Introduction
Osteoporosis is a metabolic disease of the bones
characterized by low bone mass and the micro-architectural
deterioration of bone tissue (1).
This disease causes bones to break more easily in affected
individuals compared with normal, healthy patients. Osteoporosis is
particularly common in older women (2). In 2017, it was estimated that >200
million people worldwide suffer from osteoporosis (3). In the USA, ~50% of all postmenopausal
women had osteoporosis in 2018(4).
Postmenopausal osteoporosis (PMO) occurs as a result of decreased
estrogen levels and increased bone immune function, resulting in an
imbalance in bone remodeling (5).
Estrogen is a well-known regulator of the immune system and T-cell
function (6) and regulates T cell
activation via the classical estrogen receptor pathway (7). As it is currently understood, T cells
serve a pivotal role in the pathogenesis of PMO (8). In ovariectomized (OVX) mice, an animal
model of PMO, increased proliferation of activated T cells has been
observed (9). Furthermore, several
subsets of T cells, including CD4+ T cells, have been
demonstrated to be present at increased levels in the peripheral
blood of patients with osteoporosis (10). In a study where bone loss was
induced in nude mice via ovariectomy, levels were subsequently
restored by transferring wild-type T cells into the mice (8). Additionally, depletion of T cells can
be counteracted anti-CD4/CD8 antibody treatment, which protects OVX
mice from ovariectomy-associated bone loss (11). Recently, Song et al (12) discovered that vascular endothelial
cells secrete exosomes that attenuate bone loss via microRNA-155 in
OVX mice. Additional studies have reported that estrogen reduction
promoted the proliferation of T cells and that activated T cells
expressed high levels of pro-osteoclastogenic cytokines, TNF-α and
receptor activator of the NF-κB ligand (13-15),
which promote the activation of osteoclasts and break the dynamic
balance of bones (16,17). These findings indicated that the
activation of T cells promotes osteoclastogenesis.
Mesenchymal stem cells (MSCs) are ideal multipotent
stem cells for tissue regeneration due to their excellent
capacities for proliferation and differentiation. After
differentiation is induced in vitro or in vivo, MSCs
can differentiate into several types of tissues, including fat,
muscle, bone, cartilage, tendon, ligament, nerve and liver tissue
(18-20).
As a type of MSC, bone marrow mesenchymal stem cells (BMMSCs) are
widely used in studies of bone regeneration due to their properties
of multipotency and active proliferation (21). BMMSCs are a class of adult stem
cells present in the bone marrow stroma and participate in the
formation of the bone marrow microenvironment. Furthermore, BMMSCs
have the potential to differentiate into mesoderm and
neuroectoderm-derived tissue cells (22). Functional defects, including
decreased proliferative activity and decreased osteogenic
differentiation of BMMSCs, lead to the onset of PMO (23).
Current research is largely focused on the
interaction between T cells and osteoblasts and osteoclasts
(24), while there are few reports
on the effects of T cells on the proliferation and differentiation
of BMMSCs (25,26). In the present study, an osteoporosis
model was established in order to elucidate the effects of T cells
on the proliferation and differentiation of BMMSCs and to further
explore the pathogenesis of PMO.
Materials and methods
Reagents and chemicals
A variety of reagents and chemicals were used in the
current study, including: α-minimum essential medium (MEM; Gibco;
Thermo Fisher Scientific, Inc.), trypsin (Gibco; Thermo Fisher
Scientific, Inc.), FBS (Tianhang; Zhejiang Tianhang Biotechnology
Co., Ltd.), phenol red-free α-MEM (Gibco; Thermo Fisher Scientific,
Inc.), RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.), a
total RNA extraction kit (Takara Bio, Inc.), a one-step reverse
transcription-PCR kit (Takara Bio. Inc.) and magnetic beads coated
with anti-mouse CD4 antibodies (Miltenyi Biotec., Inc.), CD3 and
CD28 (both, BD Biosciences), a volumetric microscope, an inverted
phase contrast microscope and camera system (Olympus Corporation),
a flow cytometer (Beckman Coulter, Inc.), an Alkaline Phosphatase
Staining kit (cat. no. P0321; Beyotime Institute of Biotechnology),
a mouse TNF-α ELISA kit (cat. no. MTA00B; R&D Systems, Inc.),
anti-TNF-α antibodies (cat. no. AF-410-NA; R&D Systems, Inc.),
FITC-conjugated anti-CD3 (cat. no. 11-0032-82; eBioscience; Thermo
Fisher Scientific, Inc.), allophycocyanin (APC)-conjugated anti-CD4
(cat. no. 17-0042-82; eBioscience; Thermo Fisher Scientific, Inc.),
phycoerythrin (PE)-conjugated anti-CD69 (cat. no. 12-0691-82;
eBioscience; Thermo Fisher Scientific, Inc.) and Armenian hamster
IgG isotype control (cat. no. 12-4888-81; eBioscience; Thermo
Fisher Scientific, Inc.). Furthermore, anti-runt related
transcription factor 2 (Runx2; cat. no. ab76956), anti-osteocalcin
(OCN; cat. no. ab93876), anti-p-ERK (cat. no. ab217322), anti-ERK
(cat. no. ab17942), anti-p-JNK (cat. no. ab4821), anti-JNK (cat.
no. ab112501), anti-p-P38 (cat. no. ab47363), anti-P38 (cat no.
ab197348) and anti-β-actin (cat. no. ab8227) were purchased from
Abcam and used for the current research.
PMO mouse model
Animal studies were reviewed and approved by the
Animal Care and Use Committee of Chongqing Medical University
(Chongqing, China; approval no. 2018101702) and carried out
according to their guidelines. A total of 30 female C57BL/6 mice
(age, 6-8 weeks; weight, 21-25 g) were housed in a room at a
humidity of 50±10% and a controlled temperature of 25±1˚C with 12-h
light/dark cycles. The mice were maintained in an individually
ventilated cage system and provided with free access to sterile
food and water. The mice were randomly divided into either the OVX
group (n=15) or the sham group (n=15). Mice were anesthetized with
an intraperitoneal injection of 50 mg/kg sodium pentobarbital.
Bilateral ovaries of the mice in the OVX group were removed, while
in the sham group, 1 g of adipose tissue surrounding bilateral
ovaries (distance from ovaries, ~0.5 cm) was removed. After one
month, a micro-CT was performed to validate the success of the
model. Mice were sacrificed by cervical dislocation and 1 ml of
blood was collected from the abdominal aorta of each mouse. For
serum collection, blood was allowed to coagulate for 30 min at room
temperature, followed by centrifugation at 2,000 x g for 10 min at
4˚C. Serum estradiol levels were determined using a mouse estrogen
ELISA kit (cat. no. KGE014; R&D Systems, Inc.), according to
the manufacturer's protocol.
Flow cytometry
The mice from the OVX and sham group were sacrificed
by cervical dislocation and soaked in ethanol for 5 min. Spleens
were excised, mixed with Hanks' balanced salt solution (Gibco;
Thermo Fisher Scientific, Inc.) and tissues were crushed through
the mesh filter for mincing. Following washing with PBS and
treatment with a Red Blood Cell Lysis buffer (cat. no. C3702;
Beyotime Institute of Biotechnology), a total of 2x106
cells were suspended in flow tubes with 100 µl of FACS staining
buffer (2% FBS in PBS) and incubated with FITC conjugated anti-CD3
(1:200), APC-conjugated anti-CD4 (1:150), PE-conjugated anti-CD69
(1:100) and IgG isotype control (1:100) antibodies in the dark at
4˚C for 20 min. Following fixation in 2% formalin at room
temperature for 20 min, cells were assessed using a FACScan
Analyzer with FACSDiva software (version 6.2.1; BD Biosciences).
Splenocytes were initially gated using forward and side scatter
properties and CD4+ T cells were subsequently gated.
Isolation and culture of
CD4+ T cells
Spleen single cell suspensions from the OVX and sham
groups were incubated with magnetic beads coated with an anti-mouse
CD4 antibodies. CD4+ T cells were isolated strictly
according to the manufacturer's protocol. These purified cells were
then subjected to RNA isolation in order to determine the
expression levels of the proinflammatory cytokines IL-2, IFN-γ and
TNF-α.
Purified T cells were maintained in RPMI-1640 medium
(containing 10% FBS) at 37˚C with 5% CO2 and
seeded into a 12-well plate at a density of 1x105
cells/well. Following 24 h of culturing, the TNF-α level in the
culture supernatant of T cells was determined using a TNF-α ELISA
kit, according to the manufacturer's protocol. The detection limit
of this kit was 10.9-700 pg/ml.
T cells co-culture with BMMSCs
BMMSCs were isolated from the tibia and femur of
mice, as previously described (27). Following three passages, BMMSCs were
collected. The positive surface markers (CD29 and CD90) and the
negative surface markers (CD34 and CD45) for BMMSCs (27,28)
were analyzed by flow cytometry. MSCs were immunostained with
FITC-conjugated antibodies against CD90 (cat. no. 11-0909-42;
1:20), CD45 (cat. no. 11-0451-82; 1:100), CD34 (cat. no.
11-0341-82; 1:100) and CD29 (cat. no. 11-0291-82; 1:50) at room
temperature for 1 h. All antibodies were purchased from
eBioscience, Thermo Fisher Scientific, Inc. Analysis was performed
using a FACScan Analyzer and FACSDiva software (version 6.2.1; BD
Biosciences) and the results demonstrated that the isolated BMMSCs
were a relatively pure population of stromal cells that were
negative for CD34 and CD45, and positive for CD29 and CD90
(Fig. S1). The single cell
suspension from whole bone marrow was briefly cultured at 37˚C with
5% CO2 in α-MEM (containing 20% FBS). Following 24 h of
culturing, the medium was replaced every 3 days after discarding
the unattached cells and passaging was performed when cell
confluence reached 80%.
A total of 2x106 CD4+ T cells
from the sham group and OVX group were collected in cell culture
plates, which were pre-coated with anti-CD3 (5 µg/ml) antibodies
for 4 h and anti-CD28 (2 µg/ml) antibodies for 12 h, as previously
described (28-30),
and treated with anti-TNF-α (1 ng/ml) antibodies for 2 h at room
temperature. At a concentration of 1x105 cells/well, T
cells with anti-TNF-α antibody treatment or an equal volume of PBS
from the sham group or OVX group were then transferred to 96-well
culture plates pre-populated with BMMSCs (2x104
BMMSCs/well) and cultured for 7 days. The control group was the
well that contained only BMMSCs.
MTT assay
A total of 1x103/well T cells, with or
without anti-TNF-α pretreatment, from the sham group and OVX group
were added to BMMSC-coated wells (1.5x103 cells/well) in
96-well plates. At the indicated time (1, 2, 3, 4, 5, 6 or 7 days),
20 µl of MTT solution (5 g/l) was added to each well. Following
incubation for 4 h, the supernatant was discarded, 150 µl of DMSO
was added to each well and the solution was mixed for 10 min. OD
values at 450 nm were determined using a Biotek Synergy H1 plate
reader (BioTek Instruments, Inc.).
Cell cycle analysis
Cells were gathered and fixed with 70% ethanol at
4˚C overnight. Cells were subsequently treated with ribonuclease A
(20 µg/ml; Sigma-Aldrich; Merck KGaA) and incubated with propidium
iodide (50 µg/ml; Sigma-Aldrich; Merck KGaA) for 30 min at 37˚C.
The population of cells in the G2-M, S and G0-G1 phases were
determined using a FACScan Analyzer with FACSDiva software (version
no. 6.2.1; BD Biosciences).
Alkaline phosphatase staining and
Alizarin red staining assays
A total of 1x105 T cells with or without
anti-TNF-α treatment from the sham and OVX groups were co-cultured
with 5x105 BMMSCs using osteogenic medium into 6-well
plates. Following 7 days of culturing, the osteogenic medium was
discarded. BMMSCs were washed twice with PBS and fixed with 4%
paraformaldehyde at room temperature for 30 min. After being washed
twice with PBS, the cells were treated with a BCIP/NBT solution in
the dark for 30 min or stained with alizarin red staining solution
for 30 min at 37˚C.
Micro-CT detection
The distal femurs of mice were placed parallel to
the long axis of the scanning bed, fixed with transparent tape and
subjected to micro-CT scanning. The obtained microstructural
imaging data were reconstructed and structural parameters,
including bone volume/tissue volume (BV/TV), trabecula thickness,
trabecula number and bone mineral density (BMD) were calculated
using Inveon Research Workplace software (version no. 2.2; Siemens
Healthineers).
RT-quantitative (RT-qPCR)
Prior to extracting total RNA from bone fragments,
collected bone tissues were placed in an RNase-free mortar with
liquid nitrogen and ground into powder with a pestle. The obtained
powders were then transferred into a tube containing
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.) and
a Polytron® (Kinematica AG) homogenizer was used to
further powder bone. Total RNA was isolated from bone fragments,
CD4+ T cells or BMMSCs that had been co-cultured with T
cells, both with and without anti-TNF-α, using TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. RT-qPCR was performed using the one-step
RT-PCR kit, according to the manufacturer's protocol. RT-qPCR was
conducted using the following thermocycling conditions: RT step at
55˚C for 20 min; initial denaturation at 95˚C for 5 min; 40 cycles
of 95˚C for 20 sec and 60˚C for 1 min. The primers used in the
current study were as follows: IL-2 forward,
5'-CCCAAGCAGGCCACAGAATTGAAA-3' and reverse,
5'-TGAGTCAAATCCAGAACATGCCGC-3'; IFNγ forward,
5'-TCAAGTGGCATAGATGTGGAAGAA-3' and reverse,
5'-TGGCTCTGCAGGATTTTCATG-3'; TNF-α forward,
5'-TCTTCTCATTCCTGCTTGTGG-3' and reverse,
5'-GGTCTGGGCCATAGAACTGA-3'; Runx2 forward,
5'-ATTGGCACCATCTTTACTGTTACC-3' and reverse,
5'-CTCCTTAGAATCTGTTTGCTCTCATA-3'; OCN forward,
5'-CTGACAAAGCCTTCATGTCCAA-3' and reverse, 5'-CCG
CACGACAACCGCACCAT-3'; and β-actin forward,
5'-TGGCACCCAGCACAATGAA-3' and reverse,
5'-CTAAGTCATAGTCCGCCTAGAAGCA-3'. Relative expression was calculated
for each gene using the 2-ΔΔCq method (31) following normalization against
β-actin expression. The methods used for measuring Runx2 and OCN
mRNA expression from bone tissues were same as the methods for
RT-qPCR using RNA from cells.
Western blotting
Following co-culturing for 7 days, BMMSCs were
collected and assessed via western blotting. Cells were lysed in
RIPA containing 1 mM phenylmethylsulfonyl fluoride (Beyotime
Institute of Biotechnology). A Bradford protein assay (Bio-Rad
Laboratories, Inc.) was used to quantify protein concentrations.
Protein samples (25 µg/well) were separated using 10% SDS-PAGE.
Proteins were electroblotted onto PVDF membranes (0.45 mm; EMD
Millipore). Following this, the membranes were blocked using 5%
non-fat dry milk in TBS with 0.1% Tween-20 for 1 h at room
temperature. The membranes were then incubated with anti-Runx2
(1:500), anti-OCN (1:500), anti-p-ERK (1:500), anti-ERK (1:500),
anti-p-JNK (1:500), anti-JNK (1:500), anti-p-P38 (1:500), anti-P38
(1:500) and anti-β-actin (1:1,000) antibodies at 4˚C overnight.
This was followed by the incubation of membranes with horseradish
peroxidase-conjugated secondary antibodies (1:5,000) at room
temperature for 1 h. Membranes were visualized using an ECL system
and protein expression levels were normalized to β-actin protein
levels. The phosphorylated proteins were normalized to their
corresponding total proteins.
Statistical analysis
Statistical analysis was performed using SPSS
software (version no. 11.0; SPSS, Inc.). All data were expressed as
mean ± standard deviation of ≥3 independent experiments. Unpaired
Student's t-test or one-way ANOVA followed by a Tukey's post hoc
test was used to analyze data. P<0.05 was considered to indicate
a statistically significant difference.
Results
Bone formation and osteoblast
differentiation is reduced in OVX mice
A month following resection, serum estradiol levels
in the OVX group were significantly lower compared with the sham
group (P<0.05; Table I). MicroCT
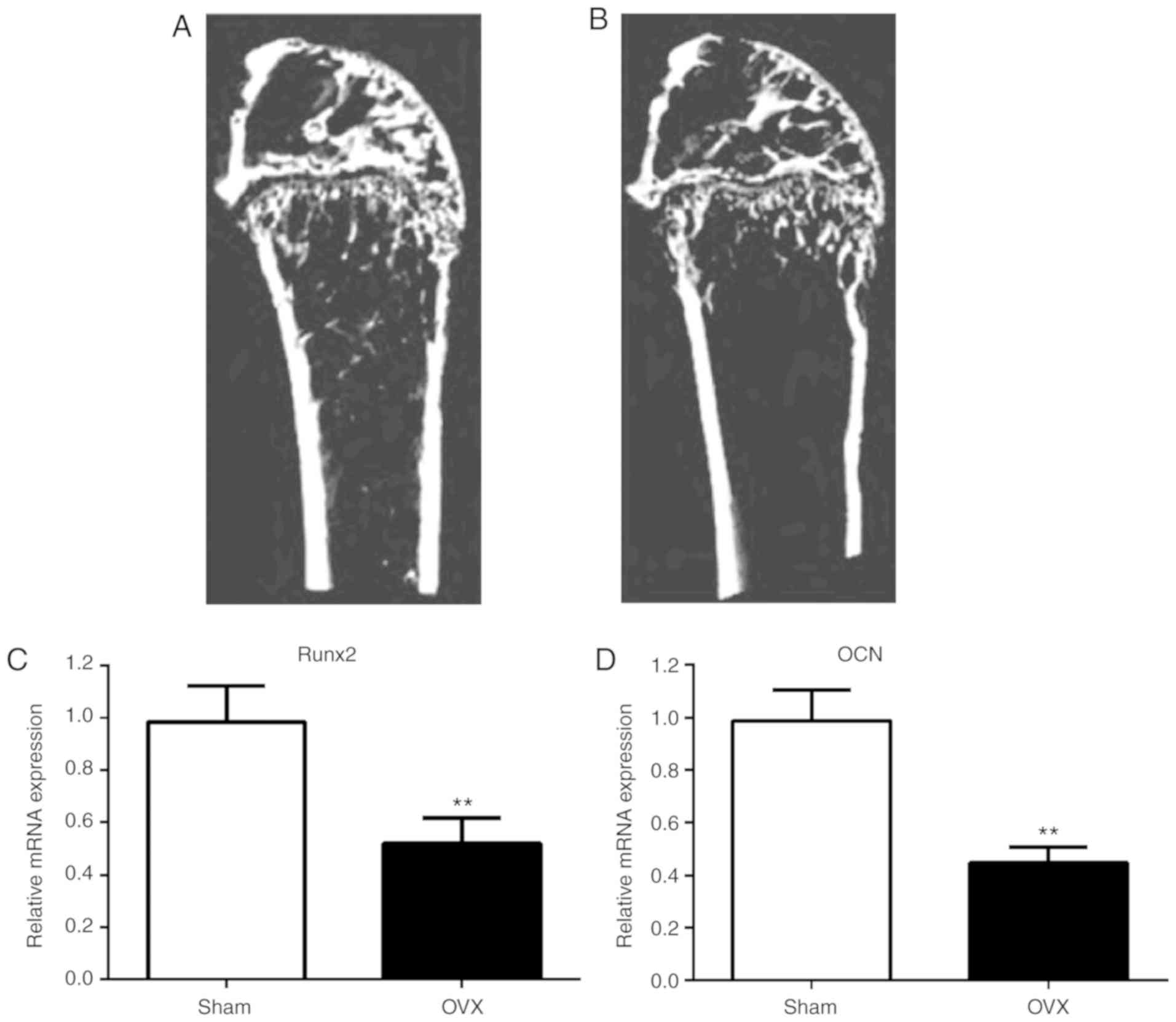
images displayed significantly lower bone mass in the OVX group
compared with the sham group (Fig.
1A and B). Furthermore,
compared to the sham group, BV/TV, trabecular thickness, trabecular
number and BMD were significantly reduced in the OVX group
(P<0.05). These results indicated that the mouse PMO model,
following ovarian ablation, was successfully constructed (Table II).
 | Table IThe level of serum estradiol. |
Table I
The level of serum estradiol.
| Group | Number | Serum estradiol
(pg/ml) |
|---|
| Sham | 10 | 48.88±17.89 |
| OVX | 10 |
10.44±3.80a |
 | Table IIMetaphyseal morphological parameters
in sham and OVX group. |
Table II
Metaphyseal morphological parameters
in sham and OVX group.
| Group | BV/TV (%) | Tb.Th (mm) | Tb.N (1/mm) | BMD
(mg/cm3) |
|---|
| Sham | 20.13±3.24 | 0.68±0.07 | 4.61±0.40 | 409.80±21.55 |
| OVX |
4.95±1.29b |
0.08±0.02b |
2.82±0.70a |
260.45±18.79a |
In order to assess osteoblast differentiation in
vivo, the expression of markers of osteogenesis progression,
Runx2 and OCN (32), in bone tissue
was determined using RT-qPCR (Fig.
1C and D). Compared to the sham
mice, lower levels of Runx2 and OCN were observed in the bone
tissue of the OVX mice, indicating that osteoblast differentiation
was reduced in OVX mice.
CD4+ T cells are activated
in OVX mice
The proportions of CD4+CD69+ T
cells in the spleen were analyzed using flow cytometry.
CD4+ T cells were gated as demonstrated in Fig. 2A. In the OVX group, the spleen
exhibited a significant increase in the proportion of
CD4+CD69+T cells compared with the sham group
(P<0.01; Fig. 2B). To further
explore the activation of T cells, the expression of
proinflammatory cytokines in T cells was determined using RT-qPCR.
The results indicated that the expression levels of IL-2, IFN-γ and
TNF-α were all significantly higher (P<0.05) in T cells of the
OVX group compared with the sham group (Fig. 2C).
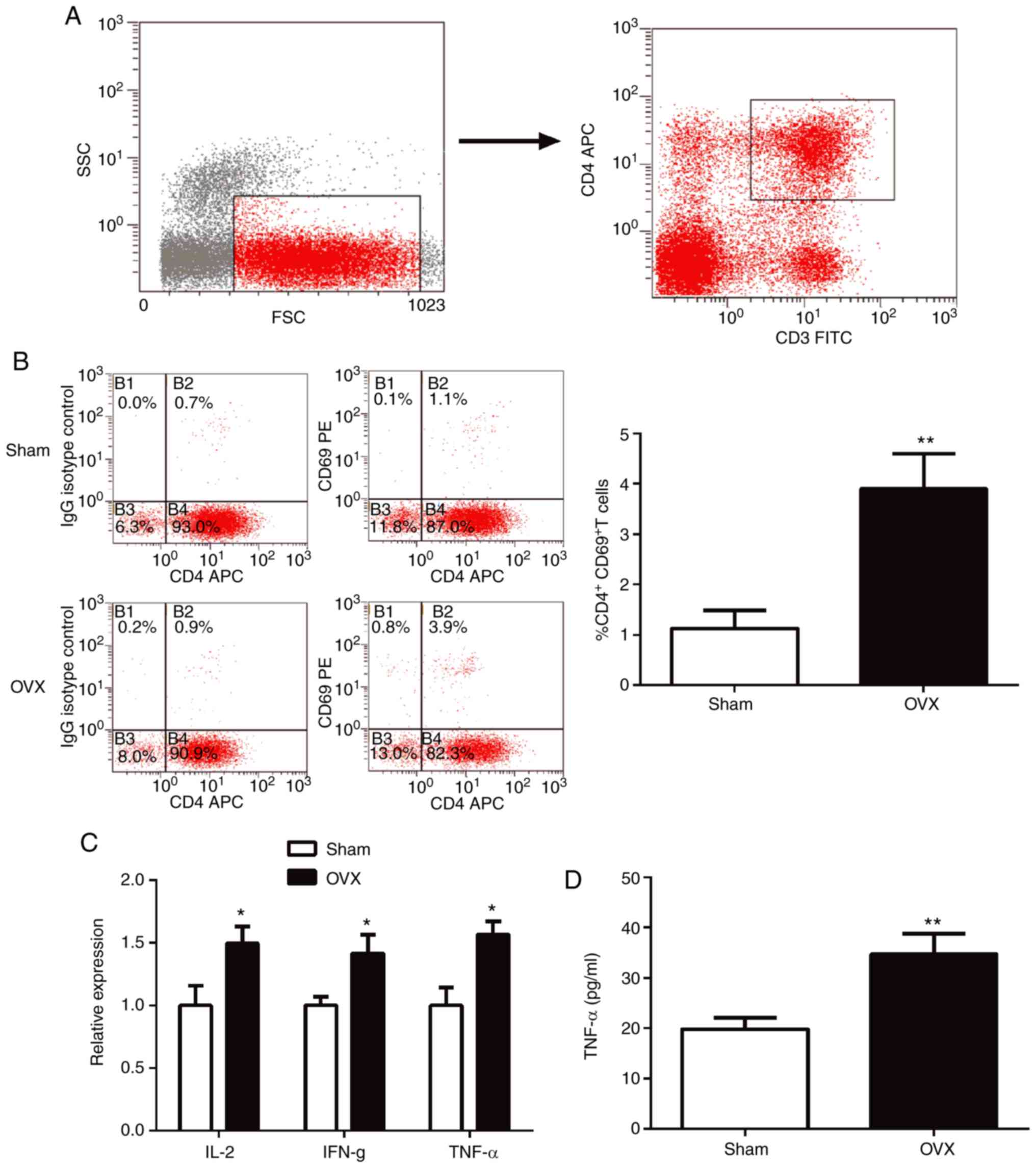 | Figure 2CD4+ T cells are activated
in OVX mice. (A) The gating strategies used for CD4+ T
cells. (B) Percentage of CD4+CD69+ cells in
spleens tested using flow cytometry. (C) Expression levels of
proinflammatory cytokines, IL-2, IFN-γ and TNF-α, in purified
CD4+ T cells were determined using reverse
transcription-quantitative-PCR. (D) TNF-α levels in the supernatant
from purified CD4+ T cell cultures were measured by
ELISA. *P<0.05, **P<0.01 vs. sham. CD,
cluster of differentiation; OVX, ovariectomized; IL-2, interleukin
2; IFN-γ, interferon- γ; TNF-α, tumor necrosis factor-α; IgG,
immunoglobulin; APC, allophycocyanin. |
ELISA results indicated that the expression of the
pro-osteoclastogenic cytokine TNF-α in T lymphocytes of OVX mice
was significantly higher compared with the sham group (Fig. 2D; P<0.05).
CD4+ T cells from OVX mice
reduce BMMSC proliferation via TNF-α
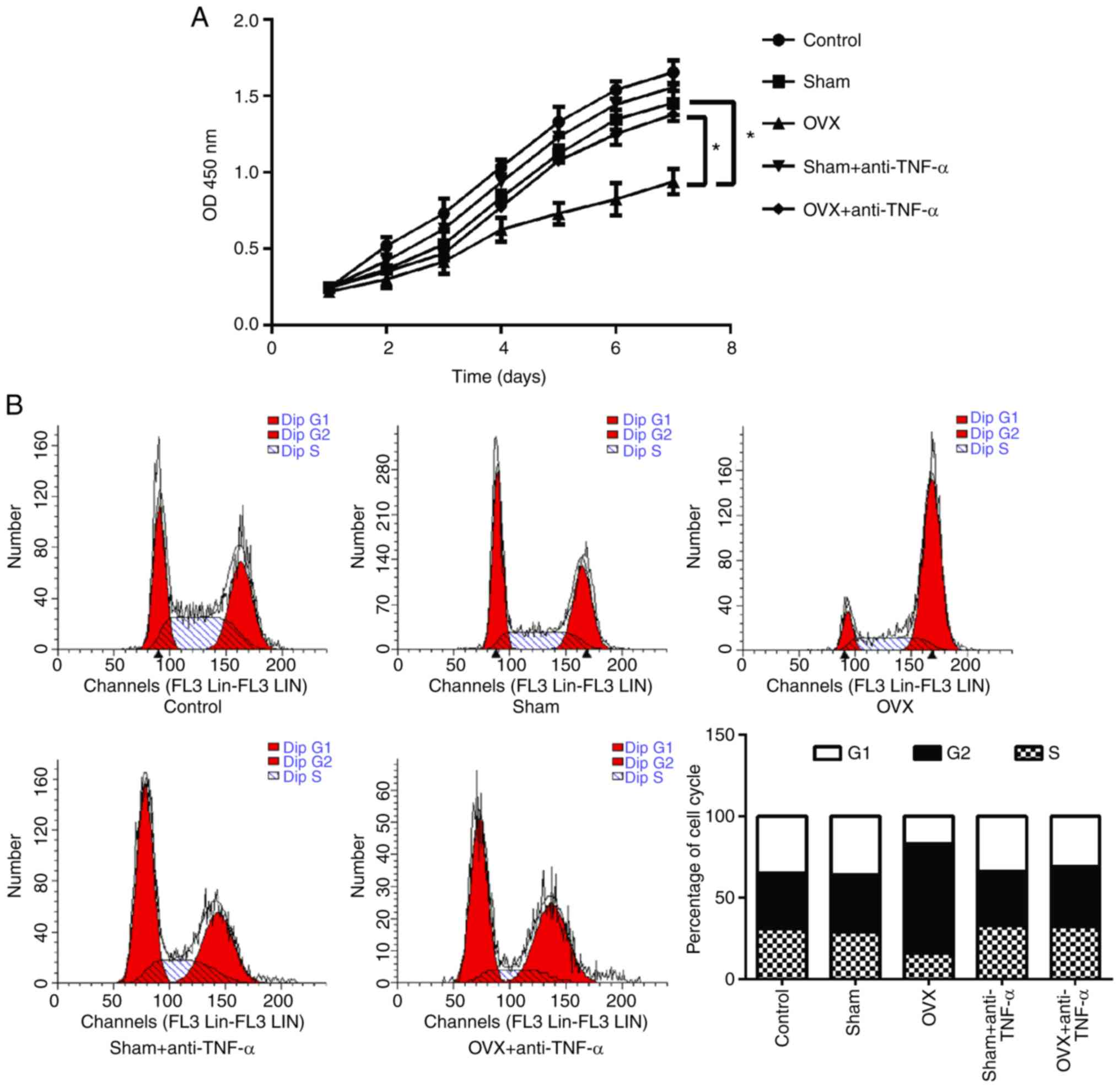
To investigate the effects of CD4+ T
cells on the proliferation of BMMSCs, MTT assays were performed
(Fig. 3A). The results indicated
that the proliferative ability of BMMSCs co-cultured with
CD4+ T cells from OVX mice was significantly decreased
compared with the sham group (P<0.05). However, this decreased
proliferation ability was not observed in T cells that had received
anti-TNF-α treatment (P<0.05). Furthermore, the cell cycle of
BMMSCs was analyzed using flow cytometry and the results revealed
that CD4+ T cells from OVX mice arrested the cell cycle
of BMMSCs at the G2/M phase, an effect that was not observed in T
cells that had received anti-TNF-α treatment (Fig. 3B).
TNF-α inhibits osteogenic
differentiation of BMMSCs
In order to elucidate the effects of CD4+
T cells on BMMSCs osteogenic differentiation, alkaline phosphatase
staining and alizarin red staining were performed. The results
revealed that compared with the sham group, BMMSCs co-cultured with
CD4+ T cells from OVX mice demonstrated less ALP
activity and calcium deposition. Following T cell pre-treatment
with anti-TNF-α, the ALP activity and calcium deposition levels
were increased in BMMSCs co-cultured with CD4+ T cells
from OVX mice, as well as in those from the sham group, compared to
cells co-cultured with T cells that had not been pre-treated
(Fig. 4A and B).
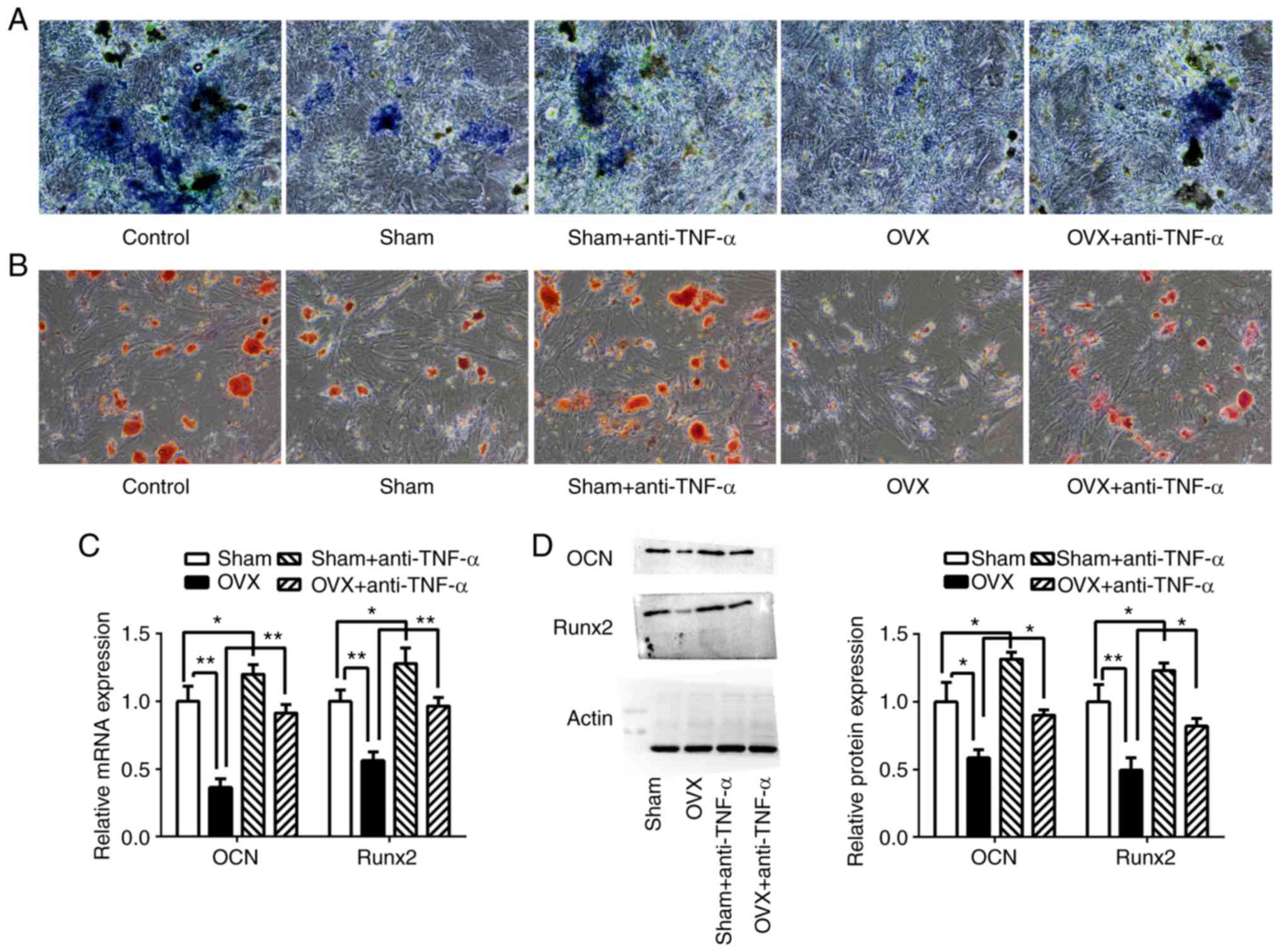 | Figure 4CD4+ T cells from OVX mice
inhibited BMMSC osteogenic differentiation. CD4+ T cells
with or without anti-TNF-α treatment from the sham group and the
OVX group were co-cultured with BMMSCs in osteogenic medium.
Following 7 days of culturing, (A) ALP staining and (B) Alizarin
red staining were performed. (C) Relative mRNA expression of
osteogenic marker genes in BMMSCs, Runx2 and OCN, were determined
using reverse transcription-quantitative PCR. (D) Western blotting
of Runx2 and OCN. *P<0.05. **P<0.01.
CD, cluster of differentiation; OVX, ovariectomized; BMMSCs, bone
marrow mesenchymal stem cells; TNF-α, tumor necrosis factor-α.;
ALP, alkaline phosphatase; BMMSCs, bone marrow mesenchymal stem
cells; Runx-2, runt related transcription factor 2; OCN,
osteocalcin. |
The osteoblastic differentiation of BMMSCs was
further evaluated by examination of the expression of the
osteoblastic markers Runx2 and OCN using RT-qPCR and western
blotting (Fig. 4C and D). The results indicated that compared to
the sham group, the expression of Runx2 and OCN were significantly
decreased in BMMSCs co-cultured with CD4+ T cells from
the OVX group (P<0.01). The expression levels of Runx2 and OCN
were increased in BMMSCs co-cultured with CD4+ T cells
from OVX mice that had been pretreated with anti-TNF-α (P
<0.01), as well as in those in the sham group compared with
BMMSCs co-cultured with cells that had not been pretreated
(P<0.05).
Influence of CD4+ T cells
on osteogenesis-related signaling pathways
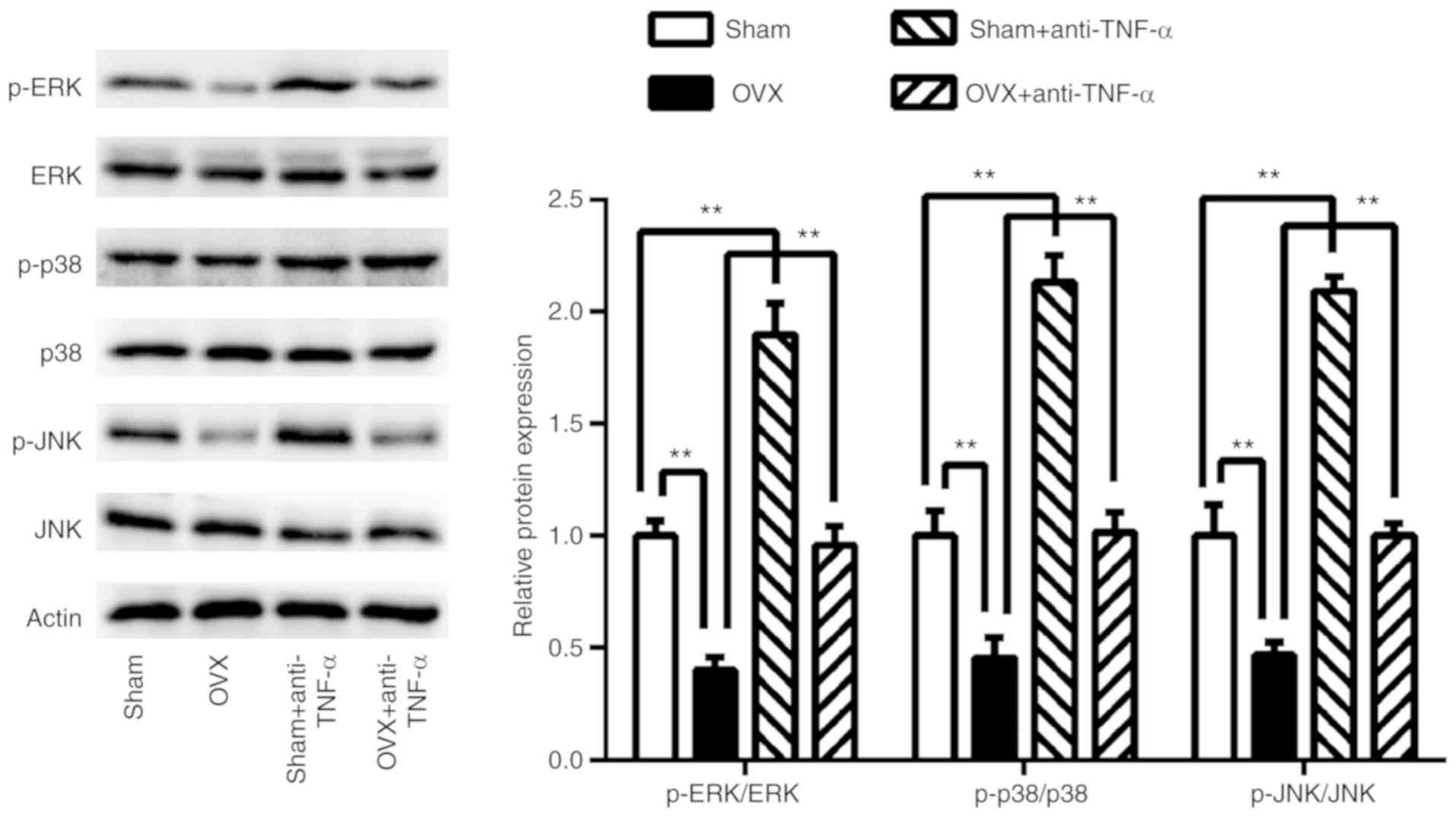
To examine which signaling pathways were involved in
CD4+ T cells exhibited decreased BMMSCs osteogenic
differentiation in OVX mice, 7 days post-co-culturing the ERK, JNK
and p38 MAPK osteogenesis-related signaling pathways were assessed
(Fig. 5). The results revealed that
these signaling pathways were significantly suppressed in BMMSCs
co-cultured with CD4+ T cells from the OVX group
(P<0.01), whereas they were restored following anti-TNF-α
treatment (P<0.01). In T cells that had received pre-treatment
with anti-TNF-α, these three signaling pathways were further
activated in BMMSCs co-cultured with CD4+ T cells from
the sham group (P<0.01) compared with controls.
Discussion
In PMO, T cells have been identified as a critical
cell population in promoting osteoclastogenesis (33). Moreover, as the principle source of
osteogenic cells, the proliferation and osteogenic differentiation
abilities of BMMSCs are important factors to understand in order to
develop improved osteoporosis treatments (34). The purpose of the present study was
to investigate the effect of ovariectomy-induced CD4+ T
cells on the proliferation and osteogenic differentiation of
BMMSCs.
Previous studies have demonstrated that
ovariectomy-induced osteoclastogenesis and bone loss are associated
with increased T cell activation (35,36)
and pro-osteoclastogenic cytokine TNF-α production (16,37).
In the present study, the results indicated that the activation of
CD4+ T cells was enhanced in OVX mice. This was
determined by analyzing the percentage of
CD4+CD69+ T cells in the spleen and the gene
expression levels of the proinflammatory cytokines, IL-2, IFN-γ and
TNF-α in CD4+ T cells isolated from OVX mice spleens.
Furthermore, increased TNF-α levels were observed in cultured
supernatants of CD4+ T cells isolated from OVX mice
spleens.
In order to assess whether CD4+ T cells
had an impact on the proliferation and osteogenic differentiation
of BMMSCs, BMMSCs were co-cultured with CD4+ T cells
from the OVX or sham group. A decrease in BMMSC proliferation
ability was induced using CD4+ T cells from OVX mice.
CD4+ T cells from OVX mice were also revealed to arrest
the cell cycle of BMMSCs at the G2/M phase. Similarly, the
osteogenic potential of BMMSCs was reduced using CD4+ T
cells from the OVX group and demonstrated lower ALP activity, less
calcium deposition and reduced expression of the osteoblastic
marker genes, Runx2 and OCN.
Regarding the importance of TNF-α in
ovariectomy-induced osteoclastogenesis, it was hypothesized that
TNF-α secreted by activated CD4+ T cells mediated the
inhibition of proliferation and osteogenic differentiation of
BMMSCs. Following pre-treatment with CD4+ T cells with
anti-TNF-α, the proliferation and osteogenic differentiation
abilities of BMMSCs were fully restored. It has been confirmed by
numerous studies that TNF-α stimulates the production of
osteoclasts (38-40);
however, the effects of TNF-α on stem cell proliferation and
osteogenic differentiation are still being explored. Recently,
TNF-α has been reported to suppress the osteogenic differentiation
of MSCs (41). The results from the
present study indicated that CD4+ T cells inhibited the
proliferation and osteogenic differentiation of BMMSCs by producing
high levels of TNF-α.
There are a number of common crossover mechanisms
between the immune response and bone remodeling. In the case of
inflammation or injury, immune cells receive transmission
information to act on osteoblasts and osteoclasts (42). Similarly, BMMSCs have an impact on T
cells (43). Studies have found
that BMMSCs affect the proliferation and differentiation of T cells
through the use of exocrine and paracrine cytokines (44,45).
The present study demonstrated that T cells also affected the
proliferation and osteogenic differentiation of BMMSCs.
Previous studies have indicated that several MAPKs
are essential components of the signal transduction machinery,
which serve an important role in the differentiation process
(46-48).
Several MAPKs, including ERKs, JNK and p38 MAPK have been confirmed
to be involved in the differentiation of MSCs into osteoblasts
(41,49). In the present study, these signaling
pathways were revealed to be critical for the osteogenesis of
BMMSCs. CD4+ T cells from OVX mice inhibited the ERK,
JNK and MAPK p38 signaling pathways, which suppressed the
osteogenic differentiation of BMMSCs. Furthermore, this inhibition
was remedied with an anti-TNF-α treatment.
As numerous studies have confirmed that the
OVX-BMMSC have decreased proliferative ability and osteogenic
differentiation in comparison to those from control animals
(50-52),
the phenotypes of BMMSC from OVX mice in the present study were not
assessed. Furthermore, evaluating the effects of activated T cells
on OVX-BMMSC would be challenging due to the reduced abilities of
proliferation and osteogenic differentiation in OVX-BMMSC. In
future studies, the influence of OVX-BMMSC on T cells will be
evaluated, which will provide information about the underlying
mechanisms between the immune response and bone remodeling in
PMO.
In conclusion, to the best of our knowledge, the
present study is the first to indicate that CD4+ T cells
inhibit the proliferation and osteogenic differentiation of BMMSCs
during the pathogenesis of OVX-induced osteoporosis and that this
inhibition may be, at least partially, mediated via the enhanced
expression of TNF-α by CD4+ T cells. These results may
provide novel insight into the dysfunction of BMMSCs caused by
estrogen deficiency.
Supplementary Material
Phenotype identification of BMSCs. The
percentages of BMSCs expressing (A) CD34, (B) CD44, (C) CD29 and
(D) CD90 were 1.3, 1.8, 97.6 and 93.9%, respectively. BMSC, bone
marrow stromal cells; CD, cluster of differentiation.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Medical
Scientific Research Project of Chongqing Health Bureau (grant no.
2012-2-129), the National Natural Science Foundation of China
(grant no. 81700958), the National Natural Science Foundation of
China (grant no. 81800979) and the Science and Technology Project
of Yubei District, Chongqing [grant no. 2017 (agriculture society)
45].
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BYS, NG and WMH conceived and designed the
experiments. BYS and LW conducted the experiments. BYS, YY, LC and
WMH performed data analysis, interpretation and discussion. BYS
wrote the manuscript. LW, NG and WMH revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Animal studies were approved by the Ethics Committee
of Chongqing Medical University (Chongqing, China; approval no.
2018101702).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lane JM, Russell L and Khan SN:
Osteoporosis. Clin Orthop Relat Res 139-150, 2000.
|
|
2
|
McCormick RK: Osteoporosis: Integrating
biomarkers and other diagnostic correlates into the management of
bone fragility. Altern Med Rev. 12:113–145. 2007.PubMed/NCBI
|
|
3
|
De Martinis M, Sirufo MM and Ginaldi L:
Osteoporosis: Current and emerging therapies targeted to
immunological checkpoints. Curr Med Chem: Jul 30, 2019 (Epub ahead
of print). doi: 10.2174/0929867326666190730113123.
|
|
4
|
Aggarwal BB, Shishodia S, Takada Y,
Banerjee S, Newman RA, Bueso-Ramos CE and Price JE: Curcumin
suppresses the paclitaxel-induced nuclear factor-kappaB pathway in
breast cancer cells and inhibits lung metastasis of human breast
cancer in nude mice. Clin Cancer Res. 11:7490–7498. 2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Datta HK, Ng WF, Walker JA, Tuck SP and
Varanasi SS: The cell biology of bone metabolism. J Clin Pathol.
61:577–587. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Khosla S, Oursler MJ and Monroe DG:
Estrogen and the skeleton. Trends Endocrinol Metab. 23:576–581.
2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Adori M, Kiss E, Barad Z, Barabás K,
Kiszely E, Schneider A, Kövesdi D, Sziksz E, Abrahám IM, Matkó J
and Sármay G: Estrogen augments the T cell-dependent but not the
T-independent immune response. Cell Mol Life Sci. 67:1661–1674.
2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang N, Gui Y, Qiu X, Tang W, Li L, Gober
HJ, Li D and Wang L: DHEA prevents bone loss by suppressing the
expansion of CD4(+) T cells and TNFa production in the OVX-mouse
model for postmenopausal osteoporosis. Biosci Trends. 10:277–287.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Faienza MF, Ventura A, Marzano F and
Cavallo L: Postmenopausal osteoporosis: The role of immune system
cells. Clin Dev Immunol. 2013(575936)2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Eastell R, O'Neill TW, Hofbauer LC,
Langdahl B, Reid IR, Gold DT and Cummings SR: Postmenopausal
osteoporosis. Nat Rev Dis Primers. 2(16069)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li JY, Tawfeek H, Bedi B, Yang X, Adams J,
Gao KY, Zayzafoon M, Weitzmann MN and Pacifici R: Ovariectomy
disregulates osteoblast and osteoclast formation through the T-cell
receptor CD40 ligand. Proc Natl Acad Sci USA. 108:768–773.
2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Song H, Li X, Zhao Z, Qian J, Wang Y, Cui
J, Weng W, Cao L, Chen X, Hu Y and Su J: Reversal of osteoporotic
activity by endothelial cell-secreted bone targeting and
biocompatible exosomes. Nano Lett. 19:3040–3048. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chen X, Zhi X, Wang J and Su J: RANKL
signaling in bone marrow mesenchymal stem cells negatively
regulates osteoblastic bone formation. Bone Res.
6(34)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Uehara IA, Soldi LR and Silva MJB: Current
perspectives of osteoclastogenesis through estrogen modulated
immune cell cytokines. Life Sci. 256(117921)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sang C, Zhang J, Zhang Y, Chen F, Cao X
and Guo L: TNF-α promotes osteoclastogenesis through JNK
signaling-dependent induction of Semaphorin3D expression in
estrogen-deficiency induced osteoporosis. J Cell Physiol.
232:3396–3408. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Roggia C, Gao Y, Cenci S, Weitzmann MN,
Toraldo G, Isaia G and Pacifici R: Up-regulation of TNF-producing T
cells in the bone marrow: A key mechanism by which estrogen
deficiency induces bone loss in vivo. Proc Natl Acad Sci USA.
98:13960–13965. 2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li J, Wang Q, Yang R, Zhang J, Li X, Zhou
X and Miao D: BMI-1 mediates estrogen-deficiency-induced bone loss
by inhibiting reactive oxygen species accumulation and T cell
activation. J Bone Miner Res. 32:962–973. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bi H, Ming L, Cheng R, Luo H, Zhang Y and
Jin Y: Liver extracellular matrix promotes BM-MSCs hepatic
differentiation and reversal of liver fibrosis through activation
of integrin pathway. J Tissue Eng Regen Med. 11:2685–2698.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Phinney DG: Biochemical heterogeneity of
mesenchymal stem cell populations: Clues to their therapeutic
efficacy. Cell Cycle. 6:2884–2889. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bi H and Jin Y: Current progress of skin
tissue engineering: Seed cells, bioscaffolds, and construction
strategies. Burns Trauma. 1:63–72. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kim KI, Park S and Im GI: Osteogenic
differentiation and angiogenesis with cocultured adipose-derived
stromal cells and bone marrow stromal cells. Biomaterials.
35:4792–4804. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Pilz GA, Ulrich C, Ruh M, Abele H, Schäfer
R, Kluba T, Bühring HJ, Rolauffs B and Aicher WK: Human term
placenta-derived mesenchymal stromal cells are less prone to
osteogenic differentiation than bone marrow-derived mesenchymal
stromal cells. Stem Cells Dev. 20:635–646. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gimble JM and Nuttall ME: The relationship
between adipose tissue and bone metabolism. Clin Biochem.
45:874–879. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Singh A, Mehdi AA, Srivastava RN and Verma
NS: Immunoregulation of bone remodelling. Int J Crit Illn Inj Sci.
2:75–81. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu Y, Wang L, Kikuiri T, Akiyama K, Chen
C, Xu X, Yang R, Chen W, Wang S and Shi S: Mesenchymal stem
cell-based tissue regeneration is governed by recipient T
lymphocytes via IFN-γ and TNF-α. Nat Med. 17:1594–1601.
2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Martinet L, Fleury-Cappellesso S,
Gadelorge M, Dietrich G, Bourin P, Fournié JJ and Poupot R: A
regulatory cross-talk between Vgamma9Vdelta2 T lymphocytes and
mesenchymal stem cells. Eur J Immunol. 39:752–762. 2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Biver G, Wang N, Gartland A, Orriss I,
Arnett TR, Boeynaems JM and Robaye B: Role of the P2Y13 receptor in
the differentiation of bone marrow stromal cells into osteoblasts
and adipocytes. Stem Cells. 31:2747–2758. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yamaza T, Miura Y, Bi Y, Liu Y, Akiyama K,
Sonoyama W, Patel V, Gutkind S, Young M, Gronthos S, et al:
Pharmacologic stem cell based intervention as a new approach to
osteoporosis treatment in rodents. PLoS One.
3(e2615)2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Eljaafari A, Tartelin ML, Aissaoui H,
Chevrel G, Osta B, Lavocat F and Miossec P: Bone marrow-derived and
synovium-derived mesenchymal cells promote Th17 cell expansion and
activation through caspase 1 activation: Contribution to the
chronicity of rheumatoid arthritis. Arthritis Rheum. 64:2147–2157.
2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Duffy MM, Pindjakova J, Hanley SA,
McCarthy C, Weidhofer GA, Sweeney EM, English K, Shaw G, Murphy JM,
Barry FP, et al: Mesenchymal stem cell inhibition of T-helper 17
cell-differentiation is triggered by cell-cell contact and mediated
by prostaglandin E2 via the EP4 receptor. Eur J Immunol.
41:2840–2851. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Duan L, Zhao H, Xiong Y, Tang X, Yang Y,
Hu Z, Li C, Chen S and Yu X: miR-16-2* Interferes with
WNT5A to Regulate Osteogenesis of Mesenchymal Stem Cells. Cell
Physiol Biochem. 51:1087–1102. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kelleher FC and O'Sullivan H: Monocytes,
macrophages, and osteoclasts in osteosarcoma. J Adolesc Young Adult
Oncol. 6:396–405. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kang SK, Shin IS, Ko MS, Jo JY and Ra JC:
Journey of mesenchymal stem cells for homing: Strategies to enhance
efficacy and safety of stem cell therapy. Stem Cells Int.
2012(342968)2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
D'Amelio P, Grimaldi A, Di Bella S,
Brianza SZM, Cristofaro MA, Tamone C, Giribaldi G, Ulliers D,
Pescarmona GP and Isaia G: Estrogen deficiency increases
osteoclastogenesis up-regulating T cells activity: A key mechanism
in osteoporosis. Bone. 43:92–100. 2008.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhang JL, Qiu XM, Zhang N, Tang W, Gober
HJ, Li DJ and Wang L: BuShenNingXin decoction suppresses
osteoclastogenesis by modulating RANKL/OPG imbalance in the
CD4+ T lymphocytes of ovariectomized mice. Int J Mol
Med. 42:299–308. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Aoki K, Saito H, Itzstein C, Ishiguro M,
Shibata T, Blanque R, Mian AH, Takahashi M, Suzuki Y, Yoshimatsu M,
et al: A TNF receptor loop peptide mimic blocks RANK ligand-induced
signaling, bone resorption, and bone loss. J Clin Invest.
116:1525–1534. 2006.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kudo O, Fujikawa Y, Itonaga I, Sabokbar A,
Torisu T and Athanasou NA: Proinflammatory cytokine
(TNFalpha/IL-1alpha) induction of human osteoclast formation. J
Pathol. 198:220–227. 2002.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Algate K, Haynes DR, Bartold PM, Crotti TN
and Cantley MD: The effects of tumour necrosis factor-α on bone
cells involved in periodontal alveolar bone loss; osteoclasts,
osteoblasts and osteocytes. J Periodont Res. 51:549–566.
2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Marahleh A, Kitaura H, Ohori F, Kishikawa
A, Ogawa S, Shen WR, Qi J, Noguchi T, Nara Y and Mizoguchi I: TNF-α
Directly Enhances Osteocyte RANKL Expression and Promotes
Osteoclast Formation. Front Immunol. 10(2925)2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Du D, Zhou Z, Zhu L, Hu X, Lu J, Shi C,
Chen F and Chen A: TNF-α suppresses osteogenic differentiation of
MSCs by accelerating P2Y2 receptor in estrogen-deficiency induced
osteoporosis. Bone. 117:161–170. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kumar G and Roger PM: From Crosstalk
between Immune and Bone Cells to Bone Erosion in Infection. Int J
Mol Sci. 20(5154)2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lecarpentier Y, Schussler O, Sakic A,
Rincon-Garriz JM, Soulie P, Bochaton-Piallat ML and Kindler V:
Human bone marrow contains mesenchymal stromal stem cells that
differentiate in vitro into contractile myofibroblasts controlling
t lymphocyte proliferation. Stem Cells Int.
2018(6134787)2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Lu X, Wang X, Nian H, Yang D and Wei R:
Mesenchymal stem cells for treating autoimmune dacryoadenitis. Stem
Cell Res Ther. 8(126)2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Rios C, Garbayo E, Gomez LA, Curtis K,
D'Ippolito G and Schiller PC: Stem cells and their contribution to
tissue repair. Stem Cell Regenerative Medicine. 1:9–22. 2010.
|
|
46
|
Sun Y, Liu WZ, Liu T, Feng X, Yang N and
Zhou HF: Signaling pathway of MAPK/ERK in cell proliferation,
differentiation, migration, senescence and apoptosis. J Recept
Signal Transduct Re. 35:600–604. 2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Cai TY, Zhu W, Chen XS, Zhou SY, Jia LS
and Sun YQ: Fibroblast growth factor 2 induces mesenchymal stem
cells to differentiate into tenocytes through the MAPK pathway. Mol
Med Rep. 8:1323–1328. 2013.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Hwang JH, Byun MR, Kim AR, Kim KM, Cho HJ,
Lee YH, Kim J, Jeong MG, Hwang ES and Hong JH: Extracellular Matrix
Stiffness Regulates Osteogenic Differentiation through MAPK
Activation. PLoS One. 10(e0135519)2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Huang RL, Yuan Y, Tu J, Zou GM and Li Q:
Opposing TNF-α/IL-1β- and BMP-2-activated MAPK signaling pathways
converge on Runx2 to regulate BMP-2-induced osteoblastic
differentiation. Cell Death Dis. 5(e1187)2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zhou S, Zilberman Y, Wassermann K, Bain
SD, Sadovsky Y and Gazit D: Estrogen modulates estrogen receptor
alpha and beta expression, osteogenic activity, and apoptosis in
mesenchymal stem cells (MSCs) of osteoporotic mice. J Cell Biochem
Suppl. 36:144–155. 2001.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Yang N, Wang G, Hu C, Shi Y, Liao L, Shi
S, Cai Y, Cheng S, Wang X, Liu Y, et al: Tumor necrosis factor
alpha suppresses the mesenchymal stem cell osteogenesis promoter
miR-21 in estrogen deficiency-induced osteoporosis. J Bone Miner
Res. 28:559–573. 2013.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Sang C, Zhang Y, Chen F, Huang P, Qi J,
Wang P, Zhou Q, Kang H, Cao X and Guo L: Tumor necrosis factor
alpha suppresses osteogenic differentiation of MSCs by inhibiting
semaphorin 3B via Wnt/β-catenin signaling in estrogen-deficiency
induced osteoporosis. Bone. 84:78–87. 2016.PubMed/NCBI View Article : Google Scholar
|