Introduction
Bladder cancer is one of the most common malignant
tumors in the urogenital system. The incidence of bladder cancer
currently ranks 11th among all malignant tumors and ~15,000 people
succumb to bladder cancer each year worldwide (1). In China, bladder cancer is the
genitourinary tumor with the highest morbidity and mortality. The
incidence rate in males is ~7.3/100,000, and in females it is
~2.0/100,000. The incidence of bladder cancer in China is also
increasing year by year (2,3). Consequently, bladder cancer has
attracted increased attention due to its high incidence and
recurrence rates, which threaten human health.
The human RAB1A, member RAS oncogene family (Rab1A)
gene is located at chromosome 2q14 and is primarily expressed in
the endoplasmic reticulum and Golgi apparatus (4); it is known to be involved in vesicle
trafficking between the endoplasmic reticulum and the Golgi
apparatus (5,6). Rab1A, a member of the Ras-associated
binding (Rab) family, is a small guanosine triphosphate (GTP)
enzyme that is an activator of mammalian rapamycin target protein
complex 1 (mTORC1) (7,8), and has been demonstrated to be
involved in the regulation of vesicle transport from the
endoplasmic reticulum to the Golgi (9,10). A
number of previous studies identified that Rab1A protein was also
involved in the regulation of signal transduction (11), cell migration (12) and autophagy (13,14).
Concomitantly, the abnormal expression of Rab1A was also associated
with the development of certain clinical diseases, including
Parkinson's disease (15) and
primary cardiomyopathy (16). In
recent years, studies have begun to examine to the role of Rab1A in
tumorigenesis and development, and have identified that Rab1A was
increased in a number of malignant tumors and served an important
role in the development of tumors, including tongue (17), breast (18), lung (19), liver (20) and colorectal cancer (21,22).
However, the expression pattern of Rab1A in bladder cancer remains
unknown.
In the present study, the expression levels of Rab1A
protein in the cells and tissues of bladder cancer were measured,
and its clinical significance for patients with bladder cancer was
analyzed. It was identified that Rab1A protein was highly expressed
in bladder cancer cells and tissues, and was associated with the
development of bladder cancer, and that it may be a potential
target for the treatment of bladder cancer.
Materials and methods
Study objects and clinical
specimens
A total of 153 patients with bladder cancer (121
males and 32 females) were selected as the patient cohort from
January 2011 to December 2012 in The First Affiliated Hospital of
Hebei North University (Zhangjiakou, China). The cancer tissues and
adjacent cancer normal tissues were collected following surgical
resection. Each tissue sample was divided into 3 sections; 1 was
stored as paraffin sections for use in the immunohistochemistry
(IHC) analysis, and 2 sections were stored in liquid nitrogen. The
age range of the 153 patients with bladder cancer was 45-72 years
(median age, 65 years); additional clinical data is summarized in
Table I. Exclusion criteria were as
follows: i) Incomplete age, sex, disease history and tumor
information; ii) postoperative follow-up loss or unknown cause of
mortality; iii) presence of other malignancies, or malignancies
that could not be identified as primary liver cancer due to
mortality from other sudden diseases (including cardiovascular and
cerebrovascular diseases), or evidence of poor postoperative mental
status affecting patient prognosis; iv) patients who were pregnant
or breast-feeding; and v) patients with chronic infectious diseases
(human immunodeficiency virus, hepatitis B or tuberculosis).
 | Table IAssociation between Rab1A expression
and clinical data of patients with bladder cancer. |
Table I
Association between Rab1A expression
and clinical data of patients with bladder cancer.
| | Rab1A expression | |
|---|
| Characteristics | N | Low | High | χ2 | P-value |
|---|
| Sex |
|
Female | 32 | 18 | 14 | 0.178 | 0.673 |
|
Male | 121 | 63 | 58 | | |
| Age, years |
|
<60 | 62 | 32 | 30 | 0.114 | 0.736 |
|
≥60 | 91 | 49 | 42 | | |
| Tumor size, cm |
|
<2.5 | 81 | 51 | 30 | 6.149 | 0.013 |
|
≥2.5 | 72 | 30 | 42 | | |
| Histological
grade |
|
I | 72 | 42 | 30 | 15.282 | <0.001 |
|
II | 43 | 29 | 14 | | |
|
III | 38 | 10 | 28 | | |
| Tumor number |
|
Single | 92 | 49 | 43 | 0.009 | 0.922 |
|
Multiple | 61 | 32 | 29 | | |
| TNM |
|
0a-II | 85 | 66 | 19 | 48.856 | <0.001 |
|
III-IV | 68 | 15 | 53 | | |
| Lymph node
metastasis |
|
Yes | 28 | 8 | 20 | 8.170 | 0.004 |
|
No | 125 | 73 | 52 | | |
| Remote
metastasis |
|
Yes | 10 | 0 | 10 | 12.037 | 0.001 |
|
No | 143 | 81 | 62 | | |
| Tumor category |
|
Urothelium | 136 | 70 | 66 | 1.063 | 0.303 |
|
Squamous
cell carcinoma | 17 | 11 | 6 | | |
In addition, all subjects (or their guardians)
included in the present study consented to the study protocol and
provided written informed consent. The Ethics Committee of The
First Affiliated Hospital of Hebei North University approved the
study.
Cell and cell culture
The normal human bladder epithelial SV-HUC-1 cell
line, and human bladder cancer ScaBER (HTB-3), 5637 (HTB-9) and T24
(HTB-4) cell lines were obtained from the American Type Culture
Collection and were cultured in Dulbecco's modified Eagle medium
(Thermo Fisher Scientific, Inc.), to which 10% fetal bovine serum
(Thermo Fisher Scientific, Inc.) and 1% penicillin-streptomycin
(Thermo Fisher Scientific, Inc.) were added.
Western blot analysis
Firstly, RIPA buffer (Beijing Solarbio Science &
Technology Co., Ltd.) was used to exact total protein from tissues
and cells, where a Bicinchoninic Acid Assay kit (Beyotime Institute
of Biotechnology) was used to quantify protein concentration. A
total of 40 µg protein was subsequently separated by 10% SDS-PAGE
and transferred to polyvinylidene fluoride membranes. The membranes
were blocked using 5% bovine serum albumin (Sigma-Aldrich; Merck
KGaA) solution for 1 h at room temperature, and block solution was
used to dilute primary and secondary antibodies. The primary
antibodies used were as follows: Anti-Rab1A (cat. no., ab97956;
1:1,000; Abcam) or anti-GAPDH (cat. no., ab9484; 1:3,000; Abcam).
The secondary antibodies were as follows: Horseradish peroxidase
(HRP)-conjugated goat anti-Rabbit IgG H&L (cat. no. ab205718;
1:30,000; Abcam) or horseradish peroxidase-conjugated goat
anti-mouse (cat. no., ab6789; 1:3,000; Abcam). The membranes were
incubated with the primary antibodies overnight at 4˚C, followed by
incubation with corresponding second antibodies for 1 h at room
temperature. Western Lightening™ Plus-ECL (PerkinElmer,
Inc.) was used to visualize the protein bands, and Image J version
1.50d (National Institutes of Health) was used to perform
densitometric analysis.
IHC
Paraffin-embedded sections of preserved bladder
cancer tumor and adjacent cancer tissues were selected, and IHC was
used to detect the expression of Rab1A protein. All experimental
protocols were performed according to the protocols of
VECTASTAIN® Elite® ABC kit (Vector
Laboratories, Inc.) and analyzed using a Leica TCS SP5 light
microscope (Leica Microsystems, Inc.) using the LAS AF Lite image
browser software (version 4.0; Leica Microsystems, Inc.).
Anti-Rab1A (cat. no., ab97956; 1:100; Abcam) was selected as the
primary antibody and the horseradish-conjugated goat anti-rabbit
IgG (H&L) (cat. no., ab6702; 1:2,000; Abcam) was selected as
the secondary antibody. Sections were incubated with the primary
antibody overnight at 4˚C and with the secondary antibody for 2 h
at 37˚C. The scoring method used from five sections per condition
in the present study as described previously by Shimada et
al (17), which was calculated
based on the staining intensity and percentage of stained cells: 0,
No appreciable staining in cells; 1, weak staining in cells
comparable to stromal cells; 2, intermediate staining; 3, strong
staining. The fraction of positive cells was scored as 0-100%. The
IHC score was calculated by multiplying the intensity and the
fraction scores, producing a total range of 0-300.
Statistical analysis
SPSS 20.0 software (IBM Corp.) was used to perform
the statistical analysis on the data. Measurement data are
presented as the mean ± standard deviation. Differences between two
groups was compared by a paired Student's t-test for paired data,
and data from multiple groups was analyzed using a one-way analysis
of variance followed by Duncan's post hoc test. Counts were
recorded as proportions, and the differences between groups were
compared using a χ2 test. Kaplan-Meier curves were used
to analyze the survival data, and a log-rank test was used to
compare the survival of patients with high and low Rab1A expression
levels. A Cox regression model was used to analyze factors that
affected the survival of patients with bladder cancer. P<0.05
was considered to indicate a statistically significant
difference.
Results
Rab1A is highly expressed in human
bladder cancer tissues and cells
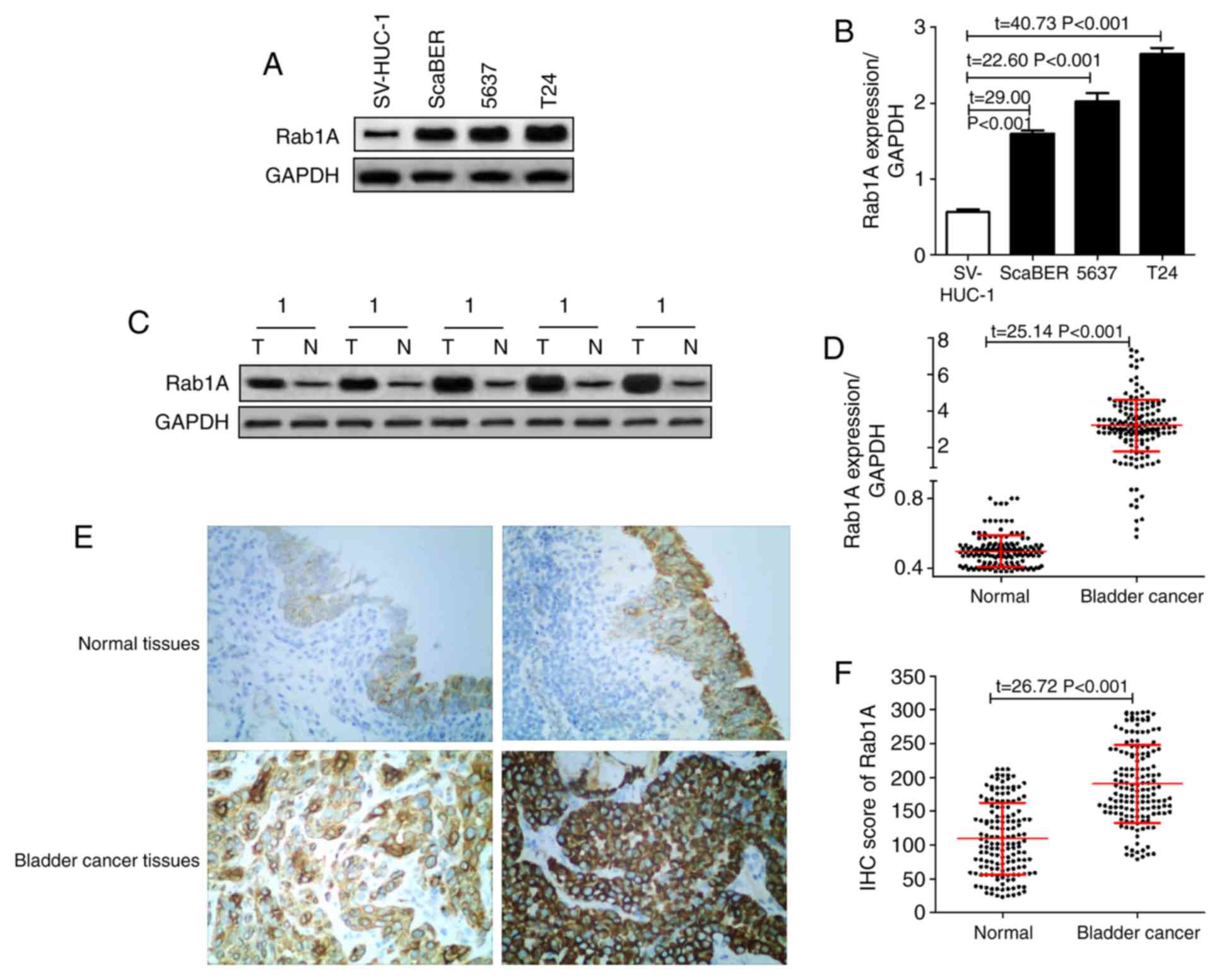
As demonstrated in Fig.
1A and B, it was identified
that the expression of Rab1A protein in the normal human bladder
epithelial SV-HUC-1 cell line was significantly decreased compared
with that in the human bladder cancer ScaBER, 5637 and T24 cell
lines. In addition, the Rab1A expression levels in 153 pairs of
bladder cancer tissues and adjacent normal tissues were detected by
western blot analysis. The results revealed that the relative
expression of Rab1A in tumor tissues was 3.20±1.42, which was
significantly increased compared with that in the adjacent normal
tissues (0.50±0.09) (P<0.05; Fig.
1C and D).
In addition, IHC was also used to measure the
expression of Rab1A in tissues and determine the patterns of
distribution in bladder cancer cells; it was identified that the
IHC score of the 153 bladder cancer tissues was 190.65±57.77, which
was significantly increased compared with that in the adjacent
normal tissues (109.57±53.12) (P<0.05; Fig. 1E and F).
Association between Rab1A and clinical
data of patients with bladder cancer
A total of 153 patients with bladder cancer were
divided into two groups depending on the expression of Rab1A
protein in cancer tissues, which was measured by western blot
analysis, using the median value as the cut-off: Low Rab1A
expression was identified in 81 cases [<3.17 (median) of 153
patients with bladder cancer] and 72 cases exhibited high Rab1A
expression [≥3.17 (median) of 153 patients with bladder cancer]. In
addition, the association between Rab1A expression and
clinicopathological features in patients with bladder cancer was
analyzed. As demonstrated in Table
I, the expression of Rab1A was not associated with age, sex,
tumor number and tumor category, but was significantly associated
with tumor size, histological grade, tumor-node-metastasis (TNM)
stage (2002 version) (23,24), lymph node metastasis and remote
metastasis.
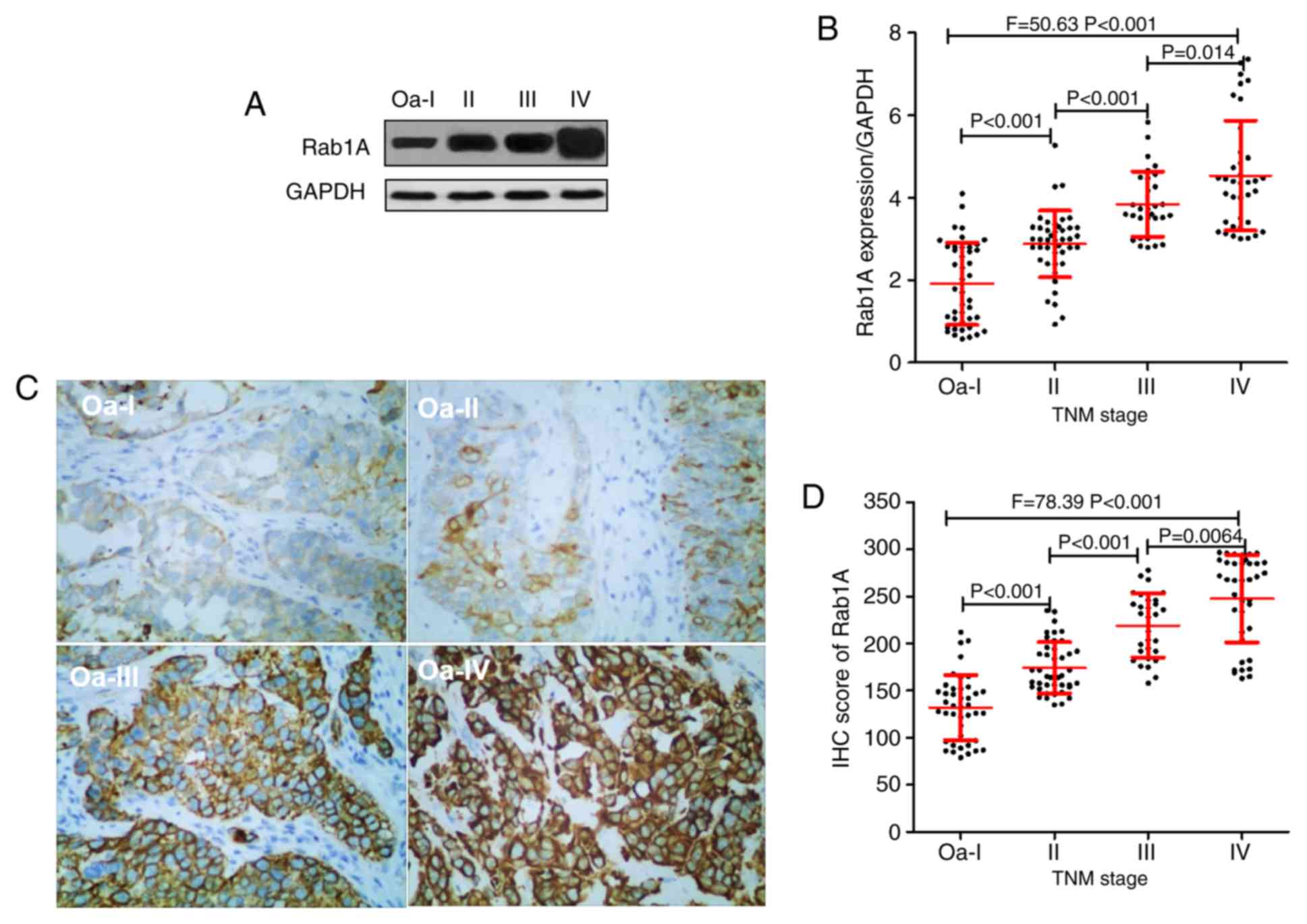
Rab1A protein expression and TNM stage
of bladder cancer
The TNM stages of 153 patients with bladder were
assessed; 42, 43, 30 and 38 patients were diagnosed with grade
0a-I, II, III and IV, respectively. The results of the western blot
analysis suggested that the expression of Rab1A protein in 0a-I,
II, III, and IV bladder cancer tissues were 1.92±1.00, 2.88±0.81,
3.85±0.79 and 4.54±1.33, respectively (Fig. 2A and B). IHC analysis indicated that the Rab1A
protein IHC scores were 131.86±34.47, 174.37±27.16, 219.23±34.06
and 247.74±46.44 in the grade 0a-I, II, III and IV bladder cancer
tissues, respectively (Fig. 2C and
D).
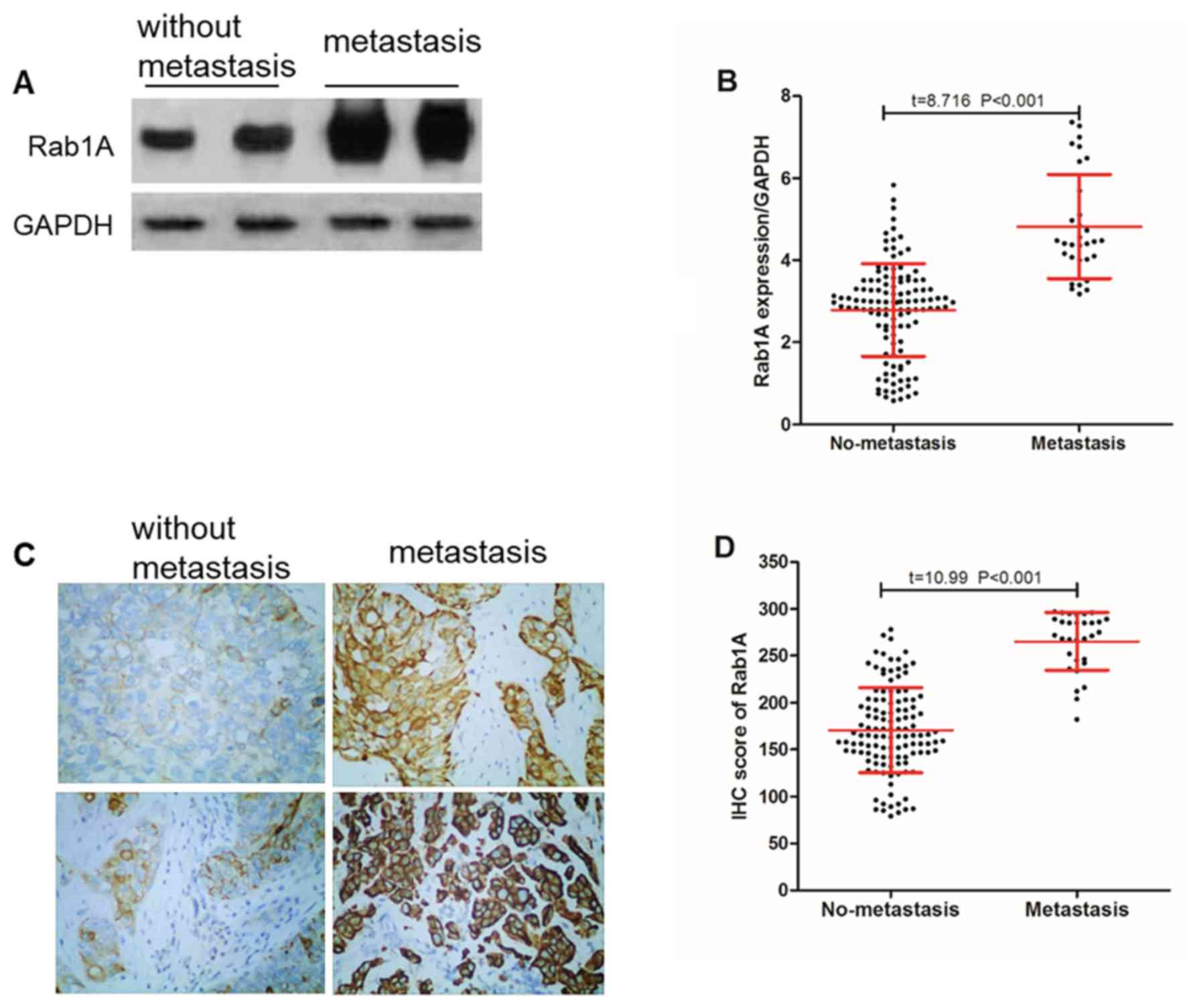
Rab1A protein expression and bladder
cancer cell metastasis
A total of 31 patients with bladder cancer were
identified to exhibited cancer cell metastasis (lymph node
metastasis and distant metastasis), and the expression of Rab1A
protein in tumor tissues of patients with metastatic and
non-metastatic bladder cancer were compared. The results of the
western blot analysis demonstrated that the relative expression of
Rab1A protein was (4.82±1.27) in the metastatic bladder cancer
tissues, which was significantly (P<0.001) increased compared
with that in the non-metastatic bladder cancer tissues (2.79±1.13;
Fig. 3A and B).
In addition, IHC data suggested that (Fig. 3C and D) the Rab1A protein IHC score was
265.16±30.83 in the metastatic bladder cancer tissues, which was
significantly (P<0.001) increased compared with that in the
non-metastatic bladder cancer tissues (170.54±40.28).
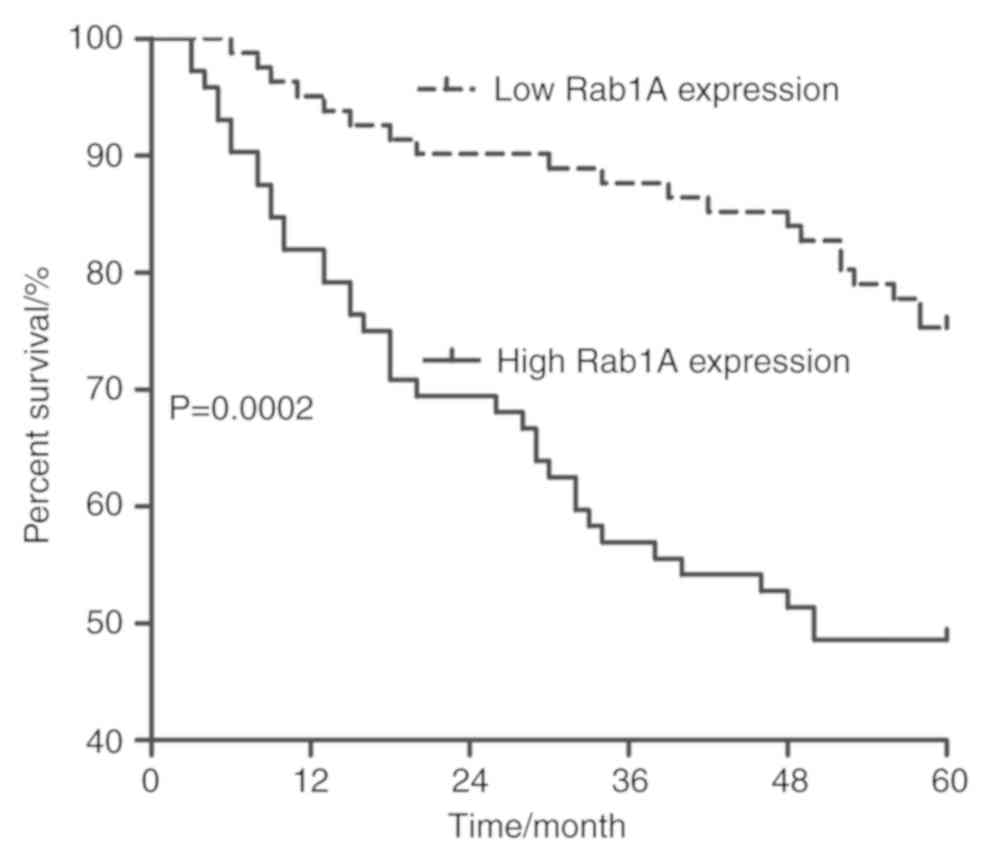
Effect of Rab1A protein on the
prognosis of patients with bladder cancer
The results of the univariate and multivariate Cox
regression models indicated that Rab1A was an independent risk
factor for survival in patients with bladder cancer (odds
ratio=0.549; 95% confidence interval=0.139-0.916) (Tables II and III). In addition, a 5-year follow-up of
the 153 patients with bladder cancer following surgery was
performed, and it was identified that the 5-year overall survival
rate of patients with low expression of Rab1A protein was 75.31%,
while that of patients with high expression of Rab1A was only
48.61%, as demonstrated in Fig.
4.
 | Table IICox univariate regression model
analysis of survival of 153 patients with bladder cancer. |
Table II
Cox univariate regression model
analysis of survival of 153 patients with bladder cancer.
| Variable | N | 95% CI | OR | P-value |
|---|
| Sex | | | | |
|
Female/male | 32/121 | 1.056-4.512 | 2.134 | 0.213 |
| Age, years | | | | |
|
<60/≥60 | 62/91 | 0.298-1.569 | 0.935 | 0.328 |
| Tumor size, cm | | | | |
|
<2.5/≥2.5 | 81/72 | 0.650-2.634 | 1.025 | 0.309 |
| Histological
grade | | | | |
|
I/II-III | 72/81 | 0.802-2.688 | 1.596 | 0.022 |
| Tumor number | | | | |
|
Single/Multiple | 92/61 | 0.782-3.697 | 2.264 | 0.139 |
| TNM | | | | |
|
0a-I/II-III | 85/61 | 1.652-5.126 | 9.105 | <0.001 |
| Cancer cells
metastasis | | | | |
|
Yes/No | 31/122 | 2.069-4.008 | 3.269 | <0.001 |
| Smoking | | | | |
|
Yes/No | 103/50 | 0.894-2.956 | 1.027 | 0.042 |
| Total
cystectomy | | | | |
|
Yes/No | 112/41 | 1.023-4.781 | 2.308 | 0.047 |
| Rab1A
expression | | | | |
|
Low/High | 81/72 | 1.229-5.224 | 2.941 | 0.012 |
 | Table IIICox multivariate regression model
analysis of survival of 153 patients with bladder cancer. |
Table III
Cox multivariate regression model
analysis of survival of 153 patients with bladder cancer.
| Variables | 95% CI | OR | P-value |
|---|
| Histological
grade | 0.981-4.028 | 2.021 | <0.001 |
| TNM | 1.154-4.218 | 2.302 | <0.001 |
| Cancer cells
metastasis | 1.102-8.648 | 3.741 | 0.019 |
| Smoking | 0.549-3.694 | 2.103 | 0.412 |
| Total
cystectomy | 0.143-1.542 | 0.512 | 0.089 |
| Rab1A
expression | 0.139-0.916 | 0.549 | 0.027 |
Discussion
Rab1A is a member of the Rab protein family, which
is a family of at least 30 GTPases like Ras. The majority of the
functions comprise the recognition and fusion processes of membrane
vesicles, regulating the membrane transport process between cells
(21,25). A previous study has indicated that
small molecule GTPases including the Ras, Rho, Rac and Ral families
served important roles in tumorigenesis (26). In particular, Rab1A has been
identified to be highly expressed in a variety of malignant tumor
tissues as a small molecule GTPase and a member of the Ras oncogene
superfamily (20,21).
The present study identified that the expression
levels of Rab1A protein in human bladder cancer cell lines were
significantly increased compared with that in a human normal
bladder epithelial cell line, and its expression level in human
bladder cancer tissues was also significantly increased compared
with that in adjacent normal tissues. In 2005, Rab1A was first
protein identified to be highly expressed in tongue cancer
(17); subsequently, the study of
Rab1A in the field of malignant tumors has become more intense, and
has demonstrated different degrees of increased expression of Rab1A
in various malignant tumors. It is widely hypothesized that its
high expression promotes the development of cancer by regulating
the mTOCRC1 signaling pathway (27,28),
which directly leads to poor prognoses in colorectal (21) and liver cancer (20). Combined with the results of the
present study, these observations suggest that Rab1A protein, which
was highly expressed in bladder cancer tissues, may be involved in
the regulation of bladder cancer development.
Subsequent analysis performed in the present study
revealed that Rab1A protein expression was significantly associated
with histological grade, TNM stage and cancer metastasis in
patients with bladder cancer, and that its expression was increased
with later TNM stages. It was also demonstrated that Rab1A
expression was significantly increased in patients with metastatic
cancer compared with patients without metastasis. In addition, it
was identified to be an independent risk factor affecting the
prognosis of patients with bladder cancer. The occurrence and
development of malignant tumors is a multi-step process in which
multiple factors are combined through internal and external
effects. The incidence of tumor cells transference from the primary
site to other sites and the continuation of growth is not only a
criterion for distinguishing between benign and malignant tumors,
but also a major cause of treatment failure and mortality in
patients with malignant tumors. The epithelial-mesenchymal
transition process is a key step in conferring the ability of tumor
cells to invade and migrate, and is one of the most important
causes of mortality in patients with malignant tumors (29,30).
mTOR is an evolutionarily conserved serine/threonine protein kinase
with two complexes, mTORC1 and mTORC2. Rab1A, a member of the Rag
GTPase family, activates the mTORC1 signaling pathway. A number of
previous studies have demonstrated that Rab1A may participate in
tumor cell growth, apoptosis, invasion and migration by activating
the mTORC1 signaling pathway: Xu et al (8) identified that in hepatocellular
carcinoma, Rab1A was highly expressed and regulated the
proliferation, growth, invasion and metastasis of hepatoma cells by
activating the mTORC1 signaling pathway, and that the inhibition of
Rab1A expression caused EMT inhibition. In addition, Yang et
al (28) revealed that Rab1A
and Rab1B, which were highly expressed in esophageal squamous cell
carcinoma, inhibited autophagy in cancer cells and promoted cancer
cell survival by activating the mTORC1 signaling pathway.
Previous studies have demonstrated that the loss or
decreased expression of PTEN in the bladder mucosal epithelium that
regulated the PI3K/Akt/mTOR signaling pathway led to dysfunction of
this signaling pathway, causing the bladder cell epithelium to lose
its normal cell cycle regulation and hyperproliferation and
malignant transformation (31).
Becker et al (32)
identified that the combination of a mTORc1/TORc2 inhibitor and
lapatinib inhibited the proliferation, invasion and migration of
bladder cancer cells in vitro. These results suggested that
Rab1A may participate in the development of bladder cancer by
regulating the mTORC1 signaling pathway.
In conclusion, the expression of Rab1A in bladder
cancer was upregulated, and its expression increased with
increasing TNM stage. It is associated with the metastasis of tumor
cells and negatively affects the survival time of patients with
bladder cancer, and may be a potential target for the treatment of
bladder cancer. However, the function of Rab1A at the cellular and
whole organism level was not examined in bladder cancer. In order
to improve the understanding of the function of Rab1A in bladder
cancer, future studies are required.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HS made substantial contributions to the conception
and design, acquisition of data and revising the critically for
important intellectual content of this study. TL, XL, HL, CL and
XDL analyzed and interpreted the data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All subjects (or their guardians) included in the
present study consented to the study protocol and provided written
informed consent. The Ethics Committee of The First Affiliated
Hospital of Hebei North University approved the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. Ca Cancer J Clin. 62:10–29. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wei F, Wu Y, Tang L, Xiong F, Guo C, Li X,
Zhou M, Xiang B, Li X, Li G, et al: Trend analysis of cancer
incidence and mortality in China. Sci China Life Sci. 60:1271–1275.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Quan Y, Song Q, Wang J, Zhao L, Lv J and
Gong S: MiR-1202 functions as a tumor suppressor in glioma cells by
targeting Rab1A. Tumor Biol. 39(101042831769756)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Allan BB, Moyer BD and Balch WE: Rab1
recruitment of p115 into a cis-SNARE complex: Programming budding
COPII vesicles for fusion. Science. 289:444–448. 2000.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Satoh A, Wang Y, Malsam J, Beard MB and
Warren G: Golgin-84 is a rab1 binding partner involved in golgi
structure. Traffic. 4:153–161. 2010.
|
|
7
|
Thomas JD, Zhang YJ, Wei YH, Cho JH,
Morris LE, Wang HY and Zheng XF: Rab1A is an mTORC1 activator and a
colorectal oncogene. Cancer Cell. 26:754–769. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Xu BH, Li XX, Wang HY and Zheng XS:
Overexpressed Rab1A is associated poor prognosis and promotes
oncogenic growth and metastasis through mTORC1 activation in
hepatocellular carcinoma. AACR, 2015.
|
|
9
|
Mukhopadhyay A, Nieves E, Che FY, Wang J,
Jin L, Murray JW, Gordon K, Angeletti RH and Wolkoff AW: Proteomic
analysis of endocytic vesicles: Rab1a regulates motility of early
endocytic vesicles. J Cell Sci. 124:765–775. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mukhopadhyay A, Quiroz JA and Wolkoff AW:
Rab1a regulates sorting of early endocytic vesicles. Am J Physiol
Gastrointest Liver Physiol. 306:G412–G424. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Charng WL, Yamamoto S, Jaiswal M, Bayat V,
Xiong B, Zhang K, Sandoval H, David G, Gibbs S, Lu HC, et al:
Drosophila tempura, a novel protein prenyltransferase α subunit,
regulates notch signaling Via Rab1 and Rab11. PLoS Biol.
12(e1001777)2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang C, Yoo Y, Fan H, Kim E, Guan KL and
Guan JL: Regulation of Integrin β 1 recycling to lipid rafts by
Rab1a to promote cell migration. J Biol Chem. 285:29398–29405.
2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tanaka M, Mun S, Harada A, Ohkawa Y,
Inagaki A, Sano S, Takahashi K, Izumi Y, Osada-Oka M, Wanibuchi H,
et al: Hsc70 contributes to cancer cell survival by preventing
Rab1A degradation under stress conditions. PLoS One.
9(e96785)2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Salem A, Almahmoudi R, Listyarifah D,
Siponen M, Maaninka K, Al-Samadi A, Salo T and Eklund KK: Histamine
H4 receptor signalling in tongue cancer and its
potential role in oral carcinogenesis-a short report. Cell Oncol
(Dordr). 40:621–630. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Coune PG, Bensadoun JC, Aebischer P and
Schneider BL: Rab1A over-expression prevents Golgi apparatus
fragmentation and partially corrects motor deficits in an
alpha-synuclein based rat model of Parkinson's disease. J
Parkinsons Dis. 1:373–387. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wu G, Yussman MG, Barrett TJ, Hahn HS,
Osinska H, Hilliard GM, Wang X, Toyokawa T, Yatani A, Lynch RA, et
al: Increased myocardial Rab GTPase expression: A consequence and
cause of cardiomyopathy. Circ Res. 89:1130–1137. 2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shimada K, Uzawa K, Kato M, Endo Y, Shiiba
M, Bukawa H, Yokoe H, Seki N and Tanzawa H: Aberrant expression of
RAB1A in human tongue cancer. Br J Cancer. 92:1915–1921.
2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Xu H, Qian M, Zhao B, Wu C, Maskey N, Song
H, Li D, Song J, Hua K and Fang L: Inhibition of RAB1A suppresses
epithelial-mesenchymal transition and proliferation of
triple-negative breast cancer cells. Oncol Rep. 37:1619–1626.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang X, Liu F, Qin X, Huang T, Huang B,
Zhang Y and Jiang B: Expression of Rab1A is upregulated in human
lung cancer and associated with tumor size and T stage. Aging
(Albany NY). 8:2790–2798. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Xu BH, Li XX, Yang Y, Zhang MY, Rao HL,
Wang HY and Zheng XF: Aberrant amino acid signaling promotes growth
and metastasis of hepatocellular carcinomas through Rab1A-dependent
activation of mTORC1 by Rab1A. Oncotarget. 6:20813–20828.
2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Thomas JD, Zhang YJ, Wei YH, Cho JH,
Morris LE, Wang HY and Zheng XFS: Rab1A Is an mTORC1 activator and
a colorectal oncogene. Cancer Cell. 26:181–182. 2016.
|
|
22
|
Fan SJ, Snell C, Turley H, Li JL,
McCormick R, Perera SM, Heublein S, Kazi S, Azad A, Wilson C, et
al: PAT4 levels control amino-acid sensitivity of
rapamycin-resistant mTORC1 from the Golgi and affect clinical
outcome in colorectal cancer. Oncogene. 35:3004–3015.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kirkali Z, Chan T, Manoharan M, Algaba F,
Busch C, Cheng L, Kiemeney L, Kriegmair M, Montironi R, Murphy WM,
et al: Bladder cancer: Epidemiology, staging and grading, and
diagnosis. Urology. 66 (6 Suppl 1):S4–S34. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Herr HW: Pathologic evaluation of radical
cystectomy specimens. Cancer. 95:668–669. 2002.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hutagalung AH and Novick PJ: Role of Rab
GTPases in membrane traffic and cell physiology. Physiol Rev.
91:119–149. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li Y, Wang HY and Zheng XF: Rab1 GTPases
as oncogenes. Aging (Albany NY). 7:897–898. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
RAB1A Promotes Oncogenesis in Colorectal
Cancer via mTORC1 Activation. Cancer Discovery. 4:1366. 2014.
|
|
28
|
Yang XZ, Wang R, Li XX, Yang Y, Wang HY
and Zheng XS: Rab1A and Rab1B promote esophageal squamous cell
carcinoma through activating mTORC1 signaling and inhibiting
autophagy. AACR, 2016.
|
|
29
|
Zavadil J, Haley J, Kalluri R, Muthuswamy
SK and Thompson E: Epithelial-mesenchymal transition. Cancer Res.
68:9574–9577. 2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Seton-Rogers S: Epithelial-mesenchymal
transition: Untangling EMT's functions. Nat Rev Cancer.
16(1)2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hay N and Sonenberg N: Upstream and
downstream of mTOR. Genes Dev. 18:1926–1945. 2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Becker MN, Wu KJ, Marlow LA, Kreinest PA,
Vonroemeling CA, Copland JA and Williams CR: The combination of an
mTORc1/TORc2 inhibitor with lapatinib is synergistic in bladder
cancer in vitro. Urol Oncol. 32:317–326. 2014.PubMed/NCBI View Article : Google Scholar
|