Introduction
Multi-infarct dementia (MID) is a common type of
vascular dementia that is characterized by multiple lesions and
infarction of small arteries in the cerebral gray-white matter
(1). The brain is an organ that
consumes a high amount of energy and requires stable blood flow to
deliver a sufficient amount of energy to maintain synaptic activity
(2,3). A number of studies have reported that
cerebral hypoperfusion is caused by cardiac arrest, arrhythmia and
heart failure (4,5), decreased cerebral blood flow induced
by hyperlipidemia, atherosclerosis and diabetes (6), as well as ageing and the
apolipoprotein E gene (7), which
are major risk factors for cognitive impairment. Insufficient or
low cerebral blood flow, especially acute cerebral ischemia, can
cause insufficient glucose uptake, significantly reduced ATP
content (8) and the accumulation of
lactic acid in the brain (9). The
mitochondria is the main site of ATP synthesis (10) and an excessive accumulation of
lactic acid can cause mitochondrial dysfunction (11), inducing oxidative stress,
mitochondrial autophagy and apoptosis (12,13),
which in turn exacerbate MID lesions and cognitive decline.
Therefore, regulating energy metabolism in the brain may be an
effective strategy to improve cognitive impairment in MID.
Saponin, which is the main active constituent of
ginseng and astrogalus, has also been reported to significantly
improve symptoms in ischemic disease animal models (14-17)
and cognitive decline in dementia animal models (18-21).
However, the use of a combination of ginseng and astrogalaus (CGA)
saponin in vascular dementia has not yet been reported. Therefore,
the present study aimed to investigate the effect of CGA on
cognitive function in rats with MID and to explore the potential
mechanisms of action from the aspect of energy regulation.
Materials and methods
Animals
A total of 65 male specific pathogen-free
Spraque-Dawley rats (weight, 200±20 g; age, 6 weeks) were purchased
from Chengdu Dashuo Experimental Animal Co., Ltd. All rats were
maintained at 22±2˚C with 65±5% humidity and had free access to
drinking water and feed on a 12-h light/dark cycle. The
experimental procedure was approved by the Institute of Materia
Medica Integration and Transformation for Brain Disorders Ethical
Committee.
Drugs and reagents
The ginseng and astrogalus total saponins were
purchased from Baoji Herbest Bio-Tech Co., Ltd. (batch nos.
20171120 and 20171121, respectively). CGA is a combination of
ginseng total saponin (cat. no. 20171120; purity, 90% determined by
supplier using HPLC.) and astragaloside total saponin (cat. no.
20171121; purity, 90% determined by supplier using HPLC) at 1:1.
ATP, ADP and AMP disodium salt were purchased from Sigma-Aldrich;
Merck KGaA.
Model preparation and grouping
After 3 days of adaptive feeding, the MID rat model
was prepared using the micro-thromboembolic method, as previously
described (24-25). The
sham-operation group received the same surgical procedure, but the
thrombus was replaced with normal saline. At 2 weeks
post-operation, Y maze was used to evaluate the success of
modeling. Animals were assigned to treatment groups following the
Y-maze task; groups were counterbalanced based on the number of
errors animals made during the Y-maze task, such that there was no
difference in error times between treatment groups (P=0.999 for
error times) The evaluation criteria (26) were as follows: If the number of
errors was >5, the modeling was deemed successful, animals that
were not successful were excluded; successful model rats were
ranked from low to high according to the number of errors and then
put into five cages in sequence according to a zigzag method
(27) to ensure that the number of
errors by rats in each cage was not significantly different. The
cages were randomly assigned to the following groups (n=10 per
group): Model, Hydergine (0.7 mg/kg; Tianjin Huajin Pharmaceutical
Co., Ltd.), high-dose CGA (CGAH; 20 mg/ml), low-dose CGA (CGAL; 10
mg/ml) (22,23) and control groups. The control group
consisted of 10 sham-operated rats. The rats in the drug treatment
groups were intragastrically administered with the corresponding
drugs once per day for 60 days, at a dose of 10 ml/kg. The rats in
the sham-operation and model groups were intragastrically
administered with 10 ml/kg of normal saline once per day for 60
consecutive days.
Morris water maze
Hidden platform experiment. On day 54 of drug
intervention, the five-day hidden platform experiment was performed
in all groups of rats. The rats were trained twice a day, during
which the time taken to find the platform within 60 sec (escape
latency period) was recorded for each rat. If a rat failed to find
the platform within 60 sec, it was guided to and kept on the
platform for 10 sec, and the escape latency was recorded as 60
sec.
Spatial probe test. The spatial probe test
was implemented on day 59 of drug intervention. The platform was
removed. Subsequently, the rats were placed into the maze in the
same position and their movement trajectories, as well as their
frequency of crossing the position that formerly held the platform,
within 60 sec were recorded.
Open field test
The open field test was performed with an OFT-100
opening experiment system (TechMan Software, WMT-100; Chengdu
Taimeng Software Co., Ltd.). The movement time, immobile period and
frequency of standing in a 5 min session were recorded and used as
evaluating indicators.
Mitochondrial swelling and membrane
potential detection
After the water maze experiment, a total of 24 rats
were sacrificed and 80 mg of brain tissue was collected from the
hippocampus of each rat. Manual grinding with PBS using a glass
homogenizer and divided the homogenate into two parts, then
centrifuged (600 x g; 5 min; 4˚C) with one part of the homogenate,
and the supernatant carefully transferred to another tube for
further centrifugation (1,200 x g; 10 min; 4˚C). The supernatant
was discarded and the precipitate resuspended in 500 µl
Mitochondria Storage Buffer (cat. no. C3609; Beyotime Institute of
Biotechnology) at a concentration of 1x105 cells/ml per
tube, mixed for the detection of mitochondrial swelling. The second
part of homogenate was collected and the concentration was adjusted
to 1x106 cells/ml. Subsequently, 5 µl of a mitochondrial
membrane potential detection JC-1 kit (cat. no. 551302;
Becton-Dickinson and Company) was added to 100 µl sample, incubated
at 37˚C for 15 min and then washed with PBS. After centrifugation
(350 x g; 5 min; 4˚C).The precipitate was collected. The
precipitate was resuspended in 300-400 µl JC-1 dilution, mixed and
mounted for the detection of mitochondrial membrane potential.
Mitochondrial swelling and membrane potential were analyzed using a
flow cytometer (CytoFLEX; Beckman Coulter, Inc.) and Kaluza
software (version 2.1; Beckman Coulter, Inc.)
Detection of hippocampus energy
load
A total of 65 mg of hippocampus tissue was
collected, mixed with normal saline (weight of hippocampus: Normal
saline volume, 1:10) and homogenized in an ice bath. Subsequently,
0.6 ml homogenate was collected, mixed with an equal volume of 0.5
mol/l perchloric acid in a vortex mixer, and centrifuged at 4˚C and
12,000 x g for 10 min. Furthermore, 0.8 ml supernatant was
collected, and the pH was neutralized using NaOH (5 mol/l). The
supernatant was allowed to stand for 30 min and was then
centrifuged at 4˚C and 12,000 x g for 10 min. Subsequently, the
supernatant was directly sampled and analyzed using
high-performance liquid chromatography (Agilent1260; Agilent
Techonologies, Inc.) to determine the contents of AMP, ADP and ATP.
ATP, ADP and AMP disodium salts were used as internal standards.
The conditions used were as follows: Hypersil™ ODS C18 column
(particle size, 5 µm; 250x4.6 mm2); column temperature,
25˚C; The mobile phase is phosphate buffer and methanol, 0.05 mol/l
PBS (pH 6.5); flow rate, 1.0 ml/min; sample temperature, 10˚C;
sampling volume, 10 µl; and ultraviolet wavelength, 254 nm. The
energy load was calculated as follows: EC = ([ATP] +
0.5[ADP])/([ATP] + [ADP] + [AMP]).
Hematoxylin and eosin staining
The rats were sacrificed and placed in an ice bath
to harvest the brain. Part of the left hemisphere was fixed with 4%
paraformaldehyde at room temperature for 24 h, replaced with new 4%
paraformaldehyde, and then fixed for 48 h at room temperature,
dehydrated and embedded with paraffin. Subsequently, the tissue was
cut into 5 µm slices, stained with hematoxylin for 30 min and eosin
for 5 min at room temperature, vitrified with xylene, sealed with
neutral resin, and then placed under a optical microscope (x200;
model, Olympus Optical CX22; Olympus Corporation) to observe the
morphology of neurons in the hippocampal CA1 region in 3 visual
fields of each sample. Pathological scoring was performed as
previously described (28), the
pathological scoring standard is shown in Table I.
 | Table IThe pathological grading
standard. |
Table I
The pathological grading
standard.
| Grade | Microscopic
description |
|---|
| - | No lesion. |
| | 0. |
| + | 1. Focal edema of
gray and white matter, cells dissolved; |
| | 2. Focal atrophy of
gray and white matter, hyperchromatic nuclei and cytoplasm; |
| | 3. Focal slight
cellular proliferation (mainly gliacyte); |
| | 4. Slight
perivascular edema; |
| | 5. Slight vascular
engorgement and hemorrhage; |
| | 6. Slight
inflammatory cell infiltration; |
| | 7. Slight gray
matter atrophy; |
| | 8. Slight ependymal
cells proliferation. |
| | It was graded as
‘+’ when conformed to one of all above, score of which was 1. |
| ++ | 1. Multifocal edema
of gray and white matter, cells dissolved; |
| | 2. Focal atrophy of
gray and white matter, hyperchromatic nuclei and cytoplasm; |
| | 3. Focal cellular
proliferation (mainly gliacyte); |
| | 4. Medium vascular
edema and hemorrhage; |
| | 5. Medium
inflammatory cells infiltration; |
| | 6. Medium
perivascular edema. |
| | It was graded as
‘++’ when conformed to one of all above, score of which was 2. |
| +++ | 1. diffuse edema of
gray and white matter, cells dissolved; |
| | 2. Multifocal
atrophy of gray and white matter, hyperchromatic nuclei and
cytoplasm; |
| | 3. Focal mass
cellular proliferation (mainly gliacyte); |
| | 4. Severe vascular
edema and hemorrhage; |
| | 5. Severe
inflammatory cell infiltration. |
| | It was graded as
‘+++’ when conformed to one of all above, score of which was
3. |
Western blot analysis
Thirty milligrams of brain tissue was weighed, mixed
with RIPA buffer (cat. no. WB020; 1 mg: 10 µl; Multisciences
(Lianke) Biotech, Co., Ltd.) homogenized in an ice bath and
centrifuged at 4˚C and 12,000 x g for 10 min. The resulting
supernatant was collected for protein determination by BCA. Equal
amounts (50 µg) of the total protein were separated by 8% SDS-PAGE,
transferred to a PVDF membrane and blocked with 5% BSA (cat. no.
EZ3416D317; BioFroxx; neoFroxx GmbH) at room temperature for 90
min. Subsequently, the membrane was incubated with the following
primary antibodies at 4˚C overnight: anti-PI3K (cat. no. 4257S;
1:1,000; Cell Signaling Technology, Inc.), anti-phosphorylated
(p)-PI3K (cat. no. 4228S; 1:1,000; Cell Signaling Technology,
Inc.), anti-AKT (cat. no. 4685S; 1:1,000; Cell Signaling
Technology, Inc.), anti-p-AKT (cat. no. 4060S; 1:1,000; Cell
Signaling Technology, Inc.) and GAPDH (cat. no. 180411; 1:5,000;
Wuhan Servicebio Technology Co., Ltd.). Subsequently, the membrane
was washed with TBST solution three times and incubated with the
horseradish peroxidase-conjugated goat anti-rabbit IgG secondary
antibody [cat. no. A8040064; 1:5,000; Hangzhou Multisciences
(Lianke) Biotech Co., Ltd.] at room temperature for 90 min. The
membrane was washed with TBST solution three times. Protein bands
were visualized using a hypersensitive ECL kit (cat. no.
4AW011-100; Beijing 4A Biotech Co., Ltd.). Protein expression was
quantified using Quantity One (version 4.6.2; Bio-Rad Laboratories,
Inc.) software with GAPDH as the loading control.
ELISA
A total of 50 mg of brain tissue was weighed and
mixed with PBS containing 1% PMSF (1 mg: 9 µl), homogenized in an
ice bath and centrifuged at 4˚C and 5,000 x g for 10 min. The
supernatant was collected. Subsequently, 100 µl supernatant was
used to determine the concentrations of insulin (Rat insulin ELISA
kit; cat. no. E-EL-R2466c ; Elabscience Biotechnology Co., Ltd.),
glutamate (glutamic acid measurement kit; cat. no. A074-1-1;
Nanjing Jiancheng Bioengineering Institute.) and γ-aminobutyric
acid (GABA) (γ-aminobutyric acid assay kit; cat. no. H168; Nanjing
Jiancheng Bioengineering Institute.), according to the
manufacturers' protocols.
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analyses of the data was performed in a double-blinded
fashion. Morris water maze escape latency data were analyzed using
a two-way ANOVA followed by Tukey's post-hoc test. The other data
in line with normal distribution were analyzed using a one-way
ANOVA followed by Tukey's post-hoc test . The pathological results
were analyzed using Kruskal Wallis followed by Dunn's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
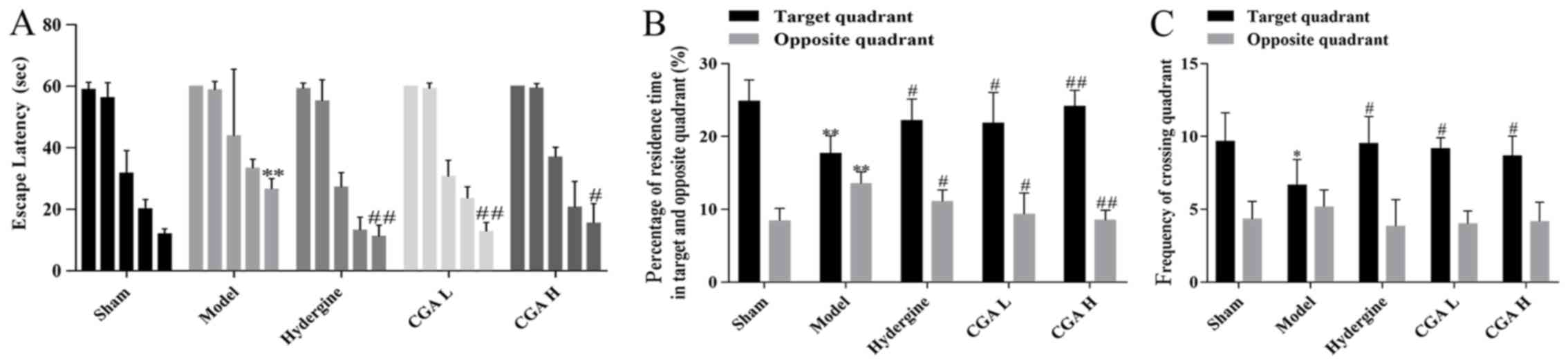
Effect of CGA on learning and memory
in rats with MID
The escape latency of each group displayed a
time-dependent pattern, and was indicated to decrease as training
time increased (Fig. 1A). The
escape latency of the rats was significantly increased in the model
group compared with the sham group, while the escape latency of the
rats in the CGA group was significantly shorter than the model
groups (Fig. 1A). Meanwhile, the
percentage of residence time in the target quadrant in the model
group was decreased compared with the sham, hydergine and CGA
groups, but the percentage in the opposite quadrant was increased
compared with the sham and CGA H groups (Fig. 1B). Similarly, the frequency of
crossing the target quadrant in the model group was significantly
reduced compared with the sham, hydergine and CGA groups, while the
frequency of crossing the opposite quadrant displayed no
statistical difference between all the groups (Fig. 1C).
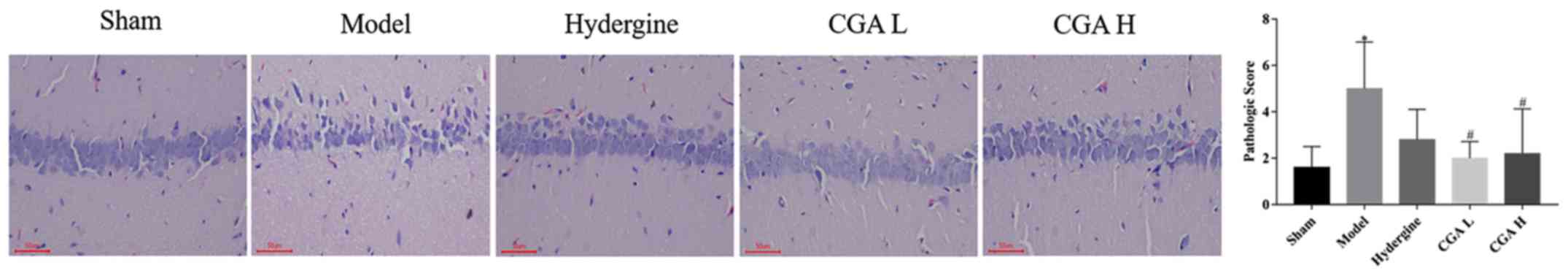
Effect of CGA on the pathomorphology
of hippocampal CA1 area in rats with MID
The vertebral nerve cells in the hippocampal CA1
area were sparsely arranged, and the number of cells with
incomplete structure, nerve cell necrosis and degeneration was
increased in the model group compared with the sham group
(P<0.05; Fig. 2). The sparse
arrangement, necrosis and degeneration of the nerve cells were
significantly improved in the CGA groups compared with the model
group (P<0.05; Fig. 2).
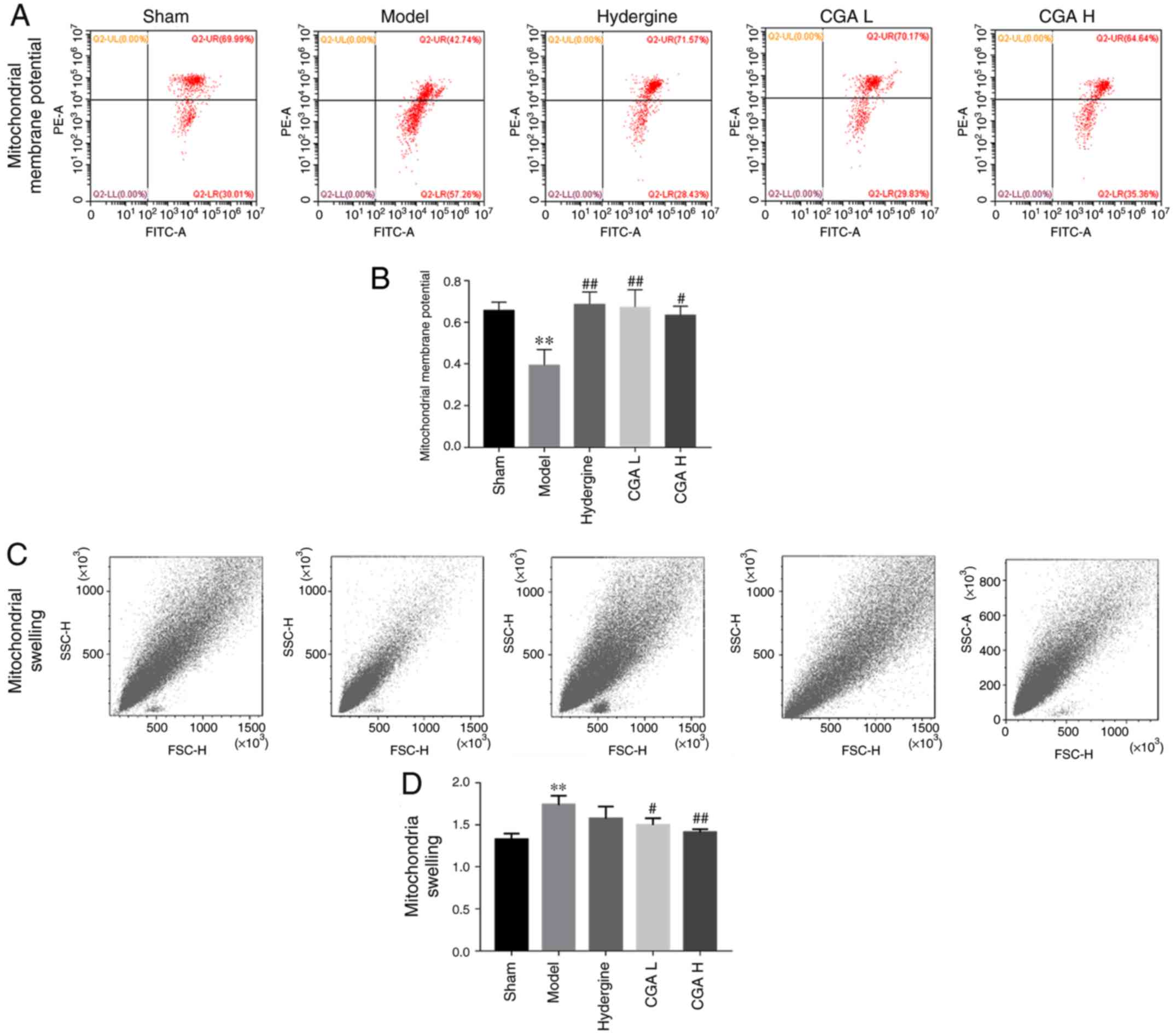
Effect of CGA on hippocampal
mitochondrial function in rats with MID
The mitochondrial membrane potential in the
hippocampus decreased significantly in the model group compared
with the sham group (P<0.01; Fig.
3A and B). The CGA groups
displayed significantly reversed effects of MID on the
mitochondrial membrane potential compared with the model group
(P<0.05; Fig. 3A and B). Furthermore, the mitochondrial swelling
in the hippocampus was significantly increased in the model group
compared with the sham group (P<0.01; Fig. 3C and D), while the mitochondrial swelling was
significantly decreased in the CGA H and CGA L groups compared with
the model group (P<0.05; Fig. 3C
and D).
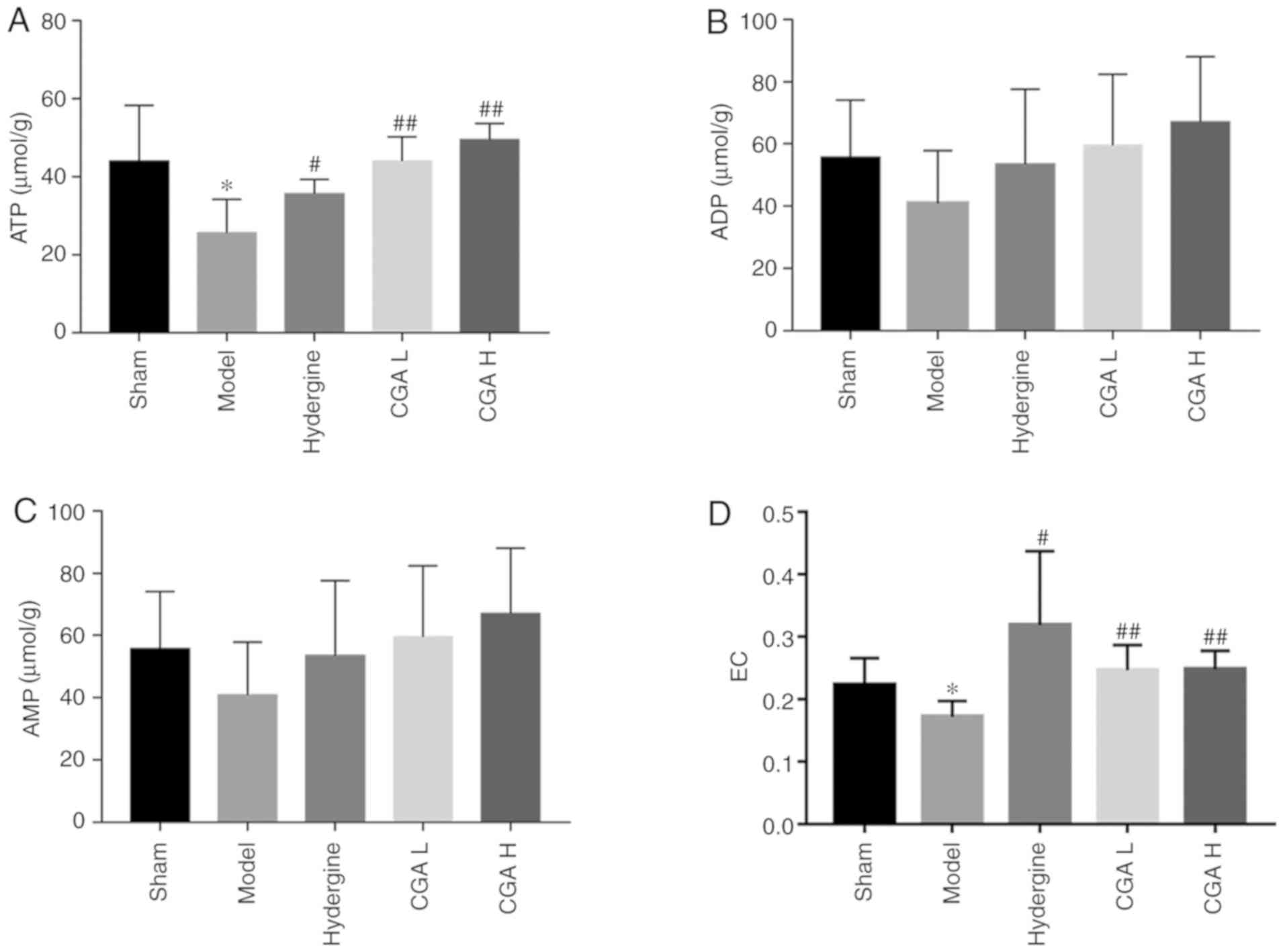
Effect of CGA on brain energy load in
rats with MID
ATP content in the brain tissue was significantly
decreased (P<0.05; Fig. 4A), AMP
and ADP contents were not significantly altered (P>0.05;
Fig. 4B and C), and the brain energy load was
significantly decreased (P<0.05; Fig. 4D) in the model group compared with
the sham group. The ATP content in the brain tissue was
significantly increased (P<0.05; Fig. 4A), AMP and ADP contents were not
significantly altered (P>0.05; Fig.
4B and C), and the brain energy
load was significantly increased (P<0.05; Fig. 4D) in the CGA and Hydergine groups
compared with the model group.
Effect of CGA on the PI3K/AKT
signaling pathway in rats with MID
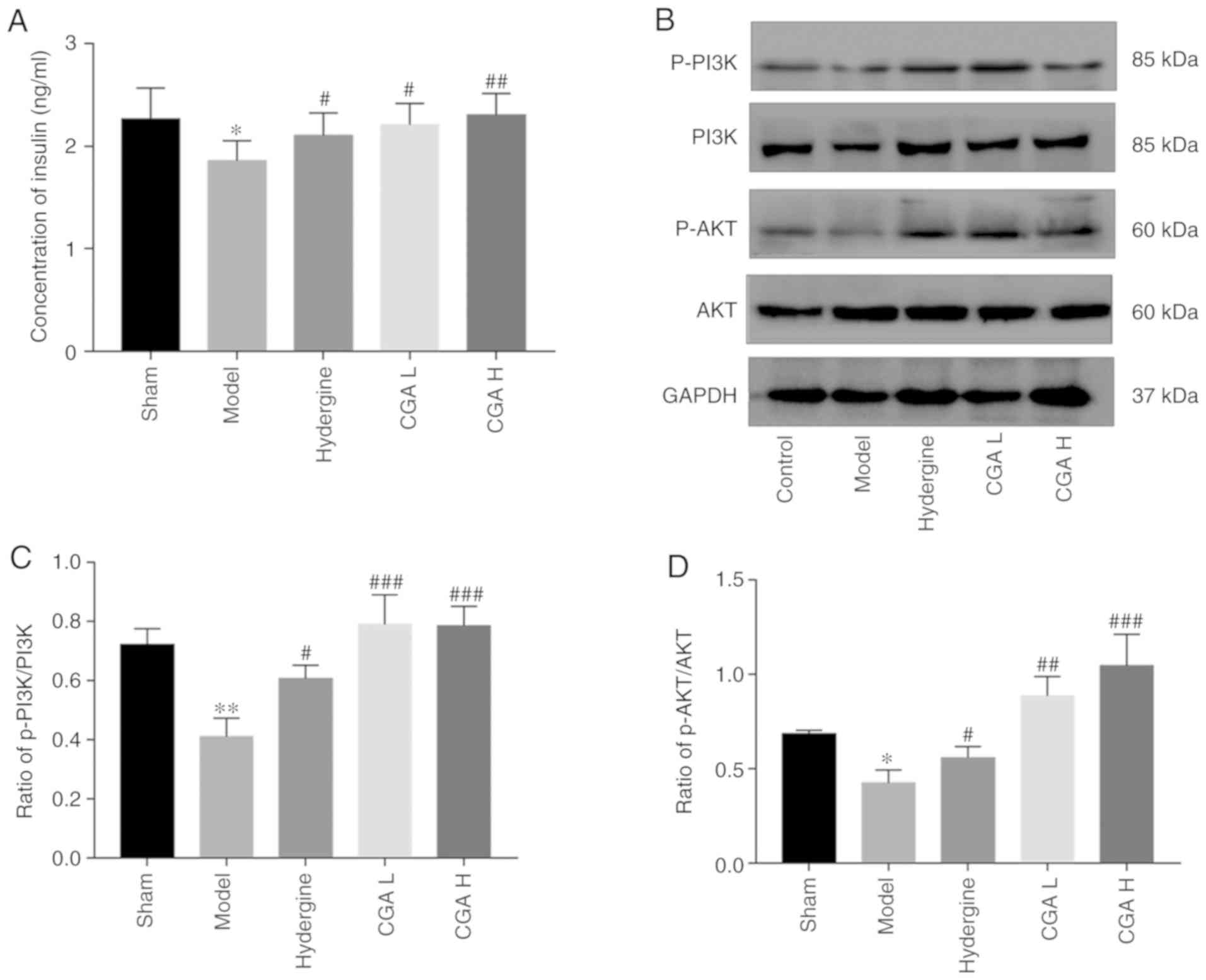
The insulin content in the brain was significantly
lower in the model group compared with the sham group (P<0.05;
Fig. 5A). However, the insulin
content in the brain was significantly increased in the CGA and
Hydergine groups compared with the model group (P<0.05; Fig. 5A). The ratio of p-PI3K/PI3K and
p-AKT/AKT in the brain was significantly decreased in the model
group compared with the sham group (Fig. 5B and C). In contrast, the ratio of p-PI3K/PI3K
and p-AKT/AKT was significantly increased in the CGA and Hydergine
groups compared with the model group (Fig. 5B and C).
Effect of CGA on the concentration of
neurotransmitters
A number of emotional behavioral changes were
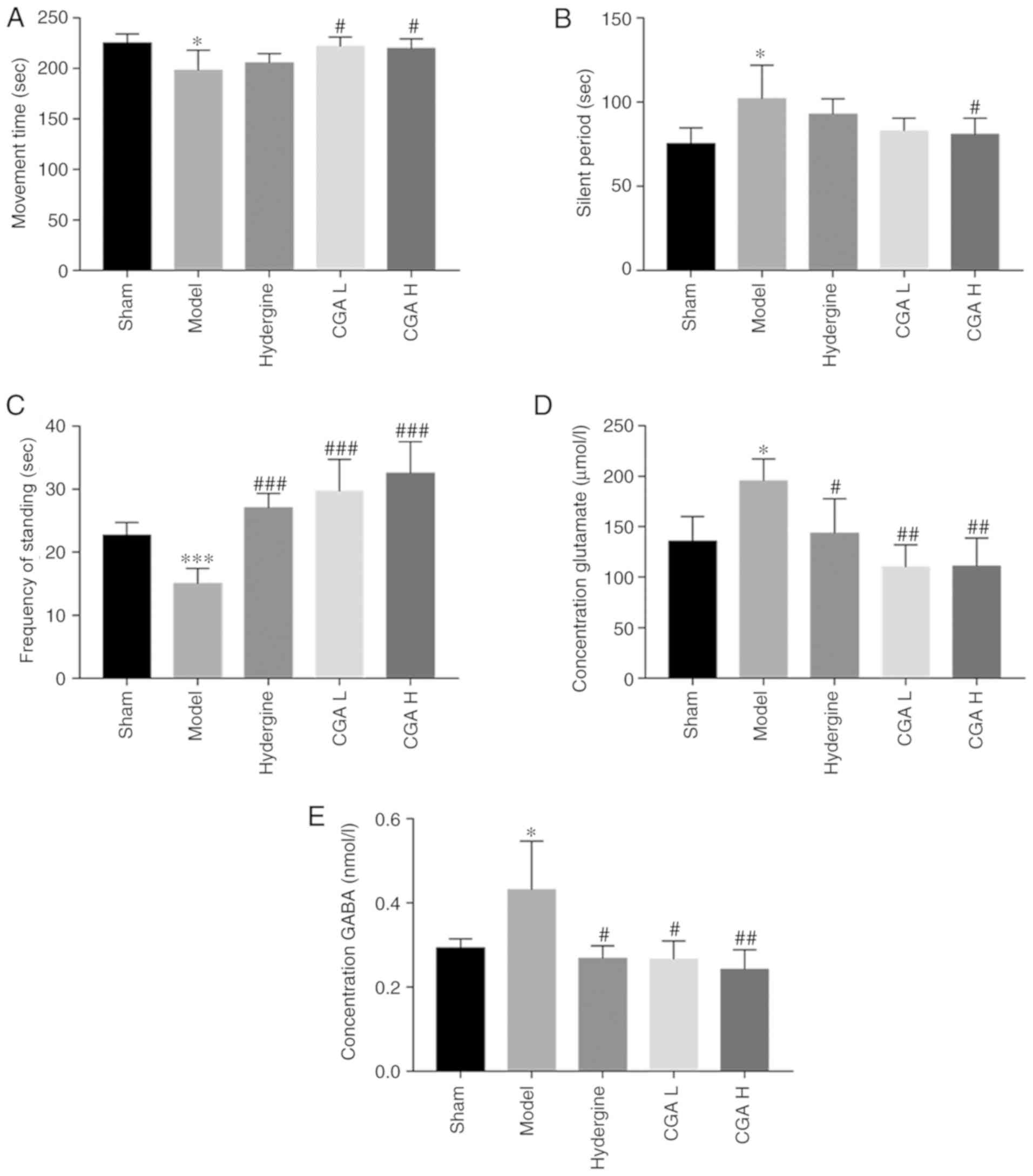
observed in the model rats, except cognitive decline. The movement
time was significantly lower in the model group compared with the
sham group (P<0.05; Fig. 6A).
Furthermore, the silent period was significantly increased in the
model group compared with the sham group (P<0.05; Fig. 6B). However, the effect of the MID
model on both movement time and immobile period were reversed by
administering CGA H. Meanwhile, the MID model-induced lower
frequency of standing was also reversed by the administration of
CGA (P<0.05; Fig. 6C).
Subsequently, the concentration of GABA and glutamate in the brain
tissues was detected. The concentration of glutamate in the brain
tissue of model rats was higher compared with the sham rats
(P<0.05; Fig. 6D). When compared
with the model group, the concentration of glutamate in the
Hydergine and CGA groups decreased significantly (P<0.05;
Fig. 6D). CGA also significantly
reduced the GABA content in the brain of MID model rats when
compared with the model group (P<0.01; Fig. 6E).
Discussion
Worldwide, vascular dementia is the second most
common form of dementia accompanied by obvious cognitive
dysfunction after Alzheimer's disease (29), and MID is a common type of vascular
dementia. Currently, no licensed drugs are available for the
treatment of vascular dementia (30), and the reported therapeutic
strategies for the disease primarily focus on inhibiting oxidative
stress, apoptosis and inflammation (31,32).
Multiple and cortical microinfarctions are closely related to
dementia and cognition (33), which
is associated with their direct influence on the production of ATP
in the brain (7). Therefore,
regulating the energy metabolism in the brain and maintaining the
physiological function of normal nerve cells in patients with MID
may be effective therapeutic strategies in the treatment of this
disease. The present study suggested that CGA significantly
improved the cognitive function, and disordered arrangement and
sparseness of vertebral cells in the hippocampal CA1 area in rats
with MID. Furthermore, CGA regulated mitochondrial swelling and
membrane potential, and significantly increased ATP content and
brain energy load in rats with MID. In addition, CGA significantly
increased the insulin content and the expression of PI3K and AKT in
the brain of rats with MID.
The presence of multiple arteriolar vascular
infarctions in the gray matter is a key characteristic of MID
(34). Injection of a small amount
of thrombus (diameter, 50-100 µm) into the internal carotid artery
can simulate multiple arterial infarctions in cognitive areas,
including the cortex and hippocampus (35), making it an ideal method for
modeling MID. The present study displayed significantly declined
cognitive function, lesions in the hippocampal CA1 area, structural
and functional impairment of the mitochondria, and brain ATP and
energy load in rats with MID. Continuous low-level brain energy not
only affects neuronal function, but also induces autophagy and
apoptosis via the mTOR, PI3K, peroxisome proliferator activated
receptor-γ and AMP-activated protein kinase signaling pathways
(36). The PI3K/AKT signaling
pathway is a key regulator involved in the metabolism, growth,
proliferation and survival of cells, which can be activated by
multiple receptors or proteins, including insulin, insulin-like
growth factor-1, low-density lipoprotein-related receptor 1 and
toll-like receptors (37-39).
After being activated by insulin, the PI3K signaling pathway is
involved primarily in energy metabolism in vivo. The present
study suggested that the insulin content and expression of PI3K and
AKT proteins was significantly decreased in the brain of rats with
MID compared with control rats.
Saponin is the main active substance of ginseng and
Huangqi, and has been reported to improve learning and memory
functions in a number of studies (14,15,17,20). The present study
suggested that CGA significantly improved the decline in the
learning and memory of rats with MID and improved the degeneration
and necrosis of nerve cells in the hippocampal CA1 area. A number
of studies have reported that the active ingredients of ginseng,
including Rb1, Rg1, Rh2E2, Re, Rg3, Rd and Rf, can regulate energy
metabolism (40-46).
Similarly, the saponin ingredient of Huangqi exerts a similar
effect to Astragaloside IV, which regulates energy metabolism by
regulating glycolytic pathways (47,48).
The present study suggested that CGA regulated the structure and
function of the mitochondria, increased brain ATP content and
energy load, and significantly increased insulin content in the
brain of rats with MID. It further revealed that CGA significantly
upregulated the phosphorylation levels of PI3K and AKT, therefore,
CGA may activate the PI3K/AKT signaling pathway to regulate energy
metabolism. However, identifying the specific saponin ingredients
that activate the suppressed PI3K/AKT signaling pathway in MID
requires further investigation.
Glutamate and GABA are excitatory and inhibitory
neurotransmitters, respectively (49). A large number of studies have
indicated a role for both neurotransmitters in the occurrence and
development of dementia, similar to other neurotransmitters,
including acetylcholine (50-52).
Similarly, glutamic acid and GABA in the nerves located in the
ventral tegmental region display a regulating effect on risk
factors for dementia, including insomnia and insufficient sleep
(53). The present study suggested
that the activity and standing times of MID model animals decreased
significantly, and the contents of glutamate and GABA in the brain
tissue increased abnormally compared with control rats, which was
similar to the results reported in the aforementioned studies. GABA
is derived primarily from glutamate metabolism, indicating why the
two neurotransmitters should display an opposite pattern of
expression (54). The relationship
between GABA and glutamate in the present study was consistent with
previous findings (55-57),
suggesting that either the source of glutamate increased or the
elimination of GABA decreased. However, further investigation is
required to clarify this.
In conclusion, improvements in brain energy
metabolism in rats with MID by CGA was closely associated with the
regulatory effect of CGA on the PI3K/AKT signaling pathway and
neurotransmitter systems. The majority of drugs that have been
reported to improve dementia display antioxidant, anti-inflammatory
and antiapoptotic mechanisms. However, the present study focused on
energy metabolism and to the best of our knowledge, suggested for
the first time that CGA regulated energy metabolism via the
PI3K/AKT signaling pathway to achieve an anti-vascular dementia
effect. The results of the present study suggested that the use of
CGA may be beneficial for the treatment of vascular dementia.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Key Research
and Development Plan of Sichuan Province (grant no. 19ZDYF0600),
the program of Traditional Chinese Medicine Bureau of Sichuan
Province (grant no. 2018JC013), the Program of Education Department
of Sichuan Province (grant no. 17ZA0149), the Program of Chengdu
University of Traditional Chinese Medicine Science and Technology
Development Fund (grant no. ZRYY1727).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SX conceived, designed the study and critically
reviewed, edited, and revised the paper; YW conceived and revised
the final manuscript. YF performed the experiments and drafted the
original manuscript. JW performed the experiments, data analysis
and revised the manuscript. BL guided the partial experimental
methods and critically reviewed and revised the original
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The experimental procedure was approved by the
Institute of Meterial Medica Integration and Transformation for
Brain Disorders Ethical Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Iadecola C: The pathobiology of vascular
dementia. Neuron. 80:844–866. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Iadecola C: Neurovascular regulation in
the normal brain and in Alzheimer's disease. Nat Rev Neurosci.
5:347–360. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Harris JJ, Jolivet R and Attwell D:
Synaptic energy use and supply. Neuron. 75:762–777. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Stefansdottir H, Arnar DO, Aspelund T,
Sigurdsson S, Jonsdottir MK, Hjaltason H, Launer LJ and Gudnason V:
Atrial fibrillation is associated with reduced brain volume and
cognitive function independent of cerebral infarcts. Stroke.
44:1020–1025. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Marshall RS: Effects of altered cerebral
hemodynamics on cognitive function. J Alzheimers Dis. 32:633–642.
2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
de la Torre JC: Cerebral hemodynamics and
vascular risk factors: Setting the stage for Alzheimer's disease. J
Alzheimers Dis. 32:553–567. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Qian J, Wolters FJ, Beiser A, Haan M,
Ikram MA, Karlawish J, Langbaum JB, Neuhaus JM, Reiman EM, Roberts
JS, et al: APOE-related risk of mild cognitive impairment and
dementia for prevention trials: An analysis of four cohorts. PLoS
Med. 14(e1002254)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tian J, Fu F, Geng M, Jiang Y, Yang J,
Jiang W, Wang C and Liu K: Neuroprotective effect of
20(S)-ginsenoside Rg3 on cerebral ischemia in rats. Neurosci Lett.
374:92–97. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Feng Y, Zhang W and Guo J: The alterations
of brain lactate, lactate dehydrogenase, creatine phosphokinase and
its influence on these of peripheral blood or liver tissue and
entero-barrier during brain hypoperfusion. Zhongguo Bing Li Sheng
Li Xue Hui. 12:1106–1109. 1999.(In Chinese).
|
|
10
|
van der Bliek AM, Sedensky MM and Morgan
PG: Cell Biology of the Mitochondrion. Genetics. 207:843–871.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Parihar MS and Brewer GJ: Amyloid-β as a
modulator of synaptic plasticity. J Alzheimers Dis. 22:741–763.
2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li H, Liu Y, Lin LT, Wang XR, Du SQ, Yan
CQ, He T, Yang JW and Liu CZ: Acupuncture reversed hippocampal
mitochondrial dysfunction in vascular dementia rats. Neurochem Int.
92:35–42. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Park YS, Choi SE and Koh HC: PGAM5
regulates PINK1/Parkin-mediated mitophagy via DRP1 in CCCP-induced
mitochondrial dysfunction. Toxicol Lett. 284:120–128.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wan Q, Ma X, Zhang ZJ, Sun T, Xia F, Zhao
G and Wu YM: Ginsenoside reduces cognitive impairment during
chronic cerebral hypoperfusion through brain-derived neurotrophic
factor regulated by epigenetic modulation. Mol Neurobiol.
54:2889–2900. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
1Dong X, Zheng L, Lu S and Yang Y:
Neuroprotective effects of pretreatment of ginsenoside Rb1 on
severe cerebral ischemia-induced injuries in aged mice: Involvement
of anti-oxidant signaling. Geriatr Gerontol Int. 17:338–345.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tang B, Wang D, Li M, Wu Q, Yang Q, Shi W
and Chen C: An in vivo study of hypoxia-inducible factor-1α
signaling in ginsenoside Rg1-mediated brain repair after
hypoxia/ischemia brain injury. Pediatr Res. 81:120–126.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li M, Li H, Fang F, Deng X and Ma S:
Astragaloside IV attenuates cognitive impairments induced by
transient cerebral ischemia and reperfusion in mice via
anti-inflammatory mechanisms. Neurosci Lett. 639:114–119.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zong W, Zeng X, Chen S, Chen L, Zhou L,
Wang X, Gao Q, Zeng G, Hu K and Ouyang D: Ginsenoside compound K
attenuates cognitive deficits in vascular dementia rats by reducing
the Aβ deposition. J Pharmacol Sci. 139:223–230. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang G, Liu A, Zhou Y, San X, Jin T and
Jin Y: Panax ginseng ginsenoside-Rg2 protects memory impairment via
anti-apoptosis in a rat model with vascular dementia. J
Ethnopharmacol. 115:441–448. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chang CP, Liu YF, Lin HJ, Hsu CC, Cheng
BC, Liu WP, Lin MT, Hsu SF, Chang LS and Lin KC: Beneficial effect
of astragaloside on Alzheimer's disease condition using cultured
primary cortical cells under β-amyloid exposure. Mol Neurobiol.
53:7329–7340. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li WZ, Wu WY, Huang DK, Yin YY, Kan HW,
Wang X, Yao YY and Li WP: Protective effects of astragalosides on
dexamethasone and Aβ25-35 induced learning and memory impairments
due to decrease amyloid precursor protein expression in 12-month
male rats. Food Chem Toxicol. 50:1883–1890. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang Y, Su H, Zhang J and Kong J: The
effects of ginsenosides and anserine on the up-regulation of renal
aquaporins 1-4 in hyperuricemic mice. Am J Chin Med. 47:1133–1147.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Qiu LH, Zhang BQ, Lian MJ, Xie XJ and Chen
P: Vascular protective effects of and its main constituents in rats
with chronic hyperhomocysteinemia. Exp Ther Med. 14:2401–2407.
2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yang JW, Wang XR, Ma SM, Yang NN, Li QQ
and Liu CZ: Acupuncture attenuates cognitive impairment, oxidative
stress and NF-κB activation in cerebral multi-infarct rats.
Acupunct Med. 37:283–291. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang X, Wu B, Nie K, Jia Y and Yu J:
Effects of acupuncture on declined cerebral blood flow, impaired
mitochondrial respiratory function and oxidative stress in
multi-infarct dementia rats. Neurochem Int. 65:23–29.
2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ying S, Lai X, Guan C, Xie LL, Wu LN and
Tang CZ: Effect of electroacupuncture on learning-memory ability of
vascular dementia rats with concomitant hypertension and
hyperlipemia. Zhen Ci Yan Jiu. 34:368–375. 2009.(In Chinese).
PubMed/NCBI
|
|
27
|
Lin C, Jiang F, Peng T, et al:
Toxicity test of zsydo on rabbit embryo fetal development//2015
(fifth) annual meeting of pharmacotoxicology.
|
|
28
|
Ren X, Wei J and Gong D: Tongluoxingnao
effervescent tablets ameliorate learning and memory impairment in a
rat model of vascular dementia via the regulation of the p38 and
ERK MAPK signaling pathways. Int J Clin Exp Med. 9:5400–5412.
2016.
|
|
29
|
Smith EE: Clinical presentations and
epidemiology of vascular dementia. Clin Sci (Lond). 131:1059–1068.
2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
O'Brien JT and Thomas A: Vascular
dementia. Lancet. 386:1698–1706. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Gubandru M, Margina D, Tsitsimpikou C,
Goutzourelas N, Tsarouhas K, Ilie M, Tsatsakis AM and Kouretas D:
Alzheimer's disease treated patients showed different patterns for
oxidative stress and inflammation markers. Food Chem Toxicol.
61:209–214. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bagheri G, Rezaee R, Tsarouhas K, Docea
AO, Shahraki J, Shahriari M, Wilks MF, Jahantigh H, Tabrizian K,
Moghadam AA, et al: Magnesium sulfate ameliorates carbon monoxide
induced cerebral injury in male rats. Mol Med Rep. 19:1032–1039.
2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Arvanitakis Z, Leurgans SE, Barnes LL,
Bennett DA and Schneider JA: Microinfarct pathology, dementia, and
cognitive systems. Stroke. 42:722–727. 2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ferrari C, Nacmias B and Sorbi S: The
diagnosis of dementias: A practical tool not to miss rare causes.
Neurol Sci. 39:615–627. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kaneko D, Nakamura N and Ogawa T: Cerebral
infarction in rats using homologous blood emboli: Development of a
new experimental model. Stroke. 16:76–84. 1985.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hou K, Xu D, Li F, Chen S and Li Y: The
progress of neuronal autophagy in cerebral ischemia stroke:
Mechanisms, roles and research methods. J Neurol Sci. 400:72–82.
2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yang L, Wang H, Liu L and Xie A: The role
of insulin/IGF-1/PI3K/Akt/GSK3β signaling in Parkinson's disease
dementia. Front Neurosci. 12(73)2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Peng J, Pang J, Huang L, Enkhjargal B,
Zhang T, Mo J, Wu P, Xu W, Zuo Y, Peng J, et al: LRP1 activation
attenuates white matter injury by modulating microglial
polarization through Shc1/PI3K/Akt pathway after subarachnoid
hemorrhage in rats. Redox Biol. 21(101121)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lv Y, Liu W, Ruan Z, Xu Z and Fu L: Myosin
IIA regulated tight junction in oxygen glucose-deprived brain
endothelial cells via activation of
TLR4/PI3K/Akt/JNK1/2/14-3-3ε/NF-κB/MMP9 signal transduction
pathway. Cell Mol Neurobiol. 39:301–319. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Li L, Pan CS, Yan L, Cui YC, Liu YY, Mu
HN, He K, Hu BH, Chang X, Sun K, et al: Ginsenoside Rg1 ameliorates
rat myocardial ischemia-reperfusion injury by modulating energy
metabolism pathways. Front Physiol. 9(78)2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhou P, Xie W, He S, Sun Y, Meng X, Sun G
and Sun X: Ginsenoside Rb1 as an anti-diabetic agent and its
underlying mechanism analysis. Cells. 8(204)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wong VK, Dong H, Liang X, Bai LP, Jiang
ZH, Guo Y, Kong AN, Wang R, Kam RK, Law BY, et al: Rh2E2, a novel
metabolic suppressor, specifically inhibits energy-based metabolism
of tumor cells. Oncotarget. 7:9907–9924. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Nam Y, Wie MB, Shin E, Nguyen TL, Nah S,
Ko SK, Jeong JH, Jang C and Kim H: Ginsenoside Re protects
methamphetamine-induced mitochondrial burdens and proapoptosis via
genetic inhibition of protein kinase C δ in human neuroblastoma
dopaminergic SH-SY5Y cell lines. J Appl Toxicol. 35:927–944.
2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Li J, Liu T, Zhao L, Chen W, Hou H, Ye Z
and Li X: Ginsenoside 20(S) Rg3 inhibits the Warburg effect through
STAT3 pathways in ovarian cancer cells. Int J Oncol. 46:775–781.
2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhou JS, Wang JF, He BR, Cui YS, Fang XY,
Ni JL, Chen J and Wang KZ: Ginsenoside Rd attenuates mitochondrial
permeability transition and cytochrome C release in isolated spinal
cord mitochondria: Involvement of kinase-mediated pathways. Int J
Mol Sci. 15:9859–9877. 2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Shangguan WJ, Li H and Zhang YH: Induction
of G2/M phase cell cycle arrest and apoptosis by ginsenoside Rf in
human osteosarcoma MG 63 cells through the mitochondrial pathway.
Oncol Rep. 31:305–313. 2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zhang C, Cai T, Zeng X, Cai D, Chen Y,
Huang X, Gan H, Zhuo J, Zhao Z, Pan H, et al: Astragaloside IV
reverses MNNG-induced precancerous lesions of gastric carcinoma in
rats: Regulation on glycolysis through miRNA-34a/LDHA pathway.
Phytother Res. 32:1364–1372. 2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Dong Z, Zhao P, Xu M, Zhang C, Guo W, Chen
H, Tian J, Wei H, Lu R and Cao T: Astragaloside IV alleviates heart
failure via activating PPARα to switch glycolysis to fatty acid
β-oxidation. Sci Rep. 7(2691)2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Pereira AC, Mao X, Jiang CS, Kang G,
Milrad S, McEwen BS, Krieger AC and Shungu DC: Dorsolateral
prefrontal cortex GABA deficit in older adults with
sleep-disordered breathing. Proc Natl Acad Sci USA.
114:10250–10255. 2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kwakowsky A, Calvo-Flores Guzmán B, Pandya
M, Turner C, Waldvogel HJ and Faull RL: GABAA receptor subunit
expression changes in the human Alzheimer's disease hippocampus,
subiculum, entorhinal cortex and superior temporal gyrus. J
Neurochem. 145:374–392. 2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Jo S, Yarishkin O, Hwang YJ, Chun YE, Park
M, Woo DH, Bae JY, Kim T, Lee J, Chun H, et al: GABA from reactive
astrocytes impairs memory in mouse models of Alzheimer's disease.
Nat Med. 20:886–896. 2014.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Mayor D and Tymianski M: Neurotransmitters
in the mediation of cerebral ischemic injury. Neuropharmacology.
134:178–188. 2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Yu X, Li W, Ma Y, Tossell K, Harris JJ,
Harding EC, Ba W, Miracca G, Wang D, Li L, et al: GABA and
glutamate neurons in the VTA regulate sleep and wakefulness. Nat
Neurosci. 22:106–119. 2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Petroff OA: GABA and glutamate in the
human brain. Neuroscientist. 8:562–573. 2002.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Long H, Ruan J, Zhang M, Wang C and Huang
Y: Gastrodin alleviates Tourette syndrome via
Nrf-2/HO-1/HMGB1/NF-кB pathway. J Biochem Mol Toxicol.
33(e22389)2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Jiang B, Meng L, Zou N, Wang H, Li S,
Huang L, Cheng X, Wang Z, Chen W and Wang C: Mechanism-based
pharmacokinetics-pharmacodynamics studies of harmine and harmaline
on neurotransmitters regulatory effects in healthy rats: Challenge
on monoamine oxidase and acetylcholinesterase inhibition.
Phytomedicine. 62(152967)2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Liu J, Wu Y-Y, Yu X-L, Jia HY, Mao QY and
Fang JQ: Temporal effect of acupuncture on amino acid
neurotransmitters in rats with acute cerebral ischaemia. Acupunct
Med. 37:252–258. 2019.PubMed/NCBI View Article : Google Scholar
|