Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic,
irreversible and lethal interstitial lung disease (1). People diagnosed with this disease
survive for <3-5 years (2).
Following inflammation and oxidative injury, abnormal myofibroblast
activation is observed, accompanied by enhanced
epithelial-mesenchymal transition (EMT) and a large amount of
collagen is secreted for post-injury repair (3,4). This
leads to excessive deposition of the extracellular matrix (ECM),
collapse of alveolar structures, infiltration of inflammatory cells
in the alveolar cavity and eventual development of interstitial
fibrosis (5). Finally, the lung
structure is destroyed, lung function is lost and breathing becomes
difficult (6). It can lead to
irreversible respiratory failure and eventually mortality (7). At present, no treatment exists for
IPF. In addition, the anti-inflammatory and immunosuppressive
effects of traditional drugs are not clinically optimistic and
serious side effects always occur (8). Therefore, a drug with significant
efficacy and few side effects needs to be developed urgently.
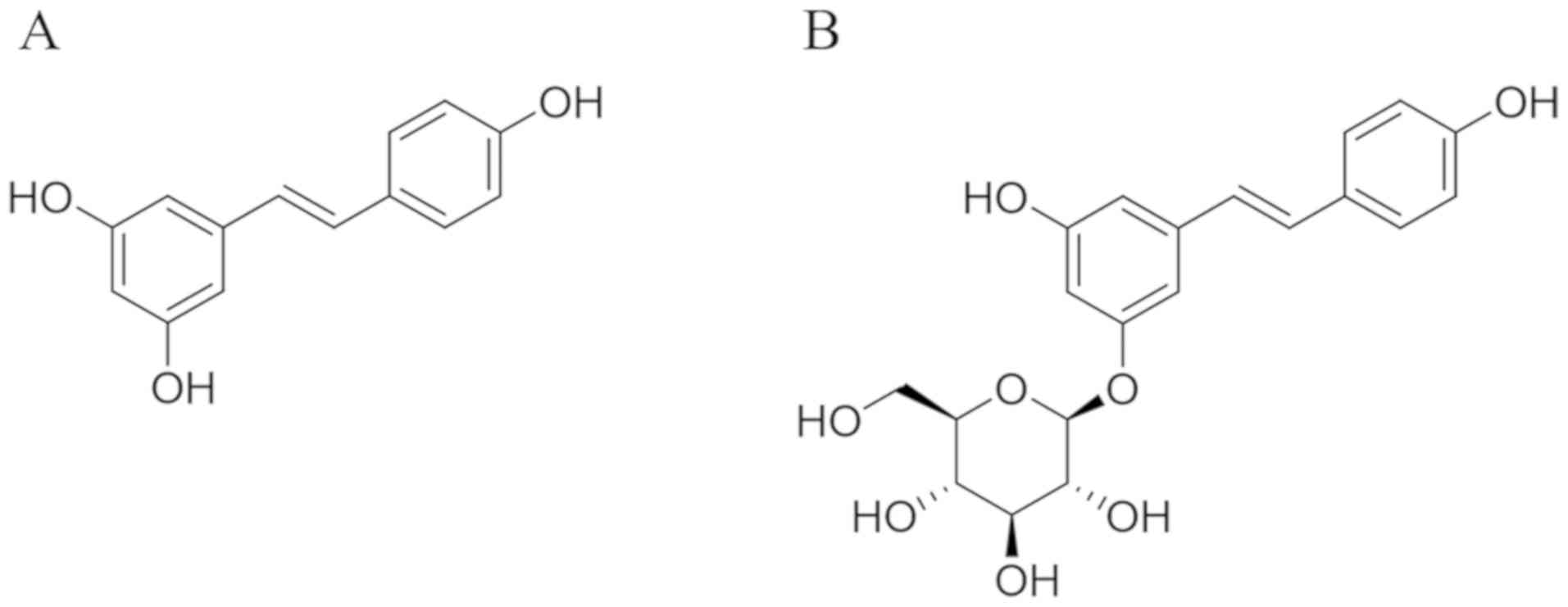
Resveratrol (3,4',5-trihydroxystilbene; Fig. 1A), a natural phytoalexin, is
abundant in a variety of plants such as grapes, peanuts and berries
and also in a number of commercial products such as grape juice and
red wine (9). It is a stilbene
compound with a strong antioxidant property owing to its polyphenol
structure (10). Previous studies
provide abundant basic data for the use of resveratrol against lung
disease (11) and fibrosis disease
(12). Further, resveratrol exerts
a protective effect against pulmonary fibrosis by inhibiting the
proliferation and differentiation of lung fibroblasts and reducing
the deposition of collagen (13).
However, it has poor water solubility due to its molecular
structure, limiting its bioavailability and pharmaceutical
applications (14).
Polydatin
(3,4',5-trihydroxystilbene-3-β-mono-D-glucoside; Fig. 1B) is a resveratrol glucoside
(15). Polydatin is usually
isolated from the roots of a Chinese medicinal herb Polygonum
cuspidatum Sieb. et Zucc. (Polygonaceae) (16). It is also abundant in several common
dietary products such as red wine, peanuts, grapes and cocoa
products, making it a promising dietary supplement combined with
other clinical antifibrotic drugs (17). Polydatin is more abundant than
resveratrol in nature and usually serves as a direct precursor of
resveratrol (18). Unlike
resveratrol, polydatin is more soluble in water owing to the
conformational changes in its structure in which the hydroxyl group
is substituted by a glucoside group on position C-3 (Fig. 1B). Polydatin has much better
bioavailability than resveratrol, benefiting from the way polydatin
enters cells via glucose carriers (19). Hence, polydatin should have a wider
range of applications and better biological properties than
resveratrol. Studies have shown that it displays strong
antioxidant, anti-inflammatory and anti-apoptotic properties.
Thanks to these properties, polydatin is frequently used for
treating health-related disorders such as cardiac disabilities
(20), various carcinomas (21,22),
hepatitis (23) and hepatic
fibrosis (24). It can also
significantly reduce lipopolysaccharide-induced lung injury
(25), protect against
PM2.5-induced respiratory system diseases (26) and alleviate reactive oxygen species
(ROS)- and bleomycin (BLM)-induced EMT and pulmonary fibrosis
(27,28).
Despite the amount of data on the role of
resveratrol against pulmonary fibrosis, the effect of resveratrol
glucoside polydatin on IPF has not been explored in any depth.
Therefore, the present study aimed to compare the efficacy of
resveratrol and its glucoside polydatin and further investigate the
possible underlying mechanism of polydatin against BLM-induced
IPF.
Materials and methods
Drugs and chemicals
Polydatin (purity >99%) and resveratrol (purity
>99%) were purchased from Guangzhou Honsea Sunshine Biotech Co.,
Ltd. Bleomycin (BLM) hydrochloride was obtained from Zhejiang Hisun
Pharmaceutical Co., Ltd. Pirfenidone was acquired from Dalian
Meilun Biotechnology Co., Ltd. Recombinant human transforming
growth factor-β1 was obtained from PeproTech Inc.; it was diluted
and stored following the manufacturer's protocol. Sodium
carboxymethylcellulose (CMC-Na) was supplied by Sigma-Aldrich
(Merck KGaA). The assay kits for measuring hydroxyproline (HYP) and
malondialdehyde (MDA) contents and total superoxide dismutase
(T-SOD) and myeloperoxidase (MPO) activities were supplied by
Nanjing Jiancheng Bioengineering Institute. Enzyme-linked
immunosorbent assay (ELISA) kits for tumor necrosis factor-α
(TNF-α), interleukin (IL)-6 and IL-13 were purchased from Shanghai
Enzyme-linked Biotechnology Co., Ltd. Reverse transcription primers
for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), collagen type
I α 1 (Col1α1), epithelial cell cadherin (E-cadherin) and α-smooth
muscle actin (α-SMA) were provided by Sangon Biotech Co., Ltd.
Antibodies against β-actin, transforming growth factor-β1 (TGF-β1),
phosphorylated Drosophila mothers against decapentaplegic
protein homolog 2/3 (p-Smad2/3), Smad2/3,
phospho-extracellular-regulated protein kinases 1/2 (p-ERK1/2) and
ERK1/2 were supplied by Affinity Biosciences. Horseradish
peroxidase (HRP) and goat anti-rabbit immunoglobulin G (H+L) were
purchased from EarthOx, LLC. All other chemicals and reagents were
at least of the analytic grade.
Cell culture
Human type II alveolar epithelial cell line A549
(American Type Culture Collection) was purchased from iCell
Bioscience Inc. It was cultured in Roswell Park Memorial
Institute-1640 (RPMI-1640) medium (Gibco; Thermo Fisher Scientific,
Inc.) containing 10% (v/v) fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and 1% (v/v) penicillin/streptomycin solution
(Gibco; Thermo Fisher Scientific, Inc.) at 37˚C, in a humidified
atmosphere of 5% CO2.
Cellular toxicity detection
The A549 cells were seeded in a 96-well plate at a
density of 4x104 cells/ml at 100 µl per well. Then, they
were treated with polydatin (0-120 µM) for 24, 48, 72 and 96 h.
Next, 20 µl of thiazolyl blue tetrazolium bromide (MTT; 5 mg/ml)
was added to each well. Following incubation for 4 h, MTT was
removed and 150 µl of dimethyl sulfoxide (DMSO) was added to each
well. The 96-well plate was shaken on a microplate reader (Thermo
Fisher Scientific, Inc.) for 10 min to dissolve the crystals
completely. The absorbance was recorded at 490 nm using a
microplate reader. The experiment was repeated three times.
Morphological observation and RNA
extraction
The A549 cells were seeded in a 6-well plate at a
density of 4x104 cells/ml at 2 ml per well. Then, they
were treated with 0, 10, 30 and 90 µM polydatin combined with
TGF-β1 (10 ng/ml) for 96 h. The cells were acquired after 96 h.
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) was added to extract the total RNA according to the
manufacturer's protocol.
Animal experiments
A total of 42, six-week-old specific-pathogen-free
male Sprague-Dawley rats (180-220 g) were provided by Guangdong
Medical Laboratory Animal Center (certificate no. SYXK2018-0085).
The rats were maintained in the animal experimental center of
Guangzhou University of Chinese medicine with five animals housed
per cage. They were placed at a constant temperature of 24˚C,
relative humidity of 65±15% and a 12-h light/dark cycle. They were
given standard food and free drinking water. Animal health and
behavior were monitored every day. All experimental protocols were
in accordance with the regulations of the Animal Protection and Use
Committee of Guangzhou University of Chinese Medicine.
BLM-induced IPF in rats
The rats were given adaptive feeding for 1 week
before starting the experiment. They were randomly divided into
seven groups: Sham control, model, pirfenidone, resveratrol and
polydatin low-, medium- and high-dose groups, with six rats in each
group. BLM-induced IPF was performed as described in a previous
study (29). The rats were
anesthetized with 50 mg/kg pentobarbital sodium by intraperitoneal
injection. Following anesthesia, the anterior neck region of the
rats was shaved and disinfected. Blunt dissection was made to
expose the trachea. Then, 5 mg/kg BLM hydrochloride was
intratracheally instilled into the rats in the six BLM-induced
groups. The sham control group received the same treatment with
sterile saline. The rats were shaken vertically for 3 min for a
uniform distribution. The subcutaneous tissue and skin were
carefully sutured.
Drug intervention
Then, 3 days following surgery (to minimize
suffering and distress of the rats), the sham control and model
groups were given 0.5% CMC-Na orally, the pirfenidone group was
given 50 mg/kg pirfenidone, the resveratrol group was given 40
mg/kg resveratrol and the polydatin low-, medium- and high-dose
groups were given 10, 40 and 160 mg/kg polydatin, respectively,
once a day for 28 days. Both polydatin and resveratrol were
dissolved in 0.5% CMC-Na solution. As described in a previous study
(30), on the 28th day following
BLM administration, the degree of IPF in rats was obvious and the
vital signs were affected. Therefore, this was chosen as the humane
endpoint. Following the last administration, the animals were
euthanized with 200 mg/kg pentobarbital sodium by intraperitoneal
injection. When the rats were anesthetized, the blood of the rats
was drained. After draining the blood and arresting the cardiac and
respiratory functions, the lung tissues were rapidly stripped for
further study.
Histopathological evaluation
The lung tissues were fixed with 4% paraformaldehyde
for 24 h at room temperature. The lung tissues were dehydrated with
an alcohol gradient (75% alcohol 4 h, 85% alcohol 2 h, 90% alcohol
2 h, 95% alcohol 1 h and anhydrous ethanol 1 h). Xylene is used to
make the tissue transparent. They were then immersed in melted
paraffin at 65˚C for 3 h, before embedding in a frame filled with
molten paraffin and allowed to solidify at -20˚C. Paraffin-embedded
tissue blocks were cut into 4-µm sections. These sections were
stained with hematoxylin and eosin (H&E) reagent, hematoxylin
(0.5%) for 5 min, followed by eosin (0.5%) for 5 min; or with
Masson's trichrome reagent, sequentially with potassium dichromate
(10%) overnight, iron-hematoxylin (1%) for 3 min, Ponceau acid (1%)
for 10 min, phosphomolybdic acid (1%) stain for 3 min and aniline
blue (1%) for 6 min; all at room temperature and observed under a
light microscope. The lung injury scores were calculated according
to the degrees of interstitial inflammation, inflammatory cell
infiltration, congestion and edema. The scores on these indicators
ranged 1-4. The final lung injury score was the sum of these scores
(31). The lung fibrosis changes
were evaluated according to the modified Ashcroft method (grades
0-8) (32). At scores 1-3, the
alveoli were partly enlarged and rarefied. Fibrotic masses appeared
from score 4. The lung structure was severely damaged with the
confluence of single fibrotic masses at score 5. Most of the lung
structure was not preserved at score 6. At score 7, the alveoli
were partially covered by fibrotic masses and at score 8, complete
occlusion occurred (33).
Reverse transcription-quantitative
(RT-q)PCR
Total RNA of the rat lung tissues was extracted
using TRIzol® reagent. The total RNA of cells
(5x105) and lung tissues was reverse transcribed to cDNA
(reaction volume, 20 µl) using the HiScript II Q RT SuperMix
(Vazyme Biotech Co., Ltd.). An Ultra-trace UV-Visible
spectrophotometer (Invitrogen; Thermo Fisher Scientific, Inc.) was
used to detect the concentration and purity of RNA. The sample
variation was amplified and quantified (reaction volume, 20 µl)
using an SYBR quantitative polymerase chain reaction Master Mix kit
(Vazyme Biotech Co., Ltd.). cDNA was subjected to a temperature of
95˚C for 30 sec; 40 cycles at 95˚C for 10 sec and 60˚C for 30 sec;
and then 95˚C for 15 sec, 60˚C for 60 sec and 95˚C for 15 sec. The
sequences of the primer are shown in Table I. GAPDH was used as an internal
reference. RNA extraction, cDNA synthesis and qPCR were performed
according to the manufacturer's protocols. The relative gene
expression of Col I, E-cadherin and α-SMA was calculated using the
following formulas:
ΔΔCq=(Cqsample-CqGAPDH)-(Cqcontrol-CqGAPDH);
fold change=2-ΔΔCq (34).
 | Table IPrimer sequences used for
quantitative PCR. |
Table I
Primer sequences used for
quantitative PCR.
| Gene name | | Primer (5'-3') |
|---|
|
Human-GAPDH | Forward |
GGCACCGTCAAGGCTGAGAAC |
| | Reverse |
GGTGGCAGTGATGGCATGGAC |
| Human-Col
1a1 | Forward |
CCTGCCGTGACCTCAAGATGTG |
| | Reverse |
CATGCTCTCGCCGAACCAGAC |
|
Human-E-cadherin | Forward |
TACAATGCCGCCATCGCTTACAC |
| | Reverse |
TGACGGTGGCTGTGGAGGTG |
|
Human-α-SMA | Forward |
TCGTGCTGGACTCTGGAGATGG |
| | Reverse |
CCGATGAAGGATGGCTGGAACAG |
|
Rat-GAPDH | Forward |
GTCCATGCCATCACTGCCACTC |
| | Reverse |
CGCCTGCTTCACCACCTTCTTG |
| Rat-Col
1a1 | Forward |
GACAGGCGAACAAGGTGACAGAG |
| | Reverse |
TGAGGTGGCTGAGGCAGGAAG |
|
Rat-E-cadherin | Forward |
GCTGCCATCGCCTACACCATC |
| | Reverse |
ACCGACCTCATTCTCAAGCACTTG |
|
Rat-α-SMA | Forward |
AGAACACGGCATCATCACCAACTG |
| | Reverse |
TGAGTCACGCCATCTCCAGAGTC |
Determination of HYP level
The content of HYP in lung tissues was measured
according to the hydroxyproline assay kit protocol (35). The data are expressed as microgram
of HYP per milligram wet lung weight (µg/mg tissue).
T-SOD, MDA and MPO assays
The activities of T-SOD and MPO and the contents of
MDA in lung tissues were determined by the hydroxylamine, hydrogen
peroxide and thiobarbituric acid methods. The levels were
determined by the colorimetric method following the manufacturer's
protocols (Nanjing Jiancheng Bioengineering Institute).
TNF-α, IL-6 and IL-13 assays
Saline was added to the lung tissues (100 mg) at a
ratio of 1:9. The tissues were then homogenized and centrifuged to
remove the supernatant. TNF-α (cat. no. ml002859), IL-6 (cat. no.
ml102828) and IL-13 (cat. no. ml003012) ELISA kits (Shanghai
Enzyme-linked Biotechnology Co., Ltd.) were used to detect the
expression of these proteins following the manufacturer's
protocols.
Western blot analysis
To collect the total proteins, the lung tissues were
homogenized using preconfigured radioimmunoprecipitation assay
(RIPA) cracking liquid [containing RIPA lysis buffer,
phenylmethanesulfonyl fluoride (PMSF), cocktail and phosphatase
inhibitors A and B at ratios of 100:1:2:1:1]. The supernatant was
collected following centrifugation at 14,000 x g for 10 min at 4˚C.
The concentrations of proteins were measured using a bicinchoninic
acid assay kit. Proteins were loaded at 50 µg per lane. The
proteins were dispersed using 8% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and then transferred
onto polyvinylidene fluoride membranes. The membranes were blocked
for 1 h at room temperature in Tris buffer solution-0.1% Tween 20
(TBST) containing 5% skimmed milk powder. Subsequently, they were
incubated with specific primary antibodies at 1:1,000 dilution
[p-ERK1/2 (cat. no. AF1015), ERK1/2 (cat. no. AF0155) and β-actin
(cat. no. AF7018)] and 1:2,000 dilution [p-Smad2/3 (cat. no.
AF3367), Smad2/3 (cat. no. AF6367) and TGF-β1 (cat. no. AF1027)]
overnight at 4˚C. The membranes were incubated for 1 h at room
temperature in TBST containing HRP and goat anti-rabbit
immunoglobulin G secondary antibody (cat. no. E03012001; EarthOx
Life Sciences). The protein bands were detected using an enhanced
chemiluminescence advanced kit (GE Healthcare). Quantity One
(v4.6.2; Bio-Rad Laboratories, Inc.) was used for densitometry.
Statistical analysis
SPSS software (version 23.0, IBM Corp.) was used for
data analysis. One-way analysis of variance and Fisher's least
significant difference test were used to analyze the significance
of different groups. Values represented as mean ± standard
deviation P<0.05 was considered to indicate a statistically
significant difference. ChemDraw Professional v16.0 was used to
draw chemical structures. GraphPad Prism software (v7; GraphPad
Software, Inc.) was used to draw the graphs.
Results
Cellular toxicity of polydatin in A549
cells
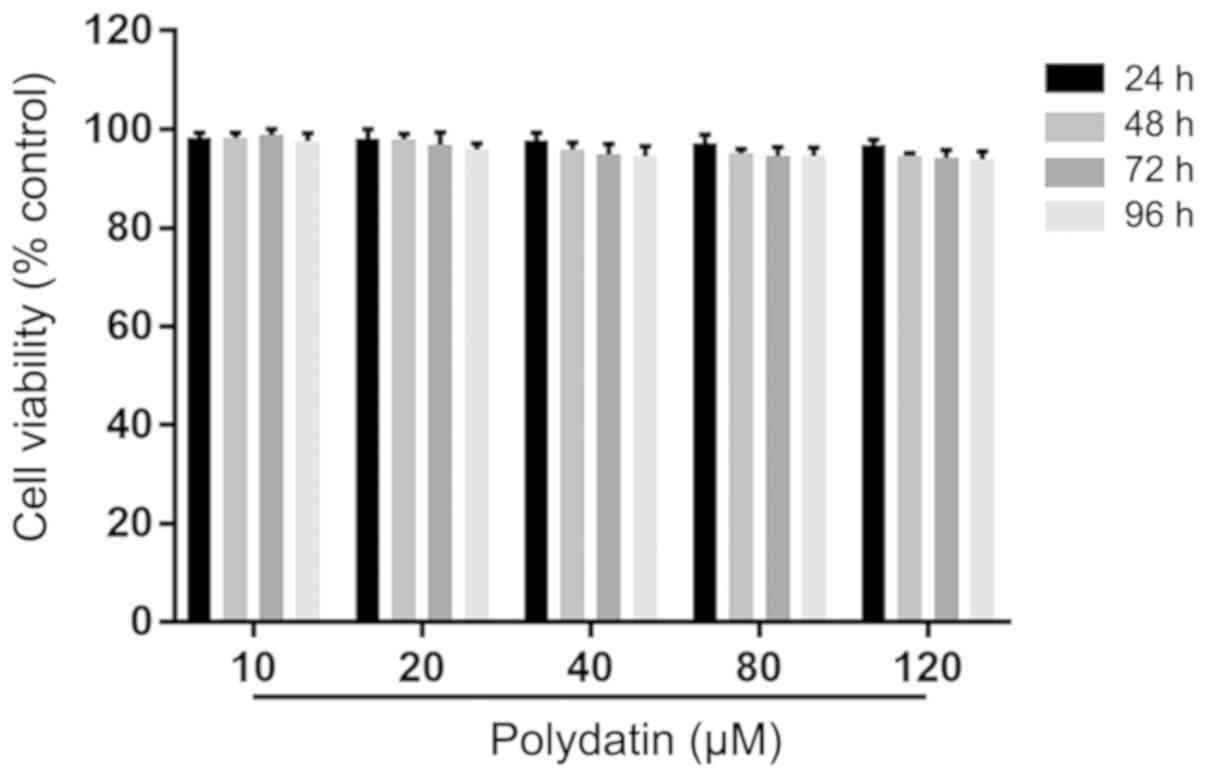
The results demonstrated that the viability of A549
cells after administering polydatin (10-120 µM) for 24, 48, 72 and
96 h was more than 90%. This suggested that polydatin had low
toxicity to A549 cells at 10-120 µM (Fig. 2).
Polydatin protects against
TGF-β1-induced phenotypic transformation of A549 cells
Based on the results of the cell toxicity
experiment, different concentrations (0, 10, 30 and 90 µM) of
polydatin combined with TGF-β1 (10 ng/ml) were subsequently
selected to investigate the effect of their independent or combined
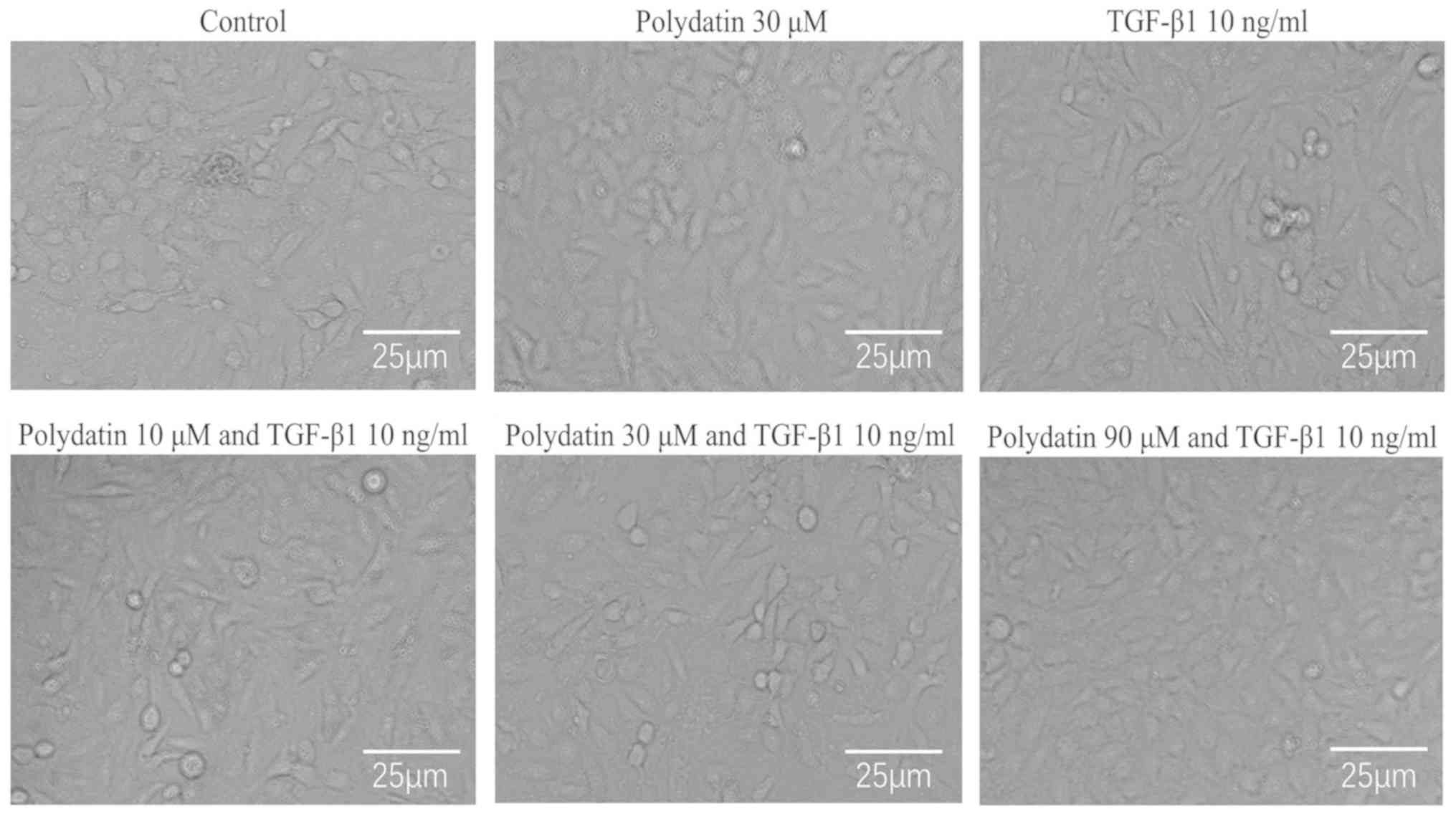
use on the phenotypic transformation of A549 cells for 96 h. The
results showed no significant difference in cell morphology between
the control and polydatin-administered groups. The epithelial cell
phenotype in these two groups was anserine nephrite type. After 96
h, significant morphological differences were observed between the
TGF-β1-treated and control groups; a number of the cells became
spindle shaped in the TGF-β1 intervention group. Little difference
in cell morphology was found between the polydatin (10 µM) combined
with TGF-β1 group and the TGF-β1 intervention group during the
experiment. However, the polydatin (30 and 90 µM) combined with
TGF-β1 group showed significant morphological differences 96 h
following the intervention; only a few cells were spindle shaped
(Fig. 3).
Effect of polydatin on EMT of A549
cells
The results of phenotypic observation showed that
the morphological differences among the groups were most obvious at
96 h following administration. Therefore, at 96 h following
culture, total RNA was extracted from the cells in the polydatin
and TGF-β1 alone or combined administration groups. The epithelial
or mesenchymal marker gene was detected to reflect the EMT of the
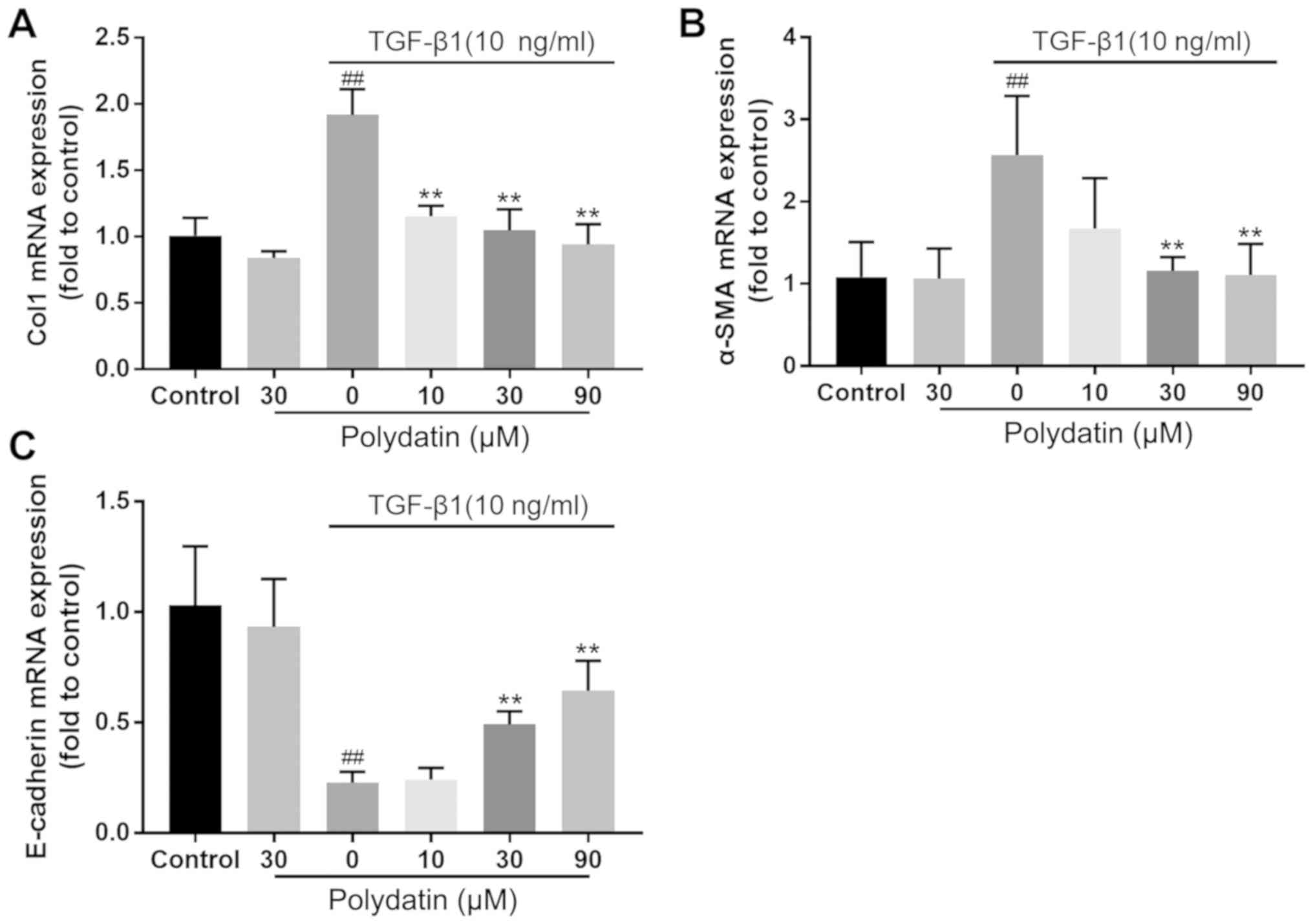
cells. The RT-qPCR results demonstrated no significant difference
in the changes in the expression levels of collagen I (Col I),
E-cadherin and α-SMA between the control and polydatin (30 µM)
treatment groups. However, the expression of E-cadherin was
significantly downregulated in the TGF-β1 (10 ng/ml) intervention
group (P<0.01) and the expression of α-SMA and Col I was
significantly increased (P<0.01), showing statistically
significant differences compared with the control group. TGF-β1 (10
ng/ml) intervention combined with polydatin (30 and 90 µM)
administration demonstrated significant differences in the
expression of Col I, E-cadherin and α-SMA compared with TGF-β1 (10
ng/ml) intervention alone (P<0.01). The administration of
polydatin inhibited the reduction in E-cadherin and the increase in
Col 1 and α-SMA induced by TGF-β1. This inhibition displayed a
dose-dependent trend (Fig. 4).
Polydatin ameliorates BLM-induced lung
pathological damages and fibrogenesis in rats
In vitro experiments confirmed that polydatin
significantly inhibited the TGF-β1-induced EMT of alveolar
epithelial cells, which plays a crucial role in the pathogenesis of
fibrosis. Therefore, a model of bleomycin-induced pulmonary
fibrosis in vivo was established to verify whether polydatin
had a protective effect on bleomycin-induced pulmonary fibrosis in
rats. H&E staining revealed the most intuitive response to
histopathological changes. The fibers in the tissue were stained
blue by Masson trichome stain, directly reflecting the degree of
organ fibrosis.
H&E staining showed that the model group had
severe pulmonary structure collapse, alveolar structure
consolidation, marked edema and congestion and inflammatory cell
infiltration compared with the sham control group. Fortunately,
pirfenidone (PFD), resveratrol (Res) and polydatin interventions
reduced the pathological injury of lung tissue. They suppressed the
alveolar wall damage and reduced inflammatory cell infiltration,
congestion and edema (P<0.01). The effect of polydatin was
dose-dependent. At the same dose, polydatin and resveratrol
produced similar effects. However, higher doses of polydatin
provided a stronger protective effect. All of these results are
shown in Fig. 5A and B. Masson trichrome staining results showed
that the lungs of rats treated with BLM had alveolar septal
thickening and collapse, massive collagen deposition and fibrous
hyperplasia compared with the sham control group (P<0.05).
However, collagen deposition in the lungs was significantly reduced
by pirfenidone (PFD), resveratrol (Res) and polydatin (P<0.01).
Polydatin exerted the effect in a dose-dependent manner
(P<0.05). The results are displayed in Fig. 5C and D.
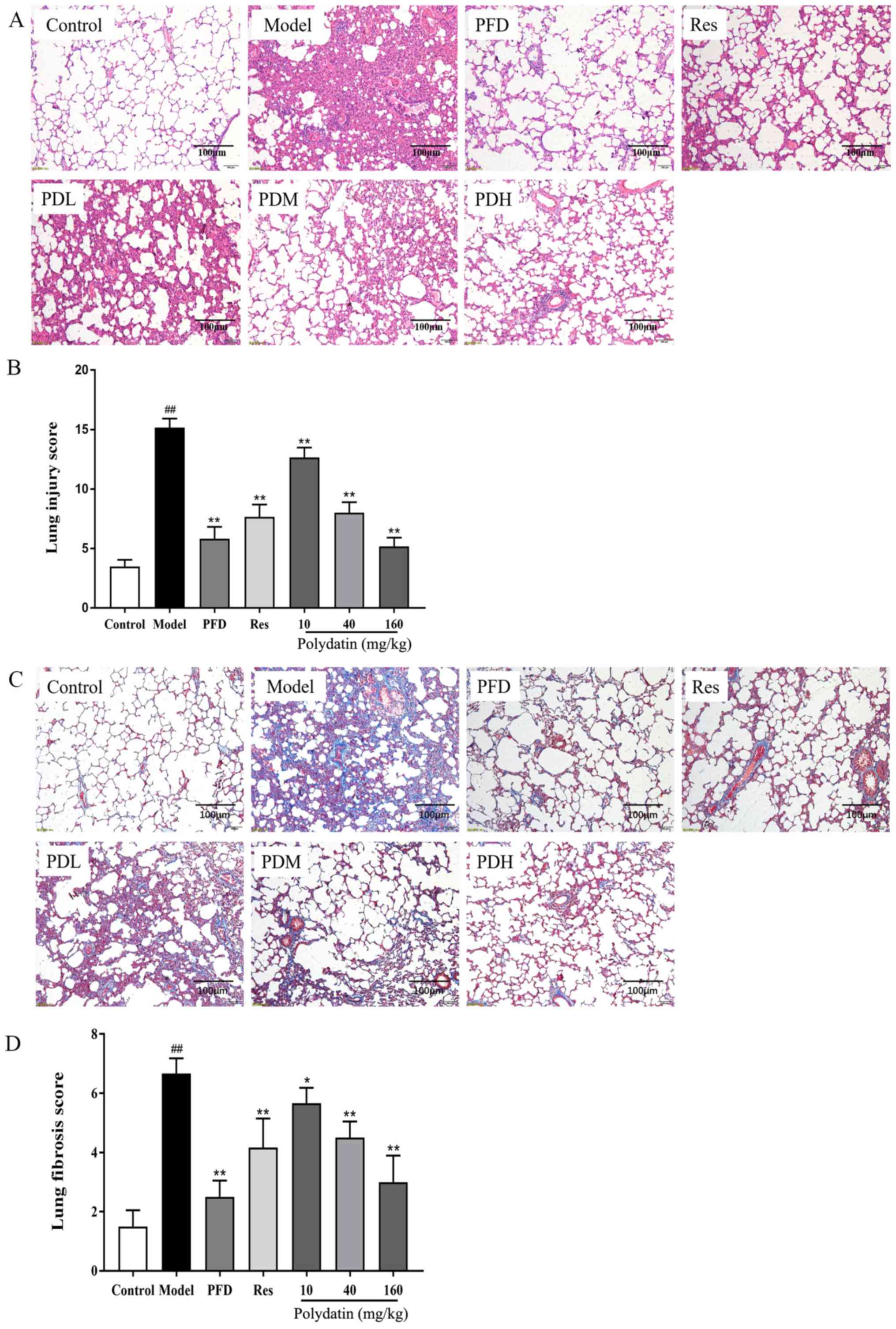 | Figure 5Effects of PFD, Res and polydatin on
BLM-induced pathological damage and fibrogenesis. (A) H&E stain
(scale bars, 100 µm). (B) Lung injury score. (C) Masson trichrome
stain (scale bars, 100 µm). (D) Lung fibrosis score. Values are
represented as mean ± standard deviation (n=6).
##P<0.01, the model group vs. the control group.
**P<0.01, *P<0.05, vs. the model group.
PFD, pirfenidone; Res, resveratrol; BLM, bleomycin; PDL, PDM and
PDH, polydatin low-, medium- and high-dose groups respectively. |
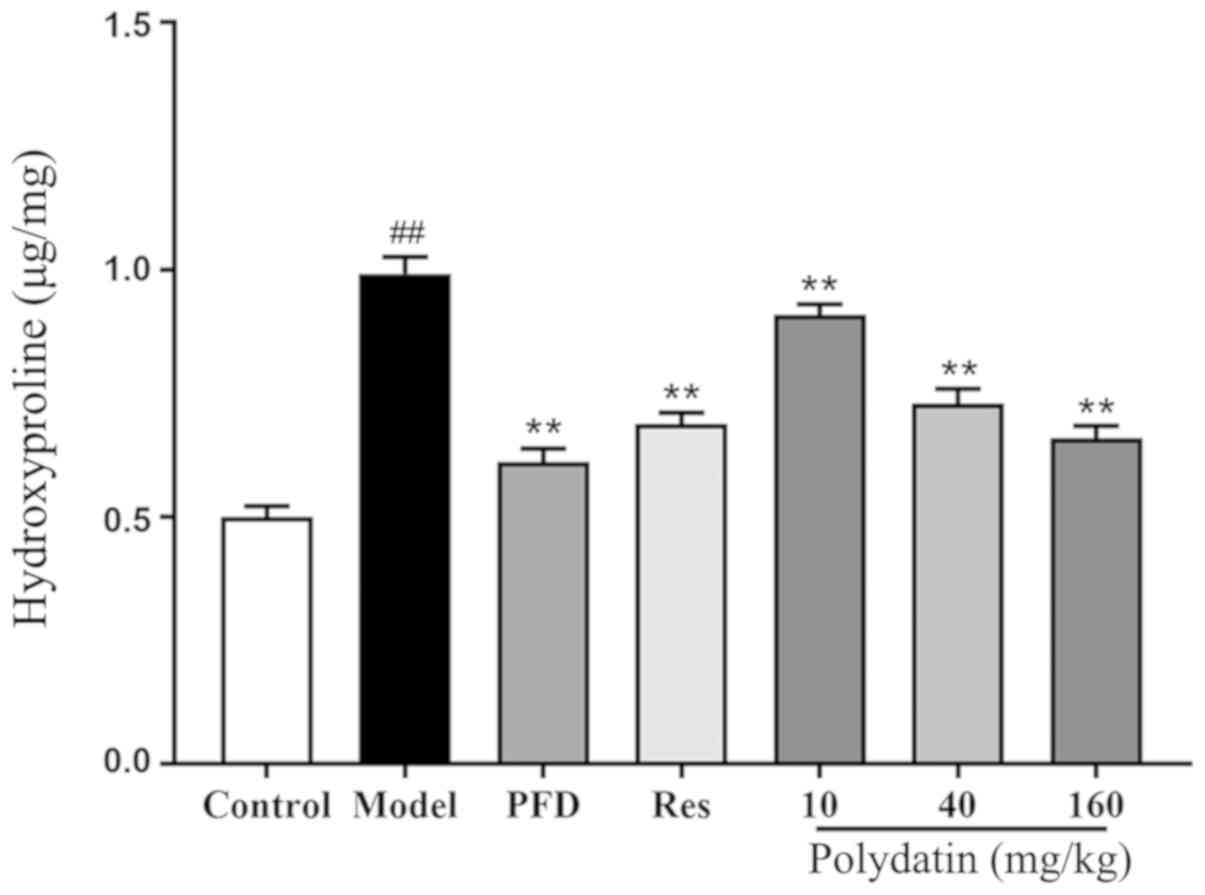
Level of HYP
The results demonstrated that the content of HYP, a
collagen deposition marker, significantly increased in the model
group compared with the sham control group (P<0.01). However,
pirfenidone (PFD) and resveratrol (Res) downregulated the
expression of HYP (P<0.01). Polydatin also significantly blocked
this induction in a dose-dependent manner (P<0.01; Fig. 6).
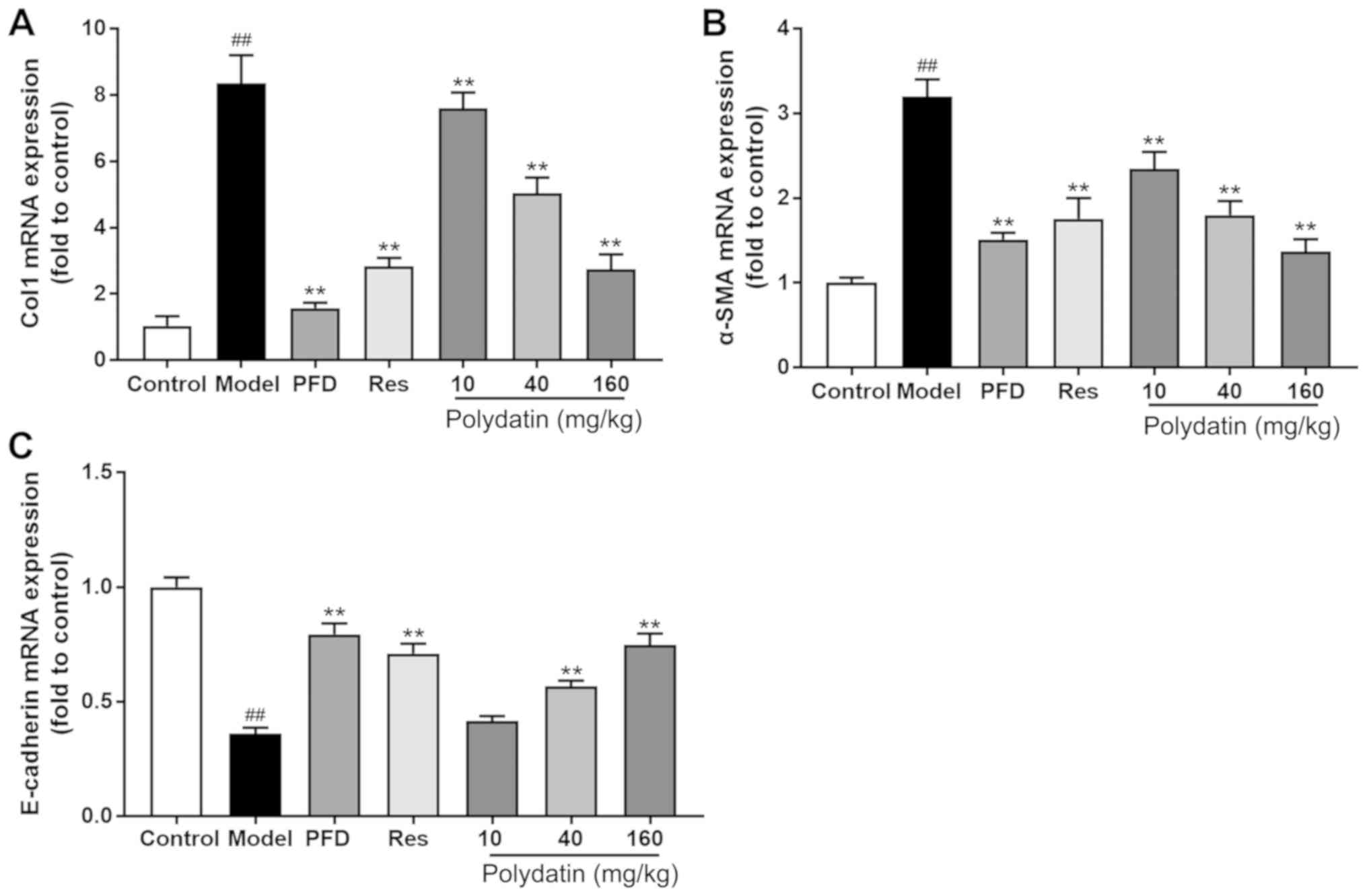
EMT and fibrosis markers are altered
by polydatin during BLM-induced IPF
Fig. 7 shows that
BLM significantly stimulated the gene expression of interstitial
markers α-SMA and Col I and decreased the gene expression level of
epithelial marker E-cadherin compared with rats having no BLM
administration (P<0.01). However, pirfenidone (PFD), high- and
medium-dose polydatin and resveratrol (Res) treatment significantly
ameliorated the magnitude of all these changes at the mRNA level
(P<0.01).
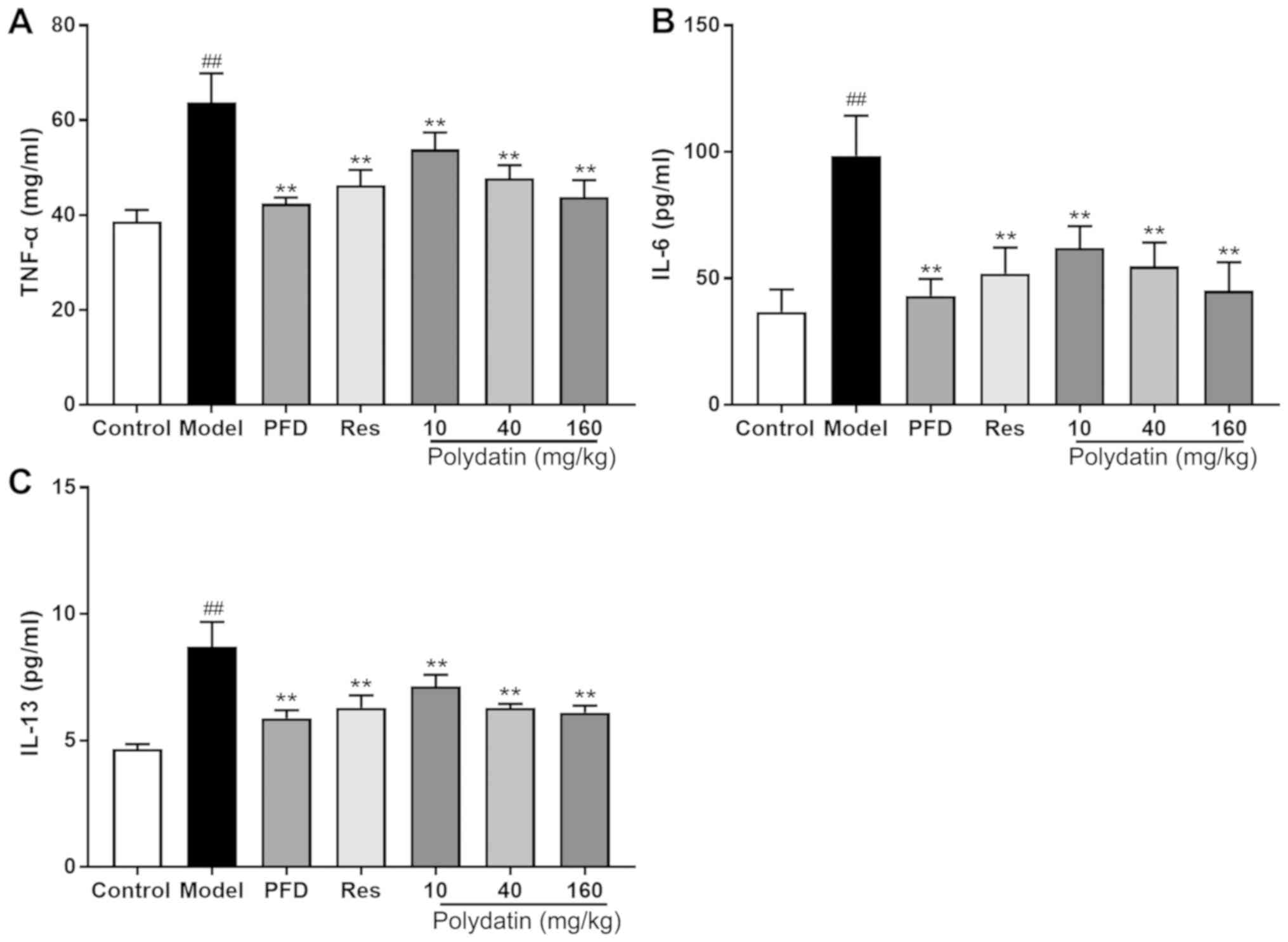
Polydatin protects against BLM-induced
inflammatory injury in rats
BLM administration significantly elevated the levels
of pro-inflammatory factors TNF-α, IL-6 and IL-13 in the lung
homogenate compared with the sham control group. However,
pirfenidone (PFD), resveratrol (Res) and polydatin (40 and 160
mg/kg) significantly decreased the elevated TNF-α, IL-6 and IL-13
levels (P<0.01) compared with the model group. Polydatin (10
mg/kg) also mitigated the lesions, but its effect was less compared
with the other two doses of polydatin (P<0.01; Fig. 8).
Polydatin protects against BLM-induced
oxidative damage in rats
Fig. 9 shows that
BLM administration in rats caused increased oxidative stress as
manifested by a significant decrease in T-SOD, and a significant
increase in MDA levels and MPO activities in the lung tissues
compared with the sham control group (P<0.01). Nevertheless,
pirfenidone (PFD), resveratrol (Res) and polydatin (40 and 160
mg/kg) significantly overcame the BLM-mediated decrease in T-SOD
and inhibited the BLM-mediated increase in MDA levels and MPO
activities in the lung tissue (P<0.01) compared with the model
group. Polydatin (10 mg/kg) also exerted the effect to a certain
extent (P<0.05).
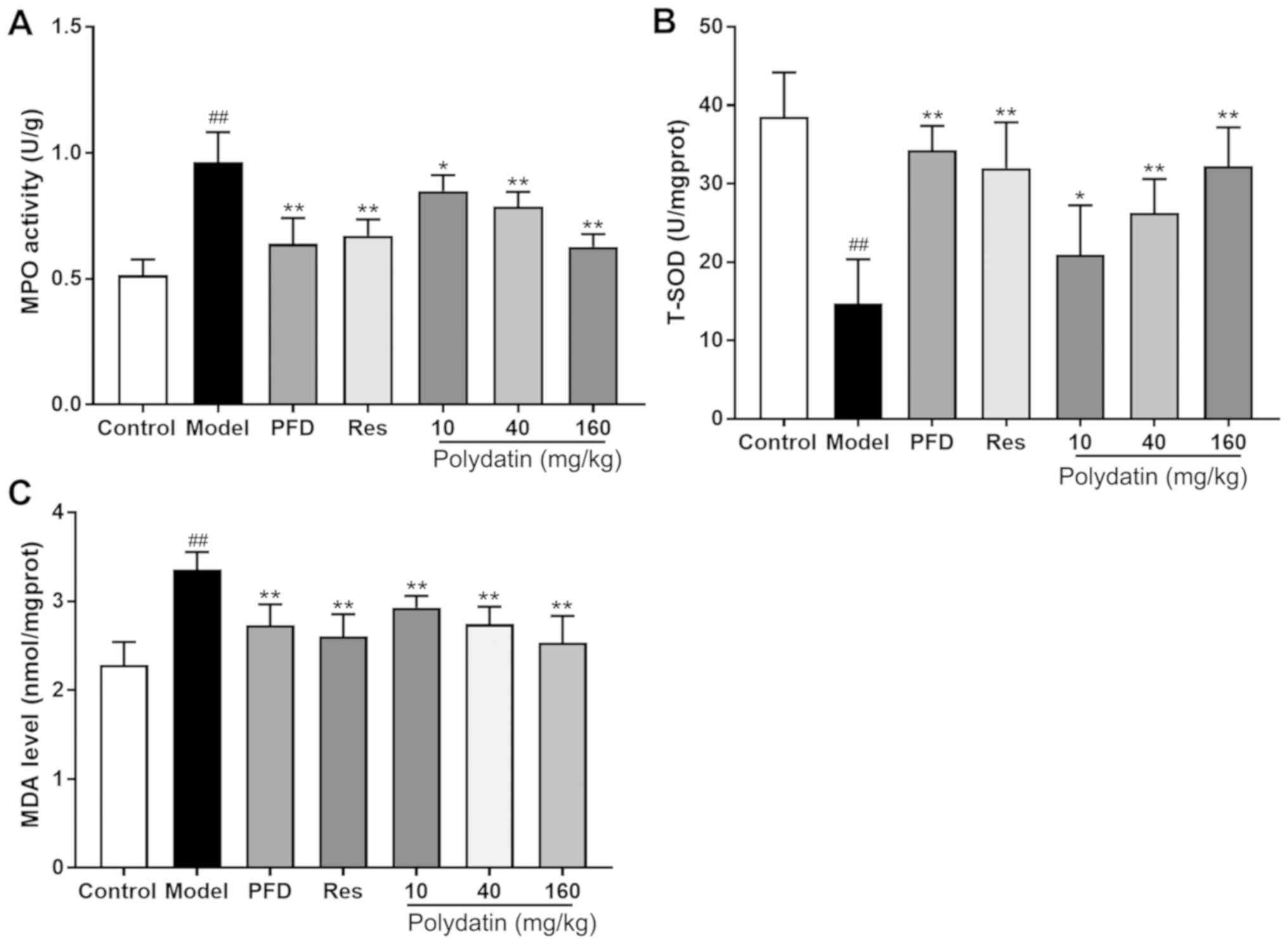 | Figure 9Effects of PFD, Res and polydatin on
oxidative damage: (A) T-SOD, (B) MDA levels and (C) MPO activities.
Values are represented as mean ± standard deviation (n=6).
##P<0.01, the model group vs. the control group.
**P<0.01, *P<0.05, vs. the model group.
PFD, pirfenidone; Res, resveratrol; T-, total; SOD, superoxide
dismutase; MDA, malondialdehyde; MPO, myeloperoxidase. |
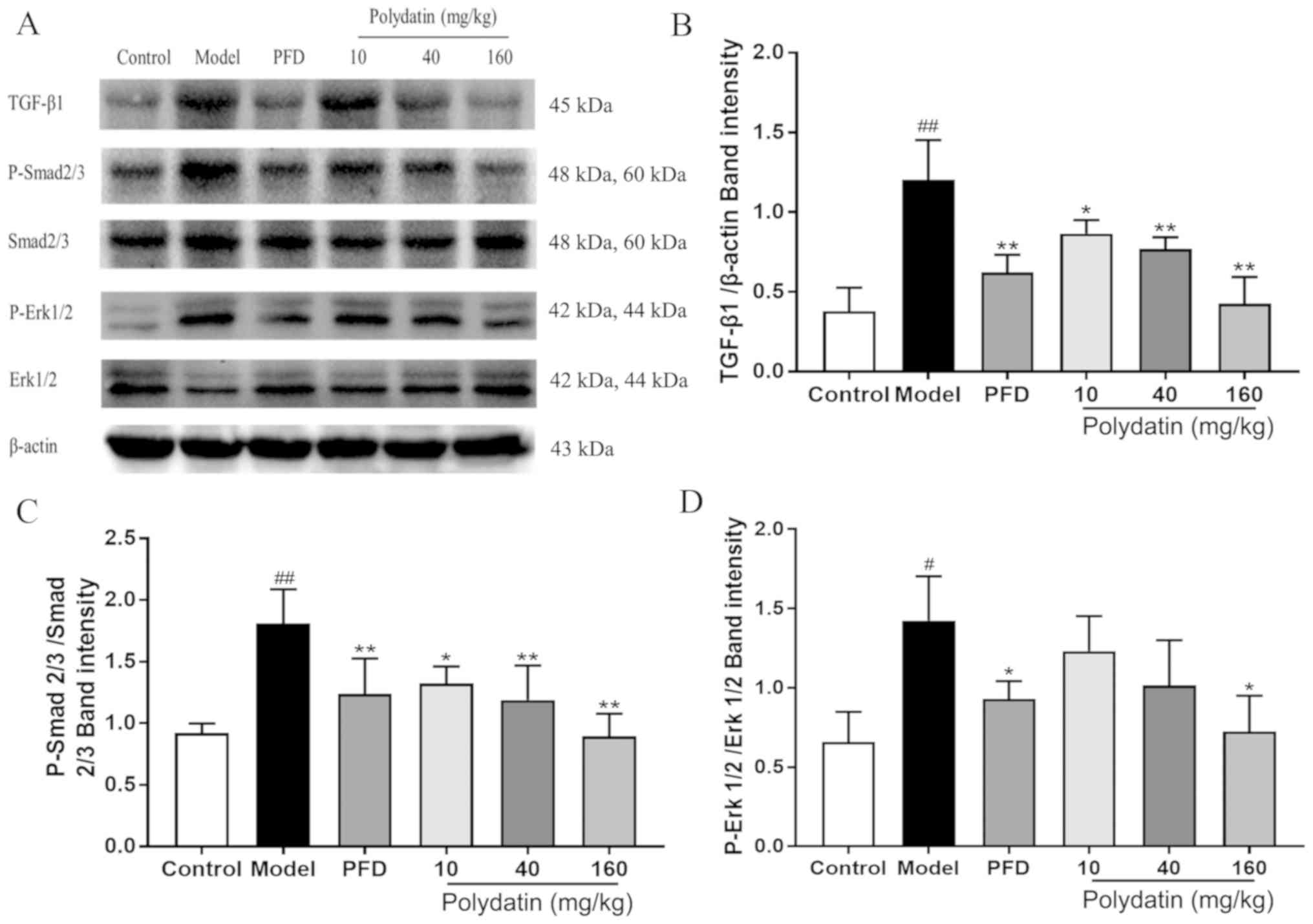
Polydatin downregulates the
TGF-β/Smad/ERK signaling pathway during pulmonary fibrosis
Fig. 10 shows that
following BLM injection, the expression level of TGF-β1 and the
phosphorylation of its downstream signals Smad2/3 and ERK1/2 were
significantly increased in rats. Promisingly, polydatin was able to
inhibit the elevation of the expression level of TGF-β1 and the
phosphorylation of Smad2/3 and ERK1/2 in a dose-dependent manner
(P<0.05).
Discussion
Idiopathic pulmonary fibrosis (IPF) is a chronic,
progressive, irreversible clinical disease with survival of 3-5
years following diagnosis (36). At
present, drugs for treating IPF are limited and no other means
exist to cure the disease (37).
IPF is characterized by the collapse and merging of alveolar
structures, significant inflammatory cell infiltration, excessive
collagen deposition and hyperplasia of fibers (38). The bleomycin (BLM)-induced pulmonary
fibrosis model simulates the process and pathological
characteristics of human pulmonary fibrosis on a larger scale
(39). BLM dripping into the lung
through the trachea directly causes severe lung injury (40). In the present study, massive
alveolar septal thickening, alveolar wall collapse, inflammatory
cell infiltration and fibrous hyperplasia occurred in the lungs of
rats in the model group, confirming the devastating effect of BLM
on the lungs. However, the treatment with polydatin effectively
prevented lung tissue damage and the progression of pulmonary
fibrosis. Its protective effect was reflected in less lung
structure collapse, inflammatory cell infiltration, congestion and
edema and prevention of collagen deposition and fiber formation in
the treatment group. At the same time, polydatin significantly
reduced the content of hydroxyproline (HYP) in vivo and
collagen I (Col I) in vitro and in vivo, indirectly
or directly reflecting its inhibitory effect on collagen synthesis.
In addition, it also increased the expression level of epithelial
marker E-cadherin and decreased the expression level of
interstitial marker α-smooth muscle actin (α-SMA) in vitro
and in vivo, reflecting its inhibitory effect on the
epithelial-mesenchymal transition (EMT) process. The effect of
polydatin was similar to that of pirfenidone and its aglycone
resveratrol. In addition, higher doses of polydatin were more
effective.
The formation of fibrosis is caused by excessive
repair following injury induced by various harmful factors. Of
these, oxidative damage and inflammatory damage are two extremely
important sources. BLM stimulation can simulate the oxidative and
inflammatory damage to human lung tissue (41,42).
BLM injection directly and markedly damages alveolar epithelial
cells (43). Inflammatory cells,
such as neutrophils and macrophages, heavily infiltrate into the
alveolar space (44), promoting the
expression of TNF-α in alveolar epithelial cells and the expression
of IL-1β, IL-13 and other pro-inflammatory factors (45). This causes a series of inflammatory
responses and severe inflammatory injuries (46). At the same time, fibroblasts are
activated in large numbers, secreting collagen proteins that can
repair damage, and accumulate in the ECM, eventually leading to the
formation of fibrotic lesions (47). In addition, the extent of
inflammatory injury beyond repair can directly induce the apoptosis
of alveolar epithelial cells (48).
Therefore, inflammatory stress plays an important role in the
development of fibrosis. However, neutrophil infiltration and
activation release large amounts of myeloperoxidase (MPO) (49). Excessive MPO catalyzes the oxidation
of protein tyrosine to produce oxidants such as 3-nitrotyrosine and
3-chlorotyrosine (50). When the
oxidant production exceeds the local antioxidant defense reaction,
it leads to oxidative stress and tissue oxidative damage (51). Meanwhile, in vitro studies
confirmed that MPO oxidation products can trigger fibrocyte
proliferation and promote fibrosis (52). In addition, BLM can directly attack
DNA. BLM combines with iron to form an activated complex that
promotes the production of oxidants such as reactive oxygen species
(ROS) (53). ROS can
indiscriminately attack DNA, proteins and lipids, causing severe
oxidative damage (54). ROS can be
oxidized by various fatty acids on the cell membrane to produce
peroxide products such as MDA, resulting in decreased membrane
stability and integrity, loss of function, cell damage and
apoptosis (55).
Previous studies have demonstrated that polydatin
possesses a strong anti-inflammatory activity. It resists
LPS-induced pneumonia (19). The
structure of polyphenol makes it possible for polydatin and
resveratrol to have a strong antioxidant activity (56). The results of the present study
demonstrated that BLM significantly increased the levels of TNF-α,
IL-6 and IL-13 in rat lungs, suggesting that BLM induced a severe
inflammatory response. The increase in the MPO level was another
indication of neutrophil infiltration. In addition, the levels of
MDA and SOD reflected the increase in oxidative stress and the
decrease in the ability to scavenge oxygen free radicals. Following
treatment with polydatin, the levels of pro-inflammatory factors
TNF-α, IL-6 and IL-13, MPO and MDA were significantly decreased and
the activity of SOD was increased. It was hypothesized that
polydatin has certain anti-inflammatory and antioxidant activities
and the mechanism may be related to inhibition of the secretion of
inflammatory oxidative factors, enhancing the scavenging of oxygen
free radicals and preventing the lipid peroxidation process. In
contrast, the anti-inflammatory and antioxidant effects of
polydatin were slightly lower compared with pirfenidone, a commonly
used drug in clinical practice. However, its effect on reducing the
levels of inflammatory factors was higher and its antioxidant
effect was close to that of resveratrol at the same dose.
TGF-β1 has been found to regulate a wide array of
cellular processes, including cell growth, differentiation,
migration, apoptosis and ECM production (57,58).
Particularly, it is considered a key mediator of fibrosis (59). The significant increase in TGF-β1
level in the lung tissue of rats with pulmonary fibrosis strongly
demonstrated that TGF-β1 was closely related to pulmonary fibrosis
(60). TGF-β could inhibit the
growth of epithelial cells, re-program epithelial cells into
mesenchymal cells and stimulate the production of ECM via
regulating downstream regulators in pulmonary fibrosis (61,62).
In vitro experiments demonstrated that 10 ng/ml TGF-β1 could
significantly induce the transformation of type II alveolar
epithelial cell A549 phenotype from cobblestone appearance into
spindle-like appearance. This phenotypic transformation was
accompanied by an increased expression level of Col I and
mesenchymal marker α-SMA and decreased expression level of
epithelial marker E-cadherin. It was proposed that TGF-β1 could
induce the EMT process of alveolar epithelial cells and promote
collagen synthesis and deposition. Following the intervention of
polydatin, alveolar epithelial cells retained their epithelioid
phenotype and the decrease in the expression level of E-cadherin
gene, an epithelial marker and the increase in the expression level
of Col I and interstitial marker α-SMA were inhibited. This
suggested that the protective effect of polydatin against pulmonary
fibrosis might be related to the regulation via the TGF-β1
signaling pathway.
ROS produced by oxidative stress and
pro-inflammatory factors, such as TNF-α, promotes the synthesis of
TGF-β1 and activates the TGF-β1 signaling pathway (63,64).
The downstream regulation of TGF-β1 is divided into Smad-dependent
and Smad-independent signaling pathways (65,66).
Following induction by TGF-β1, the downstream transduction molecule
Smad2/3 is phosphorylated and activated to form a trimer with Smad4
and conduct the signal from the cell membrane to the nucleus
(65). It can activate the
production of ECM in the nucleus (67). Previous studies indicated that
Smad3-deficient mice show significant inhibition of BLM-induced
pulmonary fibrosis (68,69). In contrast, other studies found that
the blocking of Smad3 did not entirely weaken the TGF-β1 effect and
still played a significant regulatory role in fibrosis (70,71).
These findings indicate the existence of other downstream receptors
of TGF-β1 to regulate the process of fibrosis. This downstream
signaling pathway is called the Smad-independent signaling pathway
(72). The ERK/MAPK pathway is a
Smad-independent signaling pathway. Previous studies have
demonstrated that ERK1/2 can be activated by TGF-β1 in epithelial
cells and fibroblasts (73,74) and can stimulate EMT process and ECM
production (75,76). In addition, the activation of ERK is
necessary for TGF-β1-induced fibroblast replication (77). Notably, a complex cross-talk exists
between TGF-β/Smad and TGF-β/ERK pathways. ERK1/2 can phosphorylate
the linker region of nuclear-localized Smad, increase the half-life
of p-Smad 2/3 and increase the duration of Smad target gene
transcription (78,79). In the present study, BLM upregulated
the expression of TGF-β1 and increased the phosphorylation levels
of Smad2/3 and ERK1/2, which was consistent with previous findings
(80). The results indicated that
BLM-induced IPF might correspond to the cross-talk between
TGF-β/Smad and TGF-β/ERK pathways. Pirfenidone could effectively
slow down the progression of IPF in clinical treatment and the
mechanism might be related to the regulation of TGF-β/Smad and
TGF-β/ERK pathways (81,82). Notably, following the administration
of polydatin for 28 days, the rats exhibited a low TGF-β1 level and
a significant reduction in Smad2/3 and ERK1/2 phosphorylation,
indicating that polydatin could suppress TGF-β/Smad and TGF-β/ERK
pathways effectively. In addition, the polydatin high-dose group
demonstrated a better inhibition of TGF-β1 expression and
phosphorylation of Smad2/3 and ERK1/2 compared with pirfenidone.
These pharmacological activities rendered polydatin a broader
clinical application value and might account for the protective
effect of polydatin against IPF.
The present study demonstrated that polydatin
protected against BLM-induced pulmonary fibrosis. The efficacy of
polydatin was close to that of resveratrol. The antifibrotic effect
of polydatin might be due to the relief from oxidative and
inflammatory stress and inhibition of EMT and collagen deposition
regulated by Smad-dependent and Smad-independent TGF-β signals.
These findings provided new insights into the bioactivity of
polydatin. They indicated that polydatin might have therapeutic
potential for treating IPF and could also be a promising dietary
supplement combined with other clinical antifibrotic drugs.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Pear River
Nova Program of Guangzhou (grant no. 201710010075), the Elite Youth
Education Program of Guangzhou University of Chinese Medicine and
the National Key Research and Development Plan Project ‘Special
Research Project on Modernization of Traditional Chinese Medicine’
(grant no. 2017YFC1703701).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZC, LW and YCL conceived and designed the
experiments. YLL, BC, JN and GZ performed the experiments. YLL, BC,
JZ and JY analyzed or interpreted the data for the study. YLL and
BC drafted the work. ZC, LW and YCL revised the work critically.
YLL and BC contributed equally to this work. ZC and LW contributed
equally to this work as corresponding authors. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Animal Protection and
Use Committee of Guangzhou University of Chinese Medicine
(Guangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Luppi F, Spagnolo P, Cerri S and Richeldi
L: The big clinical trials in idiopathic pulmonary fibrosis. Curr
Opin Pulm Med. 18:428–432. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang X, Ouyang Z, You Q, He S, Meng Q, Hu
C, Wu X, Shen Y, Sun Y, Wu X and Xu Q: Obaculactone protects
against bleomycin-induced pulmonary fibrosis in mice. Toxicol Appl
Pharmacol. 303:21–29. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gouda MM and Bhandary YP: Curcumin
down-regulates IL-17A mediated p53-fibrinolytic system in bleomycin
induced acute lung injury in vivo. J Cell Biochem. 119:7285–7299.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
You XY, Xue Q, Fang Y, Liu Q, Zhang CF,
Zhao C, Zhang M and Xu XH: Preventive effects of ecliptae herba
extract and its component, ecliptasaponin a, on bleomycin-induced
pulmonary fibrosis in mice. J Ethnopharmacol. 175:172–180.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pardo A and Selman M: Idiopathic pulmonary
fibrosis: New insights in its pathogenesis. Int J Biochem Cell
Biol. 34:1534–1538. 2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Manali ED, Stathopoulos GT, Kollintza A,
Kalomenidis I, Emili JM, Sotiropoulou C, Daniil Z, Roussos C and
Papiris SA: The medical research council chronic dyspnea score
predicts the survival of patients with idiopathic pulmonary
fibrosis. Respir Med. 102:586–592. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Krein PM and Winston BW: Roles for
insulin-like growth factor I and transforming growth factor-beta in
fibrotic lung disease. Chest. 122 (6 Suppl):289S–293S.
2002.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Handa T and Azuma A: Pharmacotherapy of
IPF using antifibrotic compounds. In: Idiopathic Pulmonary
Fibrosis. Nakamura H and Aoshiba K (eds). Springer, Japan,
pp147-159, 2016.
|
|
9
|
Truong VL, Jun M and Jeong WS: Role of
resveratrol in regulation of cellular defense systems against
oxidative stress. Biofactors. 44:36–49. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Brasnyó P, Molnár GA, Mohás M, Markó L,
Laczy B, Cseh J, Mikolás E, Szijártó IA, Mérei A, Halmai R, et al:
Resveratrol improves insulin sensitivity, reduces oxidative stress
and activates the Akt pathway in type 2 diabetic patients. Br J
Nutr. 106:383–389. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Vargas JE, Souto AA, Pitrez PMC, Stein RT
and Porto BN: Modulatory potential of resveratrol during lung
inflammatory disease. Med Hypotheses. 96:61–65. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Haobo L, Guangfeng Z and Xiao Z: OP0216
resveratrol ameliorates pulmonary fibrosis and inhibits human lung
fibroblasts activation via modulating SIRT1 and GLI1 signaling. Ann
Rheumatic Diseases. 74:152–153. 2015.
|
|
13
|
Chávez E, Reyes-Gordillo K, Segovia J,
Shibayama M, Tsutsumi V, Vergara P, Moreno MG and Muriel P:
Resveratrol prevents fibrosis, NF-kappaB activation and TGF-beta
increases induced by chronic CCl4 treatment in rats. J Appl
Toxicol. 28:35–43. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Di Benedetto A, Posa F, De Maria S,
Ravagnan G, Ballini A, Porro C, Trotta T, Grano M, Muzio LL and
Mori G: Polydatin, natural precursor of resveratrol, promotes
osteogenic differentiation of mesenchymal stem cells. Int J Med
Sci. 15:944–952. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhang LP, Yang CY, Wang YP, Cui F and
Zhang Y: Protective effect of polydatin against
ischemia/reperfusion injury in rat heart. Sheng Li Xue Bao.
60:161–168. 2008.PubMed/NCBI
|
|
16
|
Koneru M, Sahu BD, Gudem S, Kuncha M,
Ravuri HG, Kumar JM, Kilari EK and Sistla R: Polydatin alleviates
alcohol-induced acute liver injury in mice: Relevance of matrix
metalloproteinases (MMPs) and hepatic antioxidants. Phytomedicine.
27:23–32. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cremon C, Stanghellini V, Barbaro MR,
Cogliandro RF, Bellacosa L, Santos J, Vicario M, Pigrau M, Alonso
Cotoner C, Lobo B, et al: Randomised clinical trial: The analgesic
properties of dietary supplementation with palmitoylethanolamide
and polydatin in irritable bowel syndrome. Aliment Pharmacol Ther.
45:909–922. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Martano M, Stiuso P, Facchiano A, De Maria
S, Vanacore D, Restucci B, Rubini C, Caraglia M, Ravagnan G and Lo
Muzio L: Aryl hydrocarbon receptor, a tumor grade-associated marker
of oral cancer, is directly downregulated by polydatin: A pilot
study. Oncol Rep. 40:1435–1442. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jiang Q, Yi M, Guo Q, Wang C, Wang H, Meng
S, Liu C, Fu Y, Ji H and Chen T: Protective effects of polydatin on
lipopolysaccharide-induced acute lung injury through
TLR4-MyD88-NF-κB pathway. Int Immunopharmacol. 29:370–376.
2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liu W, Chen P, Deng J, Lv J and Liu J:
Resveratrol and polydatin as modulators of Ca2+
mobilization in the cardiovascular system. Ann N Y Acad Sci.
1403:82–91. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang YS, Zhuang ZX, Jiao Y, Xu JY, Fan SJ
and Qin SB: Polydatin inhibits metastasis of human breast cancer
and underlying mechanisms. China J Cancer Prev Treat. 21:1788–1793.
2014.
|
|
22
|
Pan JH, Wang HB, Du XF, Liu JY and Zhang
DJ: Polydatin induces human cervical cancer cell apoptosis via
PI3K/AKT/mTOR signaling pathway. Zhongguo Zhong Yao Za Zhi.
42:2345–2349. 2017.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
23
|
Mo JF, Wu JY, Zheng L, Yu YW, Zhang TX,
Guo L and Bao Y: Therapeutic efficacy of polydatin for nonalcoholic
fatty liver disease via regulating inflammatory response in obese
mice. RSC Adv. 8:31194–31200. 2018.
|
|
24
|
Li R, Li J, Huang Y, Li H, Yan S, Lin J,
Chen Y, Wu L, Liu B, Wang G and Lan T: Polydatin attenuates
diet-induced nonalcoholic steatohepatitis and fibrosis in mice. Int
J Biol Sci. 14:1411–1425. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shiyu S, Zhiyu L, Mao Y, Lin B, Lijia W,
Tianbao Z, Jie C and Tingyu L: Polydatin up-regulates clara cell
secretory protein to suppress phospholipase A2 of lung induced by
LPS in vivo and in vitro. BMC Cell Biol. 12(31)2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yan XD, Wang QM, Tie C, Jin HT, Han YX,
Zhang JL, Yu XM, Hou Q, Zhang PP, Wang AP, et al: Polydatin
protects the respiratory system from PM2.5 exposure. Sci
Rep. 7(40030)2017.
|
|
27
|
Cao K, Lei X, Liu H, Zhao H, Guo J, Chen
Y, Xu Y, Cheng Y, Liu C, Cui J, et al: Polydatin alleviated
radiation-induced lung injury through activation of Sirt3 and
inhibition of epithelial-mesenchymal transition. J Cell Mol Med.
21:3264–3276. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Qiu Y, Pan X and Hu Y: Polydatin
ameliorates pulmonary fibrosis by suppressing inflammation and the
epithelial mesenchymal transition via inhibiting the TGF-β/Smad
signaling pathway. RSC Adv. 9:8104–8112. 2019.
|
|
29
|
Gong LK, Li XH, Wang H, Zhang L, Cai Y, Qi
XM, Liu LL, Liu YZ, Wu XF, Chen FP, et al: Feitai attenuates
bleomycin-induced pulmonary fibrosis in rats. Biol Pharm Bull.
27:634–640. 2004.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhou C, Han W, Zhang P, Cai M, Wei D and
Zhang C: Lycopene from tomatoes partially alleviates the
bleomycin-induced experimental pulmonary fibrosis in rats. Nutr
Res. 28:122–130. 2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Szapiel SV, Elson NA, Fulmer JD,
Hunninghake GW and Crystal RG: Bleomycin-induced interstitial
pulmonary disease in the nude, athymic mouse. Am Rev Respir Dis.
120:893–899. 1979.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hübner RH, Gitter W, El Mokhtari NE,
Mathiak M, Both M, Bolte H, Freitag-Wolf S and Bewig B:
Standardized quantification of pulmonary fibrosis in histological
samples. Biotechniques. 44:507–511, 514-517. 2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Robbe A, Tassin A, Carpentier J, Declèves
AE, Mekinda Ngono ZL, Nonclercq D and Legrand A: Intratracheal
bleomycin aerosolization: The best route of administration for a
scalable and homogeneous pulmonary fibrosis rat model? BioMed Res
Int. 2015(198418)2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhang F, Zhang Z, Chen L, Kong D, Zhang X,
Lu C, Lu Y and Zheng S: Curcumin attenuates angiogenesis in liver
fibrosis and inhibits angiogenic properties of hepatic stellate
cells. J Cell Mol Med. 18:1392–1406. 2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Selman M, King TE Jr and Pardo A: American
Thoracic Society; European Respiratory Society; American College of
Chest Physicians. Idiopathic pulmonary fibrosis: Prevailing and
evolving hypotheses about its pathogenesis and implications for
therapy. Ann Intern Med. 134:136–151. 2001.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Fioret D: Idiopathic pulmonary fibrosis:
Diagnosis, management, and the search for a cure Electronic Theses
and Dissertations, University of Louisville. Paper.
437(394)2012.doi:10.18297/etd/437.
|
|
38
|
McCormack FX, King TE Jr, Voelker DR,
Robinson PC and Mason RJ: Idiopathic pulmonary fibrosis.
Abnormalities in the bronchoalveolar lavage content of surfactant
protein A. Am Rev Respir Dis. 144:160–166. 1991.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Williamson JD, Sadofsky LR and Hart SP:
The pathogenesis of bleomycin-induced lung injury in animals and
its applicability to human idiopathic pulmonary fibrosis. Exp Lung
Res. 41:57–73. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ramirez AM, Wongtrakool C, Welch T,
Steinmeyer A, Zügel U and Roman J: Vitamin D inhibition of
pro-fibrotic effects of transforming growth factor beta1 in lung
fibroblasts and epithelial cells. J Steroid Biochem Mol Biol.
118:142–150. 2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yu WN, Sun LF and Yang H: Inhibitory
effects of astragaloside IV on bleomycin-induced pulmonary fibrosis
in rats via attenuation of oxidative stress and inflammation.
Inflammation. 39:1835–1841. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Adegunsoye A, Balachandran J and Ivanovska
N: Inflammatory response mechanisms exacerbating hypoxemia in
coexistent pulmonary fibrosis and sleep apnea. Mediators Inflamm.
2015(510105)2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Hong JS, Ko HH, Han ES and Lee CS:
Inhibition of bleomycin-induced cell death in rat alveolar
macrophages and human lung epithelial cells by ambroxol. Biochem
Pharmacol. 66:1297–1306. 2003.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Uchida M, Shiraishi H, Ohta S, Arima K,
Taniguchi K, Suzuki S, Okamoto M, Ahlfeld SK, Ohshima K, Kato S, et
al: Periostin, a matricellular protein, plays a role in the
induction of chemokines in pulmonary fibrosis. Am J Respir Cell Mol
Biol. 46:677–686. 2012.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Li L, Wu W, Huang W, Hu G, Yuan W and Li
W: NF-κB RNAi decreases the Bax/Bcl-2 ratio and inhibits
TNF-α-induced apoptosis in human alveolar epithelial cells. Inflamm
Res. 62:387–397. 2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Dong SH, Liu YW, Wei F, Tan HZ and Han ZD:
Asiatic acid ameliorates pulmonary fibrosis induced by bleomycin
(BLM) via suppressing pro-fibrotic and inflammatory signaling
pathways. Biomed Pharmacother. 89:1297–1309. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Grounds MD: Complexity of extracellular
matrix and skeletal muscle regeneration. Adv Muscle Res. 3:269–302.
2008.
|
|
48
|
Matute-Bello G, Winn RK, Jonas M, Chi EY,
Martin TR and Liles WC: Fas (CD95) induces alveolar epithelial cell
apoptosis in vivo: Implications for acute pulmonary inflammation.
Am J Pathol. 158:153–161. 2001.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Hirano Y, Aziz M, Yang WL, Wang Z, Zhou M,
Ochani M, Khader A and Wang P: Neutralization of osteopontin
attenuates neutrophil migration in sepsis-induced acute lung
injury. Crit Care. 19(53)2015.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Van Der Vliet A, Nguyen MN, Shigenaga MK,
Eiserich JP, Marelich GP and Cross CE: Myeloperoxidase and protein
oxidation in cystic fibrosis. Am J Physiol Lung Cell Mol Physiol.
279:L537–L546. 2000.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Siqueira RF, Weigel RA, Nunes GR, Mori CS
and Fernandes WR: Oxidative profiles of endurance horses racing
different distances. Arq Bras Med Vet Zootec. 66:455–461. 2014.
|
|
52
|
Kosters M, Kothari S, Ghaly T and Dhamoon
A: Unmasking a rare rheumatological disease with the atypical
presentation of acute onset shortness of breath. Chest J. 146
(Suppl 4)(414A)2014.
|
|
53
|
Teixeira KC, Soares FS, Rocha LGC,
Silveira PCL, Silva LA, Valença SS, Dal Pizzol F, Streck EL and
Pinho RA: Attenuation of bleomycin-induced lung injury and
oxidative stress by N-acetylcysteine plus deferoxamine. Pulm
Pharmacol Ther. 21:309–316. 2008.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Lee YM, Rhee JS, Hwang DS, Kim IC,
Raisuddin S and Lee JS: Mining of biomarker genes from expressed
sequence tags and differential display reverse
transcriptase-polymerase chain reaction in the self-fertilizing
fish, kryptolebias marmoratus and their expression patterns in
response to exposure to an endocrine-disrupting alkylphenol,
bisphenol A. Mol Cells. 23:287–303. 2007.PubMed/NCBI
|
|
55
|
Culcasi M, Benameur L, Mercier A, Lucchesi
C, Rahmouni H, Asteian A, Casano G, Botta A, Kovacic H and Pietri
S: EPR spin trapping evaluation of ROS production in human
fibroblasts exposed to cerium oxide nanoparticles: Evidence for
NADPH oxidase and mitochondrial stimulation. Chem Biol Interact.
199:161–176. 2012.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Long LL, Yi YJ, Zhou JW, Cheng YD and Xia
YB: Microbial transformation of polydatin by endophytic fungi
isolated from polygonum cuspidatum and antioxidant activity
of the products. Mod Food Sci Technol. 31:76–83, and 162. 2015.
|
|
57
|
Massagué J, Blain SW and Lo RS: TGFbeta
signaling in growth control, cancer, and heritable disorders. Cell.
103:295–309. 2000.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Derynck R and Akhurst RJ: Differentiation
plasticity regulated by TGF-beta family proteins in development and
disease. Nat Cell Biol. 9:1000–1004. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
59
|
Branton MH and Kopp JB: TGF-beta and
fibrosis. Microbes Infect. 1:1349–1365. 1999.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Jin M, Wang L, Wu Y, Zang BX and Tan L:
Protective effect of hydroxysafflor yellow A on bleomycin-induced
pulmonary inflammation and fibrosis in rats. Chin J Integr Med.
24:32–39. 2018.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Khalil N and Greenberg AH: The role of
TGF-beta in pulmonary fibrosis. Ciba Found Symp. 157:194–207;
discussion 207-211. 1991.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Zhou Y, Zhang Q, Gao Y, Tan M, Zheng R,
Zhao L and Zhang X: Induced pluripotent stem cell-conditioned
medium suppresses pulmonary fibroblast-to-myofibroblast
differentiation via the inhibition of TGF-β1/Smad pathway. Int J
Mol Med. 41:473–484. 2018.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Tobar N, Villar V and Santibanez JF:
ROS-NFkappaB mediates TGF-beta1-induced expression of
urokinase-type plasminogen activator, matrix metalloproteinase-9
and cell invasion. Mol Cell Biochem. 340:195–202. 2010.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Verrecchia F and Mauviel A: TGF-beta and
TNF-alpha: Antagonistic cytokines controlling type I collagen gene
expression. Cell Signal. 16:873–880. 2004.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Feng XH and Derynck R: Specificity and
versatility in TGF-beta signaling through Smads. Annu Rev Cell Dev
Biol. 21:659–693. 2005.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-beta family signalling. Nature.
425:577–584. 2003.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Roberts AB, Tian F, Byfield SD, Stuelten
C, Ooshima A, Saika S and Flanders KC: Smad3 is key to
TGF-beta-mediated epithelial-to-mesenchymal transition, fibrosis,
tumor suppression and metastasis. Cytokine Growth Factor Rev.
17:19–27. 2006.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Zhao J, Shi W, Wang YL, Chen H, Bringas P
Jr, Datto MB, Frederick JP, Wang XF and Warburton D: Smad3
deficiency attenuates bleomycin-induced pulmonary fibrosis in mice.
Am J Physiol Lung Cell Mol Physiol. 282:L585–L593. 2002.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Shou J, Cao J, Zhang S, Sun R, Zhao M,
Chen K, Su SB, Yang J and Yang T: SIS3, a specific inhibitor of
smad3, attenuates bleomycin-induced pulmonary fibrosis in mice.
Biochem Biophys Res Commun. 503:757–762. 2018.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Wang S, Wilkes MC, Leof EB and Hirschberg
R: Imatinib mesylate blocks a non-Smad TGF-beta pathway and reduces
renal fibrogenesis in vivo. FASEB J. 19:1–11. 2005.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Zhang M, Fraser D and Phillips A: ERK,
p38, and Smad signaling pathways differentially regulate
transforming growth factor-beta1 autoinduction in proximal tubular
epithelial cells. Am J Pathol. 169:1282–1293. 2006.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Chun JN, Park S, Lee S, Kim JK, Park EJ,
Kang M, Kim HK, Park JK, So I and Jeon JH: Schisandrol B and
schisandrin B inhibit TGFβ1-mediated NF-κB activation via a
Smad-independent mechanism. Oncotarget. 9:3121–3130.
2017.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Hartsough MT and Mulder KM: Transforming
growth factor beta activation of p44mapk in proliferating cultures
of epithelial cells. J Biol Chem. 270:7117–7124. 1995.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Mucsi I, Skorecki KL and Goldberg HJ:
Extracellular signal-regulated kinase and the small GTP-binding
protein, Rac, contribute to the effects of transforming growth
factor-beta1 on gene expression. J Biol Chem. 271:16567–16572.
1996.PubMed/NCBI View Article : Google Scholar
|
|
75
|
González MN, de Mello W, Butler-Browne GS,
Silva-Barbosa SD, Mouly V, Savino W and Riederer I: HGF potentiates
extracellular matrix-driven migration of human myoblasts:
Involvement of matrix metalloproteinases and MAPK/ERK pathway.
Skelet Muscle. 7(20)2017.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Hu X, Wang H, Liu J, Fang X, Tao K, Wang
Y, Li N, Shi J, Wang Y, Ji P, et al: The role of ERK and JNK
signaling in connective tissue growth factor induced extracellular
matrix protein production and scar formation. Arch Dermatol Res.
305:433–445. 2013.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Hough C, Radu M and Doré JJ: TGF-beta
induced Erk phosphorylation of smad linker region regulates smad
signaling. PLoS One. 7(e42513)2012.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Matsuura I, Wang G, He D and Liu F:
Identification and characterization of ERK MAP kinase
phosphorylation sites in Smad3. Biochemistry. 44:12546–12553.
2005.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Kretzschmar M, Doody J, Timokhina I and
Massagué J: A mechanism of repression of TGFbeta/Smad signaling by
oncogenic Ras. Genes Dev. 13:804–816. 1999.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Wang G, Jiao H, Zheng JN and Sun X: HSP27
regulates TGF-β mediated lung fibroblast differentiation through
the Smad3 and ERK pathways. Int J Mol Med. 39:183–190.
2017.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Conte E, Gili E, Fagone E, Fruciano M,
Iemmolo M and Vancheri C: Effect of pirfenidone on proliferation,
TGF-β-induced myofibroblast differentiation and fibrogenic activity
of primary human lung fibroblasts. Eur J Pharm Sci. 58:13–19.
2014.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Li C, Rezov V, Joensuu E, Vartiainen V,
Rönty M, Yin M, Myllärniemi M and Koli K: Pirfenidone decreases
mesothelioma cell proliferation and migration via inhibition of ERK
and AKT and regulates mesothelioma tumor microenvironment in vivo.
Sci Rep. 8(10070)2018.PubMed/NCBI View Article : Google Scholar
|