Introduction
Liver transplantation is widely accepted as an
effective therapy for patients with end-stage liver disease
(1). In the past, donor organs in
China were mainly transplanted from relatives and deceased
patients. With improvements in China's legal system, the use of
deceased patients as donors was banned in 2015 (2,3). As a
consequence, there has been a severe shortage of organ donors
(4). Organ transplantation from
living relative donors has become the last resort; however, it is
not advocated (4). After lengthy
discussions and developments within the organ transplant branch of
the Chinese medical association (5), donation after citizen's death (DCD)
became the major source of organs for transplantation in
2015(3). Pediatric DCDs account for
a certain proportion of the total DCD donors (6). It is at times difficult to find
matching child recipients for the organs of older-aged normally
developed child donors (6).
Therefore, liver transplantations in adults using pediatric donor
livers have been performed to improve organ utilization (7-18).
This has broadened the source of liver donors for transplantation
in adults and certainly alleviated any shortages (7,8).
Selection of optimal recipients and appropriate surgical methods
based on the condition of the donor liver (including donor liver
volume, caliber difference between donor and recipient vessels, and
the spatial location of the donor liver in the abdominal cavity of
recipients, are crucial for successful liver transplantation in
adults using pediatric donor livers (16-18).
At present, pediatric donation in China is still in its infancy
(9-15).
The DCD donation process was implemented in the hospital of the
current study during its initial development as a pilot project. In
April 2008, a preliminary experience was obtained at the hospital
with the first case of an adult liver transplantation using a
pediatric donor liver (case 1 of the present study). Another two
transplantations wherein child donors provided organs for liver
transplantation in adults were performed in November 2015 (case 2)
and May 2016 (case 3). In the present study, these three cases of
adult liver transplantation using pediatric liver DCDs were
discussed. The relevant points and challenges discussed include
liver procurement and trimming, recipient selection, surgical tips,
prevention and treatment of small-for-size syndrome (SFSS),
selection of immunosuppressive regimens, prevention and treatment
of vascular complications and anticoagulant therapy.
Materials and methods
Patients
The study was approved by the Ethics Committee of
Jiangxi Provincial People's Hospital (Nanchang, China). Informed
consent was obtained from the legal guardians of donors for use of
their tissues and publication of associated data. Informed consent
was obtained from the recipients for receiving pediatric donor
tissue and publication of their data.
Pediatric donor data
Adult liver transplantations using pediatric donor
livers were successfully performed in three cases at Jiangxi
Provincial People's Hospital (Nanchang, China) between April 2008
and May 2016. A total of 3 pediatric donors (male, 2; female, 1;
mean age, 8.67 years; age range, 8-10 years, mean body mass index,
15.12±3.31 kg/m2) were recruited between April 2008 and
May 2016. Using the China Classification for Organ Donation
(5), one donor was designated China
Category I (organ donation consistent with international standards
for donation following brain death) and the other two donors were
designated China Category III (organ donation following brain death
awaiting cardiac death). Of the three pediatric donors, one had
died of a brain tumor and two had died of brain trauma. Age, sex
and relevant laboratory indexes, including total bilirubin (TBIL),
direct bilirubin (DBIL), aspartate aminotransferase (AST), alanine
aminotransferase (ALT), serum creatinine, warm ischemia time, cold
ischemic time and quality of the donor liver, were evaluated. All
of these parameters were within normal limits (Table I). Following trimming, the
graft/recipient weight ratio (GRWR) was 1.07-1.39% and the graft
volume/recipient standard liver volume ratio (GV/SLV) was
65.14-69.54%.
 | Table IClinical data of the pediatric liver
donors and liver grafts. |
Table I
Clinical data of the pediatric liver
donors and liver grafts.
| Donor no. | Donation
classification (China category) | Primary
disease | Age (years) | Sex | TBIL (µmol/l) | DBIL (µmol/l) | AST (IU/l) | ALT (IU/l) | CREA (µmol/l) | Warm ischemia time
(min) | Cold ischemia time
(h) | Harvested liver
mass (g) |
|---|
| 1 | I | Brain tumor | 8 | Male | 22.4 | 12.5 | 43 | 35 | 76 | 2 | 4.2 | 641 |
| 2 | III | Brain trauma | 8 | Female | 19.6 | 11.6 | 36 | 27 | 43 | 5 | 4.5 | 550 |
| 3 | III | Brain trauma | 10 | Male | 16.3 | 9.3 | 45 | 38 | 86 | 6 | 5.0 | 545 |
Clinical data of liver transplant
recipients and postoperative medications
A total of 3 liver recipients (male, 2; female, 1;
mean age, 47.67 years; age range, 39-56 years) who were confirmed
to have primary liver cancer were recruited between April 2008 and
May 2016. The liver functions were graded as Child-Pugh A in two
patients and Child-Pugh B in one patient (19). Two recipients underwent orthotopic
liver transplantation and one underwent piggyback liver
transplantation (Table II).
Post-operatively, an immunosuppressive regimen consisting of
mycophenolate mofetil and tacrolimus was scheduled for the
recipients with primary hepatocellular carcinoma. Anti-HBV
treatment was administered orally in the form of anti-HBV drugs. If
necessary, anti-HBV drugs were recommended in combination with two
antiviral drugs. Hepatitis B immunoglobulin was administered during
the hospitalization. Post-operative anticoagulant therapies,
including low molecular weight heparin sodium and aspirin, were
administered according to the recipients' liver blood flow
monitored by color Doppler ultrasound and associated coagulation
function indicators.
 | Table IIClinical data of adult liver
transplant recipients of pediatric donor livers. |
Table II
Clinical data of adult liver
transplant recipients of pediatric donor livers.
| Recipient no. | Primary
disease | Age (years) | Sex | Body height
(cm) | Body weight
(kg) | Liver function
grading | Anhepatic time
(min) | Surgical mode | Operation time
(h) |
|---|
| 1 | Primary liver
cancer | 39 | Female | 156 | 46 | Child B | 49 | Orthotopic | 6.1 |
| 2 | Primary liver
cancer | 56 | Male | 160 | 49 | Child A | 53 | Piggyback | 5.2 |
| 3 | Primary liver
cancer | 48 | Male | 162 | 51 | Child A | 58 | Orthotopic | 4.5 |
Results
In the case of recipient 1, postoperative jaundice
worsened during the course of the hospital stay following
transplantation. On the 3rd postoperative day, TBIL, DBIL, ALT and
AST levels were up to 178, 93.9, 84.1 µmol/l, 153 and 267 IU/l,
respectively, but the recipient had no chills or fever and no liver
pain, and the T-tube drainage prevented bile leakage. Recipient 1
was considered to suffer from FK506 toxicity as the FK506 blood
concentration was 12.66 ng/ml and there was no evidence of
rejection. After reducing the FK506 dose, the TBIL level gradually
decreased and the bile drainage volume gradually increased to
300-500 ml per day. Recipient 1 was discharged around postoperative
day 40. The T-tube was clamped at 2 postoperative months and
cholangiography was performed after 3 months of continuous clamping
of the T-tube. The cholangiography results indicated normal biliary
tract outflow, with no signs of obstruction and the T-tube was
removed. Postoperative chemotherapy with 5-fluorouracil and calcium
folinate was administered only once, as the white blood cell count
was low after chemotherapy. In 2011, recipient 1 died of liver
cancer recurrence and bone metastasis.
In the case of recipient 2, on the 7th postoperative
day, the patient suddenly developed abdominal distension,
prolongation of the clotting time and a sudden and dramatic rise in
transaminase levels, which peaked on the 9th postoperative day. The
TBIL, DBIL, ALT and AST levels were 89.8 µmol/l, 5,698 and 3,705
IU/l, respectively (Table III).
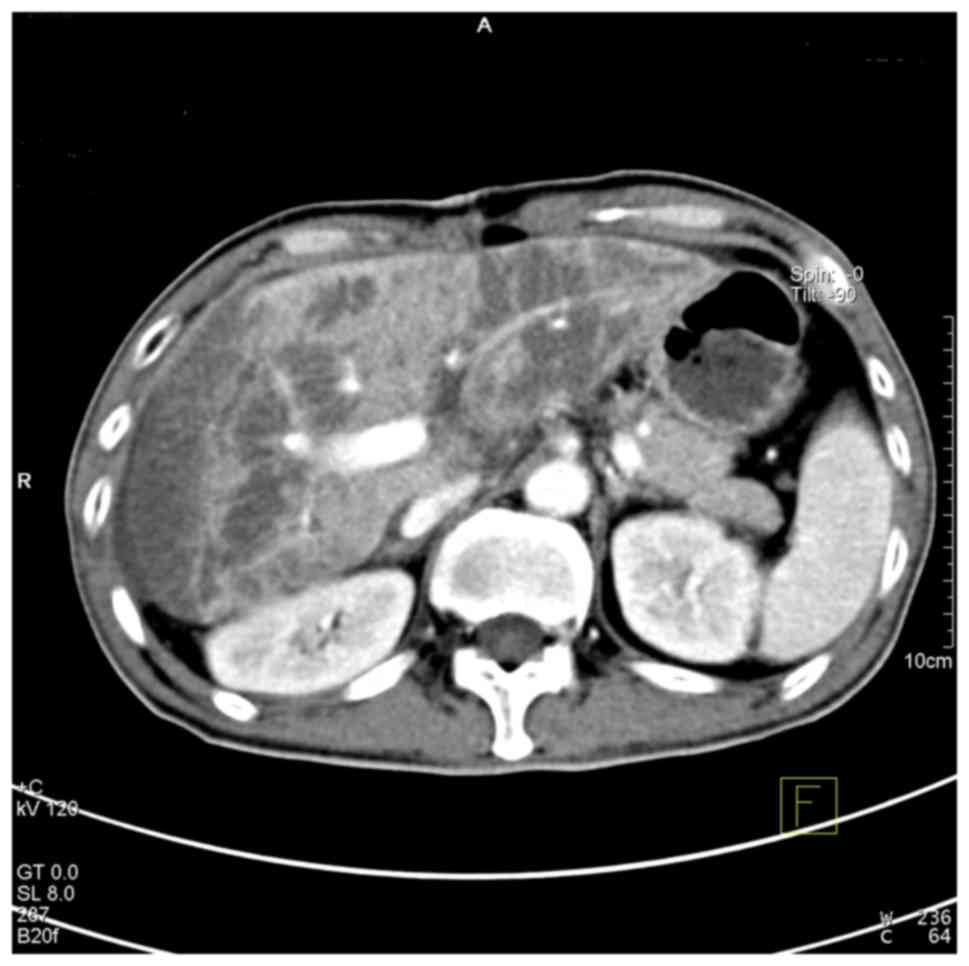
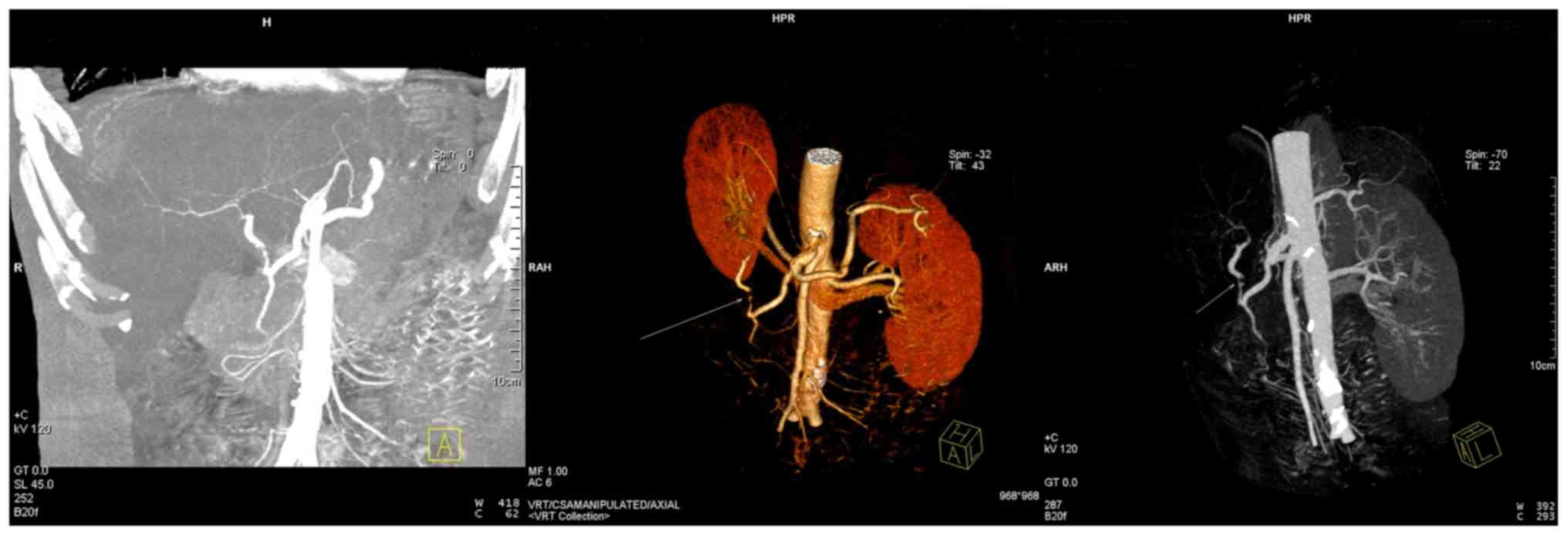
The results of CT angiography revealed multiple low-density lesions
in the liver and a weakly developed hepatic artery (Figs. 1 and 2). Recipient 2 was diagnosed with
avascular necrosis of the liver and the following measures were
taken: Withdrawal or reduction of the dosages of the drugs
considered to have caused the liver damage, adjustment of the
dosage of the immunosuppressive agents, increasing the
administration of antibacterial drugs and initiation of
somatostatin and terlipressin to improve the blood supply to the
liver. Thereafter, the clinical symptoms of recipient 2 improved
significantly on the 13th postoperative day. He experienced a
gradual recovery of his liver function and was then discharged from
hospital. As of now, recipient 2 remains in remission and is
subjected to regular follow-ups.
 | Table IIIClinical data of recipient 2
following surgery. |
Table III
Clinical data of recipient 2
following surgery.
| Number of days
after surgery | TBIL (µmol/l) | DBIL (µmol/l) | AST (U/l) | ALT (U/l) | PT (sec) | APTT (sec) | INR | Blood ammonia (µmol
/l) | Depth of ascites in
sitting position monitored by color Doppler ultrasound (cm) |
|---|
| 1 | 23.2 | 16.5 | 659 | 516 | 17.5 | 82.6 | 1.56 | 121.9 | 3.4 |
| 3 | 80.3 | 65.1 | 316 | 266 | 15.2 | 45.9 | 1.34 | 61.2 | 2.2 |
| 5 | 44.8 | 26.9 | 64 | 182 | 13.3 | 34.8 | 1.16 | 75.4 | 2.0 |
| 7 | 42.5 | 23.3 | 3,431 | 4,588 | 21.0 | 36.3 | 1.89 | 108.5 | 4.2 |
| 9 | 89.8 | 54.2 | 3,705 | 5,698 | 31.2 | 52.1 | 2.89 | 146.0 | 8.7 |
| 11 | 86.1 | 50.2 | 1,853 | 5,135 | 20.9 | 39.4 | 1.88 | 125.7 | 5.2 |
| 13 | 66.1 | 46.1 | 85I | 1,027 | 19.2 | 38.8 | 1.72 | 89.2 | 4.8 |
| 15 | 45.9 | 30.5 | 43 | 443 | 16.5 | 43.4 | 1.46 | 44.6 | 3.3 |
Recipient 3 recovered well after the liver
transplantation and his liver function returned to normal on the
3rd postoperative day. Since his discharge from hospital, the
patient has been in remission and is subjected to regular
follow-ups.
Discussion
In the present study, three cases of adult liver
transplantation using pediatric donor livers achieved good clinical
results. Pediatric donor livers have abundant blood vessels, little
connective tissue, incomplete liver parenchyma, strong regenerative
capacity and are unlikely to develop cirrhosis (20). Hepatocytes are basically mature at
the age of 8 years (6) and there is
still space for postoperative donor liver growth (9). Increased attention should be paid to
the harvesting and trimming of the donor liver, the choice of
recipients and the prevention and management of postoperative
complications.
Regarding the procurement of donor livers, it should
be observed during organ harvesting that the blood vessels and
biliary tract in the livers of the pediatric donors, particularly
those of underweight children, are thin (9,16-18).
Therefore, a modified no. 10 suction tube may be used to achieve
better perfusion instead of using a modified Foley catheter, which
is generally used for adult abdominal aortae. The donor livers from
underweight children are not necessarily mature and, therefore, the
perfusion fluid should be maintained at a moderate level so to
prevent excessive pressure that may cause liver sinus injury
(10). Furthermore, the perfusion
amount for pediatric donors is lower than that for adult donors
(9). Care must be taken to avoid
overperfusion while still ensuring adequate donor perfusion
(16). In the present study, during
the harvesting of the donor liver, the perfusion height for the
pediatric donor was 10-20 cm lower than that for the adult donor
and the perfusion amount for the pediatric donor was 1/2 to 2/3 of
that of the adult donor to reduce perfusion damage to the donor
liver.
During the trimming of the donor livers, the
pediatric donor liver used in adult liver transplantation should be
strictly assessed to avoid small-for-size donor livers (21-24).
The small-sized pediatric liver grafts may easily shift in the
abdominal cavity, causing the blood vessels and biliary tract to be
distorted (21-24).
Therefore, during trimming of donor livers, a part of the sacral
ligament may be retained to fix the donor liver after surgery. As
the wall of the inferior vena cava in the pediatric liver is
thinner, the surrounding tissue does not require to be completely
separated (21). Otherwise, blood
oozing may easily occur during and after surgery (21). The hepatic artery in the pediatric
liver is slender and complete trimming is not required (22). This reduces the probability of
vascular embolism caused by intimal injury during manipulation
(22,23). Special trimming of the common bile
duct is not generally required, which may be severed directly at
the edge of the pancreas to avoid interruption of the blood supply
(24).
Regarding the choice of recipients, patients with
tumors as the primary disease and those with lower body weights are
considered to be the optimal recipients in adult liver
transplantation using pediatric donor livers (11,16-18).
Patients who have had >1 episode of gastrointestinal bleeding,
multiple previous surgeries and/or severe portal hypertension are
not considered ideal candidates for pediatric liver
transplantations (11). In the
present study, the body masses of recipients 1, 2 and 3 were all
under 60 kg and their primary disease was primary liver cancer.
Preoperative liver function was graded as Child-Pugh A in two cases
and Child-Pugh B in one case. Therefore, the postoperative
incidence of SFSS was effectively reduced. In addition, the donor
GV/SLV may be used as a selection criterion to assess the size
matching between the liver graft and the recipient. GV may be
measured by CT or directly by the water displacement method. SLV
may be measured using the following formula: SLV (ml)=706.2x body
surface area (BSA, m2) + 2.4(25), BSA of pediatric donor
(m2)=0.0061x body height (BH, cm) + 0.0128x body weight
(BW, kg)-0.1529 and BSA of adult recipient (m2)=0.00659x
BH (cm) + 0.0126x BW (kg) -0.1603(4). The GRWR may be calculated according to
the conversion of weight to volume by the conversion factor of 1.19
ml/g (26). The currently accepted
standard is that GV/SLV should be >40% (27) and GRWR should not be <0.8-1%
(28). In the present study, the
GV/SLV was 65.14-69.54% and the GRWR was 1.07-1.39%.
Orthotopic or piggyback adult liver transplantations
from pediatric donors may be advantageous (9,16-18).
The choice of orthotopic liver transplantation falls in line with
the physiological requirements of the human body (9,16-18).
Therefore, considering the relatively small size of the pediatric
donor liver, the first porta hepatis (portal vein, hepatic artery
and the common bile duct) should be isolated carefully to preserve
the adequate length and the blood supply of the biliary tract.
Since the adult recipient has a larger abdominal cavity relative to
the child donor, orthotopic liver transplantations are relatively
better with respect to fixation of the donor liver and they impart
a lower risk of vascular complications such as outflow obstruction
(9). The use of piggyback liver
transplantations may avoid vena cava stenosis caused by the
differences in the vena cava diameters and reduce the surgical
complications caused by the blood vessel mismatches in the donor
liver (29). During liver trimming,
the donor hepatic artery may be trimmed using vascular loops for
anastomosis in order to solve the mismatch with the caliber of the
hepatic artery (11,21-24).
For the anastomosis of the common bile duct between the donor and
recipient, continuous anastomosis of the posterior wall and
intermittent anastomosis of the anterior wall have been adopted
(11). Furthermore, a T-tube may be
placed to guide the drainage if a significant difference exists in
the calibers of the common bile ducts of the donor and the
recipient (30). When entering the
blood vessels, controlling the blood pressure is necessary to
reduce damage to the donor liver (9). In the present study, orthotopic liver
transplantation was performed in two cases as there were no
significant differences in the vena cava calibers between the
recipients and donors. The ligaments of the donor livers and the
abdominal drainage tubes with balloons placed around the livers
were used in all three cases to fix the liver grafts in the
abdominal cavities (Fig. 3) . This
effectively reduced the blood vessel and biliary tract distortions
caused by position changes in the transplanted livers.
SFSS prevention is key in adult liver
transplantations using pediatric donor livers (31,32).
The mechanism underlying SFSS is that excessive perfusion of the
portal vein causes mechanical injury to the hepatic sinus and
portal vein endothelial cells, destruction of the space of Disse,
as well as flaky necrosis of liver tissues (17,33).
During liver transplantation in an adult using a pediatric donor
liver, effective control of the blood flow in the portal vein may
significantly reduce the risk of SFSS in adult recipients (34-37).
To reduce postoperative portal venous pressure, intraoperative
ligation of the splenic artery or splenectomy may be considered and
a postoperative intravenous infusion of somatostatin or
terlipressin may also be administered according to the unique
situation of the patient (38-40).
In the present study, on the 7th postoperative day, recipient 2
experienced sudden abdominal distension, developed a prolongation
of the clotting time and exhibited a sudden and marked rise in the
level of transaminase that peaked on the 9th postoperative day.
Postoperative TBIL, DBIL, ALT and AST levels in recipient 2 were
89.8 µmol/l, 5,698 and 3,705 IU/l, respectively. CT angiography
revealed multiple low-density lesions in the liver and a weakly
developed hepatic artery. Recipient 2 was thus diagnosed with
avascular necrosis of the liver, in line with the manifestations of
SFSS. Somatostatin and terlipressin were administered to improve
the blood supply in the liver. On the 13th postoperative day, the
patient's clinical symptoms improved significantly. The patient
exhibited a gradual improvement in liver function and was then
discharged from hospital. These observations indicate that the
ability of pediatric donor liver cells to regenerate is stronger
than that of adults; transplant liver function and postoperative
growth are issues of concern in adult liver transplantations using
pediatric donors (41).
Postoperative administration of terlipressin effectively alleviates
high portal venous pressure, reduces hepatic blood flow and eases
the mechanical damage to the liver sinuses. The early rise in
portal venous pressure is specifically controlled to avoid dual
injury from excessive perfusion and ischemia-reperfusion (42). This postoperative treatment provides
a novel method for effective prevention and treatment of SFSS after
liver transplantation (42,43). Terlipressin administration should
not be prolonged. Close monitoring of the patient's vital signs and
routine color Doppler ultrasound of the transplanted liver are
necessary to reduce the incidence of portal vein thrombosis and
bradycardia.
The use of immunosuppressive agents is key to
prevent rejection after liver transplantation. Tacrolimus is mainly
metabolized via the cytochrome P450 system in the liver and small
intestine and is then excreted via the biliary tract (44-47).
The use of immunosuppressive agents after liver transplantation in
an adult using a pediatric donor liver cannot be generalized.
Considering the smaller size of the donor liver in case 1, the
ability of the donor liver to metabolize the immunosuppressive
agents was less effective than that of the adult liver and
therefore, the FK506 blood concentration was maintained at a lower
level than the standard value. Combined with the results from cases
2 and 3 in the present study and the results of previous studies
(48-50),
it was indicated that pediatric donor livers have higher clearance
rates for tacrolimus and therefore, the dose of tacrolimus for
pediatric donors should be 2-4 times that of adult donors to
achieve the same concentration. This is inconsistent with the
conclusion obtained in case 1 of the present study. Therefore,
individualization of tacrolimus dosing is required after liver
transplantation in an adult using a pediatric donor liver (51,52).
Regarding the prevention and treatment of vascular
complications and anticoagulation therapy, vascular complications
associated with liver transplantation in adults using a pediatric
donor liver are mainly triggered by blood vessel volume mismatches
between donors and recipients (10). Furthermore, high portal perfusion
pressures may easily cause reactive contractions of hepatic
arterioles, which reduces the arterial blood flow and may
potentially cause the formation of a thrombus (9,16-18).
Anticoagulant therapy is given according to blood flow monitoring
on color Doppler ultrasound and laboratory indicators (8). In the present study, early
postoperative administration of low molecular weight heparin,
warfarin and aspirin anticoagulation was initiated to prevent
hepatic artery thrombosis. The anticoagulant drugs were adjusted or
stopped in accordance with the follow-up results from 3-6 months
postoperatively.
In conclusion, the present study suggested that
liver transplantations in adults using pediatric donor livers are
feasible. Systematic donor and recipient assessments, sound
surgical skills and optimal postoperative management are essential
for success in adult liver transplantations using pediatric donor
livers. Adult liver transplantations using pediatric donor livers
can widen the pool of liver donors for adult transplantation and
certainly alleviate the source shortage. Considering the condition
of the donor liver, selection of recipients and appropriate
surgical methods are particularly important for adult liver
transplantations using pediatric donor livers.
Acknowledgements
Not applicable.
Funding
The current work was supported by grants from Key
R&D Project of Scientific and Technical Supporting Program of
Jiangxi Province (grant no. 20161BBG70121) and the Science and
Technology Planning Project of Health and Family Planning
Commission of Jiangxi Province (grant no. 20181006).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LDi designed the study, analyzed and interpreted
data and wrote the manuscript. LDe and XL collected, analyzed and
interpreted data. ZX designed the study and critically revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The current study was approved by the Ethics
Committee of Jiangxi Provincial People's Hospital (Nanchang,
China). Written informed consent was obtained from the legal
guardians of donors for use of their tissues and publication of
associated data. Written informed consent was obtained from the
recipients for receiving pediatric donor tissue and publication of
their data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen ZH: New sight after a quarter
century: A long Chinese way to tackle the organ shortage in the era
of worldwide organ crises. Zhonghua Yizhi Zazhi Diziban. 4:265–272.
2010.
|
|
2
|
Zhang L, Zeng L, Gao X, Wang H and Zhu Y:
Transformation of organ donation in China. Transpl Int. 28:410–415.
2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhang GY, Liao T, Fu XB and Li QF: Organ
transplantation in China: Concerns remain. Lancet. 385:854–855.
2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yan LN: Modern Liver Transplantation. 1st
edition. Beijing, Military science publishing house, pp292-306,
2004.
|
|
5
|
Organ Transplantation Branch of the
Chinese Medical Association. National guidelines for donation after
cardiac death in China (2nd edition). Zhonghua Qiguanyizhi Zazhi
32: 756-758, 2011.
|
|
6
|
Shen XM: Clinical Pediatrics. 1st edition.
People's Medical Publishing House, Beijing, pp1010-1011, 2005.
|
|
7
|
Werner MJM, van Leeuwen OB, de Jong IEM,
Bodewes FAJA, Fujiyoshi M, Luhker OC, Scheenstra R, de Vries Y, de
Kleine RHJ and Porte RJ: First report of successful transplantation
of a pediatric donor liver graft after hypothermic machine
perfusion. Pediatr Transplant. 23(e13362)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Croome KP, Lee DD, Burns JM,
Saucedo-Crespo H, Perry DK, Nguyen JH and Taner CB: Mayo Clinic
Collaborative in Transplant Research and Outcomes: Outcomes of
liver transplantation with liver grafts from pediatric donors used
in adult recipients. Liver Transpl. 22:1099–1106. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
He XS, Ju WQ, Guo ZY, Wu LW, Tai Q, Han M,
Wang DP, Zhu XF and Huang JF: First case of pediatric donor liver
for adult liver transplantation in China. Zhonghua Waike Zazhi.
48:1597–1598. 2010.
|
|
10
|
Shi J, Luo WF, Ding LM, Xu ZD, Wang YG, Li
XC, Luo LB and Long CM: Clinical analysis of liver transplant from
a child of brain death to an adult. Zhonghua Gandan Waike Zazhi.
17:359–363. 2011.
|
|
11
|
Wei L, Zhu ZJ, Dong C, Gao W, Yang T, Sun
LY, Qu W, Rao W, Sun XY and Shen ZY: Use of liver graft from
pediatric donor of donation after cardiac death in adult recipient
(report of one case). Zhongguo Puwai Jichu Yu Linchuang Zazhi.
19:490–492. 2012.
|
|
12
|
Huang XL, Li GQ, Mu XX, Qin JJ, Zhou S, Li
MY, Pang XX Tan and SB and Sun PC: Preliminary experience of
pediatric donor liver and advanced marginal donor liver in adult
liver transplantation. Nanjing Yike Daxue Xuebao (Ziranban).
36193–196. (209)2016.
|
|
13
|
Lan C, Song JL, Yan LN, Yang JY, Wen TF,
Li B and Xu MQ: Pediatric donor to adult recipients in donation
after cardiac death liver transplantation: A single-center
experience. Transplant Proc. 49:1383–1387. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ju W, Li C, Zhang C, Ko DS, Wang D, Han M,
Schroder PM, Wang X, Jiao X, Wu L, et al: Outcome of the use of
paediatric donor livers in adult recipients: A single Chinese
Centre experience. Clin Res Hepatol Gastroenterol. 43:148–154.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li JJ, Zu CH, Li SP, Gao W, Shen ZY and
Cai JZ: Effect of graft size matching on pediatric living-donor
liver transplantation at a single center. Clin Transplant: 32,
2018. doi: 10.1111/ctr.13160.
|
|
16
|
Yasutomi M, Harmsmen S, Innocenti F,
DeSouza N and Krom RA: Outcome of the use of pediatric donor livers
in adult recipients. Liver Transpl. 7:38–40. 2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Emre S, Soejima Y, Altaca G, Facciuto M,
Fishbein TM, Sheiner PA, Schwartz ME and Miller CM: Safety and risk
of using pediatric donor livers in adult liver transplantation.
Liver Transpl. 7:41–7. 2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang R, Zhu ZJ, Sun LY, Wei L and Qu W:
Outcomes of liver transplantation using pediatric deceased donor
livers: A single-center analysis of 102 donors. Chin Med J (Engl).
131:677–683. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pugh RN, Murray-Lyon IM, Dawson JL,
Pietroni MC and Williams R: Transection of the oesophagus for
bleeding oesophageal varices. Br J Surg. 60:646–649.
1973.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shore PM, Huang R, Roy L, Darnell C, Grein
H, Robertson T and Thompson L: Potential for liver and kidney
donation after circulatory death in infants and children.
Pediatrics. 128:e631–e638. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gu L, Fang H, Li F, Zhang S, Shen C and
Han L: Impact of hepatic arterial hemodynamics in predicting early
hepatic arterial thrombosis in pediatric recipients younger than
three yr after living donor liver transplantation. Pediatr
Transplant. 19:273–278. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ackermann O, Branchereau S, Franchi-Abella
S, Pariente D, Chevret L, Debray D, Jacquemin E, Gauthier F, Hill C
and Bernard O: The long-term outcome of hepatic artery thrombosis
after liver transplantation in children: Role of urgent
revascularization. Am J Transplant. 12:1496–503. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gu LH, Fang H, Li FH, Li P, Zhu CX, Zhu JJ
and Zhang SJ: Prediction of early hepatic artery thrombosis by
intraoperative color Doppler ultrasound in pediatric segmental
liver transplantation. Clin Transplant. 26:571–576. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Guo WZ, Zhang JK, Cao SL, Wang ZH, Wen PH,
Shi XY, Yang H, Chen CQ and Zhang SJ: Experience of pediatric organ
procurement and bench surgery from donation after citizen's death.
Shiyong Qiguan Yizhi Dianzi Zazhi. 5:11–14. 2017.
|
|
25
|
Urata K, Hashikura Y, Ikegami T, Terada M
and Kawasaki S: Standard liver volume in adults. Transplant Proc.
32:2093–2094. 2000.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chan SC, Liu CL, Lo CM, Lam BK, Lee EW,
Wong Y and Fan ST: Estimating liver weight of adults by body weight
and gender. World J Gastroenterol. 12:2217–2222. 2006.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Shimada M, Ijichi H, Yonemura Y, Harada N,
Shiotani S, Ninomiya M, Yoshizumi T, Soejima Y, Suehiro T and
Maehara Y: Is graft size a major risk factor in living-donor adult
liver transplantation? Trans Int. 17:310–316. 2004.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lee HH, Joh JW, Lee KW, Kim SJ, Lee DS,
Park JH, Choi SH, Heo JS, Hyon WS, Kwak MS and Lee SK:
Small-for-size graft in adult living-donor liver transplantation.
Transplant Proc. 36:2274–2276. 2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tannuri U, Mello ES, Carnevale FC, Santos
MM, Gibelli NE, Ayoub AA, Maksoud-Filho JG, Velhote MC, Silva MM,
Pinho ML, et al: Hepatic venous reconstruction in pediatric
living-related donor liver transplantation-experience of a single
center. Pediatr Transplant. 9:293–298. 2005.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yang DS, Chen JJ, Lu Q, Huang ZY and Yang
GD: Complicated bile duct stricture and its surgical treatment.
Zhonghua Gandan Waike Zazhi. 9:73–75. 2003.
|
|
31
|
Tanaka K and Ogura Y: ‘Small-for-size
graft’ and ‘small-for-size syndrome’ in living donor liver
transplantation. Yonsei Med J. 45:1089–1094. 2004.
|
|
32
|
Marcos A: Right lobe living donor liver
transplantation: A review. Liver Transpl. 6:3–20. 2000.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Adam R, Castaing D and Bismuth H:
Transplantation of small donor livers in adult recipients.
Transplant Proc. 25:1105–1106. 1993.PubMed/NCBI
|
|
34
|
Gondolesi GE, Florman S, Matsumoto C,
Huang R, Fishbein TM, Sheiner PA, Schwartz ME, Emre S, Thung S,
Shapiro R and Miller CM: Venous hemodynamics in living donor right
lobe liver transplantation. Liver Transpl. 8:809–813.
2002.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Man K, Lo CM, Ng IO, Wong YC, Qin LF, Fan
ST and Wong J: Liver transplantation in rats using small-for-size
grafts: A study of hemodynamic and morphological changes. Arch
Surg. 136:280–285. 2001.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hill MJ, Hughes M, Jie T, Cohen M, Lake J,
Payne WD and Humar A: Graft weight/recipient weight ratio: How well
does it predict outcome after partial liver transplants? Liver
Transpl. 15:1056–1062. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
37
|
Campos BD and Botha JF: Strategies to
optimize donor safety with smaller grafts for adult-to-adult living
donor liver transplantation. Curr Opin Organ Transplant.
17:230–234. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhang YH, Zhang R and Xu J: Clinical
observation for hemodynamic changes of children DCD donor liver in
adult recipients during early stage. Shiyong Qiguan Yizhi Dianzi
Zazhi. 5:359–362. 2017.
|
|
39
|
Mack DR, Traystman MD, Colombo JL, Sammut
PH, Kaufman SS, Vanderhoof JA, Antonson DL, Markin RS, Shaw BW Jr
and Langnas AN: Clinical denouement and mutation analysis of
patients with cystic fibrosis undergoing liver transplantation for
biliary cirrhosis. J Pediatr. 127:881–887. 1995.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Noble-Jamieson G, Valente J, Barnes ND,
Friend PJ, Jamieson NV, Rasmussen A and Calne RY: Liver
transplantation for hepatic cirrhosis in cystic fibrosis. Arch Dis
Child. 71:349–52. 1994.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Song YW, Zhu ZJ, Sun LY, Wei L, Qu W, Zeng
ZG and Liu Y: Clinical study on growth and development of children
after liver transplantation. Qiguan Yizhi. 235–239. 2015.
|
|
42
|
Li LB, Chen YM, Zhang SN, Gao Y, Ren G,
Zhang CP, Xiong G and Ma J: Combination of terlipressin and FK409
for protection against small-for-size syndrome in living donor
liver transplantation. Zhongwai Jiankang Wenzhai. 25–27. 2014.
|
|
43
|
Mo LQ, Yuan BL, Xiao Y, Xiao LC, Xu KQ and
Huang WQ: Effects of terlipressin on hemodynamics and renal
function in cirrhotic patients undergoing orthotopic liver
transplantation. Shiyong Yixue Zazhi. 23:2461–2463. 2007.
|
|
44
|
Fung JJ and Starzl TE: FK506 in solid
organ transplantation. Ther Drug Monit. 17:592–595. 1995.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Spada M, Corno V, Colledan M, Segalin A,
Lucianetti A, Torre G, Riva S, Sonzogni A, Petz W and Gridelli B:
Rejection and tacrolimus conversion therapy in paediatric liver
transplantation. Transpl Int. 13 (Suppl 1):S341–S344.
2000.PubMed/NCBI View Article : Google Scholar
|
|
46
|
van Hooff JP and Christiaans MH: Use of
tacrolimus in renal transplantation. Transplant Proc. 31:3298–3299.
1999.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Crespo-Leiro MG: Tacrolimus in heart
transplantation. Transplant Proc. 35:1981–1983. 2003.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Yan LZ, Chen L, Wang LJ and Lu YX:
Metabolic differences between tacrolimus in adult and child
carcasses and living donor liver transplant recipients. Wujing
Houqin Xueyuan Xuebao (Yixueban). 23:578–582. 2014.
|
|
49
|
Neuhaus P, Blumhardt G, Bechstein WO,
Platz KP, Jonas S, Mueller AR, Langrehr JM, Lohmann R, Schattenfroh
N, Knoop M, et al: Comparison of FK506- and cyclosporine-based
immunosuppression in primary orthotopic liver transplantation. A
single center experience. Transplantation. 59:31–40.
1995.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Flanagan WM, Corthésy B, Bram RJ and
Crabtree GR: Nuclear association of a T-cell transcription factor
blocked by FK-506 and cyclosporin A. Nature. 352:803–807.
1991.PubMed/NCBI View
Article : Google Scholar
|
|
51
|
Ameyaw MM, Regateiro F, Li T, Liu X, Tariq
M, Mobarek A, Thornton N, Folayan GO, Githang'a J, Indalo A, et al:
MDR1 pharmacogenetics: Frequency of the C3435T mutation in exon 26
is significantly influenced by ethnicity. Pharmacogenetics.
11:217–221. 2001.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Jain A, Venkataramanan R, Sharma R, Kwong
T, Orloff M, Abt P, Kashyap R, Tsoulfas G, Batzold P, Williamson M
and Bozorgzadeh A: Pharmacokinetics of tacrolimus in living donor
liver transplant and deceased donor liver transplant recipients.
Transplantation. 85:554–560. 2008.PubMed/NCBI View Article : Google Scholar
|