Introduction
Carotid artery stenosis (CAS) is an important risk
factor for ischemic neurological events. Nearly 80% of strokes
occur in asymptomatic patients and the incidence increases with age
(1,2). In addition, a number of cases were
identified to suffer from coronary artery disease or peripheral
atherosclerosis in asymptomatic patients with CAS (3). CAS is initially caused by abnormal
proliferation of vascular smooth muscle cells (VSMCs), accompanied
by intimal hyperplasia caused by matrix deposition of extracellular
connective tissue to form plaques and eventually develops into
symptomatic stenosis (4,5). There are various measures to prevent
or treat CAS, including endarterectomy, endovascular stent
placement (6) and medication
(7). However, prophylactic surgery
for CAS is controversial for patients with asymptomatic CAS
(8). Statins and anti-platelet
drugs are associated with a certain degree of stroke risk (9). Therefore, it is necessary to search
for novel subclinical molecular biomarkers to reliably predict
whether asymptomatic CAS is likely to develop into symptomatic CAS
or remain stable.
MicroRNAs (miRNAs) are a group of highly conserved
small non-coding RNA molecules that act as negative regulators of
post-transcriptional regulation by inhibiting target gene
expression. miRNAs have been indicated to be abnormally expressed
in the physiological and pathological processes of various
diseases. Numerous miRNAs have been reported to be abnormally
expressed in CAS, including miR-330-5p (10) and miR-125a-3p (11), indicating that miRNAs have potential
regulatory effects in CAS. In addition, certain miRNAs are
considered potential markers of atherosclerosis and miRNAs also
have an important role in VSMCs. Bi et al (12) reported that miR-503 inhibits the
proliferation and migration of human aortic VSMCs induced by
platelet-derived growth factors via targeting insulin receptors.
Furthermore, Cremer et al (13) recently reported that miR-503-5p was
associated with MALAT1 to reduce atherosclerosis in mice. In
addition, miR-503-5p may regulate the differentiation of the
mesenchymal stem cells into VSMCs, which has a certain effect in
vascular tissue transplantation (14). However, the diagnostic value of
miR-503-5p in CAS and the effect of miR-503-5p on the proliferation
of VSMCs in CAS remains to be determined.
In the present study, the diagnostic value of
miR-503-5p in patients with CAS was evaluated by detecting the
expression levels of miR-503-5p in patients with CAS. The effect of
miR-503-5p on the proliferation of VSMCs in CAS was further
investigated.
Materials and methods
Patient recruitment and sample
collection
A total of 62 asymptomatic patients with CAS
encountered at Anqiu People's Hospital (Weifang, China) between
February 2010 and February 2014 were included. The selection
criteria were an age of ≥18 years and asymptomatic CAS identified
based on the patients' clinical data and the National Institutes of
Health Stroke Scale (15).
Exclusion criteria were a history of stroke, transient ischemic
attack, coronary instability, congestive heart failure, chronic or
acute inflammatory conditions, cancer and recent intracranial
hemorrhage. From the health check-up center, 60 healthy controls
with a similar age were selected as the control group. The
inclusion criteria were no history of stroke and no mental illness.
The patients' basic data and clinical characteristics were recorded
accordingly (Table SI) and blood
samples were obtained on the day of hospitalization. After taking
blood samples, the serum samples were collected by centrifugation
at 2,500 x g for 15 min at room temperature in a swinging-bucket
centrifuge (Centrifuge 5810R; Eppendorf) and stored at -80˚C.
Cell culture and transfection
Human (h)VSMCs were purchased from the American Type
Culture Collection and cultured in Dulbecco's modified Eagle's
medium (Gibco; Thermo Fisher Scientific, Inc.) with 10% fetal
bovine serum (Thermo Fisher Scientific, Inc.). The cells were
cultured in an incubator at 37˚C with 5% CO2. Cell
transfection was performed when the cells were ~60% confluent.
Transfected vectors were miR-503-5p mimics (cat. no.
miR10002874-1-5; Guangzhou RiboBio Co., Ltd.), miR-503-5p inhibitor
(cat. no. miR20002874-1-5; Guangzhou RiboBio Co., Ltd.), mimics
negative control (mimics NC; cat. no. miR1N0000001-1-5; Guangzhou
RiboBio Co., Ltd.) and inhibitor NC (cat. no. miR2N0000001-1-5;
Guangzhou RiboBio Co., Ltd.), respectively. The transfection
reagent was Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.).
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
TRIzol was used to extract total RNA from the
subjects' serum and miRNA was isolated using the miRNApure Mini Kit
(CWBiotech). The extracted RNA was reverse-transcribed into
complementary (c)DNA according to the specifications of the Super
cDNA First-Strand Synthesis Kit (CWBiotech). Finally, qPCR was
performed with the Ultra SYBR Mixture and the ROX Assay kit
(CWBiotech) in an ABI 7300 real-time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The reaction mixtures
were incubated at 95˚C for 10 min, followed by 40 cycles of 94˚C
for 15 sec, 55˚C for 30 sec and 70˚C for 30 sec. Primer sequences
used are as following: miR-503-5p forward
5'-CCTATTTCCCATGATTCCTTCATA-3' and reverse 5'-CTCGTTCGGCAGCACA-3';
and U6 forward 5'-AACGCTTCACGAATTTGCGT-3' and reverse
5'-CTCGTTCGGCAGCACA-3'. Using U6 RNA as the internal control, the
relative expression of miR-503-5p was calculated using the
2-ΔΔCq method (16). All
of the experiments were repeated independently three times.
Cell proliferation assay
VSMCs at the exponential growth phase after
transfection were seeded at 5x104 cells per well in
96-well plates for continuous culture for 3 days and their
proliferation capacity was determined at 0, 24, 48 or 72 h. Each
time-point was performed in triplicate. Prior to detection, 10 µl
Cell Counting kit 8 (CCK-8) reagent was added to each well,
followed by further incubation for 1 h. The absorbance value was
then detected at 490 nm using a Bio-Rad iMark plate reader (Bio-Rad
Laboratories, Inc.). The effect of miR-503-5p on the proliferation
ability of VSMCs was evaluated after 3 days of continuous
detection.
Statistical analysis
All statistical data were processed and analyzed
with SPSS 21.0 software (IBM Corp.) and GraphPad Prism 7.0
software. Student's t-test and one-way analysis of variance
followed by Tukey's test were used to detect differences between
groups. The χ2 test was used to analyze the association
between miR-503-5p expression and clinical characteristics of
patients. A receiver operating characteristic (ROC) curve was drawn
to evaluate the diagnostic value of miR-503-5p in CAS and calculate
the area under the curve (AUC). P<0.05 was considered to
indicate a statistically significant difference.
Results
Serum levels of miR-503-5p in
asymptomatic patients with CAS
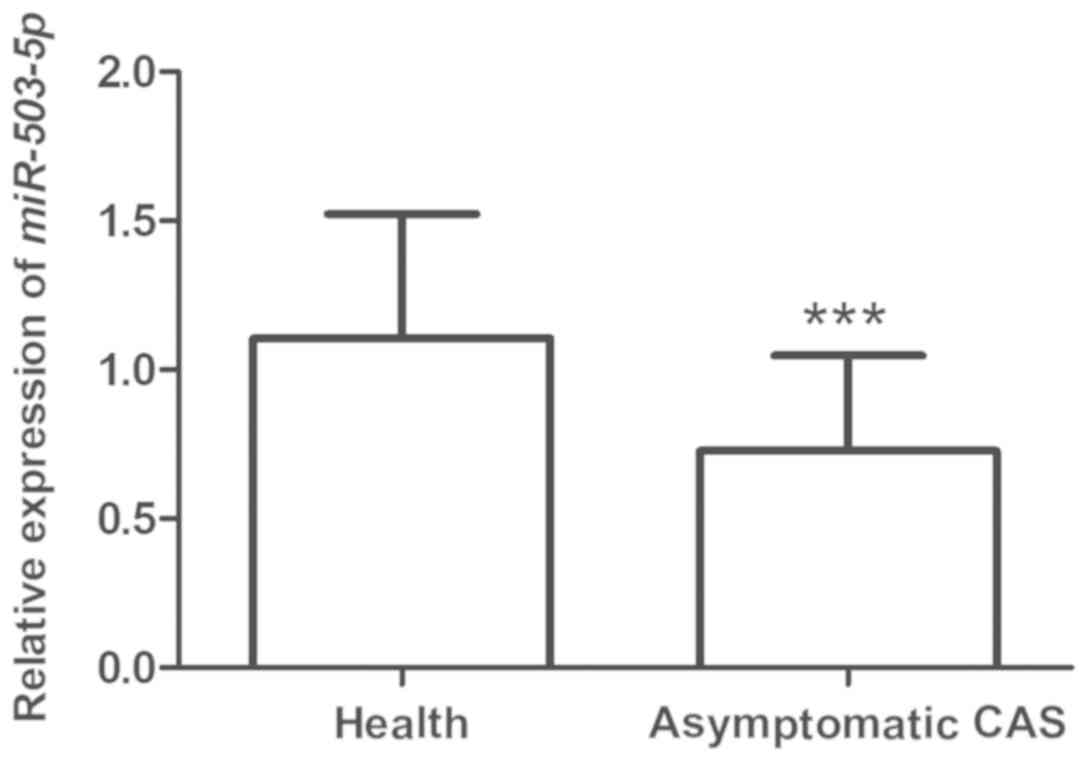
The levels of miR-503-5p in the serum of all
subjects were detected by RT-qPCR. The results indicated that the
levels of miR-503-5p in asymptomatic patients with CAS was
significantly lower than those in healthy controls (P<0.001;
Fig. 1), suggesting that miR-503-5p
may have a critical role in the development of asymptomatic
CAS.
Association between miR-503-5p and
clinicopathological features of patients
The association between the expression levels of
miR-503-5p and the clinicopathological features of asymptomatic
patients with CAS was then explored. According to the average
expression levels of miR-503-5p in asymptomatic patients with CAS,
all patients with asymptomatic CAS were divided into the high
miR-503-5p expression group (n=24) and the low miR-503-5p
expression group (n=38). As presented in Table I, the expression levels of
miR-503-5p were not significantly associated with age, gender, body
mass index (BMI), hypertension and dyslipidemia (P>0.05), but
were significantly associated with diabetes (P=0.034) and carotid
artery stenosis (P=0.017).
 | Table IAssociation of miR-503-5p with the
clinical parameters in asymptomatic patients with CAS (n=62). |
Table I
Association of miR-503-5p with the
clinical parameters in asymptomatic patients with CAS (n=62).
| | miR-503-5p
expression | |
|---|
| Parameter | Total | Low (n=38) | High (n=24) | P-value |
|---|
| Age, years | | | | 0.586 |
|
≤58 | 22 | 15 | 7 | |
|
>58 | 40 | 23 | 17 | |
| Gender | | | | 0.792 |
|
Male | 36 | 23 | 13 | |
|
Female | 26 | 15 | 11 | |
| BMI | | | | 0.444 |
|
≤25 | 32 | 18 | 14 | |
|
>25 | 30 | 20 | 10 | |
| Dyslipidemia | | | | 0.068 |
|
No | 29 | 14 | 15 | |
|
Yes | 33 | 24 | 9 | |
| Hypertension | | | | 0.063 |
|
No | 26 | 12 | 14 | |
|
Yes | 36 | 26 | 10 | |
| Diabetes | | | | 0.034 |
|
No | 23 | 10 | 13 | |
|
Yes | 39 | 28 | 11 | |
| Degree of CAS,
% | | | | 0.017 |
|
50-69 | 26 | 11 | 15 | |
|
70-99 | 36 | 27 | 9 | |
Diagnostic value of miR-503-5p in
asymptomatic patients with CAS
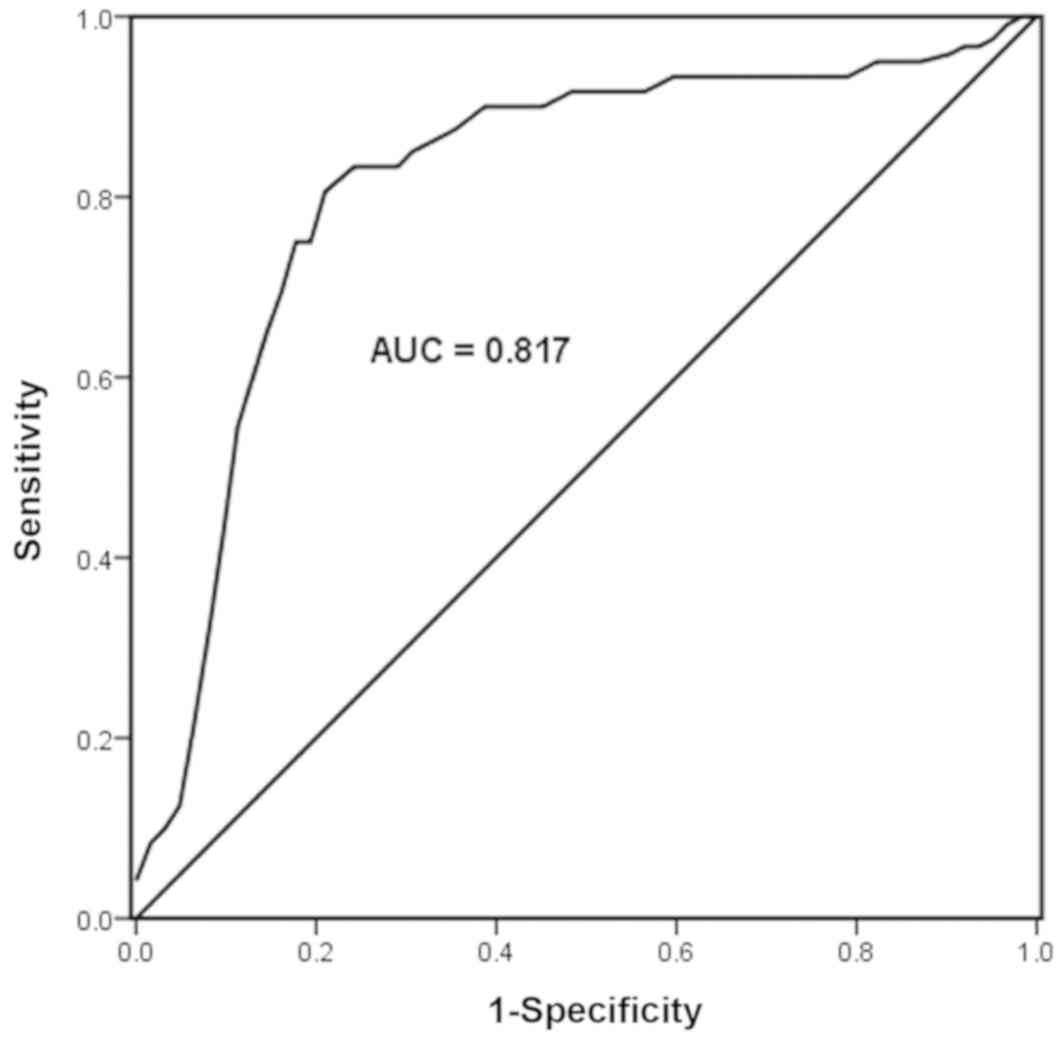
A ROC curve was drawn according to the expression
levels of miR-503-5p in asymptomatic patients with CAS and healthy
controls to evaluate the diagnostic value of miR-503-5p for CAS. As
indicated in Fig. 2, the AUC of the
ROC curve was 0.817. The sensitivity was 83.30%, the specificity
was 79.03% and the cut-off value was 0.810. The results suggested
that miR-503-5p was of high diagnostic value in asymptomatic
patients with CAS.
miR-503-5p regulates the proliferation
of hVSMCs
The occurrence of CAS is linked to the abnormal
proliferation of VSMCs. In the present study, the effect of
miR-503-5p on the proliferation of hVSMCs was verified by
transfection of miR-503-5p mimics and inhibitors. The transfection
efficiency was verified by RT-qPCR analysis. As presented in
Fig. 3A, the expression in the
miR-503-5p mimics group was significantly higher than that in the
control group (P<0.001), while the expression in the miR-503-5p
inhibitor group was significantly lower (P<0.001). The results
suggested that the overexpression and knockdown efficiency of
miR-503-5p mimics and inhibitors in hVSMCs was higher.
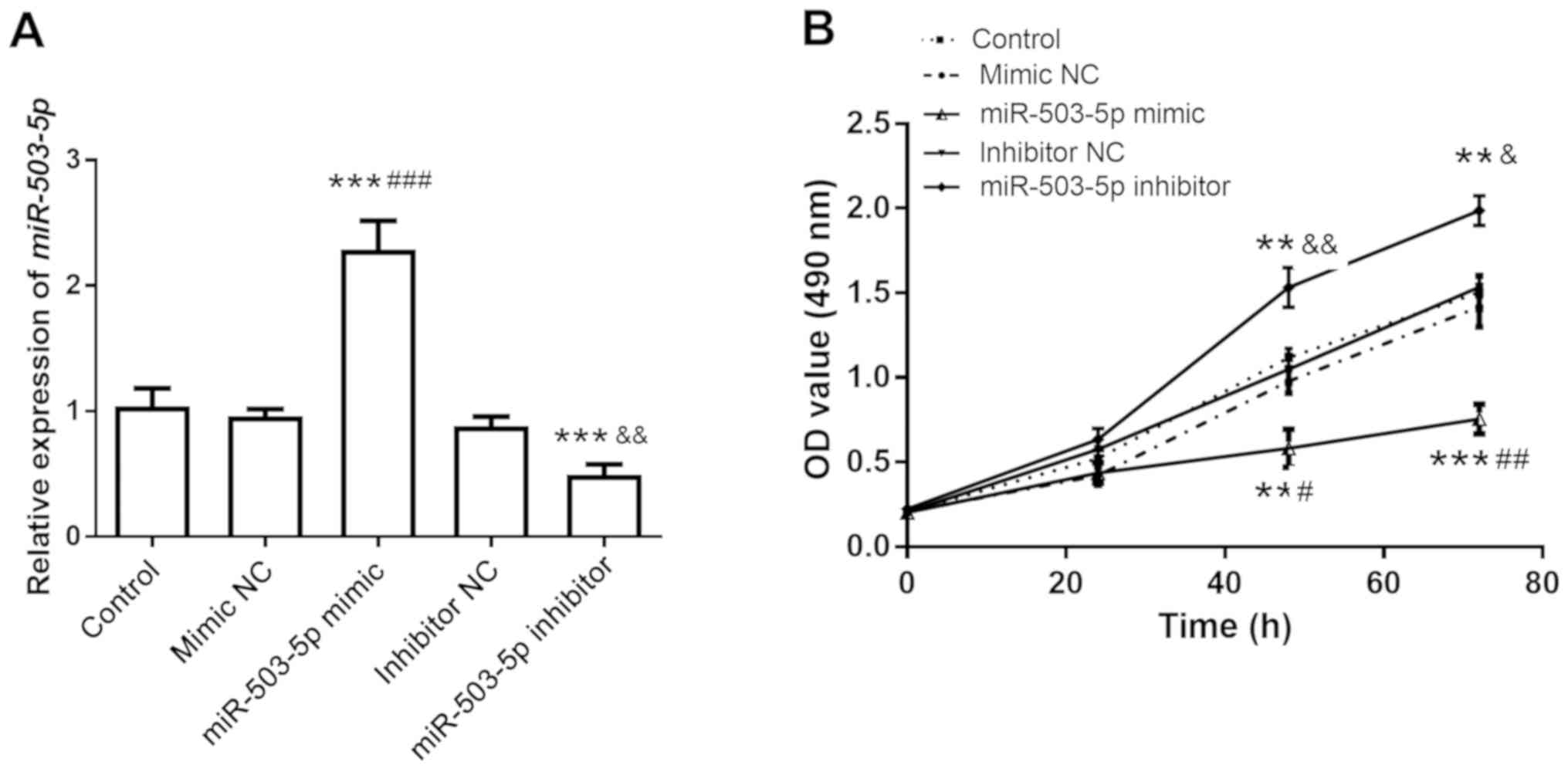 | Figure 3Detection of cell proliferation after
transfection with miR-503-5p inhibitors and mimics. (A) The
expression levels of miR-503-5p changed after transfection of
miR-503-5p inhibitor and mimics, respectively. (B) After
transfection with miR-503-5p inhibitors and mimics, the
proliferation ability of cells was detected with a Cell Counting
Kit-8. After transfection with miR-503-5p inhibitor, the
proliferative ability of the cells was significantly promoted,
whereas after transfection with miR-503-5p mimics, the
proliferation ability was significantly decreased.
**P<0.01, ***P<0.001, compared with
control group; #P<0.05, ##P<0.01,
###P<0.001, compared with mimic NC group;
&P<0.05, &&P<0.01, compared
with inhibitor NC group. miR, microRNA; NC, negative control; OD,
optical density. |
A CCK-8 assay was used to further detect the effect
of miR-503-5p on the proliferation ability of hVSMCs. As presented
in Fig. 3B, the proliferation
ability in the miR-503-5p mimics group was lower than that in the
control group, while the proliferation ability in the miR-503-5p
inhibitor group was significantly higher than that in the control
group (P<0.01).
Discussion
The carotid artery is the major blood vessel in the
neck that supplies blood from the heart to the brain and face. CAS
is a pathological condition of vascular stenosis caused by
atherosclerotic plaque (17).
Asymptomatic CAS is defined as a patient without a history of
ischemic stroke or transient ischemic attack in the ipsilateral
carotid region and without focal neurological symptoms (18). At present, CAS is diagnosed by
double ultrasound, CT angiography and MR angiography (19). Although traditional digital
subtraction angiography is the gold standard for CAS diagnosis, its
invasive nature carries the risk of stroke. Duplex ultrasound is
non-radiative and non-invasive, but its sensitivity and specificity
in diagnosing CAS are only moderate and require secondary
verification (20,21). Therefore, it is urgent to develop
novel sensitive, specific and non-invasive diagnostic markers for
CAS.
In recent years, due to its high sensitivity,
specificity and non-invasive nature, the detection of miRNAs as
biomarkers has attracted an increasing amount of attention
(22). In human cancer, certain
serum miRNAs may be used as diagnostic and prognostic markers,
including miR-1290(23),
miR-141(24) and miR-193b (25). Disorder of miRNAs is also involved
in the pathological processes of numerous cardiovascular diseases,
including atherosclerosis and CAS. For instance, inhibition of
miR-103 may reduce the inflammatory response and endoplasmic
reticulum stress in patients with atherosclerosis (10). Serum miR-638 was significantly
reduced in high-risk CAS patients undergoing carotid endarterectomy
and was indicated to serve as a non-invasive biomarker associated
with plaque vulnerability and ischemic stroke (26). In the present study, the expression
of miR-503-5p was significantly downregulated in asymptomatic
patients with CAS compared with that in healthy controls. This
confirms that miR-503-5p has a crucial role in patients with CAS.
miR-503 has been previously reported to be significantly
downregulated in the serum of patients with coronary heart disease
and to be a good prognostic marker of coronary heart disease
(27). Ezetimibe inhibits
PMA-induced monocyte/macrophage differentiation by upregulating the
expression of miRNAs, including miR-503, and exerts an
anti-atherosclerosis effect (28).
These studies are consistent with the results of the present
study.
To further confirm the potential role of miR-503-5p
in asymptomatic CAS, its diagnostic value was examined. The
experimental results indicate that miR-503-5p is highly specific
and sensitive in distinguishing asymptomatic patients with CAS from
healthy individuals. The clinical value of miR-503-5p has been
widely reported in previous studies. Circular RNA (Circrna) 0000267
promotes gastric cancer progression through sponging miR-503-5p and
regulation of high-mobility group AT-hook 2 expression (29). Low expression of miR-503 in gastric
cancer tissues and serum may be used as a diagnostic marker for
gastric cancer (30). In the
present study, the promising diagnostic value of miR-503-5p in
asymptomatic CAS patients was confirmed. Previous studies have
reported that hypertension, dyslipidemia and diabetes are risk
factors for the high prevalence of CAS (19). In addition, in patients with type II
diabetes, hypertension and dyslipidemia have been indicated to have
an accumulating effect on the carotid plaque burden (31). Recently, it has been reported that
miR-503-5p is dysregulated in diabetic nephropathy (32). Therefore, in the present study, the
clinicopathological features of patients with asymptomatic CAS and
the expression of miR-503-5p were examined and it was indicated
that the expression levels of miR-503-5p were associated with
diabetes and carotid stenosis.
Studies have reported that plaque stability during
the formation of CAS is associated with the function of VSMCs and
endothelial cells (33,34). Furthermore, miR-145 was indicated to
have a key role in CAS by regulating the function of VSMCs
(35). At the same time, miR-503
inhibits the proliferation and migration of human aortic VSMCs
induced by platelet-derived growth factors by targeting insulin
receptors (12). Therefore, in the
present study, miR-503-5p inhibitors and mimics were transfected
into hVSMCs. After confirming the sufficient overexpression and
knockdown efficiency, the proliferation ability of miR-503-5p on
VSMCs was further tested. The results suggested that inhibition of
miR-503-5p expression significantly promoted the proliferation of
VSMCs. Studies have reported that fibroblast growth factor (FGF)
expressed in 68% of cases of CAS (36). Circrna WD repeat domain 77 targets
FGF-2 and regulates the proliferation and migration of VSMCs by
stimulating miR-124(37). At the
same time, it has been reported that miR-503 inhibits tumor
angiogenesis and growth by targeting FGF2 and VEGFA (38). In pulmonary hypertension, miR-424-
and miR-503-mediated endothelial cell apelin-FGF connections are
destroyed (39). In addition, the
relevant target of miR-503-5p was detected by TargetScan and it was
indicated that epidermal growth factor 2 was the target of
miR-503-5p. Therefore, it was speculated that miR-503-5p may
regulate the functions of CAS and VSMCs by targeting FGF2. However,
how miR-503-5p exerts its role in CAS remains to be further
determined.
In conclusion, a series of experimental results have
confirmed the low expression of miR-503-5p in asymptomatic patients
with CAS and the low expression of miR-503-5p may be used as a
potential diagnostic marker of asymptomatic CAS. At the same time,
inhibition of miR-503-5p may promote the proliferation of VSMCs and
may be used as a potential therapeutic target for CAS.
Supplementary Material
Comparison of clinic characteristics
of patients with CAS with the control population.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZY, HW, JL and XL designed the study. ZY, HW, JL and
YL performed the experiments and interpreted the data. ZY, HW and
YL drafted the manuscript. JL and XL revised it critically for
important intellectual content. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The experiment was approved by the Anqiu People's
Hospital Ethics Committee (Weifang, China) and written informed
consent was obtained from all subjects.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bogousslavsky J, Van Melle G and Regli F:
The lausanne stroke registry: Analysis of 1,000 consecutive
patients with first stroke. Stroke. 19:1083–1092. 1988.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Paciaroni M, Silvestrelli G, Caso V, Corea
F, Venti M, Milia P, Tambasco N, Parnetti L and Gallai V:
Neurovascular territory involved in different etiological subtypes
of ischemic stroke in the perugia stroke registry. Eur J Neurol.
10:361–365. 2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Taussky P, Hanel RA and Meyer FB: Clinical
considerations in the management of asymptomatic carotid artery
stenosis. Neurosurg Focus. 31(E7)2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Davies MG and Hagen PO: Pathobiology of
intimal hyperplasia. Br J Surg. 81:1254–1269. 1994.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Subbotin VM: Analysis of arterial intimal
hyperplasia: Review and hypothesis. Theor Biol Med Model.
4(41)2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
O'Brien M and Chandra A: Carotid
revascularization: Risks and benefits. Vasc Health Risk Manag.
10:403–416. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bae C, Szuchmacher M and Chang JB:
Comparative review of the treatment methodologies of carotid
stenosis. Int J Angiol. 24:215–222. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
ACST-2 Collaborative Group. Halliday A,
Bulbulia R, Gray W, Naughten A, den Hartog A, Delmestri A, Wallis
C, le Conte S and Macdonald S. Status update and interim results
from the asymptomatic carotid surgery trial-2 (ACST-2). Eur J Vasc
Endovasc Surg. 46:510–518. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Abbott AL: Medical (nonsurgical)
intervention alone is now best for prevention of stroke associated
with asymptomatic severe carotid stenosis: Results of a systematic
review and analysis. Stroke. 40:e573–e583. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wei X, Sun Y, Han T, Zhu J, Xie Y, Wang S,
Wu Y, Fan Y, Sun X, Zhou J, et al: Upregulation of miR-330-5p is
associated with carotid plaque's stability by targeting Talin-1 in
symptomatic carotid stenosis patients. BMC Cardiovasc Disord.
19(149)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hu W, Chang G, Zhang M, Li Y, Yin L, Huang
Y, Feng C, Gu Y, Wen D and Wang S: MicroRNA-125a-3p affects smooth
muscle cell function in vascular stenosis. J Mol Cell Cardiol.
136:85–94. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bi R, Ding F, He Y, Jiang L, Jiang Z, Mei
J and Liu H: miR-503 inhibits platelet-derived growth
factor-induced human aortic vascular smooth muscle cell
proliferation and migration through targeting the insulin receptor.
Biomed Pharmacother. 84:1711–1716. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cremer S, Michalik KM, Fischer A,
Pfisterer L, Jaé N, Winter C, Boon RA, Muhly-Reinholz M, John D,
Uchida S, et al: Hematopoietic deficiency of the long noncoding RNA
MALAT1 promotes atherosclerosis and plaque inflammation.
Circulation. 139:1320–1334. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gu W, Hong X, Le Bras A, Nowak WN, Issa
Bhaloo S, Deng J, Xie Y, Hu Y, Ruan XZ and Xu Q: Smooth muscle
cells differentiated from mesenchymal stem cells are regulated by
microRNAs and suitable for vascular tissue grafts. J Biol Chem.
293:8089–8102. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lal BK, Dux MC, Sikdar S, Goldstein C,
Khan AA, Yokemick J and Zhao L: Asymptomatic carotid stenosis is
associated with cognitive impairment. J Vasc Surg. 66:1083–1092.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rao X, Huang X, Zhou Z and Lin X: An
improvement of the 2^(-delta delta CT) method for
quantitative real-time polymerase chain reaction data analysis.
Biostat Bioinforma Biomath. 3:71–85. 2013.PubMed/NCBI
|
|
17
|
Beckman JA: Management of asymptomatic
internal carotid artery stenosis. JAMA. 310:1612–1618.
2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Goldstein LB: Screening for asymptomatic
carotid artery stenosis: Evidence-based opinion. JAMA Intern Med.
176:633–634. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Dharmakidari S, Bhattacharya P and
Chaturvedi S: Carotid artery stenosis: Medical therapy, surgery,
and stenting. Curr Neurol Neurosci Rep. 17(77)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Nederkoorn PJ, van der Graaf Y and Hunink
MG: Duplex ultrasound and magnetic resonance angiography compared
with digital subtraction angiography in carotid artery stenosis: A
systematic review. Stroke. 34:1324–1332. 2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jahromi AS, Cina CS, Liu Y and Clase CM:
Sensitivity and specificity of color duplex ultrasound measurement
in the estimation of internal carotid artery stenosis: A systematic
review and meta-analysis. J Vasc Surg. 41:962–972. 2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Shi HB, Yu JX, Yu JX, Feng Z, Zhang C, Li
GY, Zhao RN and Yang XB: Diagnostic significance of microRNAs as
novel biomarkers for bladder cancer: A meta-analysis of ten
articles. World J Surg Oncol. 15(147)2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sun H, Wang L, Zhao Q and Dai J:
Diagnostic and prognostic value of serum miRNA-1290 in human
esophageal squamous cell carcinoma. Cancer Biomark. 25:381–387.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li Z, Ma YY, Wang J, Zeng XF, Li R, Kang W
and Hao XK: Exosomal microRNA-141 is upregulated in the serum of
prostate cancer patients. Onco Targets Ther. 9:139–148.
2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chan CM, Lai KKY, Ng EKO, Kiang MN, Kwok
TWH, Wang HK, Chan KW, Law TT, Tong DK, Chan KT, et al: Serum
microRNA-193b as a promising biomarker for prediction of
chemoradiation sensitivity in esophageal squamous cell carcinoma
patients. Oncol Lett. 15:3273–3280. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Luque A, Farwati A, Krupinski J and Aran
JM: Association between low levels of serum miR-638 and
atherosclerotic plaque vulnerability in patients with high-grade
carotid stenosis. J Neurosurg. 131:72–79. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Fei Y, Hou J, Xuan W, Zhang C and Meng X:
The relationship of plasma miR-503 and coronary collateral
circulation in patients with coronary artery disease. Life Sci.
207:145–151. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Muñoz-Pacheco P, Ortega-Hernández A, Miana
M, Cachofeiro V, Fernández-Cruz A and Gómez-Garre D: Ezetimibe
inhibits PMA-induced monocyte/macrophage differentiation by
altering microRNA expression: A novel anti-atherosclerotic
mechanism. Pharmacol Res. 66:536–543. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Cai X, Nie J, Chen L and Yu F:
Circ_0000267 promotes gastric cancer progression via sponging
MiR-503-5p and regulating HMGA2 expression. Mol Genet Genomic Med.
8(e1093)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wu D, Cao G, Huang Z, Jin K, Hu H, Yu J
and Zeng Y: Decreased miR-503 expression in gastric cancer is
inversely correlated with serum carcinoembryonic antigen and acts
as a potential prognostic and diagnostic biomarker. Onco Targets
Ther. 10:129–135. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yuan C, Lai CW, Chan LW, Chow M, Law HK
and Ying M: Cumulative effects of hypertension, dyslipidemia, and
chronic kidney disease on carotid atherosclerosis in Chinese
patients with type 2 diabetes mellitus. J Diabetes Res.
2014(179686)2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Assmann TS, Recamonde-Mendoza M, Costa AR,
Puñales M, Tschiedel B, Canani LH, Bauer AC and Crispim D:
Circulating miRNAs in diabetic kidney disease: Case-control study
and in silico analyses. Acta Diabetol. 56:55–65. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chen YC, Bui AV, Diesch J, Manasseh R,
Hausding C, Rivera J, Haviv I, Agrotis A, Htun NM, Jowett J, et al:
A novel mouse model of atherosclerotic plaque instability for drug
testing and mechanistic/therapeutic discoveries using gene and
microRNA expression profiling. Circ Res. 113:252–265.
2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Borissoff JI, Heeneman S, Kilinç E, Kassák
P, Van Oerle R, Winckers K, Govers-Riemslag JW, Hamulyák K, Hackeng
TM, Daemen MJ, et al: Early atherosclerosis exhibits an enhanced
procoagulant state. Circulation. 122:821–830. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Han Z, Hu H, Yin M, Li X, Li J, Liu L and
Liu B: miR-145 is critical for modulation of vascular smooth muscle
cell proliferation in human carotid artery stenosis. J Biol Regul
Homeost Agents. 32:506–516. 2018.PubMed/NCBI
|
|
36
|
Janczak D, Ziolkowski P, Szydelko T,
Dorobisz T, Janczak D, Dorobisz K and Chabowski M: The presence of
some cytokines and Chlamydia pneumoniae in the atherosclerotic
carotid plaque in patients with carotid artery stenosis. Postepy
Hig Med Dosw (Online). 69:227–232. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chen J, Cui L, Yuan J, Zhang Y and Sang H:
Circular RNA WDR77 target FGF-2 to regulate vascular smooth muscle
cells proliferation and migration by sponging miR-124. Biochem
Biophys Res Commun. 494:126–132. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhou B, Ma R, Si W, Li S, Xu Y, Tu X and
Wang Q: MicroRNA-503 targets FGF2 and VEGFA and inhibits tumor
angiogenesis and growth. Cancer Lett. 333:159–169. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kim J, Kang Y, Kojima Y, Lighthouse JK, Hu
X, Aldred MA, McLean DL, Park H, Comhair SA, Greif DM, et al: An
endothelial apelin-FGF link mediated by miR-424 and miR-503 is
disrupted in pulmonary arterial hypertension. Nat Med. 19:74–82.
2013.PubMed/NCBI View
Article : Google Scholar
|