuji
et al (32) has also
confirmed that miR-495 may be a potential marker of periodontitis.
Similarly, a previous study has suggested that miR-326 may be
closely related to RA in diabetic patients (33). All the aforementioned studies have
shown that miR-495 and miR-326 are closely related to bone diseases
and therefore, it is of great significance to continue to explore
miR-495 and miR-326 in RA.
ROC curve analysis showed that the combined
diagnosis of miR-495 and miR-326 has good predictive value for the
occurrence of RA and complications during treatment, suggesting
that miR-495 and miR-326 can be used as effective diagnostic
indicators for RA in the future. At present, there is still much
room for improvement in the clinical diagnosis of osteoarthritis.
The detection of inflammatory factors, although it has a very high
sensitivity, the specificity is not enough to accurately determine
whether RA occurs in patients. Regarding imaging techniques, the
testing process is very complex and cannot be used for extensive
early screening. Therefore, it is very important to find blood
markers that can accurately and rapidly reflect RA. Compared with
the traditional RA diagnostic method, the detection advantages of
miR-495 and miR-326 in peripheral blood are as follows: i) The
detection is convenient and short in cycle, and can be completed
only by extracting peripheral blood; ii) the detection results are
relatively intuitive, and no artificial re-interpretation of
results is required compared with the imaging techniques; and iii)
the samples are convenient to store and beneficial for the
long-term treatment of the patients.
The aim of the present study was to investigate the
expression of miR-495 and miR-326 in the peripheral blood of RA
patients. Due to limited experimental conditions, the study
presents some deficiencies. Because of the lack of basic
experimental support, the relevant mechanisms of miR-495 and
miR-326 involved in RA disease progression are still at the stage
of speculation. Thus, further experimental research is needed in
this direction to confirm the results presented. Moreover, due to
the short experimental period, it is still not clear whether
miR-495 and miR-326 have significant impact on the prognosis of RA
patients. This will be one of our future research directions. In
addition, due to incomplete preservation of case data, the
relationship between miR-495, miR-326 and DAS28 score was not
investigated. More representative patient data will be collected as
soon as possible for a more comprehensive analysis. For the
specific expression of miR-495 and miR-326 in different
osteoarticular diseases, meta-analysis will be conducted in our
future research in order to improve the experimental results.
In conclusion, miR-495 was highly expressed in RA
patients, whereas miR-326 was expressed at low levels. The combined
detection of miR-495 and miR-326 was shown to have a good
diagnostic value for RA.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XS and HL conceived and designed the study, and
drafted the manuscript. XS, HL and YZ collected, analyzed and
interpreted the experimental data. YZ revised the manuscript for
important intellectual content. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Yidu Central Hospital of Weifang (Weifang, China). Signed written
informed consents were obtained from the patients and/or
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mankia K and Emery P: Preclinical
rheumatoid arthritis: Progress toward prevention. Arthritis
Rheumatol. 68:779–788. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Firestein GS and McInnes IB:
Immunopathogenesis of rheumatoid arthritis. Immunity. 46:183–196.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Simon TA, Thompson A, Gandhi KK, Hochberg
MC and Suissa S: Incidence of malignancy in adult patients with
rheumatoid arthritis: A meta-analysis. Arthritis Res Ther.
17(212)2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Myasoedova E, Crowson CS, Kremers HM,
Therneau TM and Gabriel SE: Is the incidence of rheumatoid
arthritis rising?: Results from olmsted county, minnesota,
1955-2007. Arthritis Rheum. 62:1576–1582. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
McInnes IB and Schett G: Pathogenetic
insights from the treatment of rheumatoid arthritis. Lancet.
389:2328–2337. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Burmester GR and Pope JE: Novel treatment
strategies in rheumatoid arthritis. Lancet. 389:2338–2348.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Stoffer MA, Schoels MM, Smolen JS, Aletaha
D, Breedveld FC, Burmester G, Bykerk V, Dougados M, Emery P,
Haraoui B, et al: Evidence for treating rheumatoid arthritis to
target: Results of a systematic literature search update. Ann Rheum
Dis. 75:16–22. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nikiphorou E, Norton S, Young A, Carpenter
L, Dixey J, Walsh DA and Kiely P: ERAS and ERAN. Association
between rheumatoid arthritis disease activity, progression of
functional limitation and long-term risk of orthopaedic surgery:
Combined analysis of two prospective cohorts supports EULAR treat
to target DAS thresholds. Ann Rheum Dis. 75:2080–2086.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Aletaha D and Smolen JS: Diagnosis and
management of rheumatoid arthritis: A review. JAMA. 320:1360–1372.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gavrilă BI, Ciofu C and Stoica V:
Biomarkers in rheumatoid arthritis, what is new? J Med Life.
9:144–148. 2016.PubMed/NCBI
|
|
11
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Welten SM, Bastiaansen AJ, de Jong RC, de
Vries MR, Peters EA, Boonstra MC, Sheikh SP, La Monica N,
Kandimalla ER, Quax PH and Nossent AY: Inhibition of 14q32
MicroRNAs miR-329, miR-487b, miR-494, and miR-495 increases
neovascularization and blood flow recovery after ischemia. Circ
Res. 115:696–708. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yang DW, Qian GB, Jiang MJ, Wang P and
Wang KZ: Inhibition of microRNA-495 suppresses chondrocyte
apoptosis through activation of the NF-κB signaling pathway by
regulating CCL4 in osteoarthritis. Gene Ther. 26:217–229.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhou J, Xu T, Yan Y, Qin R, Wang H, Zhang
X, Huang Y, Wang Y, Lu Y, Fu D and Chen J: MicroRNA-326 functions
as a tumor suppressor in glioma by targeting the Nin one binding
protein (NOB1). PLoS One. 8(e68469)2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang J, Cao L, Wu J and Wang Q: Long
non-coding RNA SNHG1 regulates NOB1 expression by sponging miR-326
and promotes tumorigenesis in osteosarcoma. Int J Oncol. 52:77–88.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Smolen JS, Breedveld FC, Burmester GR,
Bykerk V, Dougados M, Emery P, Kvien TK, Navarro-Compán MV, Oliver
S, Schoels M, et al: Treating rheumatoid arthritis to target: 2014
Update of the recommendations of an international task force. Ann
Rheum Dis. 75:3–15. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Perkins JR, Dawes JM, McMahon SB, Bennett
DL, Orengo C and Kohl M: ReadqPCR and NormqPCR: R packages for the
reading, quality checking and normalisation of RT-qPCR
quantification cycle (Cq) data. BMC Genomics.
13(296)2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tobón GJ, Youinou P and Saraux A: The
environment, geo-epidemiology, and autoimmune disease: Rheumatoid
arthritis. Autoimmun Rev. 9:A288–A292. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chiu HY, Huang HL, Li CH, Chen HA, Yeh CL,
Chiu SH, Lin WC, Cheng YP, Tsai TF and Ho SY: Increased risk of
chronic kidney disease in rheumatoid arthritis associated with
cardiovascular complications-a national population-based cohort
study. PLoS One. 10(e0136508)2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ito H, Kojima M, Nishida K, Matsushita I,
Kojima T, Nakayama T, Endo H, Hirata S, Kaneko Y, Kawahito Y, et
al: Postoperative complications in patients with rheumatoid
arthritis using a biological agent-a systematic review and
meta-analysis. Mod Rheumatol. 25:672–678. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Clark AL, Maruyama S, Sano S, Accorsi A,
Girgenrath M, Walsh K and Naya FJ: miR-410 and miR-495 are
dynamically regulated in diverse cardiomyopathies and their
inhibition attenuates pathological hypertrophy. PLoS One.
11(e0151515)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kefas B, Comeau L, Erdle N, Montgomery E,
Amos S and Purow B: Pyruvate kinase M2 is a target of the
tumor-suppressive microRNA-326 and regulates the survival of glioma
cells. Neuro Oncol. 12:1102–1112. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lee S, Yoon DS, Paik S, Lee KM, Jang Y and
Lee JW: microRNA-495 inhibits chondrogenic differentiation in human
mesenchymal stem cells by targeting Sox9. Stem Cells Dev.
23:1798–1808. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tanaka Y: Human mesenchymal stem cells as
a tool for joint repair in rheumatoid arthritis. Clin Exp
Rheumatol. 33 (Suppl 92):S58–S62. 2015.PubMed/NCBI
|
|
25
|
Gao W, Sweeney C, Connolly M, Kennedy A,
Ng CT, McCormick J, Veale DJ and Fearon U: Notch-1 mediates
hypoxia-induced angiogenesis in rheumatoid arthritis. Arthritis
Rheum. 64:2104–2113. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang X, Yang Y and Feng Z: Suppression of
microRNA-495 alleviates high-glucose-induced retinal ganglion cell
apoptosis by regulating Notch/PTEN/Akt signaling. Biomed
Pharmacother. 106:923–929. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mao Y, Li L, Liu J, Wang L and Zhou Y:
miR-495 inhibits esophageal squamous cell carcinoma progression by
targeting Akt1. Oncotarget. 7:51223–51236. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Formosa A, Markert EK, Lena AM, Italiano
D, Finazzi-Agro' E, Levine AJ, Bernardini S, Garabadgiu AV, Melino
G and Candi E: MicroRNAs, miR-154, miR-299-5p, miR-376a, miR-376c,
miR-377, miR-381, miR-487b, miR-485-3p, miR-495 and miR-654-3p,
mapped to the 14q32.31 locus, regulate proliferation, apoptosis,
migration and invasion in metastatic prostate cancer cells.
Oncogene. 33:5173–5182. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Du C, Liu C, Kang J, Zhao G, Ye Z, Huang
S, Li Z, Wu Z and Pei G: MicroRNA miR-326 regulates TH-17
differentiation and is associated with the pathogenesis of multiple
sclerosis. Nat Immunol. 10:1252–1259. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Wernert N, Justen HP, Rothe M, Behrens P,
Dreschers S, Neuhaus T, Florin A, Sachinidis A, Vetter H and Ko Y:
The Ets 1 transcription factor is upregulated during inflammatory
angiogenesis in rheumatoid arthritis. J Mol Med (Berl). 80:258–266.
2002.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Du W, Yin L, Tong P, Chen J, Zhong Y,
Huang J and Duan S: miR-495 targeting dvl-2 represses the
inflammatory response of ankylosing spondylitis. Am J Transl Res.
11:2742–2753. 2019.PubMed/NCBI
|
|
32
|
Tomofuji T, Yoneda T, Machida T, Ekuni D,
Azuma T, Kataoka K, Maruyama T and Morita M: Micro RNA s as serum
biomarkers for periodontitis. J Clin Periodontol. 43:418–425.
2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
García-Díaz D F, Pizarro C,
Camacho-Guillén P, Codner E, Soto N and Pérez-Bravo F: Expression
of miR-155, miR-146a, and miR-326 in T1D patients from Chile:
Relationship with autoimmunity and inflammatory markers. Arch
Endocrinol Metab. 62:34–40. 2018.PubMed/NCBI View Article : Google Scholar
|