Introduction
Gliomas are the most common malignant primary brain
tumor that contribute to >50% of malignant primary brain tumor
cases worldwide (1,2). Surgical intervention, postoperative
radiotherapy and chemotherapy comprise the mainstay of treatment
for patients with high-grade glioblastoma (GBM); however, the
median survival rate remains relatively low at 12-15 months
(3,4). Thus, more antitumoral treatments are
required; therefore, further research must be performed into some
of the most promising medicinal substances to develop further
therapies to treat various types of cancer.
Considering the multi-directional differentiation
potential and other biological characteristics of glioma stem cells
(GSCs), researchers have focused on the use of GSCs for the
treatment of high-grade GBM (5). It
was demonstrated that GSCs share a metabolic pattern similar to
that of other tumor cells (6). Even
when GSCs receive sufficient oxygen, they remain highly dependent
on glycolysis to maintain their energy supply and thereby achieve
high proliferation and malignant invasion, similar to tumor cells
(7-9).
At present, the development of anticancer drugs mainly focuses on
malignant tumor cell survival/proliferation-related pathways,
aiming to inhibit tumor tissue growth by suppressing the
proliferation and migration of malignant tumor cells and inducing
cell apoptosis (10,11). Paclitaxel (PTX), among many
promising medicinal substances, is a natural diterpenoid product
used as a novel anticancer drug for patients with breast cancer,
ovarian cancer, lymphoma and non-small cell lung cancer (12). Generally, PTX regulates cell cycle
signal transduction and promotes microtubule polymerization, which
triggers G2/M-phase arrest (13).
Currently, research into the interactions between PTX and GSCs is
limited to signaling pathways of glioma genesis, whereas the
medicinal effect on the GSCs metabolism is yet to be uncovered.
In the present study, CD133+ glioma cells were
treated with PTX at various concentrations to investigate the
effect of PTX on the expression levels of relevant glycolytic genes
in stem cell-like glioma cell lines, which hopefully provided an
experimental basis for the development of novel treatments for
gliomas.
Materials and methods
Reagents
The human glioma cell line U251 was purchased from
The Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences. Other experimental materials include the
human-lyophilized CD133 MicroBead kit (cat. no. 130097049; Miltenyi
Biotec GmbH), Cell Counting Kit (CCK)-8 (Dojindo Molecular
Technologies, Inc.), glucose transporter 1 (GLUT1; cat. no. 12939),
pyruvate kinase M (PKM; cat. no. 3190), lactate dehydrogenase A
(LDHA; cat. no. 3582) and GAPDH (cat. no. 8884) primary antibodies
(Cell Signaling Technology, Inc.), goat anti-rabbit imunogobulin G
secondary antibody (cat. no. SA00001-2; ProteinTech Group, Inc.),
FBS and DMEM (Gibco; Thermo Fisher Scientific, Inc.), SYBR Green
I-based real-time PCR reagent (cat. no. 03003230001; Roche
Molecular Diagnostics), reverse transcription-quantitative PCR
(RT-qPCR) primers (Wuhan Jin Kairui Biological Engineering Co.,
Ltd.) and gelatin-coated polycarbonate membrane filters (Corning
Inc.).
Cell culture and sorting of CCD133+
U251 glioma cells with D133 immunomagnetic beads
Following resuscitation, U251 glioma cells were
added to DMEM containing 10% FBS and placed in an incubator at 37˚C
and 5% CO2, for culturing. During the exponential phase
of growth, 1 ml of culture media containing ~1x109 cells, were
collected for isolation of CD133+ cells according to the
instructions provided in the CD133 MicroBead kit.
CCK-8 assay for evaluating the
viability of PTX-treated CD133+ U251 cells
CCK8 experiments were performed according to the
manufacturer's instructions. CD133+ U251 cells were used as the
experimental group and the CD133- cells in the residue were used as
a control group. The CD133- cells were cultured in a 96-well plate,
with each well containing 1x104 cells. After 24, 48 or 72 h, CCK-8
reagent (10 µl/well) was added to each adherent culture to observe
cell growth in each well. Meanwhile, CD133+ U251 cells were
cultured in another 96-well plate at a density of 1x104 cells/well.
After becoming adherent, PTX at various concentrations (1, 2, 4 and
8 µM/well) was added to the cells. The cells were then cultured for
72 h before addition of CCK-8 reagent (10 µl/well). Finally, the
optical density in each well was measured at a wavelength of OD 450
nm to evaluate cell growth.
RT-qPCR analysis of glycolytic gene
expression levels
Following cell culture, U251 cells were plated on a
6-well plate at a density of 1x106 cells/ml. Various concentrations
of PTX (1, 2, 4, and 8 µM/well) were added once the cells became
adherent. The cells were harvested following treatment with PTX for
72 h. TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.) was used
for RNA extraction and 3 µg RNA obtained from each culture, RT kit
(cat. no. 04913850001; Roche Molecular Diagnostics) was used for
reverse transcription. cDNA was synthesized after incubation using
the following temperature protocol: 55˚C for 30 min, 85˚C for 5 min
and 4˚C for 10 min in the presence of 0.5 µl reverse transcriptase,
1.0 µl oligDT18, 4.0 µl buffer, 2.0 µl dNTPs, and 12 µl
DEPC water. NCBI primer-designer (www.ncbi.nlm.nih.gov/tools/primer-blast) was employed
for designing primers. Target genes and GAPDH were subject to
RT-qPCR using the SYBR Green I-based real-time PCR reagent with 40
cycles of the following thermocycling conditions: 95˚C for 2 min,
50˚C for 1 min and 72˚C for 1 min. The primer sequences of GLUT1,
PKM, LDHA and GAPDH used for the PCR are shown in Table I. Fluorescence collection and data
analysis were conducted using the software attached to the RT-qPCR
system. The relative expression levels of the target genes in the
different concentration subgroups were calculated using the 2-ΔΔCq
method and normalized to GAPDH (14).
 | Table ISequences of primers used in the
present study. |
Table I
Sequences of primers used in the
present study.
| Gene | Primer sequences
(5'-3') | Base pairs | GenBank accession
number |
|---|
| GLUT1 | F:
5'-CTATGGGGAGAGCATCCTGC-3' | 195 | NM_006516.3 |
| | R:
5'-CCCAGTTTCGAGAAGCCCAT-3' | | |
| PKM | F:
5'-GTGGCAAGCACACTGGATTAG-3' | 196 | NM_001206799.2 |
| | R:
5'-GAATCAATGTCCAGGCGGCA-3' | | |
| LDHA | F:
5'-CGTCGATATTCCTTTTCCACG-3' | 197 | NM_001165414.1 |
| | R:
5'-AGCAAGTTCATCTGCCAAGTC-3' | | |
| GAPDH | F:
5'-TGGACTCCACGACGTACTCAG-3' | 162 | NM_001256799.3 |
| | R:
5'-ACATGTTCCAATATGATTCCA-3' | | |
Western blot analysis for GLUT1, PKM
and LDHA protein expression detection
Cells were treated as aforementioned. Subsequently,
1 ml pre-cooled PBS was added to each well to wash the cells twice.
Protein lysis buffer (cat. no. P0013B; Beyotime Institute of
Biotechnology) was added (100 µl/well) to extract proteins from the
cells. The BCA protein assay kit was used for protein
quantification. Equal amounts of protein (30 µg/lane) were analyzed
using 10% SDS-PAGE before being electro-transferred onto a PVDF
membrane. Membranes were then blocked with 5% BSA (cat. no. P0252;
Beyotime Institute of Biotechnology) for 2 h at room temperature.
The protein bands were normalized to GAPDH (cat. no. P0063;
Beyotime Institute of Biotechnology). Membranes were incubated with
primary antibodies diluted to 1:2,000 and placed on a shaking Table
at 4˚C for overnight culture. Following primary antibody
incubation, membranes were incubated with secondary antibodies
diluted to 1:10,000 at room temperature for 1 h. Enhanced
chemiluminescent reagent (cat. no. P0018AS; Beyotime Institute of
Biotechnology) was added before exposure using an automatic
exposure system. Grayscale values were measured using ImageJ
(version no. V1.8.0.112; National Institutes of Health).
Transwell migration assay for
assessing cell invasion ability
The Transwell migration assay was performed using
the 24-well Transwell with gelatin-coated polycarbonate membrane
filter. Firstly, cells were plated on a 6-well plate at a density
of 1x106 cells/well and cultured until they reached 90-95%
confluency. 1, 2, 4, 8 µM/ml PTX solution was then added into the
each well for 48 h of incubation. Subsequently, the treated cells
were detached from the plates using 0.5 ml trypsin and then seeded
in the upper chambers which precoated with 500 ng/ml Matrigel
solution (BD Biosciences) of the Transwell chamber (~1x104
cells/well), while DMEM supplemented with 10% FBS was added into
the lower chambers. The cells were then incubated in the chambers
for 24 h at 37˚C. Finally, the chambers were stained with 0.1%
crystal violet at room temperature for 30 min, and imaged using a
light microscope at x200 magnification.
Statistical analysis
Each experiment was replicated three times, with
each concentration having internal triplicates. GraphPad Prism 6.0
(GraphPad Software, Inc.) was used for experimental drawing.
Student's t-test was used to analyze the differences between two
groups. One-way ANOVA followed by Tukey's post hoc test was used to
analyze differences among multiple groups. Experimental data were
counted with software SPSS 22.0 (IBM Corp.). P<0.05 was
considered to indicate a statistically significant difference.
Results
CD133+ U251 glioma cells exhibit
higher proliferation compared with CD133- cells
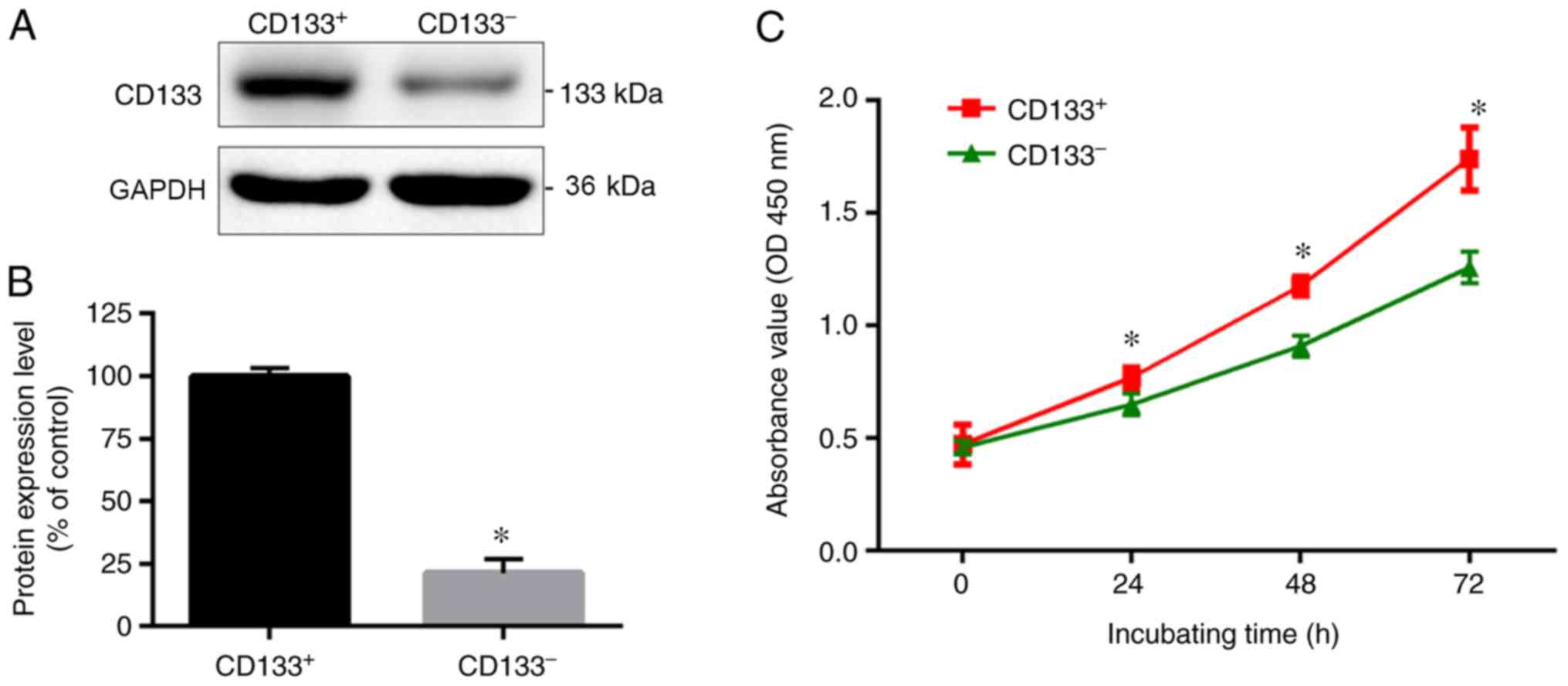
Malignant CD133+ U251 glioma cells were successfully
obtained using CD133 immunomagnetic beads. As shown in Fig. 1, the western blotting results
indicated that CD133 protein expression levels were significantly
higher in CD133+ U251 cells compared with CD133- cells. From the
CCK-8 growth curve, the optical densities of CD133+ U251 cells at
48 and 72 h were significantly higher compared with CD133- cells
(P<0.05). These data indicated that CD133+ U251 glioma cells had
a higher proliferation rate compared with CD133- cells.
PTX acts on CD133+ U251 glioma cells
as a cell growth inhibitor
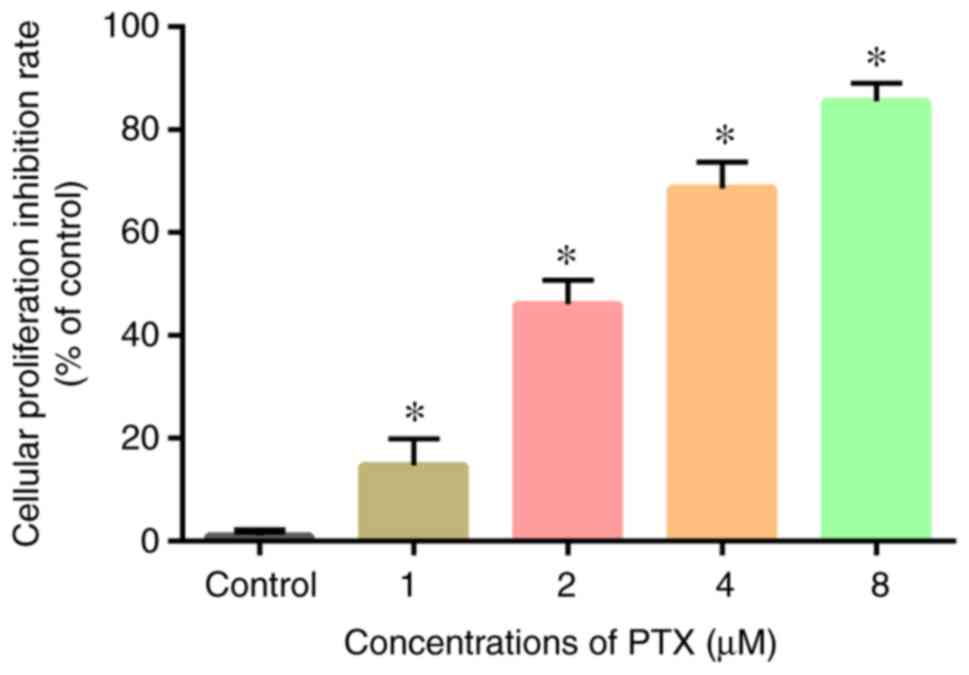
Following continuous culture and sorting of CD133+
U251 cells, PTX at various concentrations (1, 2, 4 and 8 µM/well)
was added to each group. As shown in Fig. 2, compared with the blank control
group, a higher PTX concentration resulted in greater inhibition on
the cell growth rate (P<0.05). This indicated that the cell
growth inhibition rate positively associated with the concentration
of PTX. These results show that PTX inhibited the growth of CD133+
U251 glioma cells in a dose-dependent manner.
PTX suppresses GLUT1, PKM and LDHA
mRNA expression levels in CD133+ U251 glioma cells
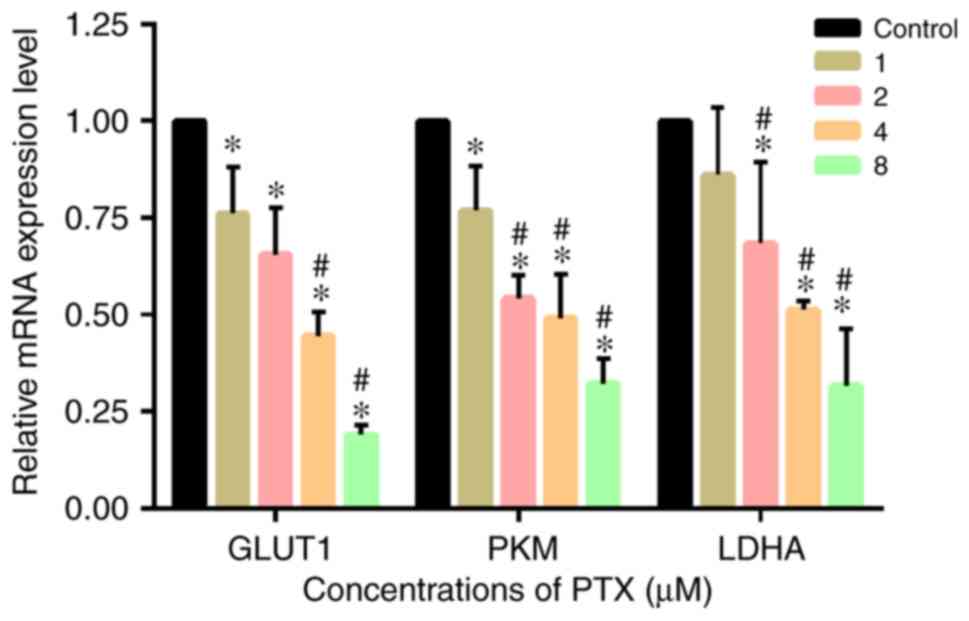
PTX at various concentrations was added to CD133+
U251 glioma cells for 72 h. RT-qPCR was performed to examine GLUT1,
PKM and LDHA mRNA expression levels. As shown in Fig. 3, compared with the control group,
the experimental groups had significantly lower mRNA expression
levels of GLUT1, PKM and LDHA (P<0.05). mRNA expression levels
decreased as the PTX concentration increased, indicating that PTX
effectively inhibited the transcription of the key glycolytic
enzymes in CD133+ glioma cells.
PTX suppresses GLUT1, PKM and LDHA
protein expression levels in CD133+ U251 glioma cells
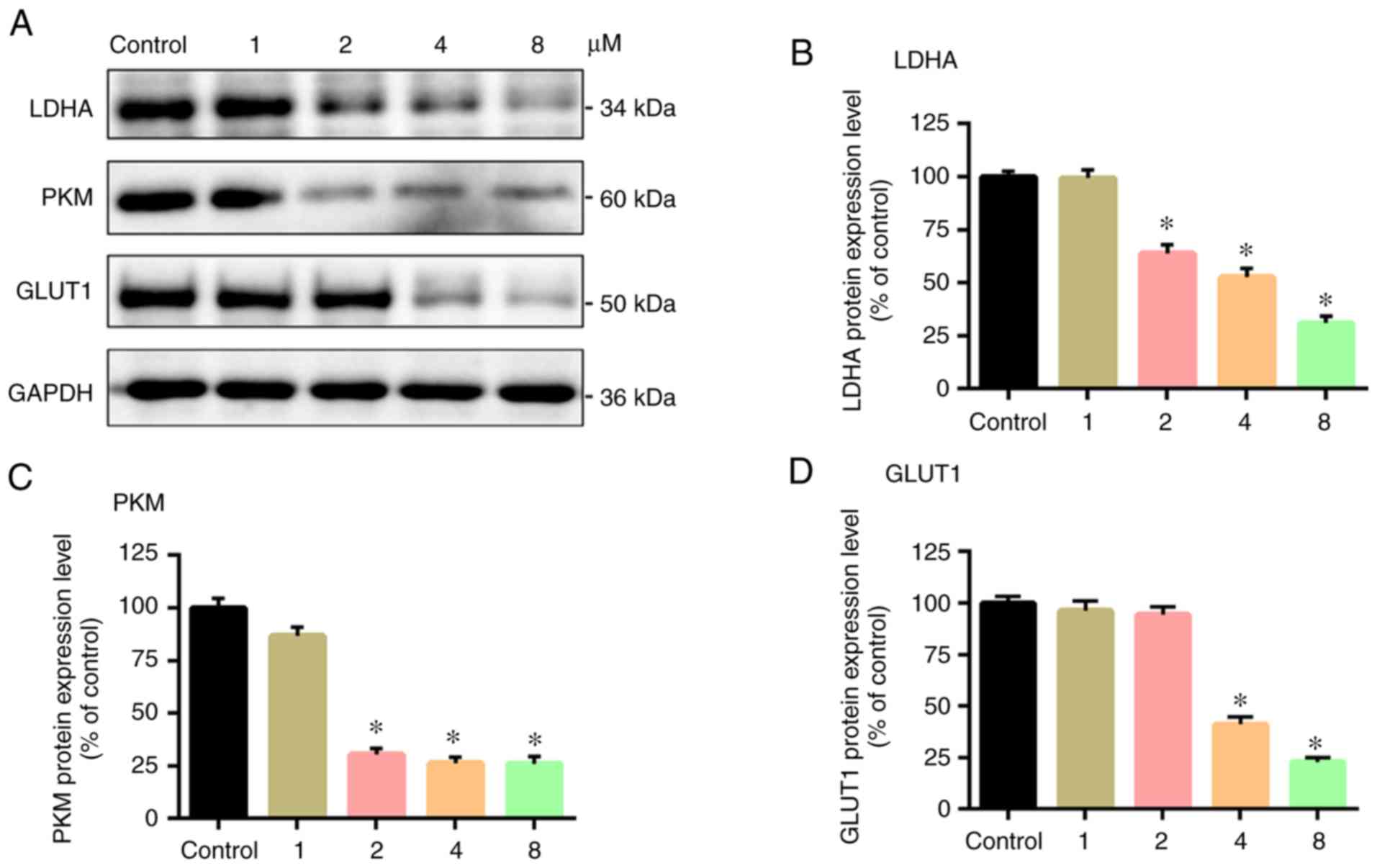
Western blot assays were performed to measure the
expression levels of the three glycolytic enzymes. As shown in
Fig. 4, GLUT1, PKM and LDHA protein
expression levels in the experimental groups were lower compared
with the control group (P<0.05), and the protein expression
levels reduced in a negative association with the PTX
concentration. This indicated that in terms of protein translation,
PTX inhibited the expression levels of the glycolytic enzymes in
CD133+ glioma cells.
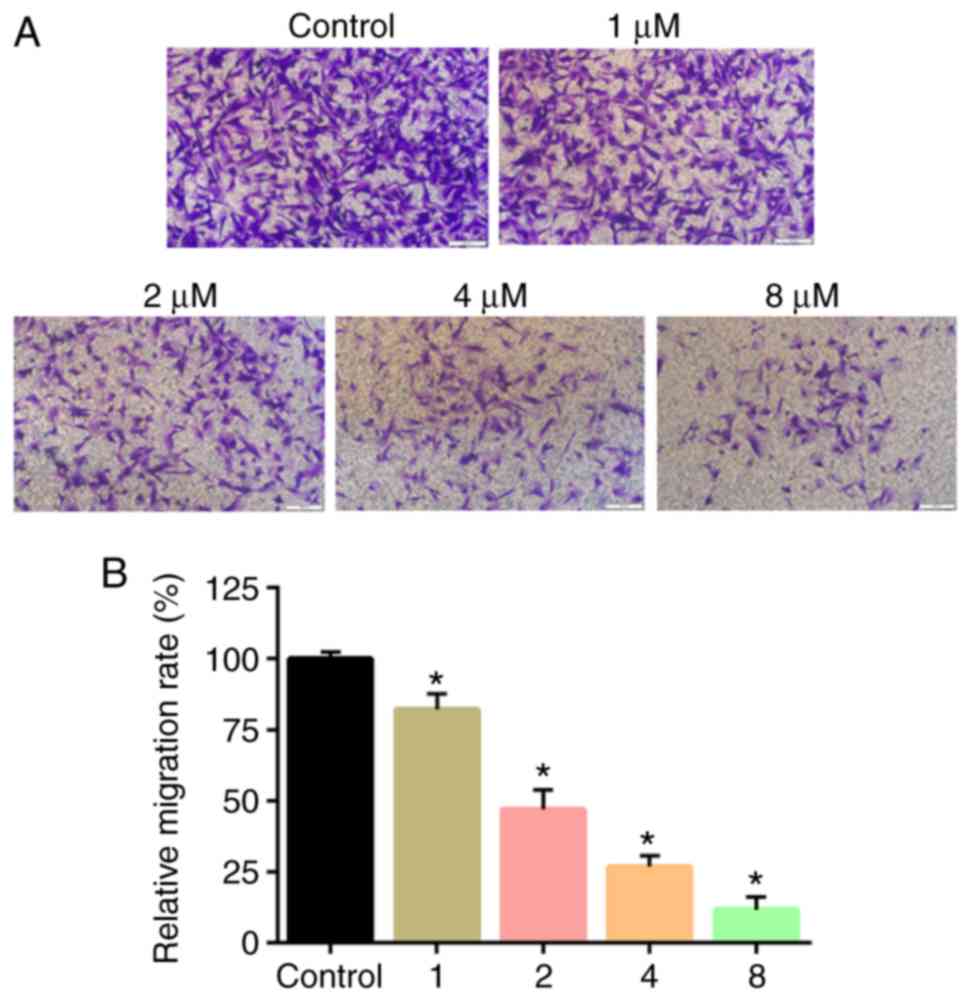
PTX treatment inhibits the migration
of CD133+ U251 cells
Transwell assays were used to evaluate in vitro cell
migration. Transwell assay results revealed that PTX treatment
significantly attenuated the migratory ability of the cells
compared with controls (Fig. 5A).
The differences in the colony count and the migratory ability was
found to be statistically significant (P<0.05; Fig. 5B).
Discussion
Cancer stem cells (CSCs) comprise a special cell
population characterized by self-renewal, cell immortalization and
a multi-directional differentiation potential (15). CSCs are present in most tumor tissue
and cancer cell populations, such as brain cancer, lymphoma, breast
cancer and lung cancer (16,17).
As a brain CSC marker, CD133 is closely associated with the
malignant proliferation and infiltration of cancer cells (18). Compared with normal cells, CD133+
CSCs are more dependent on glycolysis and rely on anaerobic glucose
metabolism via glycolysis for energy production (2). In the present study, immunomagnetic
beads were used to sort U251 glioma cells, which expressed high
levels of the CD133 protein. Based on the CCK-8 assay, it was found
that the CD133+ cells had a higher proliferative ability compared
with CD133- cells. Following PTX treatment of CD133+ cells, the
present study found that the expression levels of the genes and
proteins in the key glycolytic enzymes were significantly
suppressed, which suggested that PTX acted as a cancer cell growth
inhibitor by suppressing these genes.
The present study showed that GLUT1 expression is
negatively affected by PTX to a degree, where a low PTX dose is
less effective. According to previous reports, cancer cells require
GLUTs to transport glucose into their cytoplasm, thereby
strengthening glycolytic function and providing energy for cancer
cell growth and proliferation (19,20).
Elevated expression levels of GLUT1/3 leads to enhanced glucose
uptake in GSCs, making glycolysis the primary metabolic source of
energy for GSCs (21). In
particular, GLUT1 serves as an important regulator for the
development and progression of a range of tumors, including gliomas
and other malignant tumors (22).
It was reported that GLUT1 expression is abnormally high in many
types of cancer, including gastric, colon, bladder, liver,
colorectal and lung cancers (23).
These results suggest that GLUT1, a primary metabolic gene, is
possibly one of many critical contributors to the cancer
suppression induced by PTX.
Secondly, the present study found that PTX
effectively inhibited PKM mRNA and protein expression in GSCs. A
previous study showed that PKM plays a role in the final step of
glycolysis, namely converting the midway product into pyruvate
acid, that eventually yields ATP (24). Although PKM is necessary for cancer
cell growth, proliferation and metastasis, it has not investigated
in relation to glioma metabolism (25,26).
The effect of PTX at a lower dosage on PKM expression is much more
sensitive compared to that of GLUT1 expression. These findings not
only suggest that PTX has an antitumoral effect through interfering
with the glycolysis process, but also suggest that PTX, possibly
through a negative feedback loop from the accumulation of
intermediate products, has a more substantial effect on the late
steps in the glycolysis as opposed to directly affecting the GLUT1
expression levels. The PTX downregulated LDHA supports the idea
also.
The present study also found, in the PTX-treated
GSCs, LDHA expression was significantly inhibited. LDHA catalyzes
the conversion of pyruvic acid to lactic acid and is highly
expressed in most cancer cells (27). Studies have shown that lactic acid
produced from glycolysis is transferred out of the cells and
acidifies the local microenvironment, which not only protects
cancer cells from being killed by the host immune cells but also
impairs the effect of anticancer drugs on the cancer cells
(28). Additionally, an acidified
microenvironment is likely to induce cell matrix degradation,
microvessel formation and the Warburg effect (29). Moreover, silencing LDHA inhibited
proliferation, induced apoptosis and increased the chemosensitivity
of glioma cells to temozolomide (30). This indicates that PTX may
effectively inhibit cancer cell growth and achieve its therapeutic
effect by mediating the expression of glycolysis-related apoenzymes
in GSCs.
In conclusion, this present study designed a
PTX-treated GSC model to preliminarily explore the effect of PTX on
glycolysis in glioma cells that had high expression levels of
CD133. These results provide a theoretical basis for in vivo
experiments exploring the use of PTX for the treatment of cancers
by altering the expression of other signaling proteins leading to
EMR of glycolysis in gliomas.
Acknowledgements
Not applicable.
Funding
This study was supported by the Major State Research
Development Program of China (grant no. 2016YFC0106107), National
Natural Science Foundation of China (grant no. 81560409), Program
for Changjiang Scholars and Innovative Research Team in University
(grant no. IRT13058), Joint Fund Project of Guizhou Provincial
Science and Technology Department [grant nos. QianKeHe LH
(2016)7236 and QianKeHe (2016) support 2905].
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL and NL designed the study. SP and JJ performed
the experiments. NL analyzed the data and drafted the manuscript.
LC and MD supervised the study and drafted and revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fan X, Li Y, Shan X, You G, Wu Z, Li Z,
Qiao H and Jiang T: Seizures at presentation are correlated with
better survival outcomes in adult diffuse glioma: A systematic
review and meta-analysis. Seizure. 59:16–23. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sun X, Chen Y, Zhao H, Qiao G, Liu M,
Zhang C, Cui D and Ma L: Dual-modified cationic liposomes loaded
with paclitaxel and survivin siRNA for targeted imaging and therapy
of cancer stem cells in brain glioma. Drug Deliv. 25:1718–1727.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bastiancich C, Bianco J, Vanvarenberg K,
Ucakar B, Joudiou N, Gallez B, Bastiat G, Lagarce F, Préat V and
Danhier F: Injectable nanomedicine hydrogel for local chemotherapy
of glioblastoma after surgical resection. J Control Release.
264:45–54. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Jiapaer S, Furuta T, Tanaka S, Kitabayashi
T and Nakada M: Potential Strategies Overcoming the Temozolomide
Resistance for Glioblastoma. Neurol Med Chir (Tokyo). 58:405–421.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sakamoto D, Takagi T, Fujita M, Omura S,
Yoshida Y, Iida T and Yoshimura S: Basic Gene Expression
Characteristics of Glioma Stem Cells and Human Glioblastoma.
Anticancer Res. 39:597–607. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liebelt BD, Shingu T, Zhou X, Ren J, Shin
SA and Hu J: Glioma stem cells: Signaling, microenvironment, and
therapy. Stem Cells Int. 2016(7849890)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Seyfried TN, Flores R, Poff AM, D'Agostino
DP and Mukherjee P: Metabolic therapy: A new paradigm for managing
malignant brain cancer. Cancer Lett. 356A:289–300. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nakano I: Stem cell signature in
glioblastoma: Therapeutic development for a moving target. J
Neurosurg. 122:324–330. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Codrici E, Enciu AM, Popescu ID, Mihai S
and Tanase C: Glioma Stem Cells and Their Microenvironments:
Providers of Challenging Therapeutic Targets. Stem Cells Int.
2016(5728438)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shokoohinia Y, Jafari F, Mohammadi Z,
Bazvandi L, Hosseinzadeh L, Chow N, Bhattacharyya P, Farzaei MH,
Farooqi AA, Nabavi SM, et al: Potential Anticancer Properties of
Osthol: A Comprehensive Mechanistic Review. Nutrients. 10:36–51.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gao C, He XF, Xu QR, Xu YJ and Shen J:
Sevoflurane downregulates insulin-like growth factor-1 to inhibit
cell proliferation, invasion and trigger apoptosis in glioma
through the PI3K/AKT signaling pathway. Anticancer Drugs.
30(e0744)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Eissa IR, Bustos-Villalobos I, Ichinose T,
Matsumura S, Naoe Y, Miyajima N, Morimoto D, Mukoyama N, Zhiwen W,
Tanaka M, et al: The Current Status and Future Prospects of
Oncolytic Viruses in Clinical Trials against Melanoma, Glioma,
Pancreatic, and Breast Cancers. Cancers (Basel).
10(E356)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shen X, Liu X, Wan S, Fan X, He H, Wei R,
Pu W, Peng Y and Wang C: Discovery of coumarin as microtubule
affinity-regulating kinase 4 inhibitor that sensitize
hepatocellular carcinoma to paclitaxel. Front Chem.
7(366)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Barbato L, Bocchetti M, Di Biase A and
Regad T: Cancer stem cells and targeting strategies. Cells. 8:1–19.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Seano G: Targeting the perivascular niche
in brain tumors. Curr Opin Oncol. 30:54–60. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Katayama R, Sakashita T, Yanagitani N,
Ninomiya H, Horiike A, Friboulet L, Gainor JF, Motoi N, Dobashi A,
Sakata S, et al: P-glycoprotein Mediates Ceritinib Resistance in
Anaplastic Lymphoma Kinase-rearranged Non-small Cell Lung Cancer.
EBio Medicine. 12:54–66. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hossain M, Banik NL and Ray SK:
Synergistic anti-cancer mechanisms of curcumin and paclitaxel for
growth inhibition of human brain tumor stem cells and LN18 and
U138MG cells. Neurochem Int. 61:1102–1113. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jiang X, Xin H, Ren Q, Gu J, Zhu L, Du F,
Feng C, Xie Y, Sha X and Fang X: Nanoparticles of 2-deoxy-D-glucose
functionalized poly(ethylene glycol)-co-poly(trimethylene
carbonate) for dual-targeted drug delivery in glioma treatment.
Biomaterials. 35:518–529. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ancey PB, Contat C and Meylan E: Glucose
transporters in cancer - from tumor cells to the tumor
microenvironment. FEBS J. 285:2926–2943. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Dacevic MP, Tasic JS, Pejanovic VM, Segal
MB, Ugliesic-Kilibarda DD, Isakovic AJ, Begley DJ, Rakic LM and
Redzic ZB: The linkage of glucose to tiazofurin decreases in vitro
uptake into rat glioma C6 cells. J Drug Target. 10:633–636.
2002.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Oh S, Kim H, Nam K and Shin I: Glut1
promotes cell proliferation, migration and invasion by regulating
epidermal growth factor receptor and integrin signaling in
triple-negative breast cancer cells. BMB Rep. 50:132–137.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Madunić IV, Madunić J, Breljak D, Karaica
D and Sabolić I: Sodium-glucose cotransporters: New targets of
cancer therapy? Arh Hig Rada Toksikol. 69:278–285. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Jin X, Su H, Ding G, Sun Z and Li Z:
Exposure to ambient fine particles causes abnormal energy
metabolism and ATP decrease in lung tissues. Chemosphere.
224:29–38. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ma Z, Cui X, Lu L, Chen G, Yang Y, Hu Y,
Lu Y, Cao Z, Wang Y and Wang X: Exosomes from glioma cells induce a
tumor-like phenotype in mesenchymal stem cells by activating
glycolysis. Stem Cell Res Ther. 10(60)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tech K, Tikunov AP, Farooq H, Morrissy AS,
Meidinger J, Fish T, Green SC, Liu H, Li Y, Mungall AJ, et al:
Pyruvate kinase inhibits proliferation during postnatal cerebellar
neurogenesis and suppresses medulloblastoma formation. Cancer Res.
77:3217–3230. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Miao P, Sheng S, Sun X, Liu J and Huang G:
Lactate dehydrogenase A in cancer: A promising target for diagnosis
and therapy. IUBMB Life. 65:904–910. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Xintaropoulou C, Ward C, Wise A,
Queckborner S, Turnbull A, Michie CO, Williams ARW, Rye T, Gourley
C and Langdon SP: Expression of glycolytic enzymes in ovarian
cancers and evaluation of the glycolytic pathway as a strategy for
ovarian cancer treatment. BMC Cancer. 18:636–651. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li S, Gao J, Zhuang X, Zhao C, Hou X, Xing
X, Chen C, Liu Q, Liu S and Luo Y: Cyclin G2 Inhibits the Warburg
Effect and Tumour Progression by Suppressing LDHA Phosphorylation
in Glioma. Int J Biol Sci. 15:544–555. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Di H, Zhang X, Guo Y, Shi Y, Fang C, Yuan
Y, Wang J, Shang C, Guo W and Li C: Silencing LDHA inhibits
proliferation, induces apoptosis and increases chemosensitivity to
temozolomide in glioma cells. Oncol Lett. 15:5131–5136.
2018.PubMed/NCBI View Article : Google Scholar
|