Introduction
Pancreaticoduodenectomy (PD), first performed in
1909, was popularized by the American surgeon Professor Allen
Whipple and is therefore known as ‘Whipple operation’ (1,2). PD is
the standard surgical procedure for treating periampullary tumors
(including ampullary carcinoma, duodenal adenocarcinoma and distal
bile duct cancer) and pancreatic head carcinoma and includes two
surgical approaches: Transabdominal PD and laparoscopic PD
(3,4). PD has been historically considered to
be the most complex and promising procedure (5). Particularly in recent years, the
minimally-invasive PD technique has been extensively performed and
accepted as an effective method for treating pancreatic lesions
(6). However, the complications
post-PD, including bleeding, pseudoaneurysm, arteriovenous fistula,
infection, have constantly impeded the development of Whipple
operation (7). Among the
above-mentioned complications, delayed post-pancreatectomy
hemorrhage (PPH) is one of the relatively severe vascular adverse
events, which is usually caused by rupture of pseudoaneurysm
(8). The pseudoaneurysm is
frequently caused by the pancreatic fistula (PF) and injuries in
the surgical processes. Although pseudoaneurysm-induced bleeding is
rare in clinical practice, it has serious consequences if patients
receive improper treatment or delayed intervention. Furthermore, a
lack of proficient laparoscopic skills and training experience have
also impeded the development and application of Whipple operation
(9). However, there is insufficient
data to indicate that surgeons who lack proficient laparoscopic
skills and experience of the surgeons will lead to higher
complications.
Embolization therapy has been proven to be a
critical strategy for managing patients suffering from
cardiovascular diseases and surgical bleeding (10,11).
In the last decade, embolization therapy has been widely used in
the treatment of post-operative bleeding and delayed PPH caused by
pseudoaneurysm (12); however,
these pseudoaneurysms are managed by exploration in patients with
hemodynamically stable conditions. Ligation, pseudoaneurysm
resection and removal of the involved organ are the main methods of
surgical exploration (12).
Therefore, in the present study, the experience of intervention
treatment for pseudoaneurysm post-PD at our hospital was
retrospectively analyzed. The safety and clinical efficacy of
angiography and coil embolization in the treatment of
pseudoaneurysm after PD were investigated.
Materials and methods
Patients
The present retrospective study analyzed 17 patients
(5 females and 12 males) undergoing PD, which was followed by PPH
caused by pseudoaneurysm, between May 2011 and May 2018 at the
Affiliated Hospital of North Sichuan Medical College (Nanchong,
China). Detailed information on the patients is listed in Table I. The mean age of the patients was
61.35±8.95 years and the time between surgery and PPH was 7.41±4.56
days. The cohort comprised 13 cases of pancreatic head carcinoma, 1
case of adenocarcinoma of the duodenum and 3 cases of distal bile
duct cancer.
 | Table IDetails of the patients with bleeding
and PsA post-Whipple operation. |
Table I
Details of the patients with bleeding
and PsA post-Whipple operation.
| Patient
no./sex | Age (years) | Bleeding time
(days) | Pathological
diagnosis | Bleeding area | PsAs (n) | Technical
complications | Clinical
complications | Therapeutic
outcome |
|---|
| 1/female | 63 | 5 | Duodenal
adenocarcinoma | Digestive
tract | 2 | Distal
migration | - | Cure |
| 2/male | 57 | 7 | Pancreatic head
carcinoma | Digestive
tract | 1 | - | - | Cure |
| 3/male | 74 | 2 | Pancreatic head
carcinoma | Digestive
tract/abdominal cavity | 1 | - | - | Cure |
| 4/male | 71 | 2 | Pancreatic head
carcinoma | Digestive
tract/abdominal cavity | 1 | Rupture of GDA | Liver
dysfunction | Death |
| 5/female | 60 | 9 | Pancreatic head
carcinoma | Digestive
tract/abdominal cavity | 1 | - | - | Cure |
| 6/male | 43 | 12 | Pancreatic head
carcinoma | Digestive
tract/abdominal cavity | 1 | - | - | Cure |
| 7/male | 55 | 3 | Pancreatic head
carcinoma | Digestive
tract/abdominal cavity | 1 | - | - | Cure |
| 8/male | 52 | 6 | Pancreatic head
carcinoma | Digestive
tract/abdominal cavity | 1 | - | - | Cure |
| 9/male | 53 | 8 | Pancreatic head
carcinoma | Digestive
tract/abdominal cavity | 1 | Distal migration
and rupture of parent artery | Liver
dysfunction | Cure |
| 10/female | 59 | 13 | Distal bile duct
cancer | Digestive
tract/abdominal cavity | 1 | - | Pain | Cure |
| 11/male | 64 | 17 | Pancreatic head
carcinoma | Digestive
tract | 1 | - | Vomiting | Cure |
| 12/male | 72 | 4 | Pancreatic head
carcinoma | Digestive
tract/abdominal cavity | 1 | - | - | Cure |
| 13/male | 64 | 9 | Pancreatic head
carcinoma | Digestive
tract/abdominal cavity | 1 | - | - | Cure |
| 14/female | 56 | 5 | Distal bile duct
cancer | Digestive
tract/abdominal cavity | 1 | - | Vomiting | Cure |
| 15/male | 78 | 3 | Pancreatic head
carcinoma | Digestive
tract/abdominal cavity | 1 | - | Fever | Cure |
| 16/male | 57 | 6 | Distal bile duct
cancer | Digestive
tract/abdominal cavity | 1 | - | - | Cure |
| 17/female | 65 | 15 | Pancreatic head
carcinoma | Digestive
tract/abdominal cavity | 1 | - | - | Cure |
The present study was approved by the Ethics
Committee of the Affiliated Hospital of North Sichuan Medical
College [Nanchong, China; file no. 2019ER(A)09-02]. The treatment
methods, including exploration stent implantation and coil
embolization, were communicated to the patients and their families.
Due to economic factors and medical insurance of the patients, coil
embolization was chosen. All patients explicitly consented to
receiving this treatment.
Inclusion and exclusion criteria
The inclusion criteria were as follows: i) Patients
presented with symptoms of gastrointestinal or abdominal bleeding
after the Whipple operation; ii) patients without contraindications
underwent emergency arteriography or embolization therapy; iii) no
distant metastasis. The following exclusion criteria were applied:
i) Death due to peri-operative complications; ii) diagnosis with
peritoneal cancer; iii) patients who underwent angiography without
embolization with coils.
Arteriography and embolization
therapy
The Seldinger technique was used to percutaneously
puncture the femoral artery and insert a 5F catheter sheath. To
assess the condition of the artery of the digestive tract,
abdominal aortography was performed with a pigtail catheter.
Subsequently, a 5F catheter was inserted into the celiac, common
hepatic, splenic, gastroduodenal (GDA), superior and inferior
mesenteric arteries in all patients for angiographic analysis to
determine the location of the pseudoaneurysm(s) and the diameter of
the parent artery and collateral vessels. If any active bleeding or
pseudoaneurysm was detected, a 2.5F microcatheter (Renegade™ STC18;
Boston Science) was inserted into the target artery using a coaxial
catheter technique for super-selective angiography and
embolization. Various techniques are described for embolization of
a pseudoaneurysm using Tower or Diamond coils (Boston VortX™-18
platinum coil or VortX™-18 Diamond platinum coil; 0.018 inches;
Boston Science) and/or a detachable coil embolization system
(Interlock Fibered IDC occlusion system, 0.018 inches; Boston
Scientific). The commonly used methods of embolization of
pseudoaneurysms included simple lumen embolization (sac packing
technique), proximal embolization of the parent artery (proximal
embolization technique), inflow and outflow embolization of the
parent artery (exclusion technique or isolation technique) and
efferent artery embolization + sac packing/aneurysmal neck packing
+ afferent artery embolization (sandwich technique) (13). Sac packing is performed for saccular
pseudoaneurysms with a narrow neck, which allows for retention of
coils within the sac maintaining the patency of the parent artery.
Proximal embolization of the parent artery is applied to
pseudoaneurysms at the end of arterioles, including or excluding
pack of sac, which is essentially a special exclusion technique.
The exclusion technique is used for those pseudoaneurysms with a
small size, wide neck and short landing zone, which refers to the
area of proximal and distal stent placement and vascular
remodeling. The sandwich technique is performed for pseudoaneurysms
that are likely to have collateral inflow and outflow arteries.
Post-operative evaluation and
follow-up
The definition of bleeding and severity was
according to the Standard of International Study Group for
Pancreatic Surgery (14). The
definitions and evaluation criteria on the efficacy, as well as
technical and clinical success of embolization, were according to
the Society of Interventional Radiology guidelines (15). Achievement of hemostasis,
disappearance of the pseudoaneurysm or the occlusion of the parent
artery were considered indicative of technical success (15). Clinical success referred to the
absence of acute bleeding symptoms, stable hemodynamics, hemoglobin
decrease of no more than 15 g/l, no requirement to infuse suspended
red blood cells, no blood fluid in the drainage tube and no
re-angiography or embolization (16-18).
Technical complications included non-target vascular embolism,
iatrogenic vascular injury, puncture site bleeding, rupture of the
pseudoaneurysm, rupture of the parent artery, arterial dissection,
distal migration of the coil and straight deployment of the coil.
Clinical complications included post-embolization complications
(secondary infection) and embolization syndrome (pain, fever, liver
dysfunction and vomiting). Technical and clinical success were the
major endpoints of embolization (19-21).
The short-term clinical outcome was evaluated according to the
following points: Whether symptoms of abdominal pain or fever were
present, vital signs, bleeding and dynamic monitoring of
hemoglobin. The long-term clinical outcome was evaluated according
to the following points: Occurrence of re-bleeding, heterotopic
embolism caused liver failure or gastrointestinal ischemic
necrosis.
Results
Arteriography presentation and
embolization therapy
A total of 18 pseudoaneurysms were detected in 17
patients. The types of primary diseases, time between surgery and
PPH, bleeding time and area, number of pseudoaneurysms, technical
and clinical complications and therapeutic outcomes of all 17
patients are shown (Table I) The
angiographic findings of pseudoaneurysm were cystic, round or
irregular nodular, with spasm and rigidity of the parent artery in
the vast majority of cases (17/18; Figs. 1 and 2). Indirect signs of arteriography were
irregular and rigid morphology of the transitional area of the
common hepatic artery and the proper hepatic artery, with small
notches (Figs. 3 and 4). Sentinel hemorrhage was detected in all
patients but there was no sign of active hemorrhage at the time of
angiography. There were 17 cases of pseudoaneurysm, including 15
cases with the pseudoaneurysm located at the main trunk and
branches of the GDA, 1 case with location in the common hepatic
artery (Fig. 3A), 1 case with
location in the stump of the GDA (Fig.
4A) and 1 case with location of the pseudoaneurysm in the major
splenic artery.
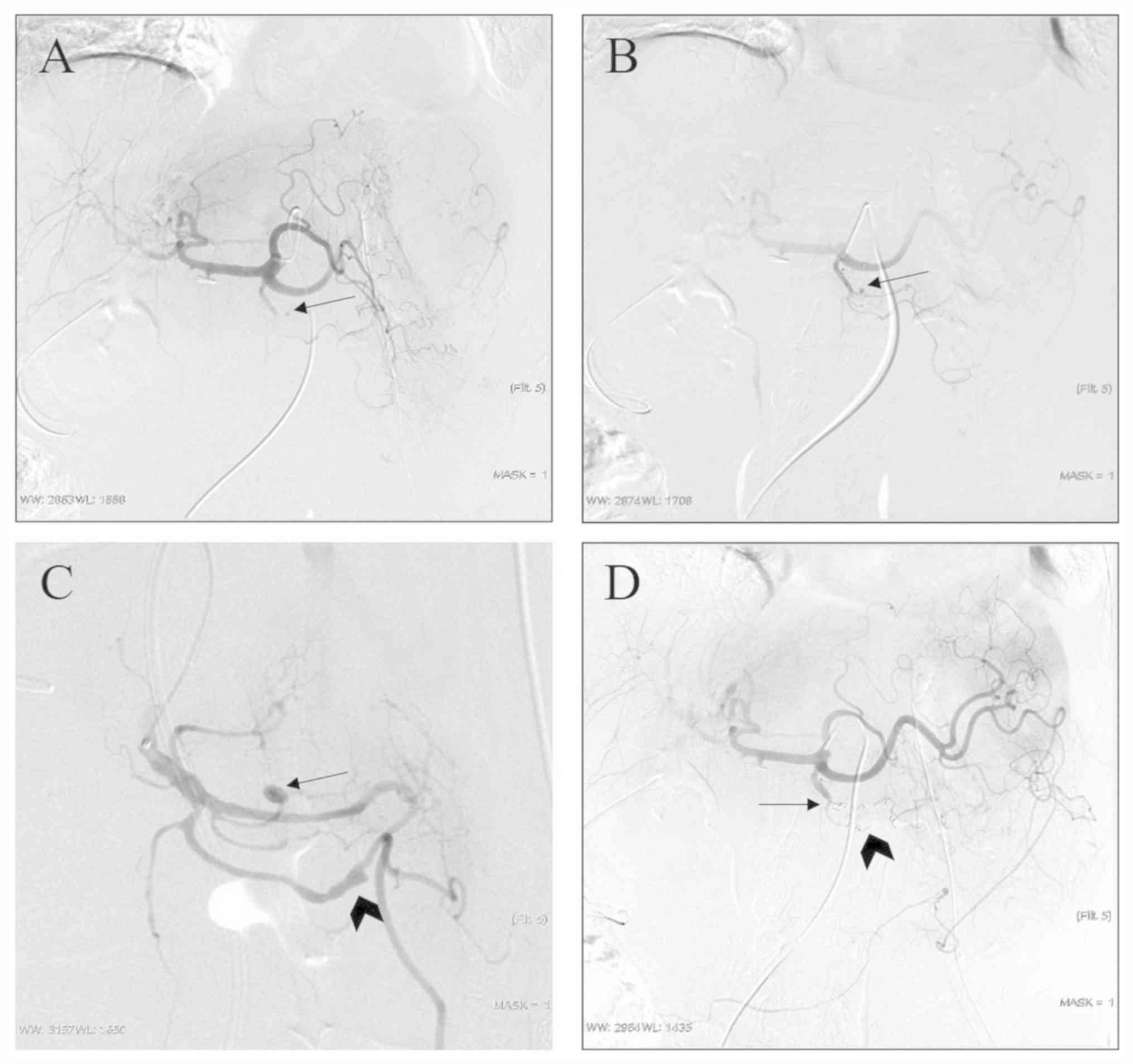
In one patient, two pseudoaneurysms were located in
different branches of the GDA, who underwent a combination of the
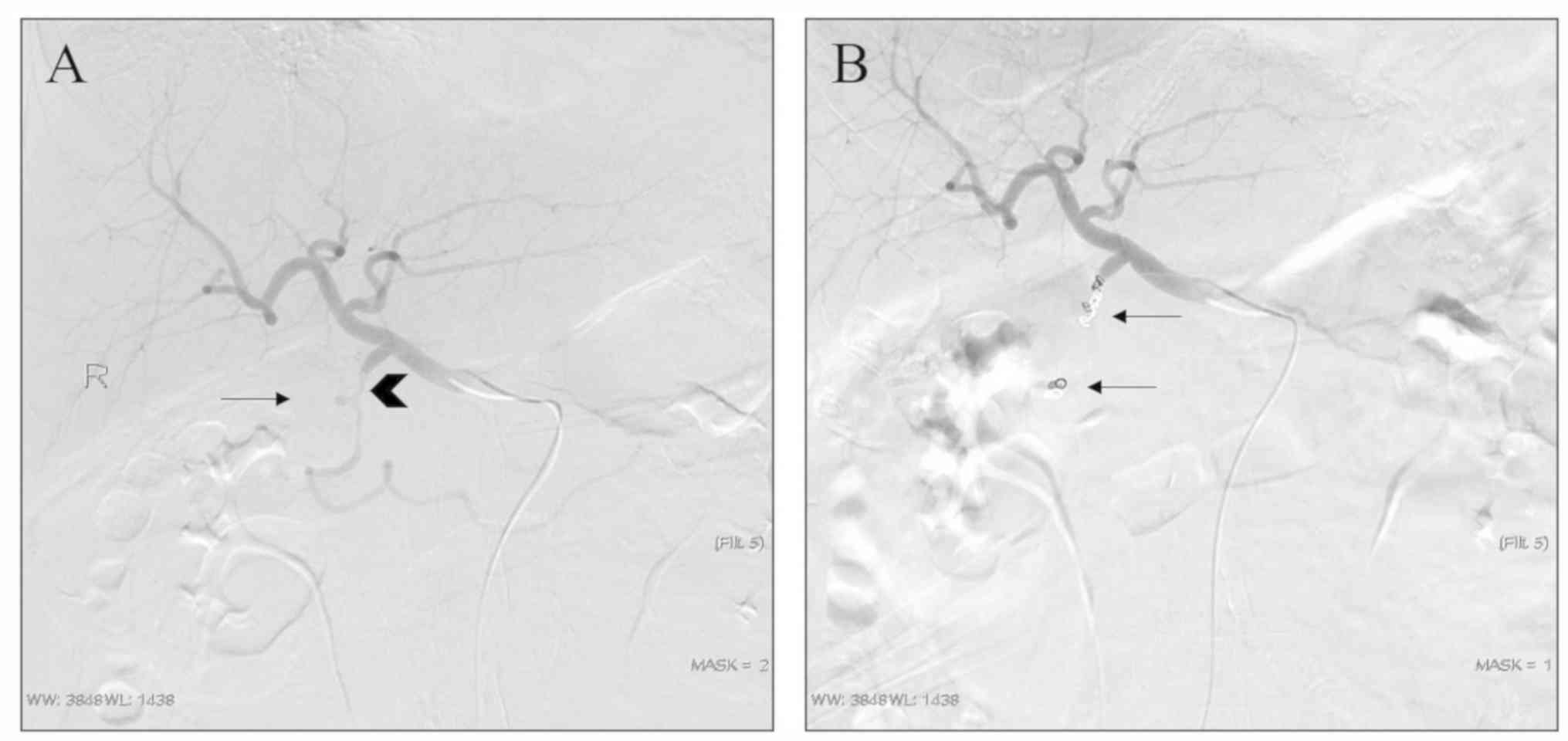
proximal embolization and exclusion techniques (Fig. 1). In 16 pseudoaneurysms, the
sandwich or exclusion technique were used for embolization, where
good therapeutic effects were achieved, as exemplified by a
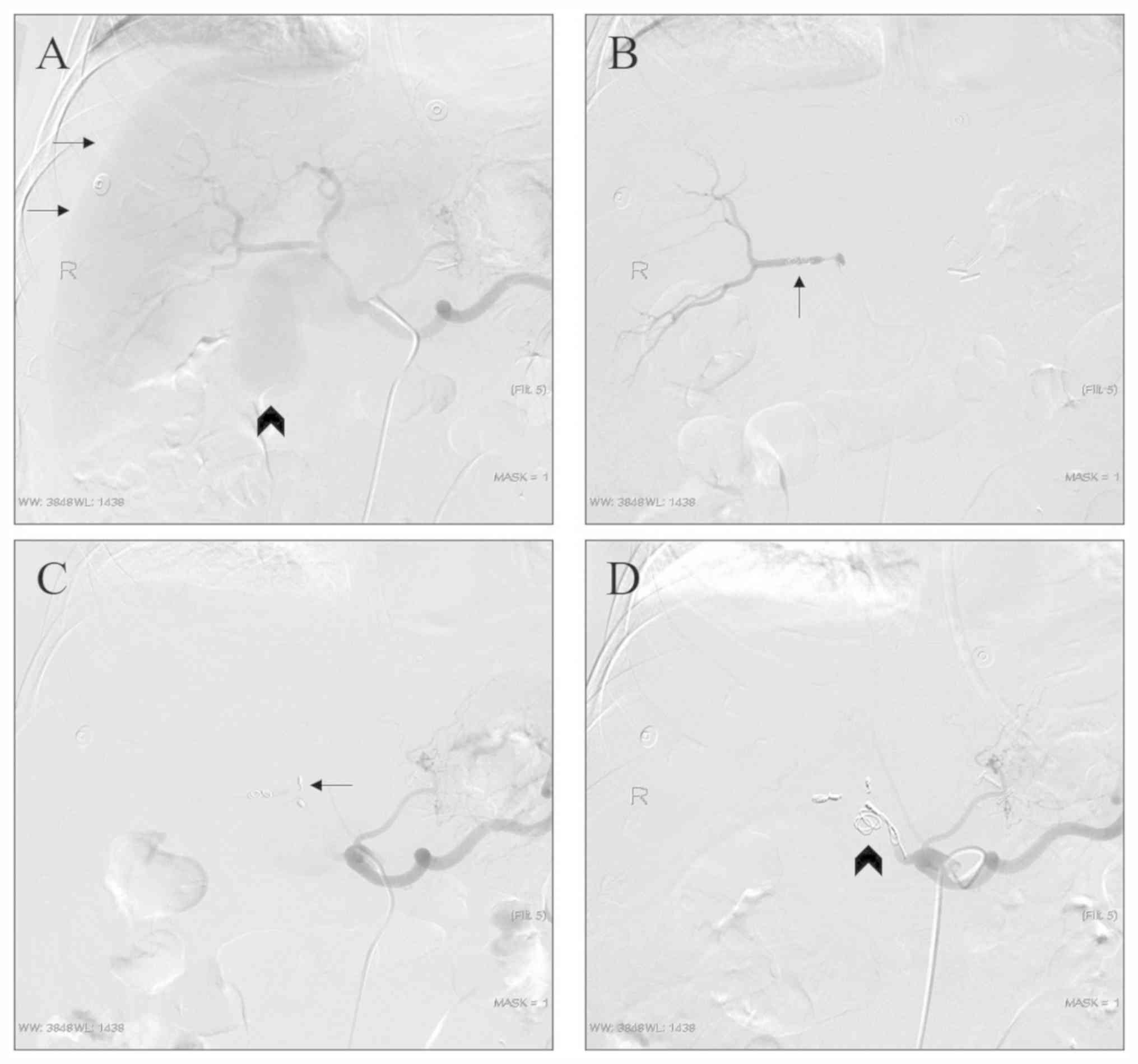
representative case with arteriography images provided in Fig. 2. Furthermore, 1 patient had
perihepatic hematocele and the liver was compressed to the midline.
Considering the large size of the pseudoaneurysm, the risk of
rupture of the pseudoaneurysm and the progressive decrease of
hemoglobin, the exclusion technique was used directly after
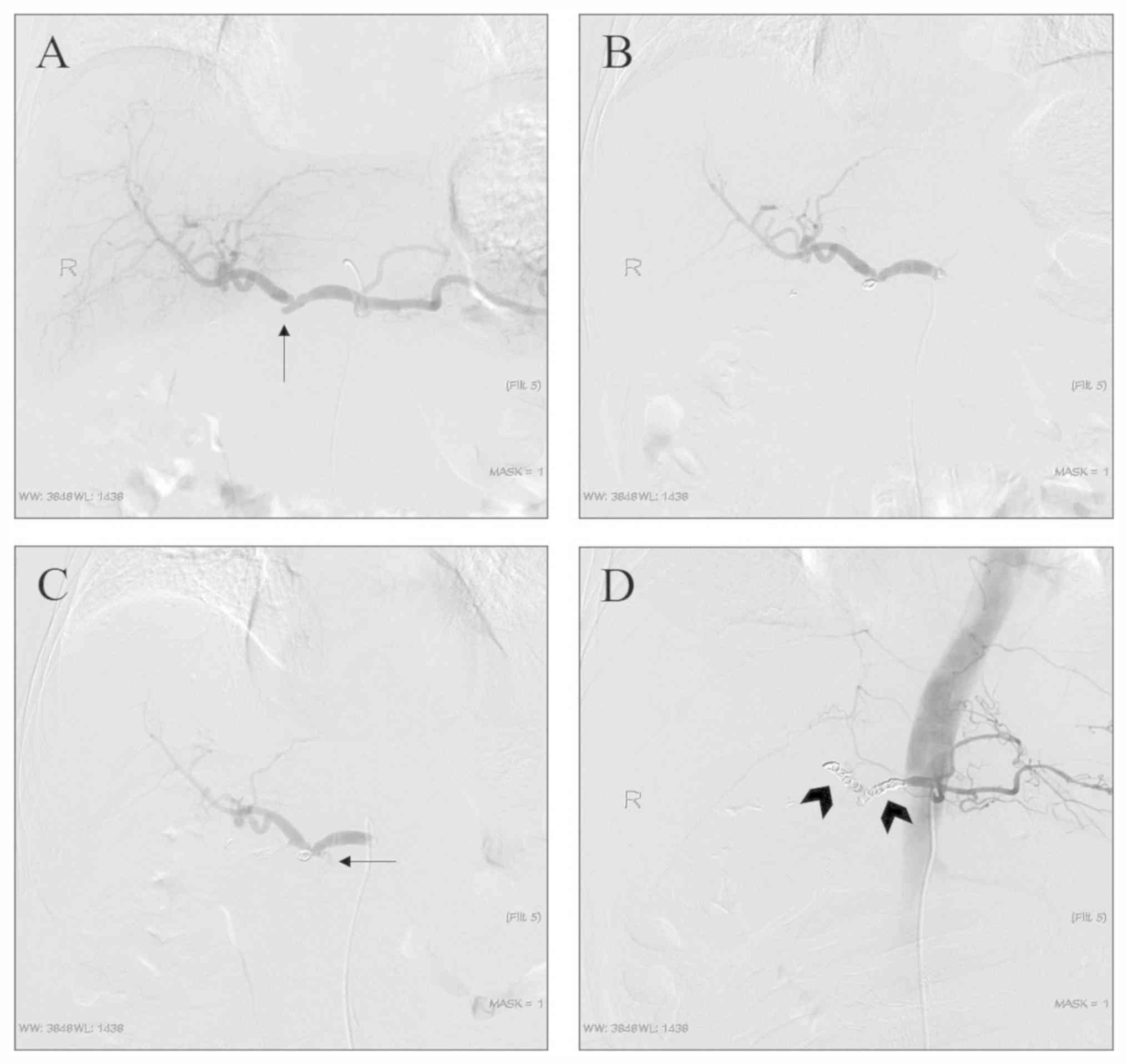
arteriography (Fig. 3). In one
case, angiography revealed an irregular GDA stump and an adjacent
hepatic artery, which was considered as a residual saccular
pseudoaneurysm. Considering that the patient had blood in the
abdominal drainage tube and no active bleeding was detected on
arteriography, the sac packing technique was used for GDA stump
embolization. Angiography revealed that the coil was in a good
position and the operation was completed (Fig. 4B). However, during the extraction of
the catheter, the patient complained of abdominal pain, which was
followed by a significant drop in blood pressure. Emergency
re-angiography revealed that the GDA pseudoaneurysm ruptured and
bled, and the sandwich technique was performed to embolize the
proper hepatic artery and common hepatic artery (Fig. 4). Despite aggressive symptomatic
treatment, the patient died 1 week later from causes including
insufficient blood volume and liver failure.
Technique, clinical complications and
success
In one patient, serious technical complications of
pseudoaneurysm rupture occurred, which resulted in death. Another
patient presented with the technical complication of coil
migration, but it did not cause any serious clinical complications.
No serious clinical complications occurred in any of the other
patients. A total of 7 patients had mild clinical complications,
including mild abdominal and dorsal pain, which was alleviated by
symptomatic management. In total, 15 patients with definite
pseudoaneurysms were successfully embolized without re-bleeding and
complications. The success rate of hemostasis was 94.1%
(16/17).
Discussion
PD has been widely used for treating pancreatic head
cancer and periampullary cancers; however, the complications still
hinder the further development of Whipple operation (22). Post-operative complications include
postoperative pancreatic fistula (POPF), hemorrhage, pseudoaneurysm
and arteriovenous fistula. Delayed post-pancreatectomy bleeding
refers to the bleeding at >24 h post-PD (17,23).
The incidence of post-pancreatectomy bleeding has been estimated to
be 4-16% and the mortality rate may be as high as 50% within 1-4
weeks (24-26).
In the present study, the mean bleeding time post-PD was ~7 days,
but no clinical manifestations such as re-bleeding appeared
post-embolization. Among numerous factors affecting the safety of
PD, POPF is the most important risk factor. For patients with
periampullary cancer, which includes duodenal, ampullary and distal
bile duct cancers, they exhibit similar clinical symptoms and
require similar treatment strategies compared with pancreatic head
carcinoma (27,28). However, pancreatic fistula after PD
for pancreatic head carcinoma is more dangerous and may cause
arterial rupture and bleeding (29,30).
With the improvement of surgery, a variety of modified Whipple
operations (such as pylorus-preserving PD) has been widely used and
the complications are gradually reduced (27). However, due to the gap in resources
between Eastern and Western China in terms of medical development,
the classic (Whipple's) PD is still the major treatment strategy at
our hospital, and therefore, its complications, including POPF and
bleeding, are not rare. POPF is the most common and serious
complication of pancreatic surgery. No matter which type of
operation is used, the incidence of POPF is as high as 20-40%
(31,32). It is not uncommon for trypsin to
erode and digest the peripancreatic artery and cause
pseudoaneurysm. Electrotome and ultrasound scalpel may damage
adventitia and result in pseudoaneurysm during lymph node removal.
In the process of tissue dissection, it is inevitable to clamp the
tissue, resulting in arterial and venous damage. Together, the
above-mentioned risk factors are common causes of bleeding and
pseudoaneurysm post-Whipple procedures. In addition, in our
experience, slippage of the ligation line of the gastroduodenal
stump may also be an important cause of bleeding (33).
Abdominal pain, abdominal distension, abdominal
hemorrhage, hematemesis and hematochezia are common clinical
symptoms of pseudoaneurysm rupture and bleeding, while sentinel
bleeding is generally regarded as a precursor of massive hemorrhage
(34). CT angiography is valuable
in the diagnosis of pseudoaneurysms with a sensitivity rate of 95%
(35,36). However, most of the patients with
early hemorrhage are not suitable to undergo this type of imaging
because of their critical and urgent condition (35,36).
Selective celiac angiography is the gold standard for the diagnosis
of bleeding and pseudoaneurysm post-PD. It may not only determine
whether there is an aneurysm and determine its size and association
with the parent artery, but also allow for interventional treatment
to be performed immediately. In digital subtraction angiography,
extravasation of contrast medium is a direct sign of bleeding.
Indirect signs of bleeding include pseudoaneurysm, vasospasm and a
rough vascular wall. A small pseudoaneurysm may be misdiagnosed as
a ligated GDA stump or the diagnosis may be missed. When
pseudoaneurysms in the major GDA or a branch are highly suspected,
the catheter should be placed as close as possible to the hepatic
artery for angiography and the injection rate, flow rate and
pressure should also be checked. A location far from the GDA neck
or a slow flow rate of the contrast agent may lead to a
false-negative result on angiography. In certain patients,
angiography may reveal difficulties in catheterization and not all
causes of the bleeding may be identified; thus, exploration and
endoscopic treatment are more appropriate for those patients with
delayed PPH and unstable hemodynamics (14,18).
Among the 17 patients in this group, 1 patient had a suspected
small pseudoaneurysm at the end of a small branch of the GDA on
celiac angiography. When common hepatic artery angiography and
amplified angiography were performed, the pseudoaneurysms and
irregular parent artery were clearly displayed. In addition,
attention should be paid to eliminate intestinal contents,
intestinal gas overlap, motion artifacts and other interference to
prevent misdiagnosis and missed diagnosis.
The common ways to deal with post-operative
hemorrhage and pseudoaneurysm are surgery and endovascular
management. The strategy during the surgical procedure is to find
the parent artery of the pseudoaneurysm and ligate it with
filaments or clamp it with titanium alloy. If PF is identified,
pancreas-intestinal anastomosis may be performed simultaneously.
Embolization, stent-graft implantation, stent-assisted coiling and
balloon remodeling techniques are commonly used endovascular
therapies. The covered stent increases the operative time and
difficulty for patients with emergency bleeding (16), and PF may lead to re-occurrence of
pseudoaneurysms at both ends of the stent. In addition, economic
factors and the minor diameter of the parent artery are also
important reasons for the limited application of covered stent. Due
to the extensive anastomosis of digestive vessels, the possibility
of re-bleeding after embolization is high, but it also associated
with the absence of large-area tissue necrosis after embolization,
and thus, it is widely used in clinical practice (37).
The embolization method of pseudoaneurysm is mainly
based on its location, size and diameter of the parent artery
(13,16). In addition, the presence of
sufficient landing zones and lateral branches also determines the
choice of embolization method. Microcoils are the preferred and
most widely used tools for embolization of a pseudoaneurysm.
Various techniques of coil embolization for pseudoaneurysm are
widely used. Pseudoaneurysms in the main GDA were similar to those
in the splenic and hepatic arteries observed in the present study.
The exclusion technique is recommended for embolization, but the
possibility of stent implantation to maintain patency of the parent
artery may reduce complications. However, in an emergency, coil
embolization is worth using to prevent the occurrence of
unfavorable events (38). The
technique of proximal embolization is widely used in the treatment
of pseudoaneurysm at the end of small branches. Sac packing is used
for saccular pseudoaneurysms with a narrow neck, which allows for
maintaining the patency of the parent artery. However,
pseudoaneurysms have a risk of secondary rupture (39). Hur et al (40) compared two embolization techniques
in the treatment of GDA stumps and concluded that the rupture rate
of the pseudoaneurysm treated by the packing technique (selective
embolization of the GDA stump and/or pseudoaneurysm sparing hepatic
arterial flow) was higher than that of the sandwich technique
(embolization of the hepatic artery proximal and distal to the GDA
stump). Ligation of the GDA and lymph node dissection during PD may
lead to the formation of pseudoaneurysm of the GDA (40). The strategy varies slightly
depending on the length of the GDA stump. In the present study, it
was assumed that if the GDA stump exhibited a beak-like change from
the normal shape and the hepatic artery was not damaged, no
embolization of the stump was required; however, a sac-like change,
regardless of its length, required intervention to prevent
secondary rupture. If the stump is long, a microcatheter may be
inserted coaxially and embolized by microcoil packing without
affecting the blood flow of the liver. If the GDA stump cannot be
embolized using the coil packing technique, it is essential to
prevent complications including coil migration when hepatic artery
embolization is performed with the exclusion technique or sandwich
technique as a last resort. In this case, it is important to choose
a microcoil with the correct diameter. However, due to bleeding,
hypotension and the use of vasoconstrictor drugs, visceral arteries
frequently constrict or spasm. If minor diameter coils are used,
distal migration and re-bleeding may occur. Similarly, if it is too
large, it may lead to artery rupture. Therefore, it may be reasoned
that the ratio of the coil diameter to parent artery diameter
should be at least 1.2-1.5 to stabilize the coil at the orifice of
the pseudoaneurysm. The coil should be stabilized in the aneurysmal
neck of a pseudoaneurysm, even if the coil packing is poor. In the
present study, there was a case of GDA residual pseudoaneurysm
(case no. 4; Table I), which was
embolized by the sac packing technique. During the extraction of
the catheter after treatment, the patient complained of abdominal
pain, which was accompanied by a significant drop in blood
pressure. Re-angiography confirmed active bleeding, which was
considered to be caused by rupture of pseudoaneurysm. Therefore,
the sandwich technique was adopted for immediate treatment.
However, the methods and strategies of embolization in this case
were initially inappropriate and resulted in irreversible technical
complications. Sac packing and sandwich technique resulted in
pseudoaneurysm rupture and hepatic artery occlusion respectively,
which were inappropriate for this patient. Covered stent placement
and assisted coiling are more suitable for this type of patient,
who had mechanically injured arteries from clamping, stretching and
removal of lymph nodes along the vessels during the operation
(41). Fluid embolization materials
have been widely used in the treatment of pseudoaneurysm, but the
risk of ectopic embolization is higher than that of coil
embolization. The combination of liquid embolization materials with
ectopic embolization is a good approach (42,43).
With the continuous improvement of technology, various approaches
are available for the management of pseudoaneurysms. The
endovascular approach, percutaneous approach and endoscopic
ultrasonography are widely used in recent years (44). In the present study, the clinical
success rate was higher (74 vs. 94.1%) and the re-bleeding rate was
lower than that reported by Pottier et al (45) (0 vs. 29%), which may have been due
to the embolic materials used, the embolic methods adopted and
different criteria for clinical success. The combination of
multiple embolic materials and methods may improve the success rate
and reduce complications and associated mortality. Although
procedures of standard clinical practice were adopted and a higher
clinical success rate and lower mortality rate (5.9%, 1/17) were
achieved in the present study as compared with those in certain
other studies; the mortality rate reported in the literature ranges
from 8.69 to 12.5% (40,46). The mortality rate in the present
study was still low, as it was within the threshold set by the
recommendations of guidelines, according to which the overall
mortality should be controlled between 2.2 and 12.8% (suggested
threshold, 10%) (15). The
mortality in one patient (case no. 4; Table I) was caused by our decision-making
mistakes. The exclusion/isolation technique or sandwich technique
is more suitable for residual saccular pseudoaneurysm of the GDA
stump, rather than the sac packing technique. A detailed analysis
of angiography images and scientific implementation measures may
avoid such incidents.
Of note, the present study had certain limitations.
Due to the nature of the patients' work and economical status, was
is difficult to perform a long-term follow-up and the data were
incomplete. In addition, the type of medical insurance and the
opinions of family members are important factors that influence the
choice of treatment. In the present study, taking individuals from
low income and low education backgrounds into consideration and
that the patients only received coil embolization, selection bias
may exist. Due to difficult catheterization of affected arteries
and high risk of migration of coils, surgical exploration may be
more appropriate for such patients. Furthermore, the present study
was a retrospective analysis and no comparative analysis was
performed. The small sample size is another shortcoming of the
present study.
In conclusion, there are abundant collateral vessels
in pseudoaneurysm of the digestive tract and the possibility of
organ necrosis after coil embolization is lower compared with that
after stent placement. A variety of embolization techniques may be
applied for the treatment of pseudoaneurysm after PD, which have
high technical and clinical success rates and small trauma. Coil
embolization is recommended in cases of emergency but care should
be taken to avoid serious technical complications.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HX and CJ made substantial contributions to the
conception and design of the study and wrote the original draft of
the manuscript. Jie Z, XLM, Jing Z, LY and YJR were responsible for
data acquisition and conducted data analysis and interpretation. HX
revised it critically for important intellectual content. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The Ethics Committee of the Affiliated Hospital of
North Sichuan Medical College approved the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
McEvoy SH, Lavelle LP, Hoare SM, O'Neill
AC, Awan FN, Malone DE, Ryan ER, McCann JW and Heffernan EJ:
Pancreaticoduodenectomy: Expected post-operative anatomy and
complications. Br J Radiol. 87(20140050)2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Qin K, Wu Z, Jin J, Shen B and Peng C:
Internal hernia following robotic assisted pancreaticoduodenectomy.
Med Sci Monit. 24:2287–2293. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Umemura A, Nitta H, Takahara T, Hasegawa Y
and Sasaki A: Current status of laparoscopic
pancreaticoduodenectomy and pancreatectomy. Asian J Surg.
41:106–114. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sato A, Masui T, Nakano K, Sankoda N,
Anazawa T, Takaori K, Kawaguchi Y and Uemoto S: Abdominal
contamination with candida albicans after pancreaticoduodenectomy
is related to hemorrhage associated with pancreatic fistulas.
Pancreatology. 17:484–489. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wang M, Cai H, Meng L, Cai Y, Wang X, Li Y
and Peng B: Minimally invasive pancreaticoduodenectomy: A
comprehensive review. Int J Surg. 35:139–146. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ammori BJ and Ayiomamitis GD: Laparoscopic
pancreaticoduodenectomy and distal pancreatectomy: A UK experience
and a systematic review of the literature. Surg Endosc.
25:2084–2099. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Smits FJ, van Santvoort HC, Besselink MG,
Batenburg MCT, Slooff RAE, Boerma D, Busch OR, Coene PPLO, van Dam
RM, van Dijk DPJ, et al: Management of severe pancreatic fistula
after pancreatoduodenectomy. JAMA Surg. 152:540–548.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Morita S, Tajima T, Yamazaki H, Sonoyama
Y, Nishina Y, Kenji O, Takagi T, Kondo T, Tanabe K and Sakai S:
Early postoperative screening by contrast-enhanced CT and
prophylactic embolization of detected pseudoaneurysms prevents
delayed hemorrhage after partial nephrectomy. J Vasc Interv Radiol.
26:950–957. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kendrick ML, van Hilst J, Boggi U, de
Rooij T, Walsh RM, Zeh HJ, Hughes SJ, Nakamura Y, Vollmer CM, Kooby
DA, et al: Minimally invasive pancreatoduodenectomy. HPB (Oxford).
19:215–224. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kim E: Embolization therapy for refractory
hemorrhage in patients with patients with chronic subdural
hematomas. World Neurosurg. 101:520–527. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Liu Y, Wang J, Lin L, Sang C, Lin Z, Pan Y
and Fu X: Clinical study on complications of intracranial reptured
aneurysm embolization by stent-assisted coil. Med Sci Monit.
24:8115–8124. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang Y and Jia P: The role of metallic
clips in transcatheter intravascular embolization for non-variceal
upper gastrointestinal bleeding cases receiving unmnageable
endoscopic therapy: A retrospective cohort study. Int J Surg.
58:26–30. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Madhusudhan KS, Venkatesh HA, Gamanagatti
S, Garg P and Srivastava DN: Interventional radiology in the
management of visceral artery pseudoaneurysms: A review of
techniques and embolic materials. Korean J Radiol. 17:351–363.
2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wente MN, Veit JA, Bassi C, Dervenis C,
Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr
MG, et al: Postpancreatectomy hemmorrhage (PPH): An international
study group of pancreatic surgery (ISGPS) definition. Surgery.
142:20–25. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Angle JF, Siddiqi NH, Wallace MJ, Kundu S,
Stokes L, Wojak JC and Cardella JF: Society of Interventional
Radiology Standards of Practice Committee. Quality improvement
guidelines for percutaneous transcatheter embolization: Society of
interventional radiology standards of practice committee. J Vascu
Interv Radiol. 21:1479–1486. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
You Y, Choi SH, Choi DW, Heo JS, Han IW,
Han S, Shin SW, Park KB, Park HS, Cho SK and Han SH: Long-term
clinical outcomes after endovascular management of ruptured
pseudoaneurysm in patients undergoing pancreaticoduodenectomy. Ann
Surg Treat Res. 96:237–249. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Meyers PM, Shcumacher HC, Higashida RT,
Derdeyn CP, Nesbit GM, Sacks D, Wechsler LR, Bederson JB, Lavine SD
and Rasmussen P: Reporting standards for endovascular repair of
saccular intracranial cerebral aneurysms. AJNR Am J Neuroradiol.
31:E12–E24. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hassold N, Wolfschmidt F, Dierks A, Klein
I, Bley T and Kickuth R: Effectiveness and outcome of endovascular
therapy for late-onset postpancreatectomy hemorrhage using covered
stents and embolization. J Vasc Surg. 64:1373–1383. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ginat DT, Saad WE and Turba UC:
Transcatheter renal artery embolization: Clinical applications and
techniques. Tech Vasc Interv Radiol. 12:224–239. 2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Belli AM, Markose G and Morgan R: The role
of interventional radiology in the management of abdominal visceral
artery aneurysms. Cardiovasc Intervent Radiol. 35:234–243.
2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cordova AC and Sumpio BE: Visceral artery
aneurysms and pseudoaneurysms-should they all be managed by
endovascular techniques? Ann Vasc Dis. 6:687–693. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xia W, Zhou Y, Lin Y, Yu M, Yin Z, Lu X,
Hou B and Jian Z: A predictive risk scoring system for clinically
relevant pancreatic fistula after pancreaticoduodenectomy. Med Sci
Monit. 24:5719–5728. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Dumitru R, Carbunaru A, Grasu M, Toma M,
Ionescu M and Dumitrascu T: Pseudoaneurysm of the splenic artery-an
uncommon cause of delayed hemorrhage after pancreaticoduodenectomy.
Ann Hepatobiliary Pancreat Surg. 20:204–210. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gao F, Li J, Quan S, Li F, Ma D, Yao L and
Zhang P: Risk factors and treatment for hemorrhage after
pancreaticoduodenectomy: A case series of 423 patients. Biomed Res
Int. 2016(2815693)2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Schäfer M, Heinrich S, Pfammatter T and
Clavien PA: Management of delayed major visceral arterial bleeding
after pancreatic surgery. HPB (Oxford). 13:132–138. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Feng J, Chen YL, Dong JH, Chen MY, Cai SW
and Huang ZQ: Post-pancreaticoduodenectomy hemorrhage: Risk
factors, managements and outcomes. Hepatobiliary Pancreat Dis Int.
13:513–522. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hatzaras I, George N, Muscarella P, Melvin
WS, Ellison EC and Bloomston M: Predictors of survival in
periampullary cancers following pancreaticoduodenectomy. Ann Surg
Oncol. 17:991–997. 2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Topal B, Fieuws S, Aerts R, Weerts J,
Feryn T, Roeyen G, Bertrand C, Hubert C, Janssens M and Closset J:
Belgian Section of Hepatobiliary and Pancreatic Surgery.
Pancreaticojejunostomy versus pancreaticogastrostomy reconstruction
after pancreaticoduodenectomy for pancreatic or periampullary
tumours: A multicentre randomised trial. Lancet Oncol. 14:655–662.
2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Saad NE, Saad WE, Davies MG, Waldman DL,
Fultz PJ and Rubens DJ: Pseudoaneurysms and the role of minimally
invasive techniques in their management. Radiographics. 25 (Suppl
1):S173–S189. 2005.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gabelmann A, Görich J and Merkle EM:
Endovascular treatment of visceral artery aneurysms. J Endovasc
Ther. 9:38–47. 2002.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kamarajah SK: Pancreaticoduodenectomy for
periampullary tumours: A review article based on surveillance, end
results and epidemiology (SEER) database. Clin Transl Oncol.
20:1153–1160. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Pedrazzoli S and Sperti C: Prevention of
clinically-relevant postoperative pancreatic fistula after
pancreticoduodenectomy. Ann Surg. 269:e7–e8. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chadha M and Ahuja C: Visceral artery
aneurysms: Diagnosis and percutaneous management. Semin Intervent
Radiol. 26:196–206. 2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yekebas EF, Wolfram L, Cataldegirmen G,
Habermann CR, Bogoevski D, Koenig AM, Kaifi J, Schurr PG, Bubenheim
M, Nolte-Ernsting C, et al: Postpancreatectomy hemorrhage:
Diagnosis and treatment: An analysis in 1669 consecutive pancreatic
resections. Ann Surg. 246:269–280. 2007.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jesinger RA, Thoreson AA and Lamba R:
Abdominal and pelvic aneurysms and pseudoaneurysms: Imaging review
with clinical, radiologic, and treatment correlation.
Radiographics. 33:E71–E96. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Dohan A, Dautry R, Guerrache Y, Fargeaudou
Y, Boudiaf M, Le Dref O, Sirol M and Soyer P: Three-dimensional
MDCT angiography of splanchnic arteries: Pearls and pitfalls. Diagn
Interv Imaging. 96:187–200. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Guo J, Yu J, Zhang Q and Song X: Clinical
efficacy and safety of uterine artery embolization (UAE) versus
laparoscopic cesarean scar pregnancy debridement surgery (LCSPDS)
in treatment of cesarean scar pregnancy. Med Sci Monit.
24:4659–4666. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Künzle S, Glenck M, Puippe G, Schadde E,
Mayer D and Pfammatter T: Stent-graft repairs of visceral and renal
artery aneurysms are effective and result in long-term patency. J
Vasc Interv Radiol. 24:989–996. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Sueyoshi E, Sakamoto I, Nakashima K,
Minami K and Hayashi K: Visceral and peripheral arterial
pseudoaneurysms. AJR Am J Roentgenol. 185:741–749. 2005.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Hur S, Yoon CJ, Kang SG, Dixon R, Han HS,
Yoon YS and Cho JY: Transcatheter arterial embolization of
gastroduodenal artery stump pseudoaneurysms after
pancreaticoduodenectomy: Safety and efficacy of two embolization
techniques. J Vasc Interv Radiol. 22:294–301. 2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kulkarni CB, Moorthy S, Pullara SK and
Kannan RR: Endovascular treatment of aneurysm of splenic artery
arising from splenomesentric trunk using stent graft. Korean J
Radiol. 14:931–934. 2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Madhusudhan KS, Gamanagatti S, Garg P,
Shalimar Dash NR, Pal S, Peush S and Gupta AK: Endovascular
embolization of visceral artery pseudoaneurysms using modified
injection technique with N-butyl cyanoacrylate glue. J Vasc Interv
Radiol. 26:1718–1725. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Leyon JJ, Littlehales T, Rangarajan B,
Hoey ET and Ganeshan A: Endovascular embolization: Review of
currently available embolization agents. Curr Probl Diagn Radiol.
43:35–53. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Piasek E, Sojka M, Kuczyńska M,
Światłowski Ł, Drelich-Zbroja A, Furmaga O and Jargiełło T:
Visceral artery aneurysms-classification, diagnosis and treatment.
J Ultrason. 18:148–151. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Pottier E, Ronot M, Gaujoux S, Cesaretti
M, Barbier L, Sauvanet A and Vilgrain V: Endovascular management of
delayed post-pancreatectomy haemorrhage. Eur Radiol. 26:3456–3465.
2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lee HG, Heo JS, Choi SH and Choi DW:
Management of bleeding from pseudoaneurysms following
pancreaticoduodenectomy. World J Gastroenterol. 16:1239–1244.
2010.PubMed/NCBI View Article : Google Scholar
|