Introduction
Chronic obstructive pulmonary disease (COPD) is an
inflammatory process that predominantly affects lung parenchyma and
small airways, resulting in progressive and largely irreversible
airflow limitation (1).
COPD-affected lungs exhibit inflammatory changes that involve
immune cells, such as neutrophils and macrophages (MPs), alongside
the activation of structural cells, including alveolar epithelial
cells (EpCs) and fibroblasts (2,3). M§Ps
are major contributors to inflammation and produce mediators that
activate the inflammatory transcriptional program of other cells
(4). For example, EpCs and MPs in
COPD-affected lung tissues have been reported to be induced by
bacteria to release inflammatory mediators, including tumor
necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6 and reactive
oxygen species, in addition to secreting elastolytic enzymes, such
as matrix metalloproteinases (3,5). In
addition, EpCs have been observed to modulate their primary
transcriptional program in response to inflammatory mediators, such
as TNF-α released by MPs stimulation (6). In fact, in one study, activated EpCs
were found to negatively regulate monocyte cytokine gene
expression, which influenced the maturation of monocytes to MPs,
and potentially further modulated cytokine release through
post-translational pathways (7).
EpCs have also been demonstrated to modulate MP phenotypes;
co-cultured A549 cells significantly increased the expression levels
of CD11b, CD14, CD54 and human leukocyte antigen-DR isotype in the
human monocyte/MP cell line, THP-1(8).
In COPD, there is an increased presence of numerous
types of inflammatory mediators, such as interleukins and
chemokines, which have derived from the inflammatory and structural
cells of the lungs and airways (5,9).
Proinflammatory cytokines are induced by numerous stimuli through
the NF-κB and Janus tyrosine kinase/STAT signaling cascades. For
example, the exacerbation of chronic respiratory diseases like COPD
is often triggered by a bacterial or viral infection (10); lipopolysaccharide (LPS), a
constituent of the outer cell membrane of Gram-negative bacteria,
binds to Toll-like receptor 4 (TLR4) located in the cell membrane
and activates the downstream transcriptional factor, NF-κB
(11), which mediates the
transcription and translation of proinflammatory mediators, such as
IL-6 and TNF-α. Thus, the present study aimed to investigate the
inflammatory response, including the release of cytokines and
signaling pathways, involved in LPS-induced EpCs/MPs
co-culture.
Materials and methods
Cell culture
The human lung cancer A549 cell line and THP-1 cells
were purchased from the American Type Culture Collection. Cells
were cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc.), supplemented with 0.05 nM β-mercaptoethanol and 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.). Cells were maintained at
37˚C in a 5% CO2 atmosphere. The A549 cells were to
represent EpCs, whereas THP-1 cells represented MPs. EpCs were
first cultured in 24-well culture plates until they had completely
adhered and subsequently the medium was removed, and the cells were
washed twice with PBS to exclude serum factor effects before
co-culturing. The MPs were plated in a Transwell insert and
physically separated with a 0.4-µm pore polyester filter (Corning,
Inc.) to avoid direct contact with the EpCs.
Cell viability assay
Following overnight culture in a 96-well plate at
37˚C, 1x104 EpCs were exposed to 0.5, 1, 2 or 4 µg/ml
LPS (Escherichia coli O55:B5; cat. no. L6529; Sigma-Aldrich, Merck
KGaA) for 6, 12, 24 and 48 h, and EpC viabilities were quantified
using MTT assays. Untreated cells were acted as control. Briefly,
100 µl MTT solution (1 mg/ml) was added to each well and following
4 h of incubation at 37˚C, the supernatants were removed and 150 µl
DMSO was added/well. The absorbance/well was measured at 570 nm
using ELX800 microplate reader (BioTek Instruments, Inc.).
Meanwhile, 5x104 MPs were plated in a
96-well plate with 10 µl Alamar Blue™ agent (cat. no. 4020ES76;
Yeasen Biotechnology Co., Ltd.) per well. At the same time, as the
cells were added to the wells, 0.5, 1, 2 or 4 µg/ml LPS was added
and the cells were exposed for 6, 12, 24 and 48 h. The
absorbance/well was measured at 570 and 600 nm using a microplate
reader. Untreated cells were used as the control.
ELISAs
From analyzing the effects of LPS on cell viability,
1 and 2 µg/ml LPS were selected as the optimum doses to use in
further experiments. EpCs/MPs (1x105 of each cell type)
were co-cultured in a 24-well Transwell plate and then stimulated
with 1 or 2 µg/ml LPS for 6, 12, 24 and 48 h. Untreated cells were
used as the control. The concentrations of IL-6, IL-1β, IL-8 and
TNF-α in conditioned media were subsequently analyzed using ELISA
kits (Boster Biological Technology; cat. no. IL-6, EK0410; IL-1β,
EK0392; IL-8, EK0413; TNF-α, EK0525), according to the
manufacturers' protocols. Subsequently, monocultures of EpCs
(1x105 cells) and MPs (1x105 cells), as well
as the co-culture of EpCs/MPs (1x105 of each cell type)
were plated in 12-well culture plate, and following stimulation
with 2 µg/ml LPS for 6, 12, 24 and 48 h, the cytokine levels were
determined using the aforementioned ELISA kits.
Detection of NF-κB DNA-binding
activity of EpCs and MPs using an electrophoretic mobility shift
assay (EMSA)
Following the induction with 2 µg/ml LPS for 6, 12,
24 and 48 h, EpCs and MPs were centrifuged for 5 min with the speed
of 500 x g at 4˚C to extract nuclear proteins using
NE-PER™ Nuclear and Cytoplasmic Extraction Reagents
(cat. no. 78833; Thermo Fisher Scientific, Inc.). Nuclear extract
protein was quantified using a bicinchoninic acid (BCA) assay
(Boster Biological Technology). The activity of NF-κB was detected
using an EMSA. The following NF-κB oligonucleotide probes were
obtained from Beyotime Institute of Biotechnology (cat. no.
1302131410): Forward, 5'-AGTTGAGGGGACTTTCCCAGGC-3' and reverse,
3'-TCAACTCCCCTGAAAGGGTCCG-5'. Binding reactions were performed at
room temperature for 20 min in 10 µl binding buffer (cat. no.
GS006; Beyotime Institute of Biotechnology), containing 10 µg
nuclear extracts, nuclease-free water, EMSA gel-shift buffer and
oligonucleotide probes. The biotin-labeled DNA oligo probe without
any protein extract was regarded as the negative control.
DNA-protein complexes were separated from free DNA probes at 120 V
on 5% polyacrylamide gels with 0.5X Tris Boric acid EDTA buffer.
Following electrophoresis, the gels were transferred to a nylon
membrane and detected using a chemiluminescent substrate (cat. no.
1419701; EMD Millipore). The signal intensity was quantified using
an image analyzer (Bio-Rad Laboratories, Inc.).
Western blotting
The expression levels of upstream proteins of the
NF-κB signaling pathway were detected using western blotting
following 24 h of EpC/MP co-culture treated with 2 µg/ml LPS. Total
protein was obtained by RIPA lysis buffer (cat. no. R0010; Beijing
Solarbio Science and Technology Co., Ltd.), and quantified using a
BCA assay. Then 20 µg protein/lane was separated via 8% SDS-PAGE.
The separated proteins were subsequently transferred to
polyvinylidene fluoride membranes (EMD Millipore) and blocked with
5% skimmed milk powder diluted in Tris-buffered saline -0.05% Tween
20 (TBST) buffer at room temperature for 1 h. The membranes were
incubated with the following primary antibodies at 4˚C overnight:
Anti-TLR4 (1:500; cat. no. 19811-1-AP; ProteinTech Group, Inc.),
anti-phosphorylated (p)-p65 (1:2,000; cat. no. ab76302; Abcam),
anti-p65 (1:2,000; cat. no. ab32536; Abcam), anti-p-ERK (1:2,000;
cat. no. ab223500; Abcam), anti-ERK (1:1,500; cat. no. ab201015;
Abcam) and anti-GAPDH (1:2,000; cat. no. ab128915; Abcam).
Following the primary antibody incubation, the membranes were
washed five times with TBST buffer and incubated with a horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody
(1:15,000; cat. no SA00001-2; ProteinTech Group, Inc.) at room
temperature for 2 h. Subsequently, the membranes were washed five
times with TBST. Protein bands were visualized with an enhanced
chemiluminescence reagent (EMD Millipore) and a Gel Doc XR+ system
(Bio-Rad Laboratories, Inc.). Expression levels were quantified
using Image Lab software version 5.1 (Bio-Rad Laboratories,
Inc.).
Investigating the effect of NF-κB
inhibition on the cytokine levels
To confirm whether the levels of proinflammatory
cytokines were regulated by the NF-κB signaling pathway,
co-cultured EpCs/MPs were pretreated with 100 µM
pyrrolidinedithiocarbamate (PDTC; Beyotime Institute of
Biotechnology), a selective NF-κB inhibitor (12), at 37˚C for 2 h prior to stimulation
with 2 µg/ml LPS for a further 24 h. The levels of IL-6, IL-1β,
IL-8 and TNF-α in the co-culture supernatant were measured using
the aforementioned commercial ELISA kits.
Statistical analysis
Statistical analysis was performed using SPSS 21.0
software (IBM Corp.) and data are presented as the mean ± SD.
Statistical differences between groups were determined using
one-way ANOVAs, followed by a Tukey's post hoc test for multiple
comparisons in data with >2 groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of LPS stimulation on cell
proliferation and viability
Cytotoxicity assays, which reflect both the level of
cell damage and alterations in cellular proliferation, were used to
investigate the cytotoxic effects of LPS-treated EpCs and MPs.
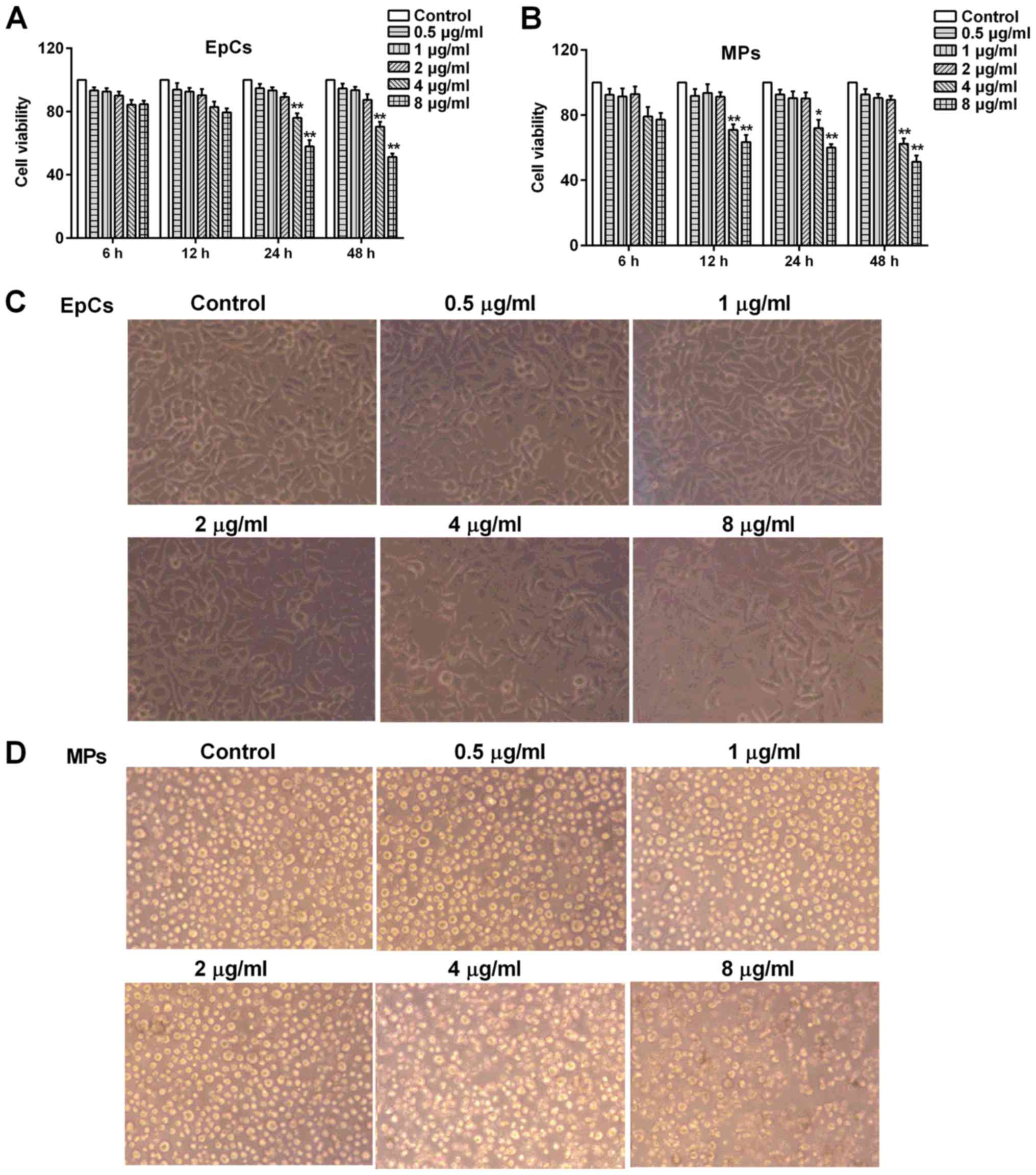
Following the treatment with LPS at various concentrations (0.5, 1,
2, 4 and 8 µg/ml) for 6, 12, 24 and 48 h, the viability of EpCs
were analyzed using an MTT assay, whereas MP viability was
determined using an alamarBlue assay. LPS was found to be non-toxic
to EpCs or MPs at concentrations between 0.5-2 µg/ml (Fig. 1A and B); however, 4 and 8 µg/ml LPS
significantly decreased the cell viability of both EpCs and MPs at
12, 24 and 48 h. In addition, 0.5-4 µg/ml LPS did not affect the
cell morphology of EpCs (Fig. 1C)
or MPs (Fig. 1D), thus 1 and 2
µg/ml LPS were selected as doses of LPS to use in subsequent
experiments.
Effect of LPS on cytokine levels in
EpC and MP monocultures, and EpC/MP co-cultures
Co-cultured EpCs/MPs were stimulated with 1 or 2
µg/ml LPS for different durations and the levels of secreted
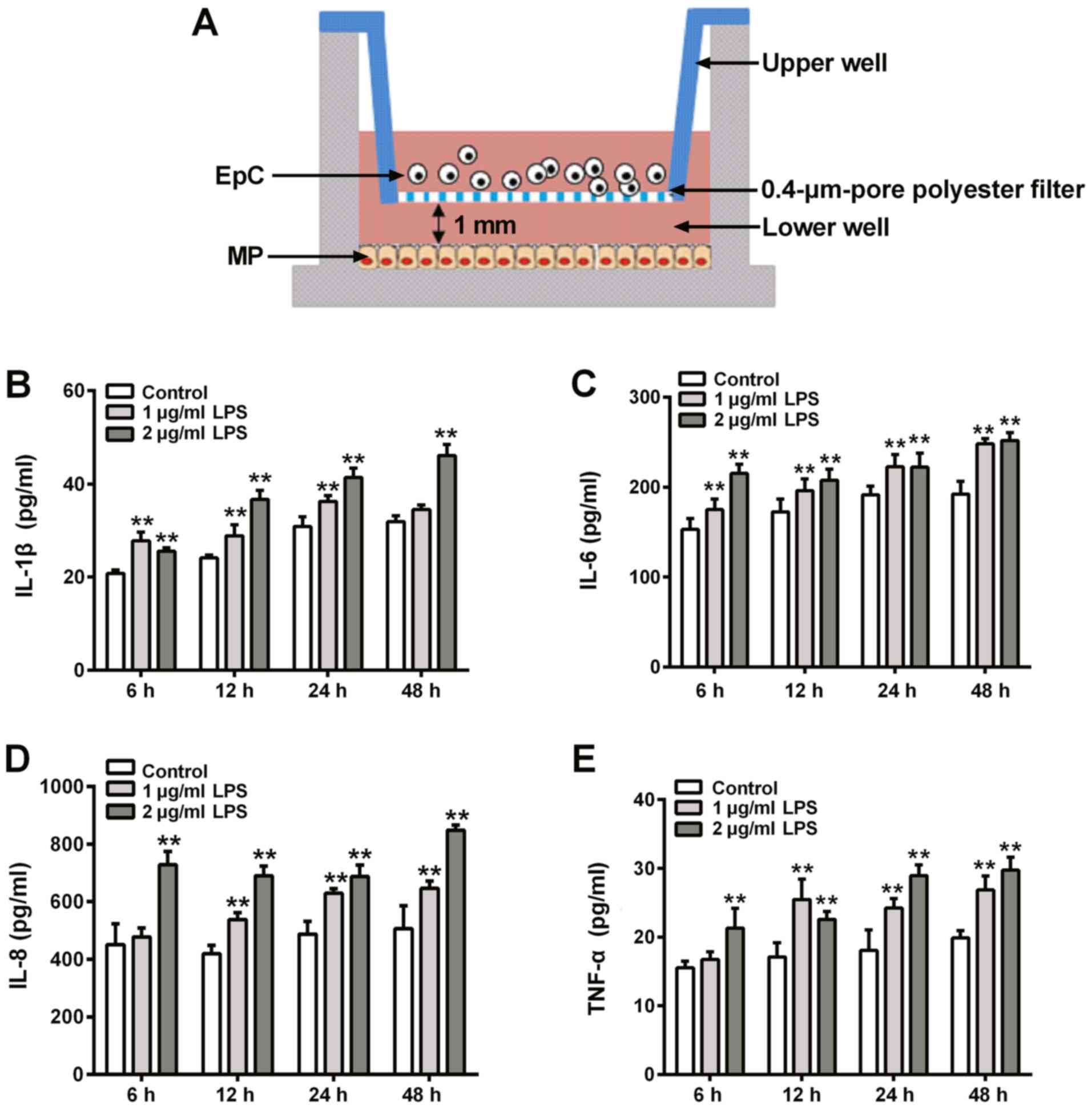
cytokines were analyzed using ELISAs. The co-culture system is
demonstrated in Fig. 2A. The data
presented in Fig. 2B-E showed that
2 µg/ml induced greater IL-1β, IL-6, IL-8 and TNF-α expression
levels at 6, 12, 24 and 48 h. Therefore, 2 µg/ml LPS constituted
the LPS dose used in subsequent experiments. Subsequently, EpCs and
MPs cultured both together, and separately, were exposed to 2 µg/ml
LPS for 6, 12, 24 and 48 h before determining the cytokine
concentrations in the supernatants. The cytokine levels of IL-1β,
IL-6, IL-8 and TNF-α were not significantly increased in EpCs
following LPS stimulation compared with the control group, except
for IL-1β levels at 48 h (Fig. 3);
however, the incubation of MPs with LPS resulted in significantly
increased levels of IL-6, IL-1β, IL-8 and TNF-α at 48 h, IL-6,
IL-1β, IL-8 at 24 h, IL-1β, IL-8 at 12 h, IL-1β and TNF-α at 6 h,
compared with the control group (Fig.
3). Upon the co-culturing of the cells, LPS stimulation
significantly increased the levels of IL-6, IL-1β, IL-8 and TNF-α
at all time points compared with the control cells (Fig. 3). Collectively, these data suggested
that LPS may not promote inflammation in EpCs, but may be able to
in MPs from 12 h. The LPS-induced inflammatory response was
amplified in the EpC/MP co-cultures, which indicated that this
co-culture system may be suitable to use as an inflammatory model
to research chronic inflammatory diseases.
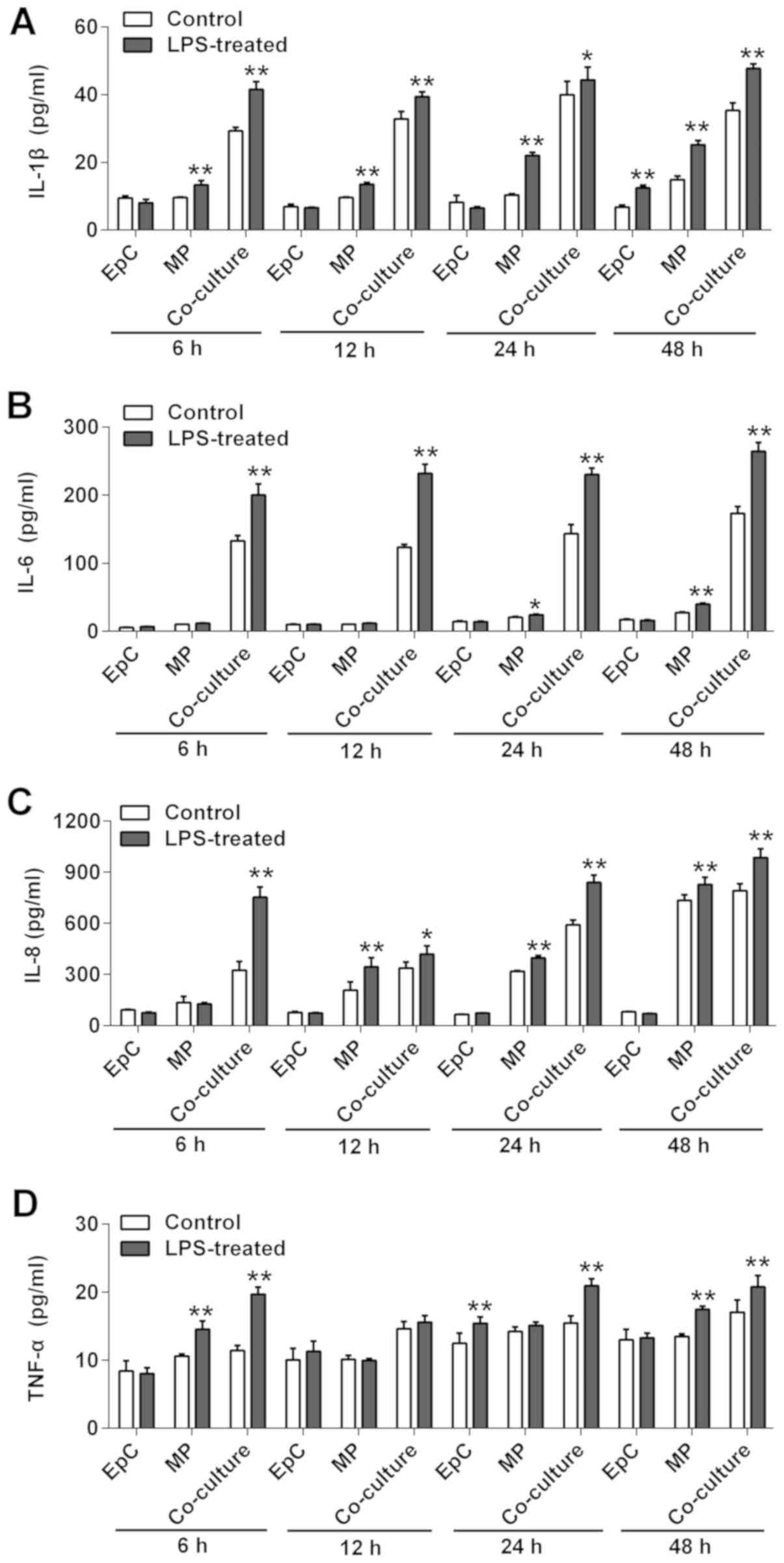 | Figure 3Effect of LPS on cytokine secretion
in EpC and MP monoculture, and EpC/MP co-culture. The levels of (A)
IL-1β, (B) IL-6, (C) IL-8 and (D) TNF-α were analyzed using ELISAs
in EpC and MP monocultures, in addition to EpC/MP co-culture,
following 2 µg/µl LPS stimulation for 48 h. Results are presented
as the mean ± SD of three independent experimental repeats.
*P<0.05, **P<0.01 vs. control group.
LPS, lipopolysaccharide; EpC, epithelial cell; MP, macrophage; IL,
interleukin; TNF, tumor necrosis factor. |
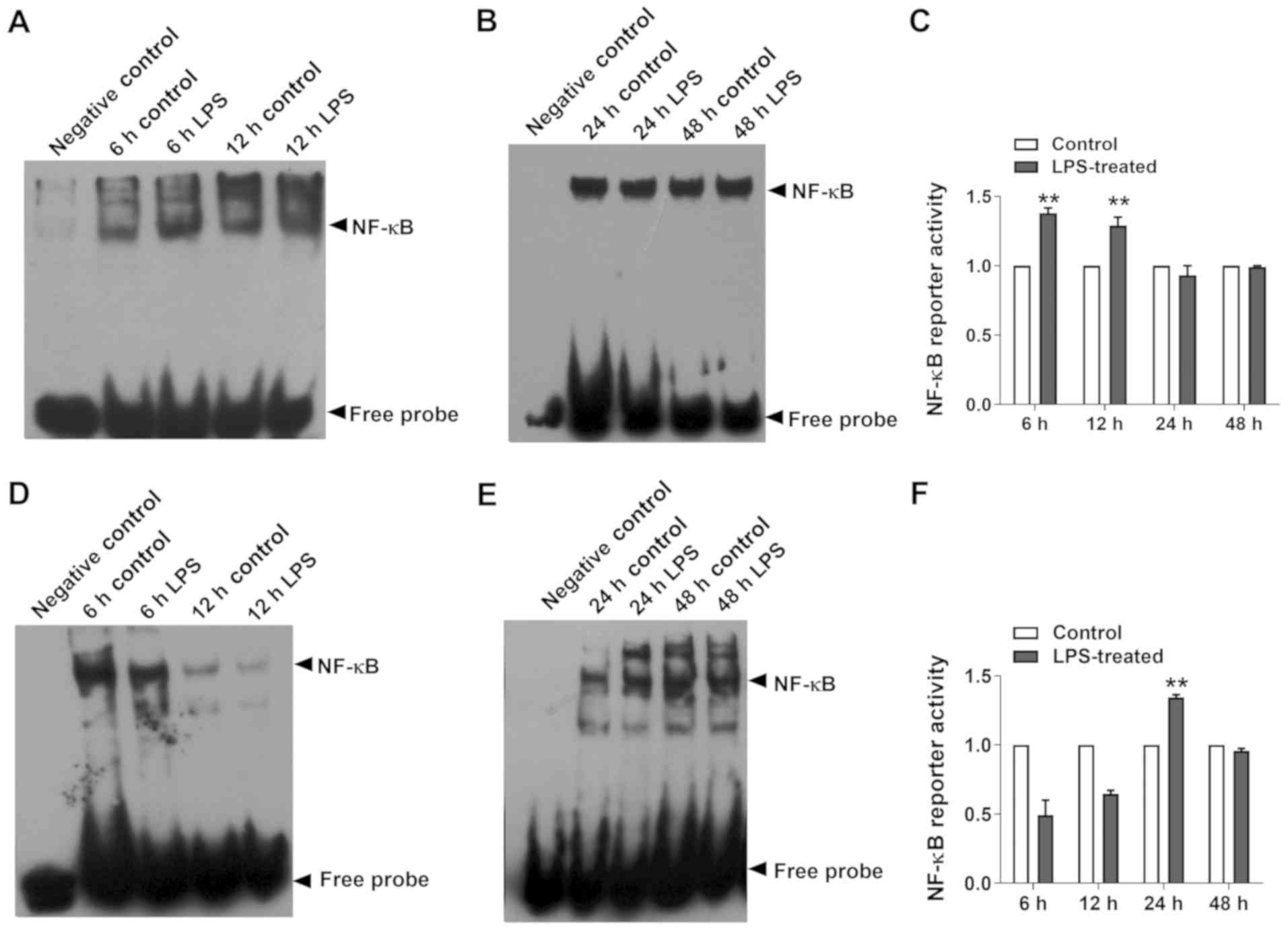
Effect on NF-κB DNA-binding
It is well established that TLR4 recognizes
bacterial LPS and triggers an inflammatory response, mainly through
the TLR4 receptor (13). TLR4
activation induces the activity of the NF-κB nuclear transcription
factor, which ultimately results in the release of proinflammatory
cytokines (14). Thus, NF-κB
DNA-binding was detected in the present study and it was revealed
that LPS significantly induced NF-κB activation in MPs at 6 and 12
h compared with the control (Fig.
4A-C), whereas NF-κB DNA-binding was significantly increased in
EpCs at 24 h compared with the control (Fig. 4D-F).
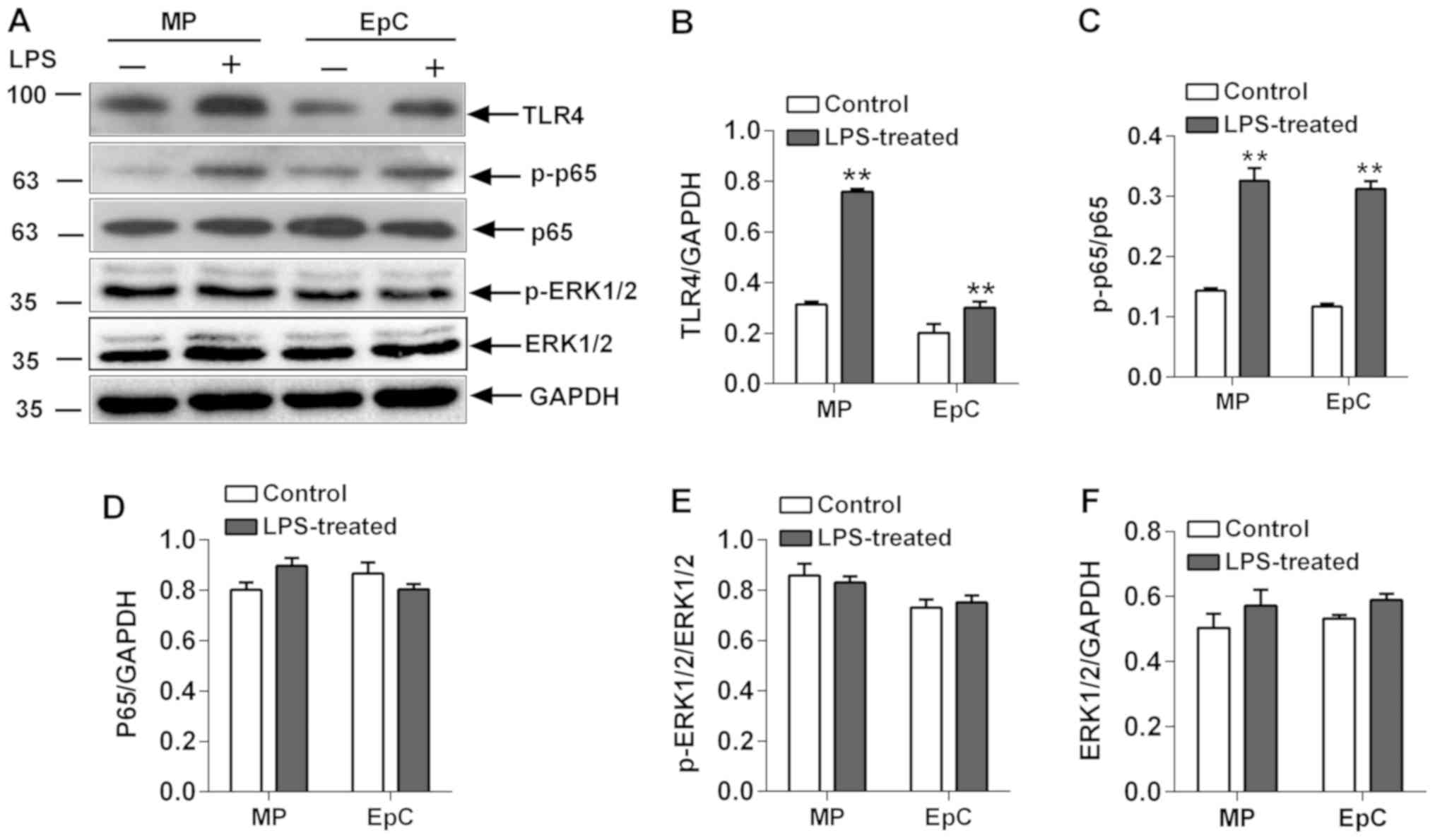
Expression levels of upstream proteins
of the NF-κB signaling pathway
As a stimulus, LPS binds to TLR4 in the cell
membrane to activate downstream factors, such as increasing p65
expression levels and subsequently, its phosphorylation levels
(15). TLR4 expression levels and
the p65 phosphorylation status were significantly increased in
LPS-induced co-cultured EpCs/MPs compared with the control cells
(Fig. 5). In addition to the
activation of NF-κB, LPS also activates a series of major
intracellular signaling transduction pathways, such as ERK, which
serves crucial roles in inflammation (16,17).
Therefore, the expression levels and phosphorylation status of
ERK1/2 in EpCs and MPs were analyzed. Notably, it was identified
that LPS had no effect on the expression levels of p-ERK1/2 or
ERK1/2 in the EpC/MP co-culture (Fig.
5). Therefore, although the evidence is lacking, it was
hypothesized that the interaction between co-cultured EpCs and MPs
may affect the expression levels of ERK1/2.
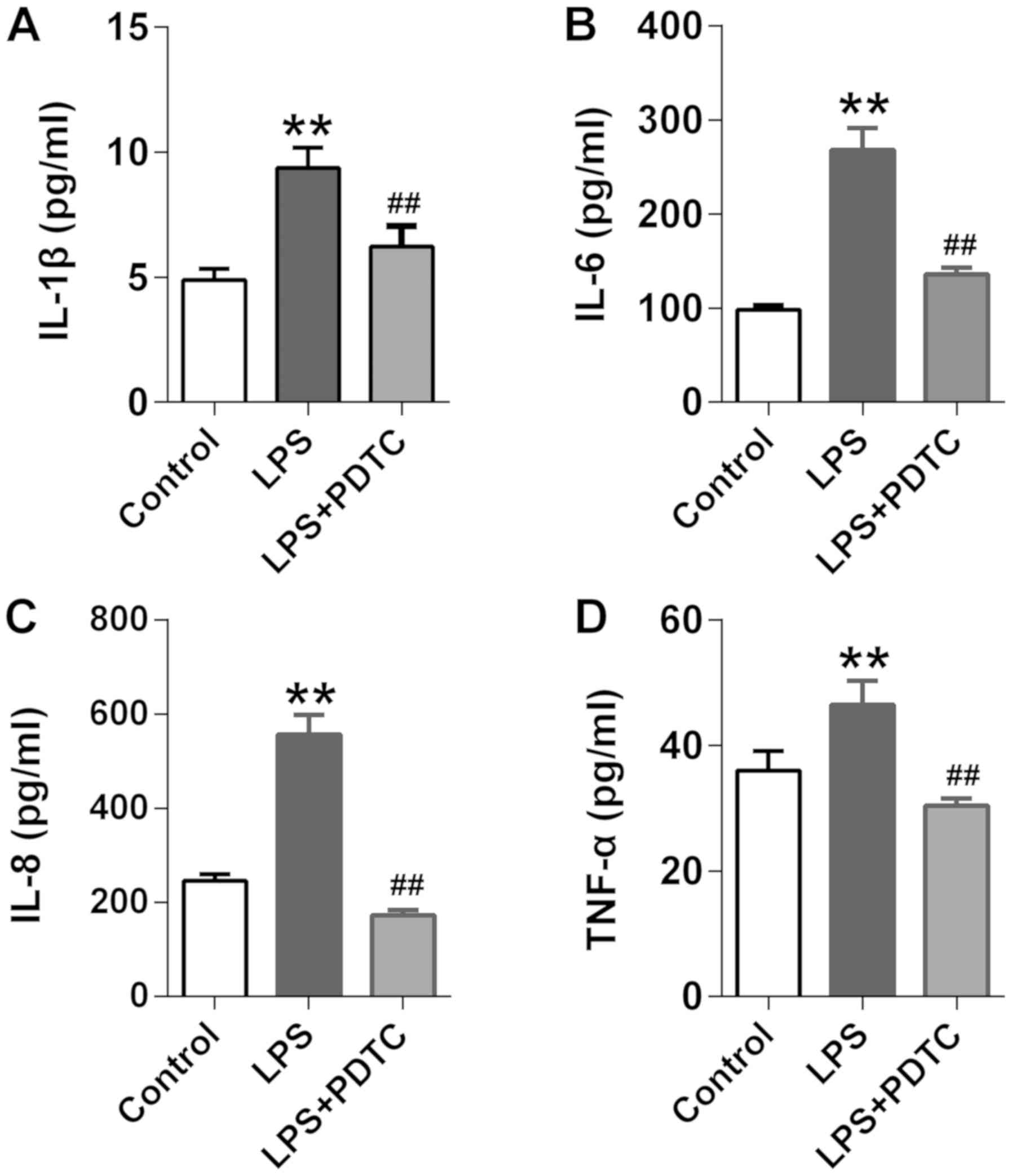
Effects of the NF-κB inhibitor on the
levels of cytokines
The above findings indicated that LPS may induce
cytokine release by activating the nuclear transcription factor,
NF-κB. To confirm its role in LPS-induced cytokine secretion, the
levels of IL-1β, IL-6, IL-8 and TNF-α were analyzed following the
incubation of co-cultured EpCs and MPs with the NF-κB inhibitor,
PDTC. It was revealed that PDTC significantly decreased the
LPS-induced release of IL-1β, IL-6, IL-8 and TNF-α (Fig. 6A-D). These findings suggested that
LPS may induce cytokine release in EpCs/MPs co-culture through the
activation of NF-κB. Collectively, the results indicated that LPS
may initially bind to TLR4, located on the MPs membrane, and
promote downstream factors, like p-p65, to contribute to NF-κB
activity. Activation of the NF-κB transcription factor may
subsequently initiate the transcription of cytokine genes, such as
TNF-α and IL-6, with the secreted cytokines then activating NF-κB
through TLR4 in EpCs to amplify the inflammatory response (Fig. 7).
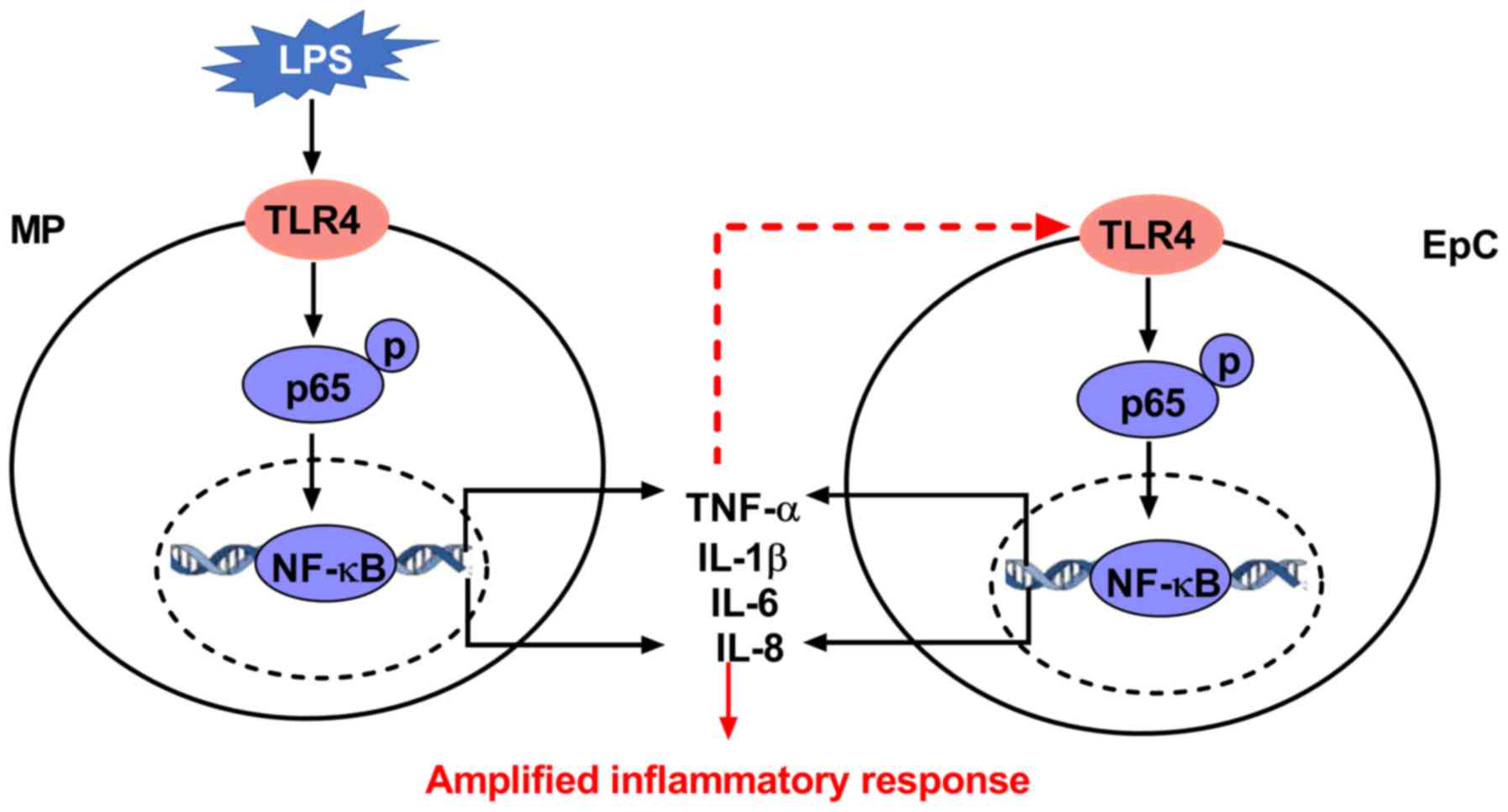 | Figure 7Schematic diagram of the hypothesized
working model of LPS-induced inflammation in EpC/MP co-culture. LPS
initially binds to TLR4 located on the MP membrane, which causes
downstream factors, such as the phosphorylation of p65, to induce
the activity of NF-κB. Activation of the NF-κB of transcription
factor initiates the transcription of cytokine genes, such as
TNF-α. These cytokines in turn activate NF-κB EpCs (shown as red
dotted lines), which contributes to the amplified inflammatory
response. The highlights in red indicate the novel findings from
the present study. Red dotted lines, hypothesized process; red
solid lines, findings from the present work. LPS,
lipopolysaccharide; IL, interleukin; TNF, tumor necrosis factor;
EpC, epithelial cell; MP, macrophage; TLR, Toll-like receptor. |
Discussion
Chronic systemic inflammation in COPD has been
associated with the constant production of cytokines by MPs,
neutrophils and lung structural cells, which is correlated with
disease progression and an exacerbated frequency of occurrence
(2,18). The air-blood barrier is mainly
composed of alveolar EpCs and MPs, with EpCs serving as diffusional
barriers and MPs providing an immunological barrier (19). In a previous study, monocultured
EpCs were treated with conditioned medium from monocytes to
investigate the inflammatory response (20). In the present study, a new
co-culture model of EpCs and MPs was established to investigate the
inflammatory response in EpCs and MPs cultured together, and
separately, in the presence and absence of LPS stimulation. The
study revealed that LPS could not promote inflammation in A549
cells (EpCs), but could in THP-1 cells (MPs), whereas an amplified
inflammatory response was observed in co-cultured EpCs/MPs.
Contradictory to the findings of the present study, a previous
study reported that the expression levels of IL-1β, IL-6 and TNF-α
were increased in A549 cells treated with 10 µg/ml LPS (21). In addition, in another study, 50 µg
LPS significantly increased the levels of proinflammatory
cytokines, including IL-1β, IL-2, TNF-α and transforming growth
factor-β in animal lung tissue lysate, in addition to increasing
the expression levels of NF-κB and histone deacetylase 3 in A549
cells (22). These paradoxical
findings may be due to the different concentrations and incubation
times of LPS.
For the co-culture experiments, the time course for
the release of cytokines was established, and a variable increase
in the levels secreted was observed following LPS treatment. As
these cytokines are regulated by the NF-κB signaling pathway, the
activity of the NF-κB nuclear transcription factor was also
analyzed (23-25).
The initiation of the inflammatory response requires various
inflammatory mediators that are regulated by inducible
transcription factors, including IL-6 and TNF-α which bind to the
promoter regions of their respective target genes (11,26,27).
The EpCs/MPs co-culture released increased levels of cytokines
compared with either EpCs or MPs monoculture following exposure to
LPS. Furthermore, only IL-1β and IL-8 levels were increased at 12 h
in monocultured MPs; although MPs released more cytokines following
the stimulation with LPS for 24 and 48 h, the levels remained much
lower compared with in the co-cultured cells. However, increased
levels of the proinflammatory cytokine, TNF-α, were observed
following 6, 24 and 48 h of LPS exposure, and increased levels of
IL-1β, IL-6 and IL-8 were observed across all time intervals from 6
to 48 h, which indicated that these cytokines may be important
factors for maintaining persistent inflammation. Some co-culture
models of chronic inflammatory have been studied for more than 7
days (19,28); in the current study, EpCs and MPs
viability following LPS treatment was monitored for 7 days;
however, the A549 (EpCs) cells began to die after 72 h (the OD
value was quite low), whereas THP-1 cells (MPs) grew well from 0 to
7 days (data not shown). In a previous study, the inflammatory
status of airway epithelial cells and MPs was determined at 24 and
48 h (29), thus in the present
study, the inflammatory effects were observed until up to 48 h of
co-culture. Therefore, in the present study, the increased
inflammatory status was investigated following the co-culture of
cells.
The results of the present study indicated that
co-cultured EpCs and MPs may amplify the inflammatory response, and
that the interactions between these two cell types might be
responsible. Consistent with these findings, a previous study also
found that co-cultured A549 cells and monocytes released increased
amounts of IL-6 and IL-8 in response to endotoxin (30). Additionally, expression levels of
the epithelial marker, E-cadherin, were discovered in the
co-culture system previously (31).
NF-κB also regulates cytokine secretions, such as
TNF-α, IL-1β, IL-6 and IL-8 in MPs and EpCs, which mediate their
expression levels by directly binding to motifs in the promoter
region of their target genes (32,33).
In the current study, the investigations into NF-κB DNA-binding
activity revealed that NF-κB was initially activated in MPs upon
being co-cultured with EpCs for 6 h. Thus, NF-κB was activated
first in MPs, suggesting that MPs may orchestrate the inflammatory
response, which is consistent with the fact that they are activated
during the early stages of chronic inflammation (34). In the present study, from the
findings it was hypothesized that LPS initially binds to TLR4
located on the membrane of THP-1 cells, promoting downstream
factors, such as NF-κB inhibitor α and p-p65, to initiate NF-κB
activity; activated NF-κB may subsequently initiate the
transcription of cytokine genes, such as TNF-α and IL-6, which in
turn may activate NF-κB in EpCs. Taking this into consideration,
NF-κB DNA binding in co-cultured EpCs/MPs was analyzed for 24 and
48 h and the EMSA data demonstrated that NF-κB DNA binding
increased in EpCs from 24 h. Based on the current study, LPS could
not induce inflammation in EpCs; however, inflammation has been
observed in TNF-α-induced EpCs (unpublished data). TNF-α levels
were increased in the co-culture system and the activity of NF-kB
was also increased in MPs, but earlier than that in EpCs.
Therefore, it was hypothesized that MP-induced TNF-α expression
levels increased the activity of NF-kB in EpCs. Consistent with the
present study, an isolated report observed a significant increase
in TNF-α, IL-1β and IL-6 expression levels in LPS-induced THP-1
cells, which was related to NF-κB activation (29).
Transcription factor activation is a multistep
process induced by various upstream signal transduction pathways
(35). TLR4 is a
pattern-recognizing receptor that identifies LPS, which is
associated with Gram-negative bacteria and is highly expressed in
MPs (36,37). Activation of the NF-κB and
ERK1/2/activator protein 1/STAT3 signaling pathways has been
reported in LPS-induced THP-1 cells (38). The current study further analyzed
the upstream factors of NF-κB in co-cultured EpCs/MPs; LPS
increased TLR4 expression levels in both EpCs and MPs and the
expression levels of phosphorylated p65 were significantly
increased. It has previously been reported that LPS activates a
series of signaling transduction pathways, including ERK, which
serves crucial roles in inflammation (25). In the present study, the expression
levels and phosphorylation status of ERK1/2 were analyzed in EpCs
and MPs; however, the current results revealed that LPS had no
effect on the expression levels or phosphorylation status of ERK1/2
in EpC/MP co-culture, which may be because the phosphorylation of
ERK1/2 occurred at an earlier stage (39). Although there is no direct evidence
to date, it was hypothesized that the interaction between
co-cultured EpCs and MPs may affect ERK1/2 expression. However, one
limitation of the present study was that as gene expression occurs
earlier than protein expression, only protein expression levels of
factors involved in the NF-κB signaling pathway were investigated.
Nonetheless, the most important and novel observation of the
present study was that in the co-culture system, the interaction
between EpCs and MPs led to an amplified inflammatory response to
LPS.
Considering these findings, the effects of
LPS-induced inflammation on cytokine levels were investigated using
the transcription factor inhibitor, PDTC. The results demonstrated
that PDTC significantly decreased IL-6, IL-1β, IL-8 and TNF-α
levels, but to varying degrees. Consistent with the findings in the
present study, Liu et al (29) reported that PDTC markedly attenuated
the expression levels of IL-1β, IL-6 and TNF-α through inhibiting
the activation of NF-κB in MPs. However, the effects of PDTC on
protein expression and the activation of nuclear transcription
factors should be further investigated in future studies.
In conclusion, the findings of the present study
suggested that LPS may promote an inflammatory response in MPs, but
not in EpCs. However, LPS may be able to amplify the inflammatory
response in co-cultured EpCs/MPs through activating the nuclear
transcription factor, NF-κB, and the expression levels of proteins
in the NF-κB signaling pathway. Previously, this research has
focused on investigating the anti-inflammatory mechanisms of
traditional Chinese herbs in treating COPD. Traditional Chinese
herbs have multiple targets and multiple levels of action, thus
from the findings of the present study, it can be suggested that
cell co-cultures may be more useful models for investigating their
mechanisms of action compared with monocultures. The present study
was the first time that the present research group had used this
combination of cells as a co-culture model, although a previously
established co-culture system using RAW 264.7/NIH3T3 cells has been
established (unpublished data). Future studies should aim to
determine the stability of this inflammatory model to help with
future in vitro studies of the chronic inflammatory
response.
Acknowledgements
Not applicable.
Funding
The research was supported by the National Natural
Science Fund of China (grant nos. 81130062 and 81603473).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL and YC designed the study; YQ, XM and CL
conceived and performed the experiments, and drafted and revised
the manuscript; and PZ, SF, XL, HD and WZ all performed the
experiments, acquired the data and performed the statistical
analysis. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Caramori G, Casolari P, Barczyk A, Durham
AL, Di Stefano A and Adcock I: COPD immunopathology. Semin
Immunopathol. 38:497–515. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Barnes PJ: Cellular and molecular
mechanisms of chronic obstructive pulmonary disease. Clin Chest
Med. 35:71–86. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wang Y, Xu J, Meng Y, Adcock IM and Yao X:
Role of inflammatory cells in airway remodeling in COPD. Int J
Chron Obstruct Pulmon Dis. 13:3341–3348. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Arora S, Dev K, Agarwal B, Das P and Syed
MA: Macrophages: Their role, activation and polarization in
pulmonary diseases. Immunobiology. 223:383–396. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Barnes PJ: Cellular and molecular
mechanisms of asthma and COPD. Clin Sci (Lond). 131:1541–1558.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ferrero MC, Fossati CA and Baldi PC:
Direct and monocyte-induced innate immune response of human lung
epithelial cells to Brucella abortus infection. Microbes Infect.
12:736–747. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kolesar L, Brabcova E, Thorburn E,
Sekerkova A, Brabcova I, Jaresova M, Viklicky O and Striz I:
Cytokine gene expression profile in monocytic cells after a
co-culture with epithelial cells. Immunol Res. 52:269–275.
2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Striz I, Slavcev A, Kalanin J, Jaresova M
and Rennard SI: Cell-cell contacts with epithelial cells modulate
the phenotype of human macrophages. Inflammation. 25:241–246.
2001.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Eapen MS, Myers S, Walters EH and Sohal
SS: Airway inflammation in chronic obstructive pulmonary disease
(COPD): A true paradox. Expert Rev Respir Med. 11:827–839.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Pavord ID, Jones PW, Burgel PR and Rabe
KF: Exacerbations of COPD. Int J Chron Obstruct Pulmon Dis. 11 Spec
Iss:21–30. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Vallance TM, Zeuner MT, Williams HF,
Widera D and Vaiyapuri S: Toll-like receptor 4 signalling and its
impact on platelet function, thrombosis, and haemostasis. Mediators
Inflamm. 2017(9605894)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wan D, Wu Q, Qu W, Liu G and Wang X:
Pyrrolidine Dithiocarbamate (PDTC) inhibits DON-induced
mitochondrial dysfunction and apoptosis via the NF-κB/iNOS pathway.
Oxid Med Cell Longev. 2018(1324173)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang P, Han X, Mo B, Huang G and Wang C:
LPS enhances TLR4 expression and IFN-γ production via the
TLR4/IRAK/NF-κB signaling pathway in rat pulmonary arterial smooth
muscle cells. Mol Med Rep. 16:3111–3116. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cichocki M, Baer-Dubowska W, Wierzchowski
M, Murias M and Jodynis-Liebert J:
3,4,5,4'-trans-tetramethoxystilbene (DMU-212) modulates the
activation of NF-kappaB, AP-1, and STAT3 transcription factors in
rat liver carcinogenesis induced by initiation-promotion regimen.
Mol Cell Biochem. 391:27–35. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shen W, Liu J, Zhao G, Fan M, Song G and
Zhang Y, Weng Z and Zhang Y: Repression of Toll-like receptor-4 by
microRNA-149-3p is associated with smoking-related COPD. Int J
Chron Obstruct Pulmon Dis. 12:705–715. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kim HS, Lee JH, Moon SH, Ahn DU and Paik
HD: Ovalbumin hydrolysates inhibit nitric oxide production in
LPS-induced RAW 264.7 macrophages. Food Sci Anim Resour.
40:274–285. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xie W, Zheng W, Liu M, Qin Q, Zhao Y,
Cheng Z and Guo F: BRF1 ameliorates LPS-induced inflammation
through autophagy crosstalking with MAPK/ERK signaling. Genes Dis.
5:226–234. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Pouwels SD, Heijink IH, ten Hacken NH,
Vandenabeele P, Krysko DV, Nawijn MC and van Oosterhout AJ: DAMPs
activating innate and adaptive immune responses in COPD. Mucosal
Immunol. 7:215–226. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kletting S, Barthold S, Repnik U,
Griffiths G, Loretz B, Schneider-Daum N, de Souza Carvalho-Wodarz C
and Lehr CM: Co-culture of human alveolar epithelial (hAELVi) and
macrophage (THP-1) cell lines. ALTEX. 35:211–222. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Qin Y, Dong H, Li J, Chen Y, Mao X and WU
M: The efects of nourishing lung and kidney formulas on
inflammatory response of alveolar epithelial cells stimulated by
monocytes conditioned medium. Chin J TCM WM Crit Care. 24:63–67.
2017.
|
|
21
|
Wang Q, Li D, Han Y, Ding X, Xu T and Tang
B: MicroRNA-146 protects A549 and H1975 cells from LPS-induced
apoptosis and inflammation injury. J Biosci. 42:637–645.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Pooladanda V, Thatikonda S, Bale S,
Pattnaik B, Sigalapalli DK, Bathini NB, Singh SB and Godugu C:
Nimbolide protects against endotoxin-induced acute respiratory
distress syndrome by inhibiting TNF-α mediated NF-κB and HDAC-3
nuclear translocation. Cell Death Dis. 10(81)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Panday A, Inda ME, Bagam P, Sahoo MK,
Osorio D and Batra S: Transcription Factor NF-κB: An Update on
Intervention Strategies. Arch Immunol Ther Exp (Warsz). 64:463–483.
2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shapouri-Moghaddam A, Mohammadian S,
Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi
A, Afshari JT and Sahebkar A: Macrophage plasticity, polarization,
and function in health and disease. J Cell Physiol. 233:6425–6440.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li C, Yang F, Liu F, Li D and Yang T:
NRF2/HO-1 activation via ERK pathway involved in the
anti-neuroinflammatory effect of Astragaloside IV in LPS induced
microglial cells. Neurosci Lett. 666:104–110. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kim KY, Lee HS and Seol GH: Eucalyptol
suppresses matrix metalloproteinase-9 expression through an
extracellular signal-regulated kinase-dependent nuclear
factor-kappa B pathway to exert anti-inflammatory effects in an
acute lung inflammation model. J Pharm Pharmacol. 67:1066–1074.
2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ko SY: Myricetin suppresses LPS-induced
MMP expression in human gingival fibroblasts and inhibits
osteoclastogenesis by downregulating NFATc1 in RANKL-induced RAW
264.7 cells. Arch Oral Biol. 57:1623–1632. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Vasanthi Bathrinarayanan P, Brown JEP,
Marshall LJ and Leslie LJ: An investigation into E-cigarette
cytotoxicity in-vitro using a novel 3D differentiated co-culture
model of human airways. Toxicol In Vitro. 52:255–264.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liu X, Yin S, Chen Y, Wu Y, Zheng W, Dong
H, Bai Y, Qin Y, Li J, Feng S and Zhao P: LPSinduced
proinflammatory cytokine expression in human airway epithelial
cells and macrophages via NFkappaB, STAT3 or AP1 activation. Mol
Med Rep. 17:5484–5491. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Striz I, Brabcova E, Kolesar L, Liu XD,
Brabcova I, Sekerkova A, Poole JA, Jaresova M, Slavcev A and
Rennard SI: Epithelial cells modulate genes associated with NF
kappa B activation in co-cultured human macrophages. Immunobiology.
216:1110–1116. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Qin Y, Zhao P, Chen Y, Liu X, Dong H,
Zheng W, Li C, Mao X and Li J: Lipopolysaccharide induces
epithelial-mesenchymal transition of alveolar epithelial cells
cocultured with macrophages possibly via the JAK2/STAT3 signaling
pathway. Hum Exp Toxicol. 39:224–234. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Truong AD, Hoang CT, Hong Y, Lee J, Lee K,
Lillehoj HS and Hong YH: Functional analyses of the interaction of
chicken interleukin 23 subunit p19 with IL-12 subunit p40 to form
the IL-23 complex. Mol Immunol. 92:54–67. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Choi HE, Kwak HJ, Kim SK and Cheon HG:
Foenumoside B isolated from Lysimachia foenum-graecum extract
suppresses LPS-induced inflammatory response via NF-κB/AP-1
inactivation in murine macrophages and in endotoxin-induced shock
model. Eur J Pharmacol. 832:120–128. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Barnes PJ: Alveolar macrophages as
orchestrators of COPD. COPD. 1:59–70. 2004.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Caramori G, Casolari P and Adcock I: Role
of transcription factors in the pathogenesis of asthma and COPD.
Cell Commun Adhes. 20:21–40. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Liao W, He X, Yi Z, Xiang W and Ding Y:
Chelidonine suppresses LPS-Induced production of inflammatory
mediators through the inhibitory of the TLR4/NF-κB signaling
pathway in RAW264.7 macrophages. Biomed Pharmacother.
107:1151–1159. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Neumann J, Ziegler K, Gelléri M,
Fröhlich-Nowoisky J, Liu F, Bellinghausen I, Schuppan D, Birk U,
Pöschl U, Cremer C and Lucas K: Nanoscale distribution of TLR4 on
primary human macrophages stimulated with LPS and ATI. Nanoscale.
11:9769–9779. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yang L, Guo H, Li Y, Meng X, Yan L, Dan
Zhang, Wu S, Zhou H, Peng L, Xie Q and Jin X: Oleoylethanolamide
exerts anti-inflammatory effects on LPS-induced THP-1 cells by
enhancing PPARα signaling and inhibiting the NF-κB and
ERK1/2/AP-1/STAT3 pathways. Sci Rep. 6(34611)2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Sa G, Liu Z, Ren J, Wan Q, Xiong X, Yu Z,
Chen H, Zhao Y and He S: Keratinocyte growth factor (KGF) induces
podosome formation via integrinErk1/2 signaling in human
immortalized oral epithelial cells. Cell Signal. 61:39–47.
2019.PubMed/NCBI View Article : Google Scholar
|