Introduction
Cardiovascular diseases such as ischemic heart
disease and stroke have been the leading cause of death globally
for the last 15 years. In 2016, 17.9 million deaths were attributed
to cardiovascular disease. High levels of cholesterol and
triglycerides, insulin resistance, obesity, smoking, unhealthy
diets and lack of exercise are the most important risk factors for
a number of cardiovascular diseases (1).
Atherosclerosis, a cause of most cardiovascular
diseases, is a chronic inflammatory process occurring at the artery
wall as a response to modified structures, particularly oxidized
low-density lipoproteins (oxLDL), stimulating both innate and
adaptive immune responses (2).
Following oxLDL migration to the subendothelial space of the intima
(the innermost layer of the artery), monocytes differentiate into
macrophages, which then ingests oxLDL via upregulated scavenger
receptors such as scavenger receptor A and CD36, leading to the
formation of ‘foam cells’ particularly at injury-prone areas such
as aortic bifurcations (3).
Subsequently, the nascent atheroma typically progresses into a more
complex lesion called a fibrous plaque via accumulation of
connective tissue with an increased number of smooth muscle cells
and lipid-laden macrophages (3).
These activated macrophages secrete numerous cytokines such as
interleukin (IL)-12, IL-1β and tumor necrosis factor (TNF)-α,
leading to the recruitment of monocytes and T lymphocytes (4). However, during early atherogenesis, a
predominant infiltration of M2 macrophages induces small
atherosclerotic lesions, suggesting that this macrophage subset may
favor an atheroprotective state mainly via the secretion of
IL-4(5). In advanced lesions, M1
macrophages cause plaque vulnerability by degrading the fibrous
layer matrix via metalloproteinases. This might lead to sudden
ruptures inside the artery (5,6),
allowing thrombi to form and therefore disrupt blood flow, leading
to life-threatening conditions such as myocardial infarction and
stroke (7,8).
Recruited T lymphocytes differentiate into different
effector cells which affect atherogenesis (9). Type 1 T helper (Th1)-related cytokines
such as interferon gamma (IFN)-γ and TNF-α are known to be
pro-inflammatory and promote the development and progression of
atherosclerosis (2). On the other
hand, type 2 T helper (Th2) anti-inflammatory cytokines, especially
IL-4 and IL-10, seem to exert anti-atherogenic effects by
inhibiting IFN-γ production (9).
Although the role of Th17 cells, which mainly secrete IL-17A, in
atherosclerosis has not yet been fully elucidated, most studies
(2,9) attribute the pro-inflammatory effects
of IL-17 to the activation of NF-κB, a hallmark transcription
factor associated with the induction of pro-inflammatory agents,
including IL-1, IL-6, TNF-α and nitric oxide synthase 2(10). In addition, IL-17, which is mainly
released by visceral adipose tissue, seems to play a role early in
atherogenesis by stimulating smooth muscle cells of the
atheromatosus arteries to secrete the chemokine eotaxin (11). Macrophages treated with IL-17
express high levels of toll-like receptor (TLR) 2 and TLR4 and
secrete high levels of inflammatory cytokines such as TNF-α and low
levels of IL-10 in the presence of oxLDL, which is a profile
similar to that of M1 macrophages, which are known to be
proatherogenic (12).
T regulatory (Treg) cells constitute another subset
of lymphocytes that play a role in atherogenesis. Treg-related
cytokines such as IL-10 and TGF-β are anti-inflammatory and promote
anti-atherogenic activities (2).
C-reactive protein (CRP) is produced by the liver in
response to tissue injury or inflammation (13). CRP exists in two isoforms: The
pentameric isoform (pCRP) and the modified monomeric isoform
(mCRP). Studies have shown that mCRP is dissociated from pCRP when
it is exposed to endothelial cells (14), neutrophils (15), cell membranes, liposomes (16) and activated platelets (17,18).
To the best of our knowledge, few studies have taken
the difference between the two isoforms of CRP into consideration
(19). The pCRP isoform has been
reported to play an anti-inflammatory role and stop the progression
of atherosclerosis, while the mCRP isoform has pro-inflammatory
roles and enhances the activation of different pathways for the
production of numerous inflammatory cells and cytokines (20). This demonstrates that the mechanisms
of mCRP dissociation could potentially be the target of
anti-inflammatory chemotherapeutics.
CRP has been shown to bind to oxLDL and promote its
uptake into the U937-derived monocyte/macrophage cell line
(21). OxLDL are a class of
lipoprotein particles, consisting of triglycerides and cholesterol
esters which form the hydrophobic core surrounded by a hydrophilic
shell formed of phospholipids, apolipoproteins and free
cholesterol. CRP, in its two isoforms, and oxLDL form a complex
with glycoproteins and are localized with macrophages in human
atherosclerotic lesions. OxLDL and CRP levels are directly
correlated in patients suffering from acute coronary syndromes
(22). The
oxLDL/CRP/lysophosphatidylcholine complex has low pro-inflammatory
activities in vitro, and is known to be antiatherogenic
(23).
The present study aimed to investigate the single
and combinatory effects of oxLDL and human CRP containing azide,
which is usually used as a preservative with CRP in its two
isoforms (mCRP and pCRP) on U937-derived macrophage release of
cytokines. The results clarified the optimal effects of CRP and
oxLDL combinations, which might help in identifying their role in
atherosclerosis.
Materials and methods
Materials and reagents
RPMI-1640 medium, PBS, L-glutamine,
penicillin/streptomycin, polymyxin B sulfate salt, FBS, and
phorbol-myristate-acetate (PMA) were purchased from Sigma-Aldrich;
Merck KGaA. CRP purified from pleural human fluid (purity >99%)
containing 0.09% sodium azide as a preservative was obtained from
Lee Biosolutions, Inc. Human oxLDL and sodium azide were purchased
from Kalen Biomedical, LLC and Sigma-Aldrich; Merck KGaA,
respectively.
Differentiation of U937 cells into
macrophages
Human monocytic U937 cells (kindly provided by Dr
Marwan El Sabban at the American University of Beirut) were
cultured in RPMI-1640 medium supplemented with 10% FBS, 2 mM
L-glutamine, 1% penicillin/streptomycin and 20 µg/ml polymexin B
(referred to as complete growth medium hereafter). The cultures
were maintained in a humidified 5% CO2 incubator at
37˚C. Cells were counted for viability using the Trypan blue
exclusion method (24). For
differentiation into macrophages, U937 cells were treated with 100
nM PMA for 24 h. Following treatment, media were removed and
adherent cells were washed twice with PBS and cultured in PMA-free
complete growth medium for 24 h. To collect adherent U937-derived
macrophages, cells were washed twice with PBS, incubated on ice for
30 min and gently detached using a cell scraper. Detached cells
were centrifuged at 250 x g at 4˚C for 5 min. Cells were then
seeded at a density of 7x105 cells/well in a 24-well
plate and allowed to adhere for 24 h prior to treatment.
CRP monomerization
Human mCRP was prepared by heating human pCRP at
80˚C for 70 min, as previously described (25). To confirm CRP monomerization, mCRP
and pCRP samples were mixed and loaded on a 12.5% polyacrylamide
gel. The gel was then stained with Coomassie brilliant blue for 1 h
at room temperature, and incubated in destain solution overnight at
room temperature. SDS-PAGE broad range protein standard (6.5-200
kDa; Bio-Rad Laboratories), was used as a marker.
Treatment of U937-derived
macrophages
U937-derived macrophages were counted and seeded in
a 24-well plate at a density of 7x105 cells/well and
cultured in a humidified 5% CO2 incubator at 37˚C for 24
h to adhere. Subsequently, cells were washed with PBS and incubated
in complete growth medium containing the corresponding treatment
reagents for 24 h. Each well contained one of the following
treatments at a concentration of 25 µg/ml for each component: mCRP,
pCRP, mCRP/pCRP, oxLDL, mCRP/oxLDL, pCRP/oxLDL, mCRP/pCRP/oxLDL,
0.09% sodium azide solution (SA1x), 0.18% sodium azide (SA2x),
SA1x, SA2x, SA1x/oxLDL, with an additional control well with no
treatment added. SA1x and SA2x served as single and double
concentration controls for sodium azide present in mCRP and
mCRP/pCRP, respectively. The adopted concentrations were chosen
based on previous literature (26).
Flow cytometry
U937 monocytes and untreated U937-derived
macrophages were collected, washed in cold PBS at and incubated at
a density of 1x105 cells per 100 µl PBS containing 10%
human serum AB (Gibco; Thermo Fisher Scientfic, Inc.) for 30 min at
4˚C to block non-specific Fc receptors. The cells were then stained
with 1 µg/ml of phycoerythrin (PE)-conjugated mouse anti-human CD11
antigen-like family member B (CD11b) antibody (5
µl/1x105 cells; cat. no. 555388; BD Biosciences) or
PE-conjugated mouse immunoglobulin G1 isotype control antibody (5
µl/1x105 cells; cat. no. 555749; BD Biosciences) for 15
min at 4˚C. Cells were then washed with cold PBS supplemented with
1% human serum AB) and finally resuspended in PBS containing 1%
human serum AB. Samples were kept on ice and CD11b expression was
analyzed within 30 min by a BD FACSCalibur™ flow cytometer and
CellQuest software (version 5.1; BD Biosciences). U937 cells and
U937-derived macrophages were identified based on their forward
scatter (FSC) and side scatter (SSC) properties. Results are
expressed as geometric mean fluorescence intensity.
ELISA
Culture supernatants from untreated and treated
U937-derived macrophages were collected and stored at -80˚C for
later cytokine analysis. Commercial ELISA kits (PeproTech, Inc.)
were used to measure the levels of IFN-γ (cat. no. 900-K27), IL-4
(cat. no. 900-K14), IL-6 (cat. no. 900-K16), IL-10 (cat. no.
900-K21), and TNF-α (cat. no. 900-K25) in culture supernatants.
ELISA was performed according to the manufacturer's
instructions.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism software (version 6.0; GraphPad Software, Inc.) by conducting
one-way ANOVA followed by Tukey's multiple comparison post hoc
test. Data are presented as the mean ± SEM. P<0.05 was
considered to indicate a statistically significant difference.
Results
Confirmation of U937 differentiation
into macrophages
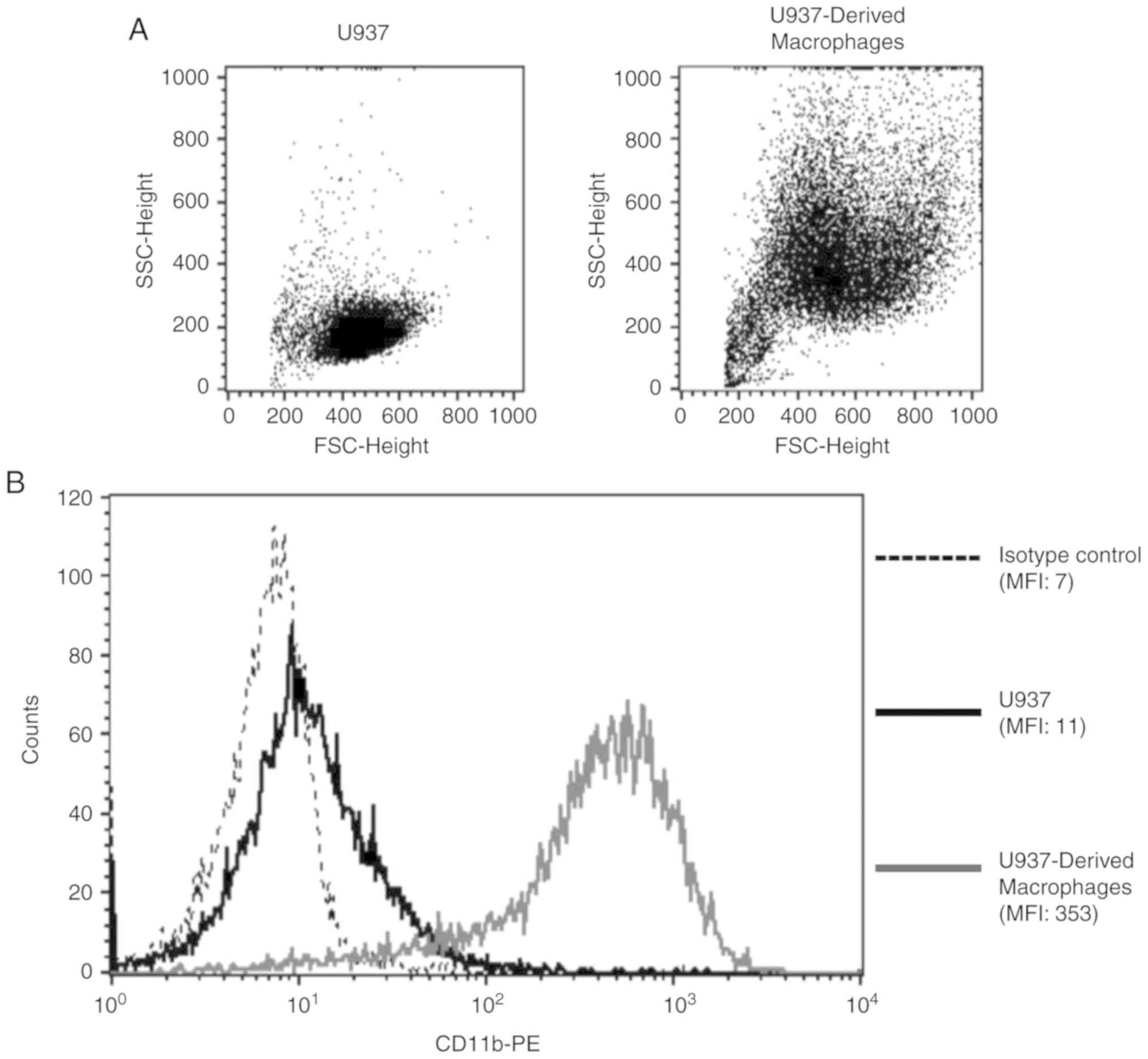
In order to validate PMA-induced differentiation of
U937 monocytes into macrophages, FSC/SSC properties and CD11b
surface expression were analyzed for both cell populations.
U937-derived macrophages were more granular and larger in size when
compared with U937 monocytes, indicative of a macrophage phenotype
(Fig. 1A). Additionally, there was
a 32-fold increase in the surface expression of the macrophage
differentiation marker, CD11b, upon the differentiation of U937
monocytes into macrophages (Fig.
1B).
Confirmation of CRP
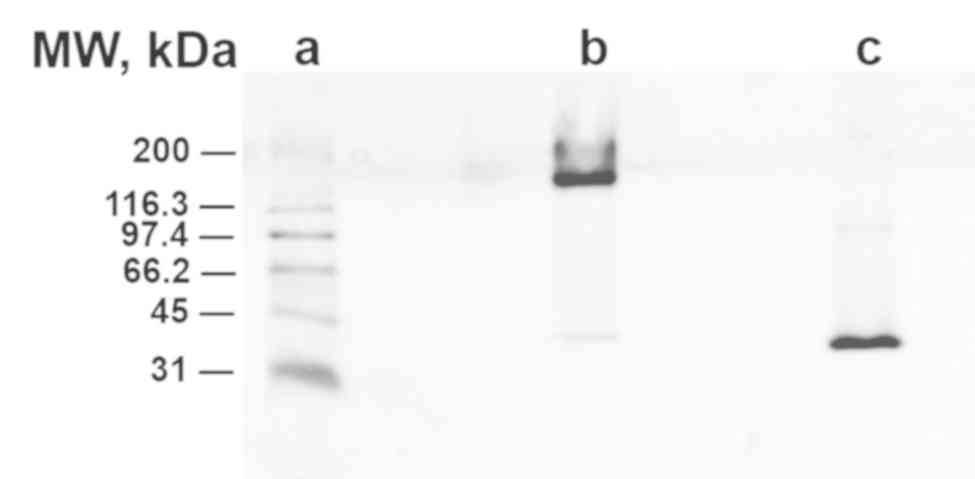
monomerization
Gel electrophoresis showed that unheated pCRP showed
slight migration at 200 kDa (lane B) while heated pCRP migrated
further (40 kDa, lane C), confirming the effective monomerization
of pCRP to mCRP (Fig. 2).
IFN-γ
Although mCRP alone had no effect on IFN-γ
production compared with control, other treatments (oxLDL,
mCRP/oxLDL, pCRP/oxLDL, mCRP/pCRP/oxLDL) reduced IFN-γ production
when compared with those of control and mCRP treatment. IFN-γ
levels for samples treated with both CRP isoforms combined with
oxLDL was significantly reduced when compared with control and mCRP
treatment. Treatment with oxLDL or a combination of pCRP/oxLDL
significantly decreased the amount of secreted IFN-γ to half, as
compared to control and mCRP treatment (P<0.001). IFN-γ was also
significantly reduced (P<0.01) in samples treated with
mCRP/oxLDL and oxLDL/SA1x when compared with levels measured in
mCRP alone and control. pCRP treatment resulted in a significant
decrease in IFN-γ levels when compared with control (P<0.05;
Fig. 3).
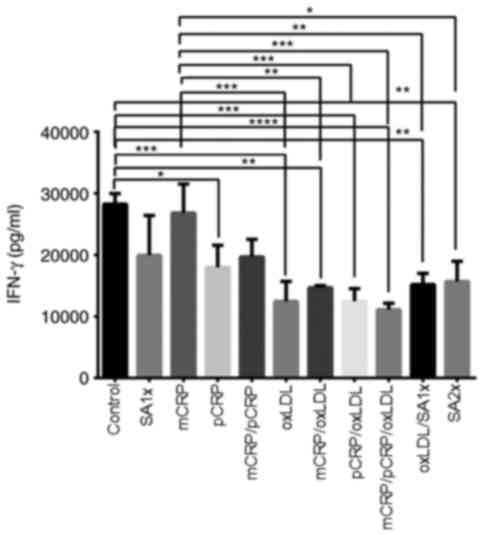 | Figure 3IFN-γ release by U937-derived
macrophages. U937-derived macrophages were cultured for 24 h in the
presence or absence of mCRP, pCRP oxLDL, SA1x and SA2x as
indicated. Data are presented as the mean values of cytokine levels
± SEM of three independent experiments. *P<0.05,
**P<0.01, ***P<0.001 and
****P<0.0001. mCRP, monomeric C-reactive protein;
pCRP, pentameric C-reactive protein; oxLDL, oxidized low-density
lipoprotein; SA1x, 0.09% sodium azide solution; SA2x, 0.18% sodium
azide solution; INF-γ, interferon-γ. |
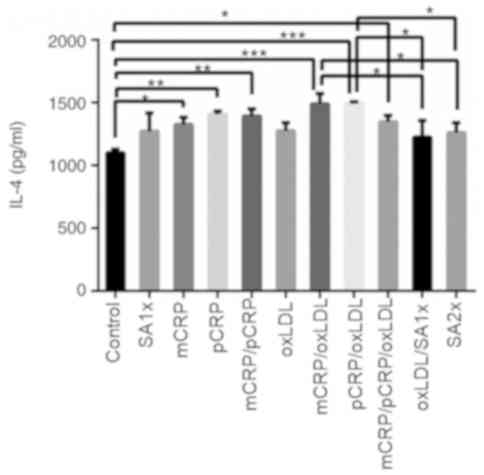
IL-4
IL-4 secretion showed significant increase when
macrophages were treated with pCRP, mCRP, or a combination of both
isoforms, as compared with control macrophages (P<0.05 and
P<0.001). Furthermore, IL-4 was highly secreted when samples
were treated with mCRP/oxLDL when compared with control
(P<0.001). A similarly significant increase in IL-4 production
was observed in macrophages treated with mCRP (P<0.05),
mCRP/pCRP/oxLDL combination (P<0.05) and pCRP/oxLDL
(P<0.001), compared with control. In addition, treatment with
SA2x or oxLDL/SA1x resulted in a slight decrease in IL-4 secretion
compared with mCRP/oxLDL and pCRP/oxLDL treatments (P<0.05;
Fig. 4).
IL-6
IL-6 release was reduced by almost 3-fold when
macrophages were treated with mCRP/pCRP/oxLDL and SA1x as compared
to untreated macrophages (P<0.0001). Similarly, treatment with
pCRP and pCRP/oxLDL downregulated IL-6 production to half the
levels measured in control cells (P<0.01). IL-6 levels were
significantly reduced upon oxLDL, mCRP/oxLDL, oxLDL/SA1x and SA2x
treatment when compared with control group (P<0.001). mCRP
treatment resulted in a significant decrease in IL-6 levels, as
compared with control cells (P<0.05). In addition, IL-6 levels
were further reduced by half when macrophages were treated with
oxLDL, pCRP/oxLDL or mCRP/pCRP/oxLDL (P<0.001), as compared with
the levels secreted by mCRP-treated macrophages. Secretion levels
of IL-6 in samples treated with mCRP/pCRP were significantly higher
in comparison with IL-6 levels after treatment with mCRP/oxLDL and
mCRP/pCRP/oxLDL (P<0.01; Fig.
5).
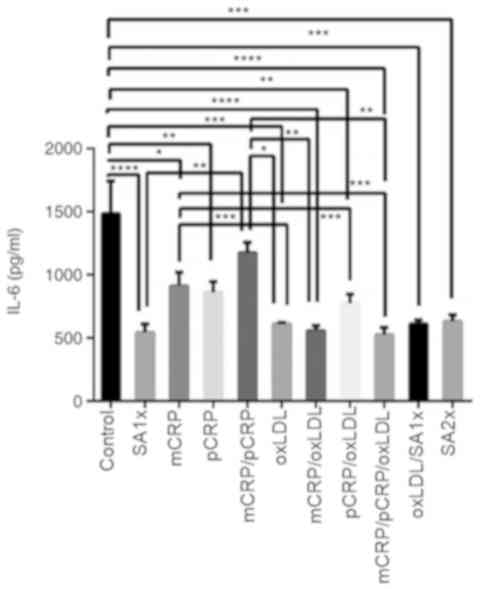 | Figure 5IL-6 release by U937-derived
macrophages. U937-derived macrophages were cultured for 24 h in the
presence or absence of mCRP, pCRP oxLDL, SA1x and SA2x as
indicated. Data are presented as the mean values of cytokine levels
± SEM of three independent experiments. *P<0.05,
**P<0.01, ***P<0.001 and
****P<0.0001. mCRP, monomeric C-reactive protein;
pCRP, pentameric C-reactive protein; oxLDL, oxidized low-density
lipoprotein; SA1x, 0.09% sodium azide solution; SA2x, 0.18% sodium
azide solution; IL, interleukin. |
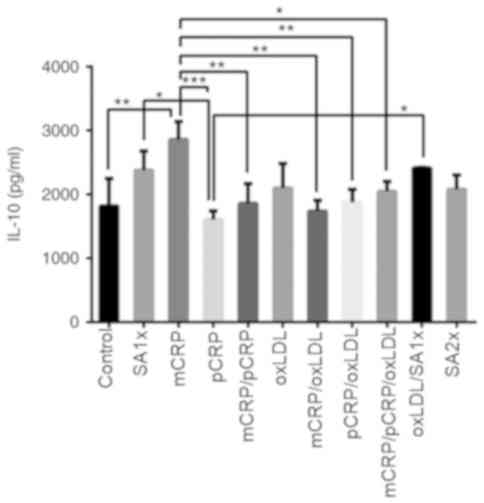
IL-10
IL-10 release increased by 50% when macrophages were
treated with mCRP compared with controls (P<0.01). By contrast,
a 2-fold reduction in IL-10 production was detected in cells
treated with pCRP as compared to mCRP treatment (P<0.001). IL-10
levels significantly decreased after treatment with mCRP/pCRP,
mCRP/oxLDL and pCRP/oxLDL compared with mCRP alone (P<0.01). The
combination of mCRP/pCRP/oxLDL significantly reduced IL-10 levels
compared with mCRP treatment. SA1x treatment and oxLDL/SA1x
resulted in a higher release of IL-10 compared with pCRP treatment
(P<0.05; Fig. 6).
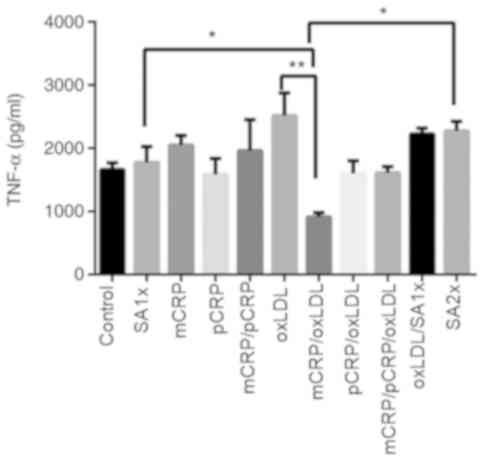
TNF-α
TNF-α levels showed an almost 3-fold decrease when
macrophages were treated with the mixture of mCRP/oxLDL compared to
the levels of TNF-α in samples treated with oxLDL alone
(P<0.01). A two-fold increase was observed in macrophages
treated with SA1x and SA2x) compared with TNF-α levels measured in
macrophages treated with mCRP/oxLDL (P<0.05; Fig. 7).
Discussion
In order to have a better understanding of the
factors affecting the activity of macrophages, which are known to
play a pivotal role in atherogenesis, the current study assessed
the single and combined effects of monomeric and pentameric CRP
azide-containing isoforms and oxLDL on the release of selected
atherosclerosis-related cytokines, including IFN-γ, IL-4, IL-6,
IL-10 and TNF-α, by U937-derived macrophages.
IFN-γ is known to be a pro-atherogenic cytokine
mainly by activating macrophages to produce other pro-inflammatory
cytokines, such as TNF-α and IL-6, via the JAK/STAT signaling
pathway (23). The present study
showed that all treatments, with the exception of mCRP alone,
caused a marked decrease in IFN-γ levels secreted by macrophages
when compared with the control group. The combination of oxLDL,
mCRP and pCRP resulted in the most significant decrease in IFN-γ
levels. This indicated that neither oxLDL or pCRP alone nor in
combination with mCRP can induce atherogenesis by upregulating
IFN-γ secreted by macrophages. On the contrary, treatments
involving oxLDL might have anti-atherogenic effects as oxLDL
treatment induced a decrease in the secretion of IFN-γ by
macrophages. Such effects might be mediated by the downregulation
of cytokines known to induce IFN-γ production, including IL-12 and
IL-18 (27,28). This was further supported by the
increased levels of IL-4, which has been previously demonstrated to
suppress IL-12/IL-18 secretion (25,26),
under most single and combined treatments, the least effect being
observed following treatment with oxLDL.
Although it was hypothesized that IL-4 acts as an
antiatherogenic cytokine, experimental data from the present study
alone are not sufficient to fully support this assumption. However,
it can be assumed that IL-4 is an immunoregulatory molecule
controlling inflammatory response by downregulating TNF production
(29). The tendency to downregulate
pro-inflammatory cytokines (especially IFN-γ) and upregulate IL-4
suggest that U937-derived macrophages tend to acquire the M2
profile (5). This was further
confirmed by the decreased IL-6 production under most treatments
especially those involving oxLDL in combination with mCRP.
This suggested scheme (acquisition of M2 profile) is
complemented by the inability of most treatments to affect the
levels of the anti-inflammatory cytokine IL-10, which suppresses
the expression of certain inflammatory cytokines, including IL-6,
IL-1 and TNF-α (23) and has been
revealed to reduce atherogenesis (30). In addition, mCRP induced a
significant upregulation of IL-10 levels when compared with control
and most other treatments. TNF-α is another potent pro-inflammatory
cytokine mainly secreted by activated macrophages. Plasma levels of
TNF-α are increased in pathologies such as atherosclerosis. This
cytokine enhances the expression of other pro-inflammatory
cytokines via NF-κB activation (31).
In line with previous studies, the present results
indicated that the secretion of TNF-α by U937-derived macrophages
increased when cells were exposed to oxLDL (26,32).
However, when oxLDL was combined with mCRP, TNF-α production
significantly decreased. This demonstrated that the effect of oxLDL
on the activity of macrophages may depend on the microenvironmental
composition.
Another important factor that might affect the
microenvironment, and therefore the results, is the presence of
azide which is usually used as a preservative for CRP. Especially
at higher doses, azide caused a significant decrease in the
secretion of IFN-γ by U937-derived macrophages. This tendency did
not appear to inhibit the effect of all other treatments (except
for mCRP) in reducing the secretion of this cytokine and even may
enhance this downregulatory effect. The presence of azide did not
appear to significantly affect the activity of macrophages in
secreting IL-4, IL-10 and TNF-α when compared with control.
However, the present study showed that azide alone at both
concentrations significantly reduced IL-6 secretion by macrophages.
In a previous study, when azide-free CRP was used, pCRP was able to
enhance IL-6 release by U937-derived macrophages which is
contradictory to the results from the present study (30).
In the present study, mCRP did not have any marked
effect on the levels of IFN-γ and TNF-α but it had the ability to
upregulate IL-4 and IL-10 levels and to downregulate IL-6 levels
secreted by U937-derived macrophages. Therefore, future studies
assessing the role of mCRP is atherosclerosis are required, as a
recent study has shown that mCRP might have contradictory
proangiogenic and antiangiogenic effects (33).
pCRP was found to be able to downregulate most
pro-inflammatory cytokines, mainly IFN-γ and IL-6 and to upregulate
the anti-inflammatory cytokine, IL-4, whilst having no notable
effects on IL-10 secretion. A similar trend was observed with the
combination of mCRP and pCRP. A significant reduction in the
secretion of the proatherogenic cytokines (IFN-γ and IL-6) was
found in samples treated with oxLDL alone and in combination with
mCRP and/or pCRP. However, no statistically significant differences
in the levels of IL-10 and TNF-α were detected between oxLDL-,
mCRP/oxLDL- or pCRP/oxLDL-treated and control macrophages.
Therefore, it was shown in the present study that, oxLDL combined
with mCRP may have an anti-atherogenic effect by modulating
cytokine production by U973-derived macrophages.
In conclusion, mCRP, pCRP and oxLDL, either
individually or in combination, can upregulate IL-4 and IL-10
secretion and downregulate IFN-γ and IL-6 secretion by U937-derived
macrophages, thus favoring M2 macrophage. Therefore, the
microenvironment of the intima, involving especially
atherosclerosis-related cytokines as IL-6, TGF-β, IL-10 and IL-17,
may be a critical factor affecting atherogenesis. Further studies
should attempt to mimic the in vivo medium of the intima to
investigate the type of macrophages, which is heavily associated
with atherogenesis, and other cell types in addition to their
behavior under different conditions.
Acknowledgements
The authors would like to thank Dr Takla El. Khoury,
Mr. Michel Zakhem and Mr. Salah El Khatib (Department of Biology,
University of Balamand, Koura, Lebanon) for their support and
technical assistance.
Funding
The present study was supported by the Balamand
Internal Research Grant (grant no. BRG 10/2013).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DJ and IK performed the experiments. DJ, IK, SB and
MK analyzed and interpreted the data. DJ wrote the first draft of
the manuscript. SB and MK reviewed and edited the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
World Health Organization. Global
tuberculosis report 2017. 2017.
|
|
2
|
Ait-Oufella H, Taleb S, Mallat Z and
Tedgui A: Recent advances on the role of cytokines in
atherosclerosis. Arterioscler Thromb Vasc Biol. 31:969–979.
2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ross R and Agius L: The process of
atherogenesis-cellular and molecular interaction: From experimental
animal models to humans. Diabetologia. 35 (Suppl 2):S34–S40.
1992.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gibson MS, Domingues N and Vieira OV:
Lipid and non-lipid factors affecting macrophage dysfunction and
inflammation in atherosclerosis. Front Physiol.
9(654)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Khallou-Laschet J, Varthaman A, Fornasa G,
Compain C, Gaston AT, Clement M, Dussiot M, Levillain O,
Graff-Dubois S, Nicoletti A and Caligiuri G: Macrophage plasticity
in experimental atherosclerosis. PLoS One. 5(e8852)2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chavez-Sanchez L, Espinosa-Luna JE,
Chavez-Rueda K, Legorreta-Haquet MV, Montoya-Diaz E and
Blanco-Favela F: Innate immune system cells in atherosclerosis.
Arch Med Res. 45:1–14. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Huang WC, Sala-Newby GB, Susana A, Johnson
JL and Newby AC: Classical macrophage activation up-regulates
several matrix metalloproteinases through mitogen activated protein
kinases and nuclear factor-κB. PLoS One. 7(e42507)2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bentzon JF, Otsuka F, Virmani R and Falk
E: Mechanisms of plaque formation and rupture. Circ Res.
114:1852–1866. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gronberg C, Nilsson J and Wigren M: Recent
advances on CD4+ T cells in atherosclerosis and its
implications for therapy. Eur J Pharmacol. 816:58–66.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kolls JK and Linden A: Interleukin-17
family members and inflammation. Immunity. 21:467–476.
2004.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tarantino G, Costantini S, Finelli C,
Capone F, Guerriero E, La Sala N, Gioia S and Castello G: Is serum
interleukin-17 associated with early atherosclerosis in obese
patients? J Transl Med. 12(214)2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
de la Paz Sanchez-Martinez M,
Blanco-Favela F, Mora-Ruiz MD, Chavez-Rueda AK, Bernabe-Garcia M
and Chavez-Sanchez L: IL-17-differentiated macrophages secrete
pro-inflammatory cytokines in response to oxidized low-density
lipoprotein. Lipids Health Dis. 16(196)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hurlimann J, Thorbecke GJ and Hochwald GM:
The liver as the site of C-reactive protein formation. J Exp Med.
123:365–378. 1966.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Khreiss T, Jozsef L, Potempa LA and Filep
JG: Conformational rearrangement in C-reactive protein is required
for proinflammatory actions on human endothelial cells.
Circulation. 109:2016–2022. 2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Khreiss T, Jozsef L, Potempa LA and Filep
JG: Loss of pentameric symmetry in C-reactive protein induces
interleukin-8 secretion through peroxynitrite signaling in human
neutrophils. Circ Res. 97:690–697. 2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ji SR, Wu Y, Zhu L, Potempa LA, Sheng FL,
Lu W and Zhao J: Cell membranes and liposomes dissociate C-reactive
protein (CRP) to form a new, biologically active structural
intermediate: mCRP(m). FASEB J. 21:284–294. 2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Eisenhardt SU, Thiele JR, Bannasch H,
Stark GB and Peter K: C-reactive protein: How conformational
changes influence inflammatory properties. Cell Cycle. 8:3885–3892.
2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Filep JG: Platelets affect the structure
and function of C-reactive protein. Circ Res. 105:109–111.
2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
McFadyen JD, Kiefer J, Braig D,
Loseff-Silver J, Potempa LA, Eisenhardt SU and Peter K:
Dissociation of C-reactive protein localizes and amplifies
inflammation: Evidence for a direct biological role of C-reactive
protein and its conformational changes. Front Immunol.
9(1351)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Caprio V, Badimon L, Di Napoli M, Fang WH,
Ferris GR, Guo B, Iemma RS, Liu D, Zeinolabediny Y and Slevin M:
pCRP-mCRP dissociation mechanisms as potential targets for the
development of small-molecule anti-inflammatory chemotherapeutics.
Front Immunol. 9(1089)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Singh SK, Suresh MV, Voleti B and Agrawal
A: The connection between C-reactive protein and atherosclerosis.
Ann Med. 40:110–120. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tabuchi M, Inoue K, Usui-Kataoka H,
Kobayashi K, Teramoto M, Takasugi K, Shikata K, Yamamura M, Ando K,
Nishida K, et al: The association of C-reactive protein with an
oxidative metabolite of LDL and its implication in atherosclerosis.
J Lipid Res. 48:768–781. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang JM and An J: Cytokines,
inflammation, and pain. Int Anesthesiol Clin. 45:27–37.
2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Strober W: Trypan blue exclusion test of
cell viability. Curr Protoc Immunol. 111:A3.B.1–A3.B.3.
2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Taylor KE and van den Berg CW: Structural
and functional comparison of native pentameric, denatured monomeric
and biotinylated C-reactive protein. Immunology. 120:404–411.
2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Krayem I, Bazzi S and Karam M: The
combination of CRP isoforms with oxLDL decreases TNF-α and IL-6
release by U937-derived macrophages. Biomed Rep. 7:272–276.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Darwich L, Coma G, Peña R, Bellido R,
Blanco EJ, Este JA, Borras FE, Clotet B, Ruiz L, Rosell A, et al:
Secretion of interferon-gamma by human macrophages demonstrated at
the single-cell level after costimulation with interleukin (IL)-12
plus IL-18. Immunology. 126:386–393. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Voloshyna I, Littlefield MJ and Reiss AB:
Atherosclerosis and interferon-γ: New insights and therapeutic
targets. Trends Cardiovasc Med. 24:45–51. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gadani SP, Cronk JC, Norris GT and Kipnis
J: IL-4 in the brain: A cytokine to remember. J Immunol.
189:4213–4219. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Frodermann V, van Duijn J, van Puijvelde
GH, van Santbrink PJ, Lagraauw HM, de Vries MR, Quax PH, Bot I,
Foks AC, de Jager SC and Kuiper J: Heat-killed staphylococcus
aureus reduces atherosclerosis by inducing anti-inflammatory
macrophages. J Intern Med. 279:592–605. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yang H, Zhao P and Tian S: Clopidogrel
protects endothelium by hindering TNFα-induced VCAM-1 expression
through CaMKKβ/AMPK/Nrf2 pathway. J Diabetes Res.
2016(9128050)2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Rios FJ, Koga MM, Pecenin M, Ferracini M,
Gidlund M and Jancar S: Oxidized LDL induces alternative macrophage
phenotype through activation of CD36 and PAFR. Mediators Inflamm.
2013(198193)2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Badimon L, Pena E, Arderiu G, Padró T,
Slevin M, Vilahur G and Chiva-Blanch G: C-reactive protein in
atherothrombosis and angiogenesis. Front Immunol.
9(430)2018.PubMed/NCBI View Article : Google Scholar
|