Introduction
Cardiac hypertrophy (CH) is closely related to a
range of cardiovascular diseases, including heart failure and
sudden cardiac death (1). CH is the
adaptive response to counteract stresses and maintain normal
cardiac function (2). In response
to external mechanical or pathological stresses, the heart
undergoes cardiac remodeling, which manifests as CH by increasing
the size and surface area of cardiomyocytes (3). Continuous CH usually coincides with
maladaptive cardiac remodeling (4).
Diverse factors are involved in the pathological progression of CH,
including altered levels of noradrenaline, angiotensin II,
interleukin-6 and long non-coding (lncRNA) (4); however, understanding the specific
molecular mechanisms underlying CH is of significance for improving
the diagnostic and therapeutic efficacies of CH.
lncRNAs are non-coding RNAs that are >200
nucleotides in length (5). lncRNAs
are involved in a number of processes, such as epigenetics, cell
cycle and cell differentiation (6).
In addition, some studies have reported that lncRNAs are also key
regulators of various cardiovascular diseases (7-9).
For example: lncRNA TINCR ubiquitin domain containing inhibits CH
by epigenetically silencing calcium/calmodulin dependent protein
kinase II (8); lncRNA myocardial
infarction-associated transcript contributed to CH by regulating
toll-like receptor 4 via microRNA (miR)-93(9); and lncRNA cardiac hypertrophy-related
factor (CHRF) promotes CH by targeting miR-93 to further regulate
Akt3 expression levels (10). It
has also been reported that lncRNA ZEB2-AS1 is a molecule involved
in tumor biology (11) that
promotes bladder cell proliferation and inhibits apoptosis by
modulating miR-27b (12). By
targeting the miR-204/high mobility group box 1 axis, ZEB2-AS1
promotes pancreatic cancer cell proliferation and invasion
(13). In gastric cancer, lncRNA
ZEB2-AS1 is upregulated, and affects cell proliferation and
invasion via the miR-143-5p/hypoxia inducible factor 1 subunit α
axis (14). However, the specific
function of ZEB2-AS1 in cardiovascular diseases, especially CH, is
not completely understood.
The present study constructed both in vivo
and in vitro CH models by TAC procedures and PE treatment,
respectively. The aim of the present study was to investigate the
role of ZEB2-AS1 in CH.
Materials and methods
Experimental animals
Specific pathogen free male C57BL6 mice (age, 8
weeks; weight, 20-25 g) were selected for constructing the in
vivo CH model. Mice in the CH group (n=8) were anesthetized
with an intraperitoneal injection of 100 mg/kg ketamine and 5 mg/kg
xylazine. Intubation was performed with a volume circulatory
ventilator and a midline incision was made above the sternum.
Muscles were carefully separated to expose the trachea. After
trachea cannula, the second rib on the left side of the thoracic
cavity was cut using surgical scissors, and both thymuses were
pushed aside to expose the ascending aortic arch. A 27G needle was
punctured into the ascending aorta in its natural growth direction.
After ligation of the ascending aorta using a 5-0 suture, the
needle was gently pulled out to perform transverse aortic
constriction (TAC) and establish pressure overload-induced cardiac
hypertrophy. Mice in the sham group (n=8) underwent anesthesia and
exposure of the ascending aortic arch without puncture and
ligation. Mice were euthanized via cervical dislocation at 6 weeks
after the cardiac hypertrophy model was established. Subsequently,
the heart and lung were harvested for subsequent experiments. Signs
of severe pain, including abnormal movement and sound, were
considered as humane endpoints requiring immediate euthanasia in
the present study. The present study was approved by the Animal
Ethics Committee of Nanjing Medical University Animal Center
(approval no. 2016NJMU-043A-23).
Cell culture of primary
cardiomyocytes
As previously described (15), primary cardiomyocytes were isolated
from newborn mice (Nanjing Medical University). Briefly, five
newborn mice were sacrificed by cervical dislocation. Following
isolation, heart tissues were cut into small pieces and digested.
After centrifugation at 4˚C and 1,050 x g for 10 min, the pellet
was resuspended in DMEM (Gibco; Thermo Fisher Scientific, Inc.) and
inoculated in collagen-coated plates. For 24 h, cardiomyocytes were
cultured in serum-free medium at 37˚C. Subsequently, the medium was
replaced with DMEM containing 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.). To induce an in vitro CH model,
primary cardiomyocytes (3x106) were treated with 100 µM
phenylephrine (PE) (Beyotime Institute of Biotechnology) for 36 h
at 37˚C. Cell surface area was measured as described previously
(3).
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from cardiomyocytes using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and the concentration of total RNA was measured using an
ultraviolet spectrophotometer (Hitachi, Ltd.). Total RNA was
reverse transcribed into cDNA at 50˚C for 45 min using a
PrimeScript RT reagent kit (Takara Biotechnology Co., Ltd.),
according to the manufacturer's protocol. qPCR was subsequently
performed using the SYBR-Green Master kit (Roche Diagnostics). The
reaction system volume was 25 µl in total and the cycling
conditions were: Pre-denaturation at 95˚C for 5 min, denaturation
at 95˚C for 30 sec, annealing at 60˚C for 45 sec, extension at 72˚C
for 3 min, with 35 cycles, and then extension at 72˚C for 5 min.
qPCR products were stored at 4˚C. The relative levels were
quantitatively analyzed using the 2-ΔΔCq method (16). GAPDH was used as internal reference.
Primer sequences were as follows: GAPDH forward,
5'-GCAAGGATACTGAGAGCAAGAG-3' and reverse,
5'-GGATGGAATTGTGAGGGAGATG-3'; ANP forward,
5'-GCCCTCATTTTGGCCATCAG-3' and reverse, 5'-TTCCCACTTGAGCAGCATTG-3';
BNP forward, 5'-TGTCCTACAGGGACCCCTTC-3' and reverse,
5'-CGCTCAGGGAACCGATTCTA-3'; PTEN forward,
5'-TGTGGTCTGCCAGCTAAAGG-3' and reverse,
5'-ACACACAGGTAACGGCTGAG-3'.
Western blotting
The cells were lysed using cell lysis buffer (cat.
no. QC25-05099; Shanghai Qincheng Biological Technology Co., Ltd.).
Total protein from cells was extracted using
radioimmunoprecipitation assay buffer and was quantified using the
bicinchoninic acid method (both reagents supplied by Beyotime
Institute of Biotechnology) method. A total of 30 ug of protein was
added into each lane for the electrophoresis. The extracted
proteins were separated using a 10% sodium dodecyl sulphate
polyacrylamide electrophoresis gel. After transfer onto
polyvinylidene fluoride membranes (EMD Millipore), the protein was
blocked in 5% skim milk for 2 h, incubated with primary antibodies
at 4˚C overnight and secondary antibodies at 20˚C for 2 h. Bands
were developed with an enhanced chemiluminescence (ECL) detection
kit (GE Healthcare) and analyzed using ImageJ Software (version
1.38; National Institutes of Health). Rabbit polyclonal ANP
antibody (1:1,000; cat. no. ab225844), rabbit monoclonal BNP
antibody (1:2,000; cat. no. ab243440), rabbit polyclonal PTEN
antibody (1:1,000; cat. no. ab170941), rabbit polyclonal GAPDH
antibody (1:500; cat. no. ab37168) and secondary goat anti-rabbit
(HRP) IgG antibody (1;2,000; cat. no. ab6721) were all purchased
from Abcam.
Vector construction and
transfection
pcDNA3.0-ZEB2-AS1 and pcDNA3.0-PTEN vectors were
constructed by cloning the cDNAs of ZEB2-AS1 and PTEN into the
mammalian expression vector pcDNA3.0 (Invitrogen; Thermo Fisher
Scientific, Inc.). Cardiomyocytes (3x106) were
transfected with pcDNA3.0-ZEB2-AS1 (100 nM), pcDNA3.0-PTEN (100
nM), pcDNA-control (NC) (100 nM), si-ZEB2-AS1 (100 nM;
5'-CAAAGGACACCTTTGGTTACCTGAA-3') or control siRNA (100 nM; all
supplied by Shanghai Qincheng Biological Technology Co., Ltd.)
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). At 48 h post-transfection, cells were used for
subsequent experiments.
Statistical analysis
Statistical analyses were conducted using SPSS
software (version 18.0; SPSS Inc.). Figure editing was performed
using GraphPad Prism software (version 6.0; GraphPad Software,
Inc.). Data are expressed as the mean ± standard deviation. The
experiments were repeated three times. Differences between two
groups were analyzed using the paired Student's t-test. Comparisons
among multiple groups were analyzed using one-way ANOVA followed by
the Bonferroni post hoc test. P<0.05 was considered to indicate
a statistically significant difference.
Results
ZEB2-AS1 upregulation in CH model
mice
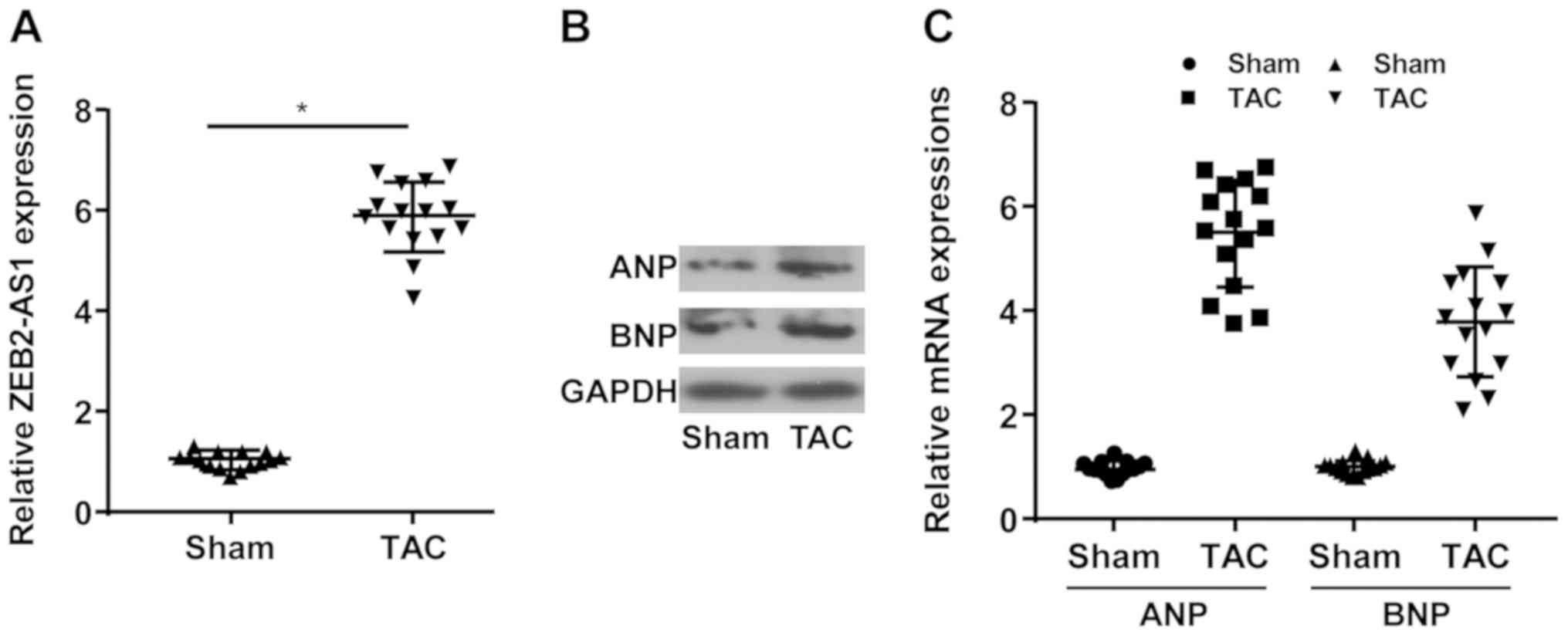
lncRNA ZEB2-AS1 expression levels in mice undergoing
TAC or sham operation were determined. Compared with the sham
group, the TAC group displayed higher expression levels of ZEB2-AS1
(Fig. 1A). Moreover, the relative
expression levels of ANP and BNP were upregulated in the TAC group
compared with the sham group (Fig.
1B and C). The results
indicated that ZEB2-AS1 may be associated with CH.
ZEB2-AS1 knockdown protects against
PE-induced CH
Following treatment with pcDNA-PTEN, the mRNA and
protein expression levels of PTEN were notably increased in primary
cardiomyocytes compared with the pcDNA-NC group (Fig. 2A and B). Following treatment with si-ZEB2-AS1 or
pcDNA-ZEB2-AS1, ZEB2-AS1 expression levels were significantly
decreased or increased in primary cardiomyocytes compared with the
si-NC and pcDNA-NC groups, respectively (Fig. 2C and D). Primary cardiomyocytes were treated
with PE (100 µM), a trigger for in vitro CH, for 36 h. PE
treatment significantly upregulated ZEB2-AS1 expression levels in
primary cardiomyocytes compared with the NC group; however,
si-ZEB2-AS1 transfection reversed PE-mediated effects on ZEB2-AS1
expression (Fig. 2E). Compared with
the NC group, cell surface area was significantly increased by PE
treatment, which was reversed by ZEB2-AS1 knockdown (Fig. 2F). ANP and BNP protein and mRNA
expression levels were notably increased by PE treatment compared
with the NC group. By contrast, si-ZEB2-AS1 transfection decreased
PE-induced ANP and BNP expression (Fig.
2G and H). The results
suggested that dysregulated ZEB2-AS1 expression may serve as a
vital factor leading to CH.
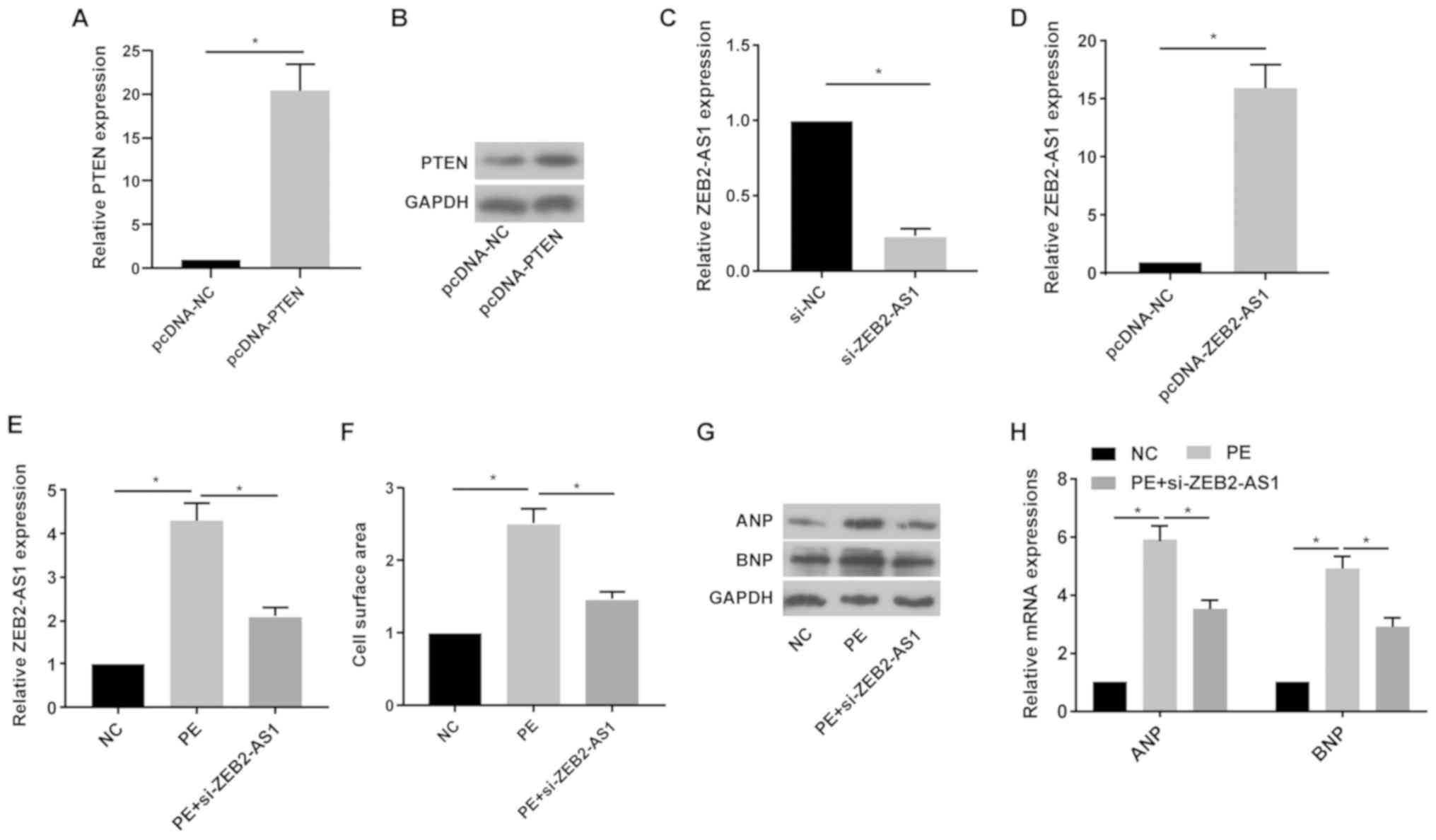 | Figure 2ZEB2-AS1 knockdown protects against
PE-induced cardiac hypertrophy. (A) mRNA and (B) protein expression
levels of PTEN in primary cardiomyocytes transfected with pcDNA-NC
or pcDNA-PTEN. ZEB2-AS1 expression levels in primary cardiomyocytes
transfected with (C) si-NC, si-ZEB2-AS1, (D) pcDNA-NC or
pcDNA-ZEB2-AS1. (E) ZEB2-AS1 expression levels in untreated,
PE-treated (100 µM) or PE-treated + si-ZEB2-AS1-transfected primary
cardiomyocytes. (F) Cell surface area in untreated, PE-treated (100
µM) or PE-treated + si-ZEB2-AS1-transfected primary cardiomyocytes.
(G) Protein and (H) mRNA expression levels of ANP and BNP in
untreated, PE-treated (100 µM) or PE-treated +
si-ZEB2-AS1-transfected primary cardiomyocytes. ZEB2-AS1, ZEB2
antisense RNA 1; PE, phenylephrine; PTEN, phosphatase and tensin
homolog; NC, negative control; si, small interfering RNA; ANP,
natriuretic peptide A; BNP, brain natriuretic peptide.
*P<0.05. |
PTEN overexpression protects against
PE-induced CH
Subsequently, the involvement of PTEN in the process
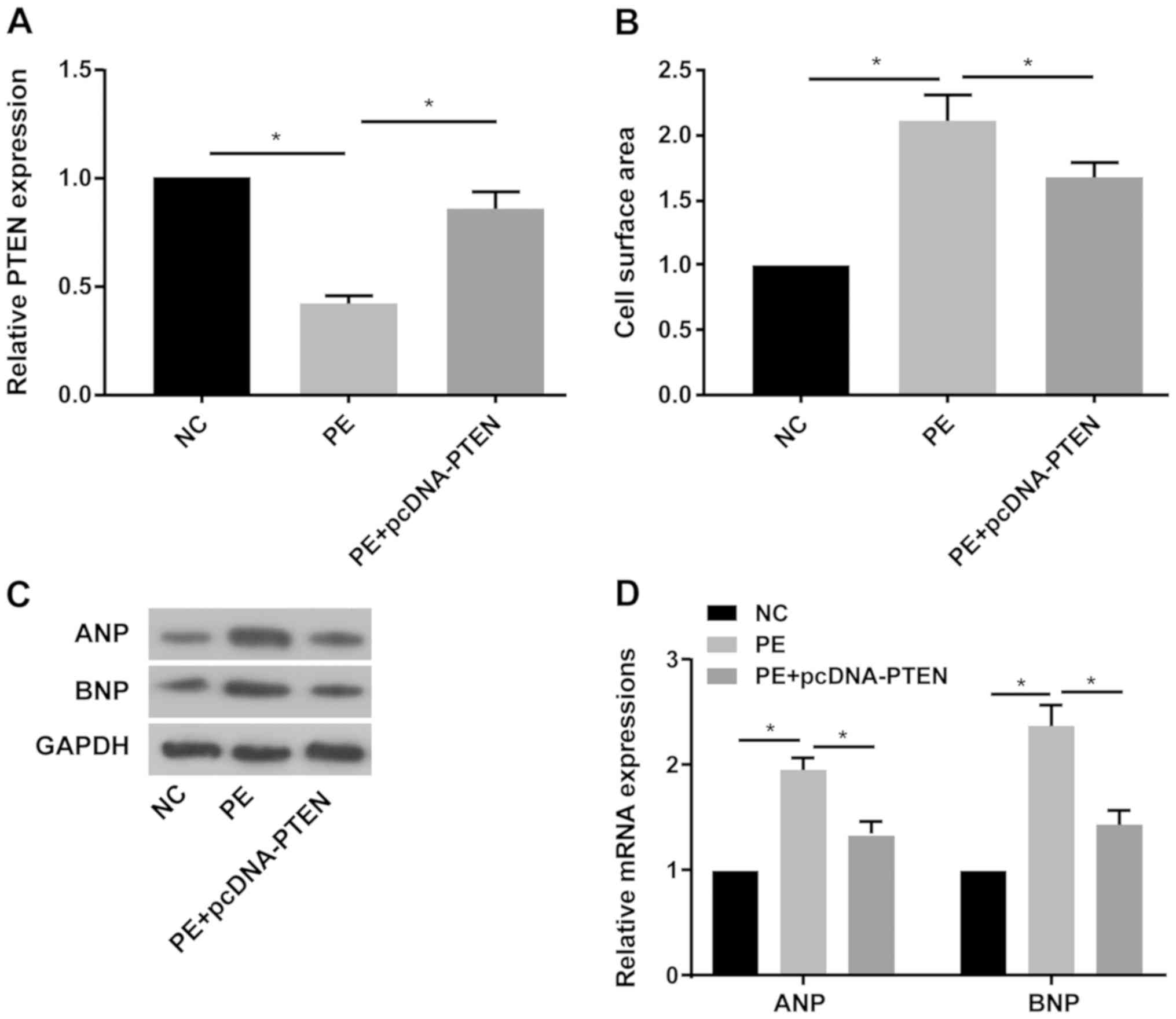
of CH was explored. The RT-qPCR results indicated that PTEN
expression was significantly decreased in PE-treated primary
cardiomyocytes compared with the NC group, and pcDNA-PTEN
transfection reversed PE-mediated downregulation of PTEN expression
(Fig. 3A). PTEN overexpression
significantly decreased PE-mediated increased cell surface area in
primary cardiomyocytes (Fig. 3B).
Furthermore, PE treatment obviously increased ANP and BNP protein
and mRNA expression levels compared with the NC group, whereas
PE-mediated effects on ANP and BNP expression were reversed by PTEN
overexpression (Fig. 3C and
D).
PTEN reverses ZEB2-AS1-mediated
effects on CH
A series of rescue assays were conducted to clarify
the effects of ZEB2-AS1/PTEN in CH. ZEB2-AS1 overexpression-induced
enlarged cell surface area in cardiomyocytes was partially reversed
by PTEN overexpression (Fig. 4A).
Additionally, ZEB2-AS1 overexpression upregulated ANP and BNP
expression levels in PE-treated cardiomyocytes, which were reversed
by co-transfection with pcDNA-PTEN (Fig. 4B). Therefore, the results indicated
that ZEB2-AS1 aggravated CH by downregulating PTEN.
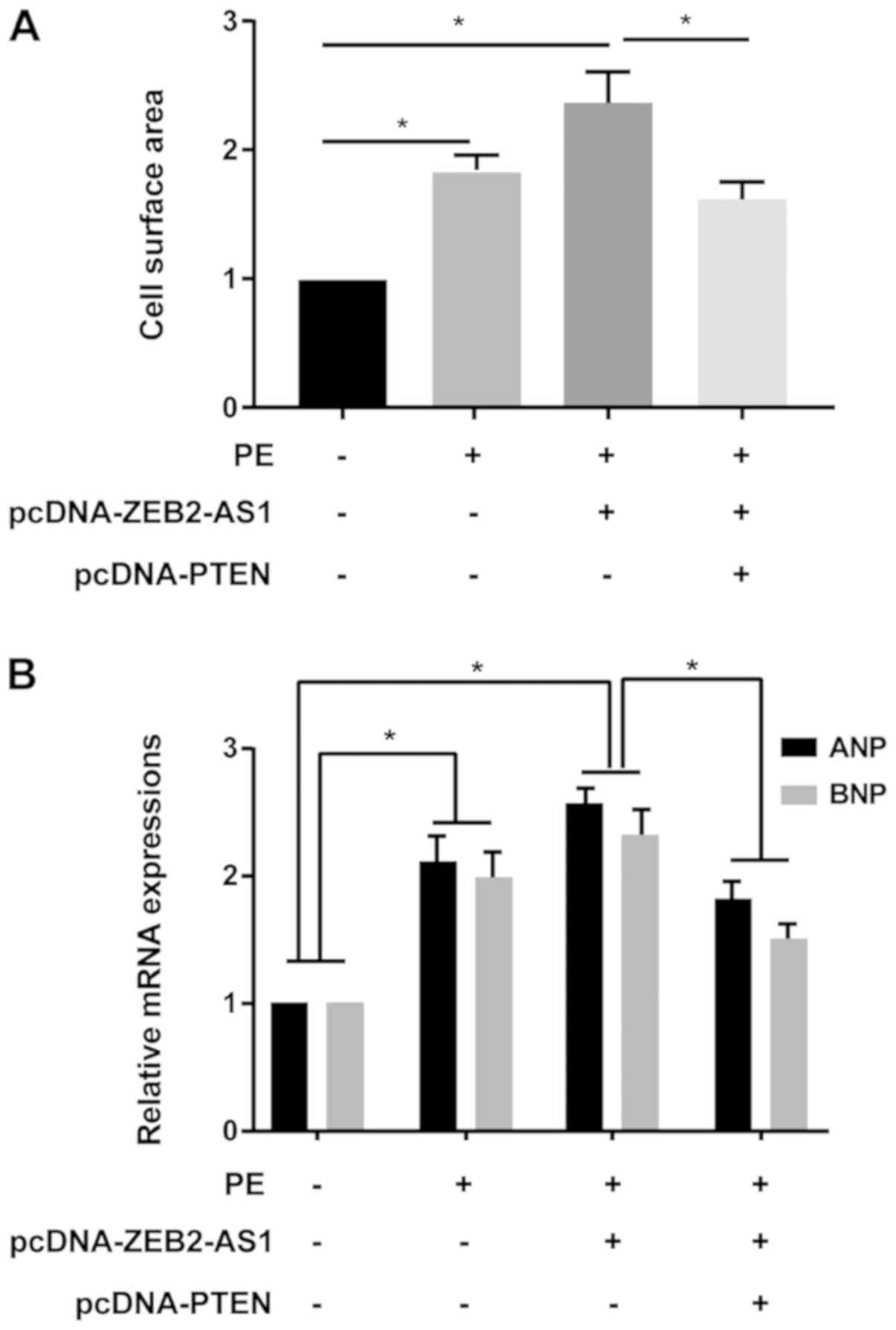 | Figure 4PTEN reverses ZEB2-AS1-mediated
effects on cardiac hypertrophy. (A) Cell surface area in untreated,
PE-treated (100 µM), PE-treated + pcDNA-ZEB2-AS1-transfected, and
PE-treated + pcDNA-ZEB2-AS1- and pcDNA-PTEN-transfected primary
cardiomyocytes. (B) ANP and BNP expression levels in untreated,
PE-treated (100 µM), PE-treated + pcDNA-ZEB2-AS1-transfected, and
PE-treated + pcDNA-ZEB2-AS1- and pcDNA-PTEN-transfected primary
cardiomyocytes. PTEN, phosphatase and tensin homolog; ZEB2-AS1,
ZEB2 antisense RNA 1; PE, phenylephrine; ANP, natriuretic peptide
A; BNP, brain natriuretic peptide. *P<0.05. |
Discussion
CH is a common heart disease (1-3).
Pathological hypertrophy of the heart leads to a decline in cardiac
function and eventually results in heart failure (17). CH is a hallmark of cardiovascular
diseases and an important predictor of adverse cardiovascular
outcomes, including hypertension and myocardial infarction
(18). CH is initially an adaptive
response to persistent overload; however, long-term progression
leads to heart failure and death (19). Due to alterations to lifestyle and
diet, the mortality and morbidity of cardiovascular diseases have
increased annually (20).
Cardiovascular diseases, such as coronary heart disease and severe
heart failure, remain the leading causes of human death worldwide
(21). CH is a precursor lesion and
independent risk factor for coronary heart disease, heart failure,
sudden cardiac death and other heart diseases (22). In particular, pathological CH leads
to impaired cardiac function and is a major determinant of common
heart diseases (23,24).
Persistent CH is closely related to the expression
of embryonic genes, including ANP and BNP (1). The present study indicated that lncRNA
ZEB2-AS1 was significantly upregulated in mice undergoing TAC
procedures compared with the sham group. ZEB2-AS1 knockdown
reversed PE-induced cardiomyocyte hypertrophy, including enlarged
cell surface area, and upregulation of ANP and BNP expression.
Therefore, it was suggested that ZEB2-AS1 may aggravate the
progression of CH.
PTEN can alleviate tumor progression by antagonizing
the activities of phosphorylases, such as tyrosine kinases
(25). It has been reported that
PTEN mutations exist in multiple types of tumors, which is
considered to be the star tumor-suppressor gene after the discovery
of p53 (26-33).
lncRNA growth arrest-specific transcript 5 induces PTEN expression
by inhibiting miR-103 in endometrial cancer cells (34). lncRNA maternally expressed 3 alters
ovarian cancer cell proliferation, invasion and migration by
regulating PTEN (35). lncRNA fer-1
like family member 4 (pseudogene) suppresses endometrial cancer
cell proliferation by regulating PTEN expression (36). However, the role of PTEN in CH is
not completely understood. The present study indicated that PTEN
expression was decreased in PE-induced hypertrophic cardiomyocytes
compared with the NC group. The results indicated that PTEN
overexpression protected against CH, manifesting as reduced cell
surface area, and downregulation of ANP and BNP expression levels
compared with the PE group. Moreover, enlarged cell surface area,
and upregulated ANP and BNP expression levels in
ZEB2-AS1-overexpression cardiomyocytes were partially reversed by
PTEN overexpression. Collectively, the results indicated that
ZEB2-AS1 aggravated CH by targeting PTEN; therefore, it was
suggested that lncRNA ZEB2-AS1 may influence the progression of CH
by downregulating PTEN.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81670421) and the
‘Six Peak Talents’ Innovation Talent Team Project (grant no.
BE2016798).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZC and QL designed the study, performed the
experiments and drafted the manuscript. ZC and LL established the
animal models. QL and LL collected the data. ZC, LL and QL analyzed
the data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Ethics
Committee of Nanjing Medical University Animal Center (approval no.
2016NJMU-043A-23).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li X, Lan Y, Wang Y, Nie M, Lu Y and Zhao
E: Telmisartan suppresses cardiac hypertrophy by inhibiting
cardiomyocyte apoptosis via the NFAT/ANP/BNP signaling pathway. Mol
Med Rep. 15:2574–2582. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Facundo H, Brainard RE, Caldas F and Lucas
A: Mitochondria and cardiac hypertrophy. Adv Exp Med Biol.
982:203–226. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tham YK, Bernardo BC, Ooi JY, Weeks KL and
McMullen JR: Pathophysiology of cardiac hypertrophy and heart
failure: Signaling pathways and novel therapeutic targets. Arch
Toxicol. 89:1401–1438. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Haque ZK and Wang DZ: How cardiomyocytes
sense pathophysiological stresses for cardiac remodeling. Cell Mol
Life Sci. 74:983–1000. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shen S, Jiang H, Bei Y, Xiao J and Li X:
Long non-coding RNAs in cardiac remodeling. Cell Physiol Biochem.
41:1830–1837. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Viereck J, Kumarswamy R, Foinquinos A,
Xiao K, Avramopoulos P, Kunz M, Dittrich M, Maetzig T, Zimmer K, et
al: Long noncoding RNA Chast promotes cardiac remodeling. Sci
Transl Med. 8(326ra22)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhou G, Li C, Feng J, Zhang J and Fang Y:
lncRNA UCA1 is a novel regulator in cardiomyocyte hypertrophy
through targeting the miR-184/HOXA9 axis. Cardiorenal Med.
8:130–139. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shao M, Chen G, Lv F, Liu Y, Tian H, Tao
R, Jiang R, Zhang W and Zhuo C: lncRNA TINCR attenuates cardiac
hypertrophy by epigenetically silencing CaMKII. Oncotarget.
8:47565–47573. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhu XH, Yuan YX, Rao SL and Wang P: lncRNA
MIAT enhances cardiac hypertrophy partly through sponging miR-150.
Eur Rev Med Pharmacol Sci. 20:3653–3660. 2016.PubMed/NCBI
|
|
10
|
Wo Y, Guo J, Li P, Yang H and Wo J: Long
non-coding RNA CHRF facilitates cardiac hypertrophy through
regulating Akt3 via miR-93. Cardiovasc Pathol. 35:29–36.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Huarte M: The emerging role of lncRNAs in
cancer. Nat Med. 21:1253–1261. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Wu X, Yan T, Wang Z, Wu X, Cao G and Zhang
C: lncRNA ZEB2-AS1 promotes bladder cancer cell proliferation and
inhibits apoptosis by regulating miR-27b. Biomed Pharmacother.
96:299–304. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gao H, Gong N, Ma Z, Miao X, Chen J, Cao Y
and Zhang G: lncRNA ZEB2-AS1 promotes pancreatic cancer cell growth
and invasion through regulating the miR-204/HMGB1 axis. Int J Biol
Macromol. 116:545–551. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang F, Zhu W, Yang R, Xie W and Wang D:
lncRNA ZEB2-AS1 contributes to the tumorigenesis of gastric cancer
via activating the Wnt/β-catenin pathway. Mol Cell Biochem.
451:73–83. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang K, Lin ZQ, Long B, Li JH, Zhou J and
Li PF: Cardiac hypertrophy is positively regulated by MicroRNA
miR-23a. J Biol Chem. 287:589–599. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Forlenza M, Kaiser T, Savelkoul HF and
Wiegertjes GF: The use of real-time quantitative PCR for the
analysis of cytokine mRNA levels. Methods Mol Biol. 820:7–23.
2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Creemers EE, Wilde AA and Pinto YM: Heart
failure: Advances through genomics. Nat Rev Genet. 12:357–362.
2011.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Frey N and Olson EN: Cardiac hypertrophy:
The good, the bad, and the ugly. Annu Rev Physiol. 65:45–79.
2003.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Braunwald E: The war against heart
failure: The Lancet lecture. Lancet. 385:812–824. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Huang Q and Cai B: Exosomes as new
intercellular mediators in development and therapeutics of
cardiomyocyte hypertrophy. Adv Exp Med Biol. 998:91–100.
2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
McMullen JR and Jennings GL: Differences
between pathological and physiological cardiac hypertrophy: Novel
therapeutic strategies to treat heart failure. Clin Exp Pharmacol
Physiol. 34:255–262. 2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bernardo BC, Weeks KL, Pretorius L and
McMullen JR: Molecular distinction between physiological and
pathological cardiac hypertrophy: Experimental findings and
therapeutic strategies. Pharmacol Ther. 128:191–227.
2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
van Rooij E, Sutherland LB, Liu N,
Williams AH, McAnally J, Gerard RD, Richardson JA and Olson EN: A
signature pattern of stress-responsive microRNAs that can evoke
cardiac hypertrophy and heart failure. Proc Natl Acad Sci USA.
103:18255–18260. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bao Q, Zhao M, Chen L, Wang Y, Wu S, Wu W
and Liu X: MicroRNA-297 promotes cardiomyocyte hypertrophy via
targeting sigma-1 receptor. Life Sci. 175:1–10. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Malaney P, Uversky VN and Dave V: PTEN
proteoforms in biology and disease. Cell Mol Life Sci.
74:2783–2794. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wise HM, Hermida MA and Leslie NR:
Prostate cancer, PI3K, PTEN and prognosis. Clin Sci (Lond).
131:197–210. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang HM, Fan TT, Li W and Li XX:
Expressions and significances of TTF-1 and PTEN in early
endometrial cancer. Eur Rev Med Pharmacol Sci. 21:20–26.
2017.PubMed/NCBI
|
|
28
|
Zhang R, Guo Y, Ma Z, Ma G, Xue Q, Li F
and Liu L: Long non-coding RNA PTENP1 functions as a ceRNA to
modulate PTEN level by decoying miR-106b and miR-93 in gastric
cancer. Oncotarget. 8:26079–26089. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Shen W, Li HL, Liu L and Cheng JX:
Expression levels of PTEN, HIF-1α, and VEGF as prognostic factors
in ovarian cancer. Eur Rev Med Pharmacol Sci. 21:2596–2603.
2017.PubMed/NCBI
|
|
30
|
Ngeow J, Sesock K and Eng C: Breast cancer
risk and clinical implications for germline PTEN mutation carriers.
Breast Cancer Res Treat. 165:1–8. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sun J, Li T, Zhao Y, Huang L, Sun H, Wu H
and Jiang X: USP10 inhibits lung cancer cell growth and invasion by
upregulating PTEN. Mol Cell Biochem. 441:1–7. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li MF, Guan H and Zhang DD: Effect of
overexpression of PTEN on apoptosis of liver cancer cells. Genet
Mol Res 15, 2016.
|
|
33
|
Beg S, Siraj AK, Jehan Z, Prabakaran S,
Al-Sobhi SS, Al-Dawish M, Al-Dayel F and Al-Kuraya KS: PTEN loss is
associated with follicular variant of Middle Eastern papillary
thyroid carcinoma. Br J Cancer. 112:1938–1943. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Guo C, Song WQ, Sun P, Jin L and Dai HY:
lncRNA-GAS5 induces PTEN expression through inhibiting miR-103 in
endometrial cancer cells. J Biomed Sci. 22(100)2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang J, Xu W, He Y, Xia Q and Liu S:
lncRNA MEG3 impacts proliferation, invasion, and migration of
ovarian cancer cells through regulating PTEN. Inflamm Res.
67:927–936. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Qiao Q and Li H: lncRNA FER1L4 suppresses
cancer cell proliferation and cycle by regulating PTEN expression
in endometrial carcinoma. Biochem Biophys Res Commun. 478:507–512.
2016.PubMed/NCBI View Article : Google Scholar
|