Introduction
Neonatal hyperbilirubinemia is common, and children
with the disease account for approximately 60% of full-term
neonates (1). With complex
pathogenesis, it causes an imbalance between the production and
elimination of bilirubin (2,3), brain
injury, and permanent neurological damage to the subcortical brain
structure, even neurological sequelae, such as abnormalities of the
vertebral body, hearing impairment and mental retardation (3-5).
Brain injury caused by bilirubin is called bilirubin-induced
neurologic dysfunction (BIND) (6),
which includes acute and chronic bilirubin encephalopathy in
neonates (7). Early prediction of
brain injury in children with severe hyperbilirubinemia is crucial
for improving prognosis. However, it is increasingly difficult to
diagnose bilirubin encephalopathy due to its unapparent symptoms in
the early stage; therefore, early diagnosis and timely treatment of
bilirubin encephalopathy are crucial to reduce the occurrence of
sequelae (8). Non-invasive magnetic
resonance imaging (MRI) can be clinically used to monitor brain
injury caused by hyperbilirubinemia (9).
As the precursor of calcitonin (10), procalcitonin (PCT) is commonly used
in the diagnosis of neonatal meningitis, septicemia and bacterial
infection (11-13).
The concentration of plasma PCT increases and reflects the activity
of systemic inflammatory responses in patients with severe fungal,
bacterial and parasitic infections and multiple organ failure
(14). A study revealed that PCT
can be used as a diagnostic indicator for early neonatal septicemia
and systemic bacterial infection in children with neonatal
hyperbilirubinemia (15).
Homocysteine (HCY) is a sulfhydryl-containing amino acid and an
intermediate product of methionine and cysteine metabolism, not
involved in protein synthesis (16). According to studies, highly
expressed HCY was an independent risk factor for cardiovascular
diseases (17), and its low
concentration triggered neuronal injury through oxidative stress,
DNA damage and activation of pro-apoptotic factors (18). Currently, there are few studies on
the diagnosis of HCY and PCT in neonatal hyperbilirubinemia
complicated with brain injury.
Since the early symptoms of hyperbilirubinemia
combined with brain injury are not evident, the diagnosis of
bilirubin nerve injury is increasingly difficult. Therefore, by
studying the MRI examination of the skull and the detection of HCY
and PCT, the value of combined diagnosis in predicting brain injury
of bilirubin in neonates was evaluated, in order to improve
diagnostic performance and enable patients to receive appropriate
treatment as soon as possible.
Patients and methods
Patients
A total of 149 children with hyperbilirubinemia
admitted to Shandong Medical Imaging Research Institute from
January 2014 to April 2016 were collected as research subjects. The
neurological function of the children was assessed based on
diagnostic criteria in practical neonatology, and the children were
divided into a brain injury group (n=67) and a non-brain injury
group (n=82). The brain injury group consisted of 37 males and 30
females, with a gestational age of (38.8±1.67) weeks, a daily age
of (8.49±1.49) days and a birth weight of (3575±73) g. The
non-brain injury group consisted of 48 males and 34 females, with a
gestational age of (39.1±1.58) weeks, a daily age of (8.65±1.54)
days and a birth weight of (3561±75) g.
Inclusion criteria were as follows: Diagnostic
criteria for bilirubin encephalopathy: i) A serum bilirubin level
≥342 µmol/l, combining typical nervous system signs and symptoms;
ii) abnormal muscle tone; iii) potential abnormalities induced by
brainstem auditory dysfunction; iv) brain magnetic resonance
imaging exhibiting bilateral globus pallidus signal abnormalities;
v) children with complete clinical data; children with normal
liver, spleen and other organs in size; children who were fed with
breast milk; children who had not received albumin, gamma globulin
and phototherapy; children without a history of intrauterine
distress and asphyxia; children without congenital
malformation.
Exclusion criteria: Children with severe dysfunction
of the heart, kidney and liver; children with nervous system
diseases caused by heredity or metabolism; children with other
severe brain diseases.
The present study was approved by the Ethics
Committee of Shandong Medical Imaging Research Institute. The
family members of the subjects were informed and signed a complete
informed consent form.
Collection and detection of serum
Fasting venous blood (2 ml) was collected within 24
h of admission and centrifuged at 4˚C and 1,000.46 x g for 10 min
to collect the supernatant, which was placed at -80˚C for testing
and prevented from repeated freezing and thawing. PCT was detected
by electrochemiluminescence (ECL) with a Roche E601 automatic ECL
analyzer and Roche reagents (Procell and Cleancell). HCY was
detected by enzymatic cycling assay (ECA) (19) with a Mindray BS-220 automatic
biochemical analyzer and HCY kit (cat. no. 181001) provided by
Zybio, Inc.
Cranial MRI
All children were induced to fall asleep 15-20 min
before examination, and administered oral 10% chloral hydrate
(30-40 mg/kg) or 10% chloral hydrate enema. During scanning,
sound-proof cotton was placed in the external auditory canal of the
children, and plastic sponge mats were used to fix the heads of the
children, so that the position line was aligned with the
radiographic base line. The children were examined using the
Phillip Achieve 1.5 T double gradient superconducting MRI system
and an 8-channel phased array head coil. Parameters of T1-weighted
imaging (T1WI): Spin echo (SE) sequence, TR=450 ms, TE=10 ms.
Parameters of T2-weighted imaging (T2WI): TR=4,600 ms, TE=110 ms,
layer thickness=5 mm, interlayer spacing=1.5 mm, imaging
matrix=220x256 mm, imaging layers=15. Parameters of
diffusion-weighted imaging (DWI): Echo planar imaging (EPI),
diffusion factor b=1,000 s/mm2. Volumetric data were
transmitted to the workstation, and images were analyzed using the
AW4.6/FuncTool software (General Healthcare). All image data were
read by two senior radiologists using the single-blind method, then
analyzed and summarized based on the results. The high-intensity
signals in the posterior part of the globus pallidus with T1 and T2
weighting were positive (20). All
subjects underwent MRI, and they were classified into an MRI
abnormal group and an MRI normal group according to the positive
signs.
Observational indexes
The expression levels of HCY and PCT in the two
groups were detected and compared. Based on the receiver operating
characteristic (ROC) curve, the maximum Youden index was determined
to obtain the sensitivity and specificity, and the corresponding
data were determined according to the ROC. Then the optimal cut-off
values of HCY and PCT were determined. Expression higher than the
optimal cut-off value indicated positive, while expression lower
than that indicated negative. The sensitivity, specificity and
accuracy of the combined detection of cranial MRI, HCY and PCT were
investigated.
Statistical analysis
SPSS 19.0 (IBM Corp.) was used to statistically
analyze the data. Count data were expressed by [n(%)], and
chi-square test was used for comparisons between groups.
Measurement data were expressed by (mean ± SD), and t-test was used
for comparisons between two groups. ROC was used to evaluate the
diagnostic value of HCY and PCT. Z=|AUC1-AUC2|/sqrt(SE1^2+SE^2) was
used to calculate Z values, and then the P-value was calculated
according to the normal distribution. P<0.05 indicated a
statistically significant difference.
Results
Comparison of general information
There was no statistically significant difference
between the brain and non-brain injury groups in terms of sex,
gestational age, daily age, average birth weight, means of
pregnancy, head circumference, the age of the mother or average
gestational week (P>0.05; Table
I).
 | Table IComparison of the general information
[n(%)]/(mean ± SD). |
Table I
Comparison of the general information
[n(%)]/(mean ± SD).
| Variable | Brain injury group
(n=67) | Non-brain injury
group (n=82) |
χ2/t-test | P-value |
|---|
| Sex | | | 0.165 | 0.685 |
|
Male | 37 (55.2) | 48 (58.5) | | |
|
Female | 30 (44.8) | 34 (41.5) | | |
| Gestational age
(weeks) | 38.80±1.67 | 39.10±1.58 | 1.124 | 0.263 |
| Daily age (days) | 8.49±1.49 | 8.65±1.54 | 0.640 | 0.523 |
| Birth weight (g) | 3575±73 | 3561±75 | 1.147 | 0.253 |
| Means of
pregnancy | | | 0.020 | 0.889 |
|
Eutocia | 36 (53.7) | 45 (54.9) | | |
|
Cesarean
delivery | 31 (46.3) | 37 (45.1) | | |
| Head circumference
(cm) | 32.87±0.57 | 33.02±0.59 | 1.567 | 0.119 |
| Age of the mother
(years) | 29.12±2.89 | 28.76±2.97 | 0.745 | 0.458 |
| Average gestational
weeks | 39.12±1.38 | 38.79±1.41 | 1.435 | 0.154 |
Comparison of the expression levels of
HCY and PCT
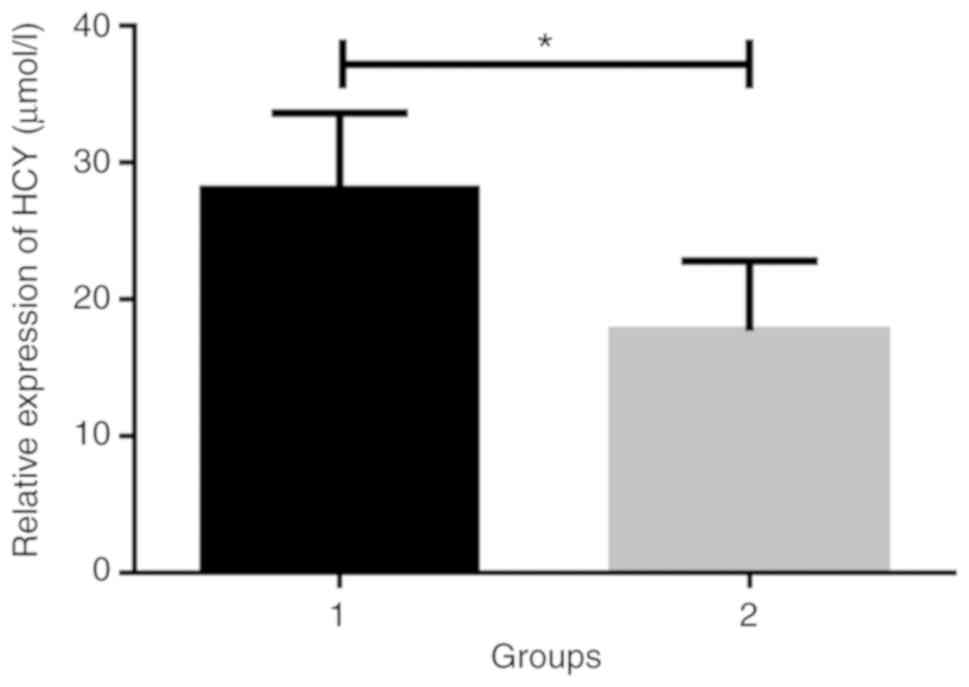
According to the results (Figs. 1 and 2), the expression levels of HCY and PCT in
the brain injury group were 27.56±6.26 µmol/l and 1.57±1.29 ng/ml,
and those in the non-brain injury group were 17.26±5.21 µmol/l and
0.34±0.31 ng/ml. The concentrations of HCY and PCT in the brain
injury group were significantly higher than those in the non-brain
injury group (t=11.75 and 9.41, P<0.001).
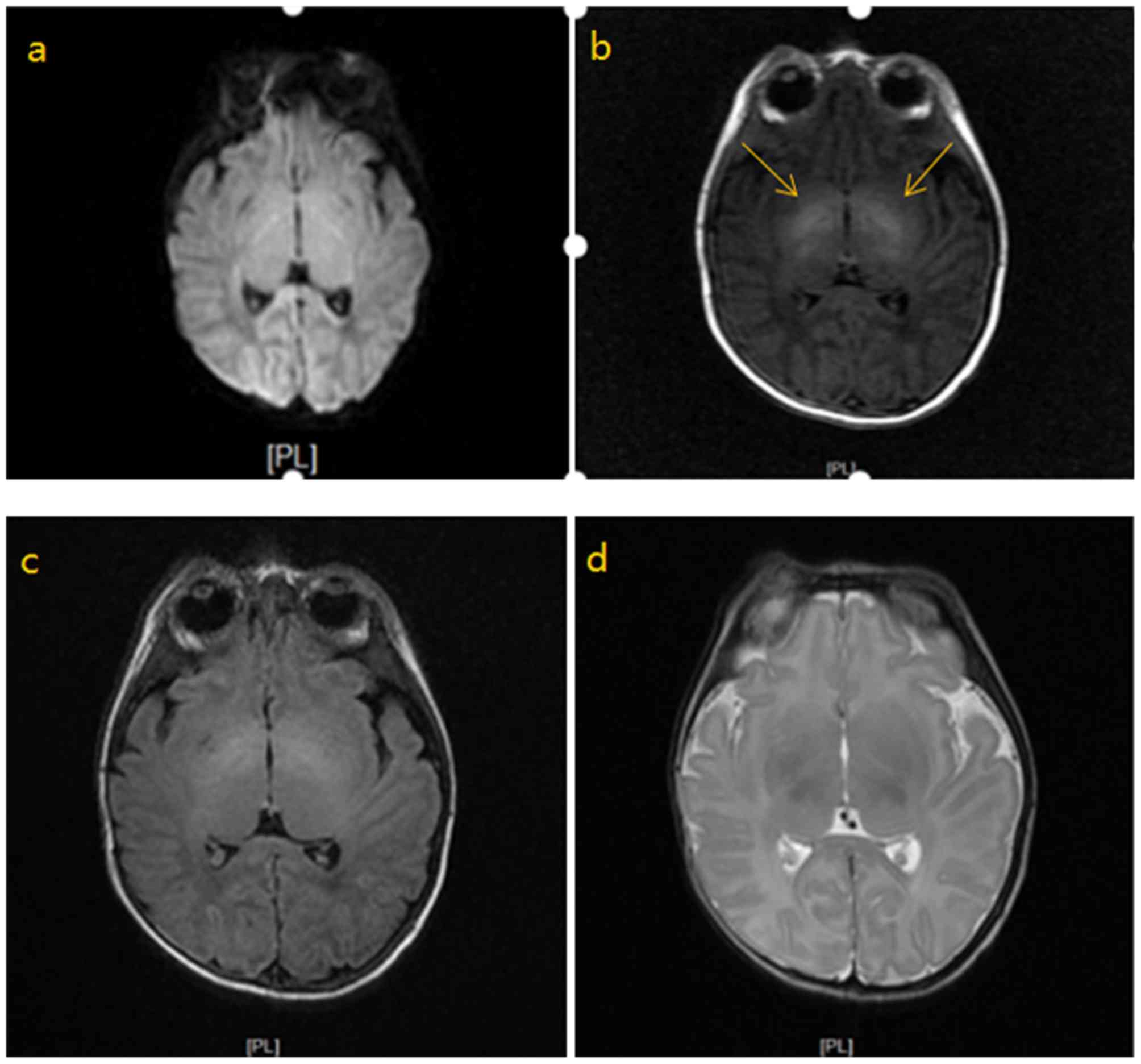
Analysis of images of MRI
Representative images of MRI are presented in
Fig. 3. The different images were
obtained from one patient. In the acute phase of neonatal bilirubin
encephalopathy, the MRI scan revealed increased symmetry of T1
signal in the bilateral globus pallidus and internal capsule; T2
showed equal signal; DWI sequence showed equal signal; the
ventricular system exhibited no expansion; there was no broadening
and deepening of cerebral sulcus, and the centerline structure was
centered.
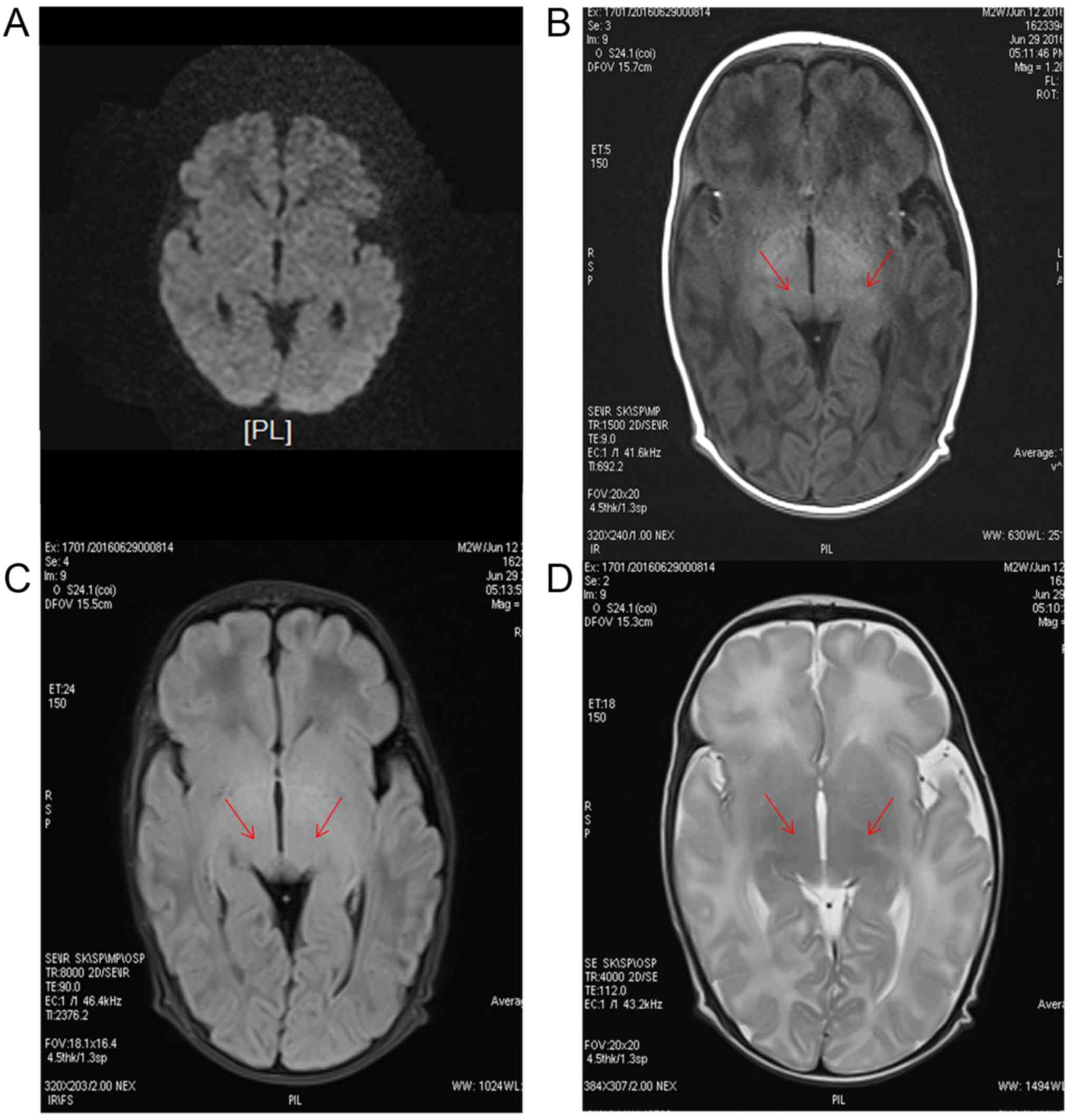
The MRI images of normal newborns can be observed in
Fig. 4. The DWI, the T1 weighted,
the T2-FLAIR and the T2 weighted sequences are presented. As
indicated by the arrows, the signals of the pale ball on both sides
of the T1 weighted sequence increased, and no obvious abnormal
signals were observed in the T2 weighted and T2-FLAIR
sequences.
Relationship between MRI results and
HCY and PCT levels in serum
According to the MRI examination results, the
patients were divided into an MRI normal group and an MRI abnormal
group. There was no significant difference in the general
characteristics between the two groups (P>0.05; Table II). In the brain injury group, the
serum HCY and PCT levels of the MRI abnormal group were
significantly higher than those of the MRI normal group, with a
statistically significant difference (P<0.05). In the non-brain
injury group, the serum HCY and PCT levels of the MRI abnormal
group were significantly higher than those of the MRI normal group,
with a statistically significant difference (P<0.05; Table III).
 | Table IIComparison of the general information
between the MRI normal group and the MRI abnormal group
[n(%)]/(mean ± SD). |
Table II
Comparison of the general information
between the MRI normal group and the MRI abnormal group
[n(%)]/(mean ± SD).
| Variable | MRI normal group
(n=84) | MRI abnormal group
(n=65) |
χ2/t-test | P-value |
|---|
| Sex | | | 0.000 | 0.980 |
|
Male | 48(57.14) | 37(56.92) | | |
|
Female | 36(42.86) | 28(43.08) | | |
| Gestational age
(weeks) | 39.11±1.57 | 38.86±1.60 | 0.960 | 0.341 |
| Daily age
(days) | 8.61±1.51 | 8.39±1.55 | 0.872 | 0.385 |
| Birth weight
(g) | 3571±78 | 3547±81 | 1.832 | 0.069 |
| Means of
pregnancy | | | 0.196 | 0.658 |
|
Eutocia | 47(55.95) | 34(52.31) | | |
|
Cesarean
delivery | 37(44.05) | 31(47.69) | | |
| Head circumference
(cm) | 32.88±0.61 | 33.01±0.57 | 1.327 | 0.187 |
| Age of the mother
(years) | 28.25±2.63 | 29.04±2.77 | 1.777 | 0.078 |
| Average gestational
weeks | 39.09±1.33 | 38.69±1.38 | 1.791 | 0.075 |
 | Table IIIAssociation between MRI results and
serum HCY and PCT levels (mean ± SD). |
Table III
Association between MRI results and
serum HCY and PCT levels (mean ± SD).
| Groups | Cases | HCY (µmol/l) | PCT (ng/ml) |
|---|
| Brain injury
group | | | |
|
MRI abnormal
group | 52 | 29.27±9.01 | 1.86±1.45 |
|
MRI normal
group | 15 | 17.88±7.87 | 0.37±0.29 |
| t-value | | 4.995 | 5.590 |
| P-value | | <0.001 | <0.001 |
| Non-brain injury
group | | | |
|
MRI abnormal
group | 13 | 25.09±6.78 | 1.32±0.87 |
|
MRI normal
group | 69 | 14.12±6.47 | 0.28±0.19 |
| t-value | | 4.663 | 3.782 |
| P-value | | <0.001 | <0.001 |
Diagnostic values of MRI, HCY and
PCT
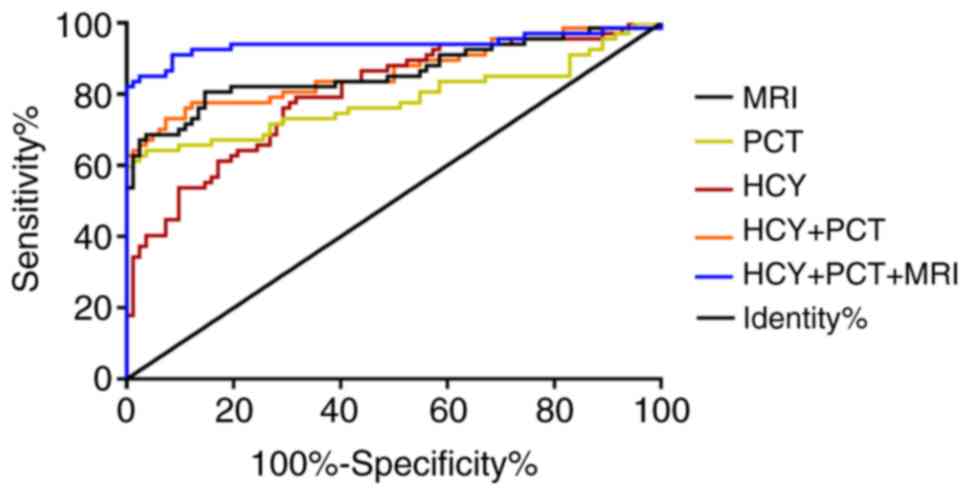
According to ROC curve, the optimal cutoff values of
HCY and PCT were respectively 19.78 and 0.89 (Fig. 5). Altogether 65 cases of
hyperbilirubinemia complicated with brain injury were detected by
MRI, including 52 cases of true positive and 69 cases of true
negative. Altogether 79 cases were detected by HCY, including 53 of
true positive and 56 of true negative. Altogether 46 cases were
detected by PCT, including 43 of true positive and 79 of true
negative. Altogether 67 cases were detected by the combination of
MRI, HCY and PCT, including 60 of true positive and 75 of true
negative (Table IV). Comparison of
the diagnostic value between single and combined detection is
presented in Table V. The
sensitivity of the combined detection was significantly higher than
that of single detection (P<0.05), the specificity was
significantly higher than that of HCY detection (P<0.05), and
the accuracy was significantly higher than that of MRI and HCY
single detection (P<0.05).
 | Table IVResult statistics on single and
combined detection. |
Table IV
Result statistics on single and
combined detection.
| Cases | + | - | Sum |
|---|
| MRI | | | |
|
+ | 52 | 13 | 65 |
|
- | 15 | 69 | 84 |
|
Sum | 67 | 82 | 149 |
| HCY | | | |
|
+ | 53 | 26 | 79 |
|
- | 14 | 56 | 70 |
|
Sum | 67 | 82 | 149 |
| PCT | | | |
|
+ | 43 | 3 | 46 |
|
- | 24 | 79 | 103 |
|
Sum | 67 | 82 | 149 |
| MRI+HCY+PCT | | | |
|
+ | 60 | 7 | 67 |
|
- | 7 | 75 | 82 |
|
Sum | 67 | 82 | 149 |
 | Table VComparison of the diagnostic value
between single and combined detection. |
Table V
Comparison of the diagnostic value
between single and combined detection.
| Indicators | Sensitivity % | Specificity % | Accuracy % |
|---|
| MRI | 77.6a | 84.1 | 81.2a |
| HCY | 79.1a | 68.3a | 73.2a |
| PCT | 64.2a | 96.3 | 81.9 |
| HCY+PCT | 73.13a | 92.68 | 83.89 |
| MRI+HCY+PCT | 90.0 | 91.5 | 90.6 |
Discussion
In recent years, neonatal hyperbilirubinemia and its
bilirubin brain damage have drawn considerable attention. Excessive
free bilirubin passes through the blood-cerebrospinal fluid barrier
and deposits in the brain tissue, resulting in bilirubin
encephalopathy, which is a brain tissue injury leading to death if
patients are not treated timely (21,22).
Therefore, early diagnosis and timely treatment are crucial for
reducing brain tissue injury and controlling disease progression
(23). MRI which is non-invasive,
non-radiative and atraumatic, is an imaging technique that uses
magnetic field signals generated by hydrogen protons in human
biological tissues to image (24).
According to a study on the analysis of MRI signal characteristics
of severe neonatal hyperbilirubinemia, T1WI images with STN/T and
GP/T ratios greater than 1.63 and 1.56 indicated bilirubin
encephalopathy, and poor prognosis usually manifested as a
symmetrical high signal on T2WI at the same position in chronic
phase (25). Therefore, early
diagnosis of neonatal hyperbilirubinemia complicated with brain
injury is of great significance.
High concentration of HCY induces the increase of
S-adenosyl homocysteine (SAH) which reduces the ratio of S-adenosyl
methionine (SAM) to SAH, and the protein expression and enzyme
activity of DNA methyltransferase, thus reducing DNA methylation
levels, inhibiting the proliferation of neural stem cells and
resulting in systemic diseases through molecular mechanisms of the
negative regulation (26). PCT,
whose expression level is very low in healthy people, is a
glycoprotein without hormone activity and produced by thyroid C
cells (27). A study revealed that
PCT was slightly increased in viral infections but significantly
increased in bacterial infections, so it is helpful to distinguish
bacterial meningitis from viral meningitis (28).
In the present study, the concentrations of HCY and
PCT in the brain injury group were significantly higher than those
in the non-brain injury group, indicating that HCY and PCT are
highly expressed in children with brain injury. According to
studies, HCY is highly expressed in diseases (29,30),
and its high expression is a risk factor for neonatal
hyperbilirubinemia (31). In a
study on hyperbilirubinemia, the expression level of PCT in the
infection group was significantly higher than that in the
non-infection group, and the combined detection had a high
diagnostic value for infection and non-infection in neonatal
hyperbilirubinemia (14). It was
therefore suggested that HCY and PCT were involved in the
occurrence and progression of hyperbilirubinemia, and have a high
diagnostic value in the complications of the disease. A study
revealed that bilateral globus pallidus of neonatal bilirubin
encephalopathy was significant, and its symmetrical high signal was
an important feature of MRI (24).
This is similar to the results of the present study. In a study by
Zhang et al on children with severe hyperbilirubinemia
(32), the NBNA score in the
abnormal MRI group was lower than that in the normal MRI group. In
a study on children with hyperbilirubinemia, MRI was correlated
with serum liver function indexes (33). Therefore, MRI may be correlated with
serum markers of neonatal hyperbilirubinemia. The specific
correlation remains to be explored in further research.
In the present study, MRI findings of bilirubin
encephalopathy patients revealed increased symmetry of T1 signals
in bilateral globus pallidus and internal sacs, equal signals in
T2, equal signals in DWI sequence, no expansion of ventricular
system, no widening or deepening of sulci and fissure, and the
centerline structure was centered. In the present study, there were
a few positive MRI signals in the non-brain injury group, which was
mainly considered as the high positive rate in the bilirubin
encephalopathy group, suggesting that the high intensity signal in
the posterior part of the MRI T1 and T2 weighted image of the
blebus was the most characteristic change in bilirubin brain
injury. In the non-brain injury group, positive signals may exist
due to the severity of the disease and abnormalities, but the
positive rate is low (20,34,35).
It has been reported that serum HCY has high sensitivity and
specificity in vascular mild cognitive dysfunction in patients with
cerebral small vessel disease, and can be used as a predictor of
vascular mild cognitive dysfunction in patients with
cerebrovascular disease (36). PCT
can be used as a rapid and accurate reference for early diagnosis
of severe brain injury complicated with pulmonary infection
(37), and serum PCT was a more
sensitive indicator for predicting the risk of multiple organ
dysfunction syndrome or death in patients with brain injury
(38). In the present study,
according to ROC, HCY and PCT can be used as diagnostic indicators
for the diagnosis of neonatal hyperbilirubinemia brain injury. The
optimal cutoff value was calculated. The sensitivity of MRI
combined with serum HCY and PCT was significantly higher than that
of single detection, and the specificity was significantly higher
than that of HCY detection, and the accuracy was significantly
higher than that of MRI and HCY single detection (P<0.05). A
study revealed that NBNA score combined with cranial MRI had a high
diagnostic value for severe neonatal hyperbilirubinemia complicated
with brain injury (32). According
to Li et al (39), the
combined detection of MRI, caspase-1 and interleukin-6 had a high
diagnostic value for white matter injury in premature infants.
Therefore, MRI combined with serum markers can significantly
improve the diagnostic rate of brain diseases.
There are currently few comparative studies on the
sensitivity and specificity of cranial MRI combined with HCY and
PCT in detecting neonatal hyperbilirubinemia complicated with brain
injury. The diagnostic value of the combined detection was
comprehensively explored in this study, however there are still
limitations. Children were not followed-up, and there was no data
on their long-term prognosis. Therefore, a multi-center and
systematic study with large samples is required to improve the
application value for the diagnosis and treatment of brain
injury.
In summary, the combination of cranial MRI, HCY and
PCT, which has a high diagnostic value for neonatal
hyperbilirubinemia complicated with brain injury, was conducive to
the early diagnosis and timely treatment of the disease and the
reduction of sequelae.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NC performed ECL, and wrote the manuscript. GW was
responsible for ECA, and revised the manuscript critically for
important intellectual content. NC and GW analyzed and interpreted
the data of the patients, and processed the statistics. Both
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Shandong Medical Imaging Research Institute. The family members of
the patients who participated in this research, signed the informed
consent and all patients had complete clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zheng J, Wei C, Zhao M and Zhao D:
Phototherapy is associated with the decrease in serum globulin
levels in neonatal hyperbilirubinemia. Biomed Rep. 10:63–69.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yu ZB, Han SP and Chen C: Bilirubin
nomograms for identification of neonatal hyperbilirubinemia in
healthy term and late-preterm infants: A systematic review and
meta-analysis. World J Pediatr. 10:211–218. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Petersen JP, Henriksen TB, Hollegaard MV,
Vandborg PK, Hougaard DM, Thorlacius-Ussing O and Ebbesen F:
Extreme neonatal hyperbilirubinemia and a specific genotype: A
population-based case-control study. Pediatrics. 134:510–515.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kuzniewicz MW, Wickremasinghe AC, Wu YW,
McCulloch CE, Walsh EM, Wi S and Newman TB: Incidence, etiology,
and outcomes of hazardous hyperbilirubinemia in newborns.
Pediatrics. 134:504–509. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Koziol LF, Budding DE and Chidekel D:
Hyperbilirubinemia: Subcortical mechanisms of cognitive and
behavioral dysfunction. Pediatr Neurol. 48:3–13. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Smitherman H, Stark AR and Bhutani VK:
Early recognition of neonatal hyperbilirubinemia and its emergent
management. Semin Fetal Neonatal Med. 11:214–225. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Maisels MJ: Neonatl jaundice. Pediatr Rev.
27:443–454. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Qian-Qian F, Shao-Hua W and Department P:
The clinical progress in early diagnosis of bilirubin
encephalopathy. Med Recap. 20:1186–1189. 2014.
|
|
9
|
Shi-Xin FU, Kai-Zhong Z, Yan B, et al:
Imaging features and clinical outcome of MRI and MRS in children
with bilirubin encephalopathy. Chin J CT MRI. 16:11–13. 2018.
|
|
10
|
Monsef A and Eghbalian F: Evaluation of
diagnostic value of procalcitonin as a marker of neonatal bacterial
infections. Iran J Pediatr. 22:314–318. 2012.PubMed/NCBI
|
|
11
|
Czyzewska M, Lachowska M and Gajewska E:
Evaluation of diagnostic value of procalcitonin (PCT) as a marker
of congenital infection in newborns. Przegl Lek. 59 (Suppl
1):S46–S49. 2002.PubMed/NCBI(In Polish).
|
|
12
|
Kawezymksi P and Piotrowski A:
Procalcitonin and C-reactive protein as a markers of neonatal
sepsis. Cinekol Pol. 75:439–444. 2004.PubMed/NCBI(In Polish).
|
|
13
|
Zahedpasha Y, Ahmadpour M, Hajiahmadi M
and Haghshenas M: Procalcitonin as a marker of neonatal sepsis.
Iran J Pediatr. 19:117–122. 2009.
|
|
14
|
Weidong HE and Laboratory DO: Application
value of immature granulocyte, D-dimer, FDP and PCT detection in
neonatal hyperbilirubinemia. China Health Stand Manage. 7:145–147.
2016.
|
|
15
|
Bo G, Feng L, Guoxin X, et al: Clinical
significance of procalcitonin, C-reactive protein and myocardial
enzymes determination in patients with neonatal hyperbilirubinemia.
Int J Lab Med. 35:2441–2443. 2014.
|
|
16
|
Joshi MB, Baipadithaya G, Balakrishnan A,
Hegde M, Vohra M, Ahamed R, Nagri SK, Ramachandra L and
Satyamoorthy K: Elevated homocysteine levels in type 2 diabetes
induce constitutive neutrophil extracellular traps. Sci Rep.
6(36362)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mangge H, Becker K, Fuchs D and Gostner
JM: Antioxidants, inflammation and cardiovascular disease. World J
Cardiol. 6:462–477. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Curro M, Gugliandolo A, Gangemi C,
Risitano R, Ientile R and Caccamo D: Toxic effects of mildly
elevated homocysteine concentrations in neuronal-like cells.
Neurochem Res. 39:1485–1495. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wu F, Yang H and Liu B: Association
between homocysteine and arterial stiffness in women with a history
of preeclampsia. J Vasc Res. 56:152–159. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Dan-Ni YE, Ling-Qing GE and Sheng-Lin YU:
Diagnostic significance of brainstem auditory evoked potential
combined with MRI in early neonatalbilirubin encephalopathy. Chin J
Child Health Care. 25:737–740. 2017.
|
|
21
|
Yan B, Kaizhong Z, Qian Z, et al:
Diagnostic performance compared of MRI and MRS in neonatal acute
bilirubin encephalopathy. Chin Imaging J Integr Tradit West Med,
2017.
|
|
22
|
Sgro M, Campbell D, Barozzino T and Shah
V: Acute neurological findings in a national cohort of neonates
with severe neonatal hyperbilirubinemia. J Perinatol. 31:392–396.
2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Iskander I, Gamaleldin R, El Houchi S, El
Shenawy A, Seoud I, El Gharbawi N, Abou-Youssef H, Aravkin A and
Wennberg RP: Serum bilirubin and bilirubin/albumin ratio as
predictors of bilirubin encephalopathy. Pediatrics.
134:e1330–e1339. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wisnowski JL, Panigrahy A, Painter MJ and
Watchko JF: Magnetic resonance imaging of bilirubin encephalopathy:
current limitations and future promise. Semin Perinatol.
38:422–428. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Xiao-Li M, Lei Z, Xiao-Hu W, et al:
Analysis of MRI signal characteristics of neonatal severe
hyperbilirubinemia. Chin J Magn Reson Imaging. 9:768–772. 2018.
|
|
26
|
Lin N, Qin S, Luo S, Cui S, Huang G and
Zhang X: Homocysteine induces cytotoxicity and proliferation
inhibition in neural stem cells via DNA methylation in vitro. FEBS
J. 281:2088–2096. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Peschanski N, Chenevier-Gobeaux C, Mzabi
L, Lucas R, Ouahabi S, Aquilina V, Brunel V, Lefevre G and Ray P:
Prognostic value of PCT in septic emergency patients. Ann Intensive
Care. 6(47)2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Velissaris D, Pintea M, Pantzaris N,
Spatha E, Karamouzos V, Pierrakos C and Karanikolas M: The role of
procalcitonin in the diagnosis of meningitis: A literature review.
J Clin Med. 7(148)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
La'ulu SL, Rawlins ML, Pfeiffer CM, Zhang
M and Roberts WL: Performance characteristics of six homocysteine
assays. Am J Clin Pathol. 130:969–975. 2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Moretti R and Caruso P: The controversial
role of homocysteine in neurology: From labs to clinical practice.
Int J Mol Sci. 20(231)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sukla KK, Tiwari PK, Kumar A and Raman R:
Low birthweight (LBW) and neonatal hyperbilirubinemia (NNH) in an
Indian cohort: Association of homocysteine, its metabolic pathway
genes and micronutrients as risk factors. PLoS One.
8(e71587)2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang HY, Qiao LX, Zhu WY and Wang Hua:
Diagnostic value of NBNA score combined head MRI in the neonates
with severe hyperbilirubinemia for brain injury. Chin J Child
Health Care. 25:164–166. 2017.
|
|
33
|
Yanming GE, Yaowu LI and Qinyan XU: The
study on the correlation between features of magnetic resonance
imaging and serum index about hyperbilirubinemia of the neonates. J
Clin Radiol. 34:1468–1471. 2015.
|
|
34
|
Chen LJ, Wang XM, Wan YZ, Li WH, Jia YG,
Yun CH and Chen TH: Superconducting MRI signal intensity in
pallidum in neonatal hyperbilirubinemia. Chin J Rehabil Theory
Pract. 22:838–840. 2016.(In Chinese).
|
|
35
|
Li XH, Zhang J, Zheng H, et al: Clinical
significance of cerebrospinal fluid bilirubin and craniocerebral
magnetic resonance imaging measurements in newborns with bilirubin
encephalopathy. Pract Clin Med. 4:76–79. 2014.
|
|
36
|
Wang T, Sun ZW, Shao LQ, Xu XB, Liu Y, Qin
M, Weng X and Zhang YX: Diagnostic values of serum levels of
homocysteine and uric acid for predicting vascular mild cognitive
impairment in patients with cerebral small vessel disease. Med Sci
Monit. 23:2217–2225. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wei W, Jinyue C, Qing Z, Chunxiao F, Min L
and Mingfen L: Early diagnosis value of procalcitonin in severe
brain damage combined with pulmonary infection. Int J Lab Med.
2934–2936. 2015.(In Chinese).
|
|
38
|
Ai-Hua F, Shu-Ming P, Ming L, et al: Value
of procalcitonin to the prediction of multiple organ dysfunction
syndrome in patients with brain injury. J Chin Pract Diagn Ther,
2011.
|
|
39
|
Li Y: Application of MRI combined with
caspase-1 and IL-6 in the diagnosis of white matter damage in
premature infants. Lab Med Clin. 14:2211–2213. 2017.
|