Introduction
Epilepsy is a prevalent and devastating neurological
disorder, which is characterized by recurrent, spontaneous and
unprovoked seizures (1). The
development and progression of epilepsy can be caused by extensive
changes in gene transcription, resulting in aberrant remodeling of
the neural network and hyperexcitability (2). Epilepsy can also originate from a wide
range of disorders of the brain and nervous system, including
tumors and non-neoplastic lesions (3-5).
Currently, a number of studies have demonstrated that epigenetic
mechanisms, including post-translational modifications, non-coding
RNA and chemical modifications to the DNA, exert significant
influence on the pathogenesis of epilepsy (6-8).
Following these observations, combined with reports implicating the
possible involvement of epigenetic mechanisms in the regulation of
metabolism underlying the neurological activities of epilepsy, the
potential for the use of epigenetic markers in epilepsy diagnosis
and prognosis has been evaluated (9-11).
Collectively, based on the characteristics of epilepsy, the
aforementioned findings may serve as a guide for the diagnosis of
epilepsy and the development of novel therapeutic strategies for
epilepsy prevention or intervention.
Clinically, multiple types of diagnoses and the
diverse set of clinical presentations that are associated with
epilepsy render accurate diagnosis and treatment difficult
(12). Epilepsy is frequently
defined as a disease that is characterized by one or more seizures
with genetic etiologies (13).
Electroencephalography is currently the most commonly applied
method for assessing epilepsy, which facilitates diagnosis, the
classification of seizure types, localization of the pathological
area, designation of treatment strategies and monitoring of
prognosis (14). Magnetic resonance
imaging (MRI) is a particularly useful technique for the evaluation
of seizure etiology and the identification of potential seizure
onset sites (15-17).
Previous MRI data suggests that epilepsy is caused by parenchymal
atrophy that is disproportionate for age, and is often associated
with aberrant hyperactivity in the cortical/subcortical T2 region
of the brain (18). MRI serves a
crucial role in the routine diagnosis of epilepsy and contributes
to etiological diagnosis in general clinical neuroscience.
Additionally, MRI data is widely applied in post-processing
procedures for the analysis of co-registered, three-dimensional
volumes from images obtained using various modalities (19). Dynamic contrast-enhanced MRI
(Dce-MRI) exhibits significant diagnostic quality in determining
the preoperative localization of epileptic foci and evaluating
prognosis in patients with epilepsy (20). Textural analysis of MRI images also
facilitates quantification during the imaging assessment of
epilepsy, which has been previously found to be essential in
understanding juvenile myoclonic epilepsy (21). Morphometric and textural analysis of
MRI images allows the detection of lesions in pediatric epilepsy
(22). Therefore, it is of
importance to review the preoperative evaluation processes of
Dce-MRI-guided lesions using morphometric and textural analysis for
patients with epilepsy.
In the present study, a quantitative evaluation was
performed on the signal-to-noise ratio (SNR) and contrast-to-noise
ratio (CNR) yielded using Dce-MRI or MRI from hippocampal images
obtained from patients with epilepsy. Grey-white matter contrast
(GWMC) and epileptogenic lesion (morphometric and textural
analysis) images taken using MRI and Dce-MRI from patients with
epilepsy were also analyzed. Additionally, images of epileptogenic
lesions acquired using MRI and Dce-MRI and the corresponding
diagnostic efficacy in patients with epilepsy were compared.
Materials and methods
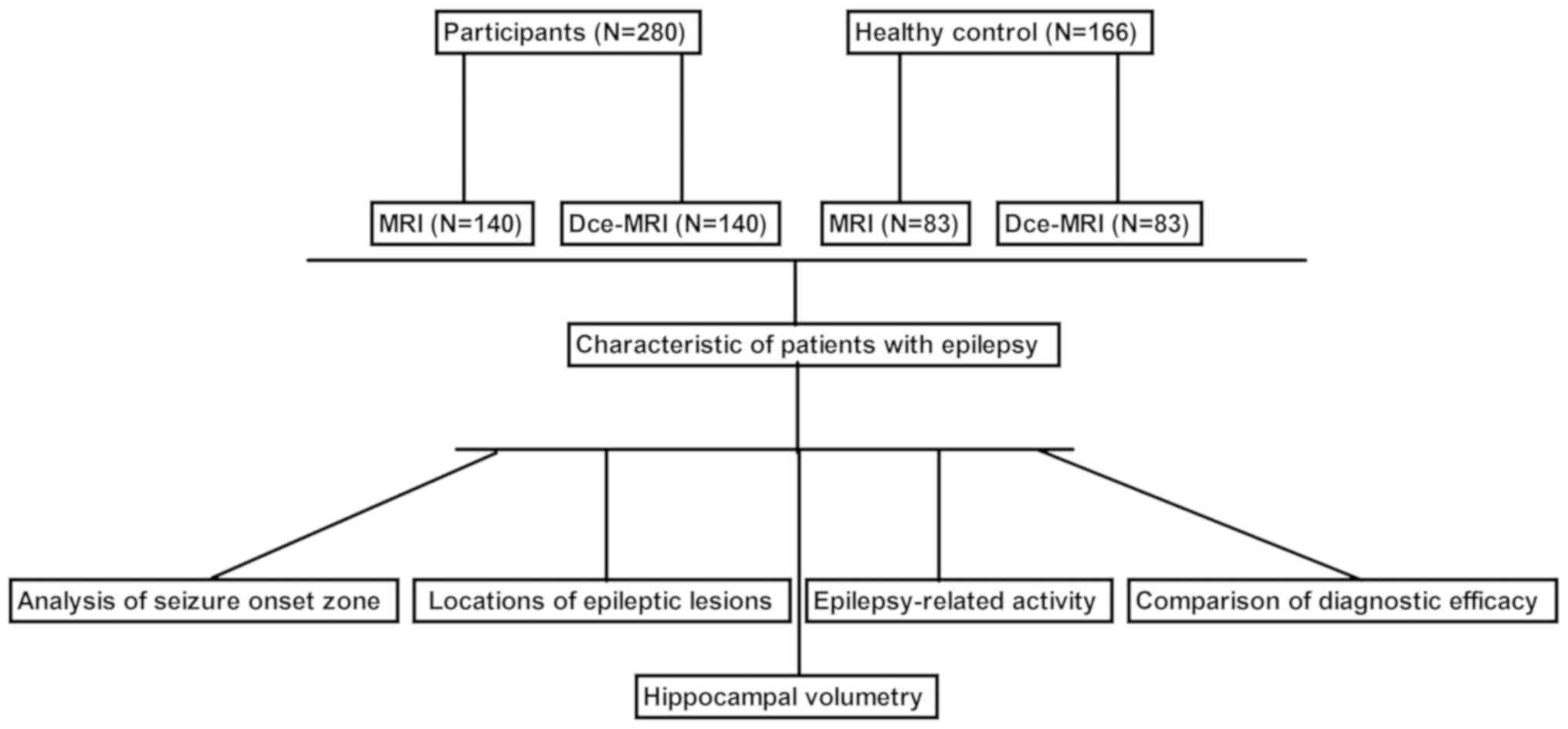
Participants and clinical scoring
A total of 280 patients with epilepsy (146 females
and 134 males; mean age, 34.7±13.5 years) and 166 healthy controls
(male/female, 86/80; mean age, 33.8±8.4 years) were recruited at
The General Hospital of Western Theater Command (Chengdu, China)
between June 2016 and July 2018. The healthy controls were
individuals who received diagnosis using MRI (n=83) and Dce-MRI
(n=83). Information on the presence of active or calcified lesions
was recorded in each patient by radiological examination based on
MRI examinations. Epilepsy in patients was diagnosed using MRI
(n=140) or Dce-MRI (n=140). The type of imaging used was according
to the patient's preference. The exclusion criteria for all
participants were as follows: i) Patients with a history of
follow-up due to neurotuberculosis, neurotoxoplasmosis, tuberous
sclerosis and surgery for temporal lobe epilepsy; and ii) patients
with brain tumor, Parkinson's disease or physical brain damage. A
schematic workflow of the present study is shown in Fig. 1. Engel classification (23) was applied for grading epilepsy.
MRI and Dce-MRI
Patients with epilepsy were imaged using a Philips
Intera Achieva 3.0T MRI scanner (Philips Medical Systems B.V.).
Acquisitions were obtained in the coronal, sagittal and axial
planes, with coronal sections also obtained perpendicularly along
the axis of the hippocampal formation. MRI was conducted using the
following parameters: i) Repetition time, 5 msec; ii) echo time, 2
msec; iii) matrix, 212x213; iv) flip angle, 90˚; v) field of view,
360x360x92 mm; vi) spatial resolution, 1.7x1.7x5.0 mm; vii) number
of slices, 19; viii) average number of signals, 1; ix) temporal
resolution, 8.3 sec; x) acquisition time, 8.5 min; and xi) number
of dynamic scans, 60. For Dce-MRI, a bolus of 0.10 mmol/kg
gadobutrol (Gadovist; Bayer AG) was intravenously injected into
each patient followed by a 20-ml saline flush. The subsequent MRI
examination consisted of turbo spin-echo T1- and T2-weighted
sequences and a three-dimensional DCE sequence. Details of sequence
parameters were as described previously (24). For Dce-MRI examinations, MR images
were obtained using the following parameters: i) Repetition time,
1,200 msec; ii) echo time, 27 msec; iii) matrix, 212x213; iv) flip
angle, 90˚; v) field of view, 360x360x92 mm; vi) spatial
resolution, 1.7x1.7x5.0 mm; vii) number of slices, 19; viii)
average number of signals, 1; ix) temporal resolution, 8.3 sec; x)
acquisition time, 8.5 min; xi) number of dynamic scans, 60; xii)
continuous phase, 24; xiii) time resolution, 2 sec; and xiv) scan
time, 48 sec. The MRI images of epileptic lesions were
retrospectively evaluated by two board-certified radiologists. In
cases of disagreements between the two radiologists regarding the
diagnosis of the same patient, pathological data were referred to a
different pair of radiologists for the final diagnostic decision.
Electrocorticography and pathological results were considered as
the gold standard for the identification and localization of
pathogenic foci and diagnostic consistency (25), in addition to the comparison of
diagnostic efficacy between Dce-MRI and MRI. Patients with diseases
including epilepsy, Tourette's syndrome, hypoglycemia and syncope
were excluded from the healthy control. All patients with epilepsy
were definitively confirmed by three independent epilepsy
specialists. The locations of epileptic lesions in MRI and Dce-MRI
images were calculated and analyzed using FrameLink software
version 1.0 (Medtronic).
MRI image analysis
All MRI images were transferred to a computer-aided
diagnostic system (Merge CADstream®; version 4.1; IBM
Corp.) and analyzed by two pathologists who specialized in
epilepsy. Signal intensity in each pixel was recorded in all
patients. Colors were assigned according to changes in pixel values
following contrast injection. The computer-aided diagnostic system
report was prospectively saved by a research assistant, where
parameters including peak signal intensity (%), SNR and CNR of the
hippocampus, area of the GWMC and epileptogenic lesions were
recorded in the MRI image analysis. MRI and Dce-MRI data were
analyzed using IDL® software version 2.2 (Exelis VIS;
Harris Geospatial Solutions, Inc.) by applying a modified Brix's
linear two-compartment pharmacokinetic model to determine the SNR,
CNR and GWMC (26).
Statistical analysis
Measurement data are expressed as the mean ±
standard deviation, whilst count data are presented as n (%).
Statistical analysis of clinical data was performed using SPSS 22.0
software (IBM Corp.). Normally distributed data were analyzed using
independent samples t-test whereas non-normally distributed data
were analyzed using the Mann-Whitney U test. Univariate analysis
for enumeration data was performed using χ2 test. A
receiver operating characteristic (ROC) curve was generated to
evaluate the diagnostic value of MRI and Dce-MRI for
differentiating patients with epilepsy from healthy controls.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Characteristics of patients with
epilepsy
Table I provides the
clinical and demographic details of the patients with epilepsy. A
total of 280 patients with epilepsy were included, who were imaged
using Dce-MRI or MRI for morphometric and textural analysis. The
ability of classic MRI (n=140) and Dce-MRI (n=140) to map to the
regions of suspected seizure onset in the brain were compared.
Among the 280 patients included in the present study, 50 were
treated with phenytoin, 60 with pregabalin, 48 with primidone, 52
with retigabine and 62 with rufinamide. No significant differences
were observed in the baseline characteristics of patients with
epilepsy between the MRI and Dce-MRI groups.
 | Table IClinical, demographic and
neuropsychological data of patients with epilepsy. |
Table I
Clinical, demographic and
neuropsychological data of patients with epilepsy.
| Patient
characteristic | MRI | Dce-MRI | P-value |
|---|
| Age (years) | 34.7±13.5 | 36.4±11.2 | 0.76 |
| Sex
(female/male) | 76/64 | 70/70 | 0.80 |
| Suspected zone of
seizure onset | | | |
|
Temporal | 40 | 45 | 0.62 |
|
Parietal | 42 | 40 | 0.82 |
|
Frontal/Temporal | 25 | 21 | 0.72 |
|
Anterior
Temporal | 14 | 17 | 0.58 |
|
Frontal | 15 | 12 | 0.63 |
|
Unknown | 4 | 5 | 0.88 |
| Antiepileptic
drugs | | | |
|
Phenytoin | 24 | 26 | 0.75 |
|
Pregabalin | 28 | 32 | 0.58 |
|
Primidone | 25 | 23 | 0.66 |
|
Retigabine | 24 | 28 | 0.56 |
|
Rufinamide | 32 | 30 | 0.64 |
Evaluation of locations of epileptic
lesions
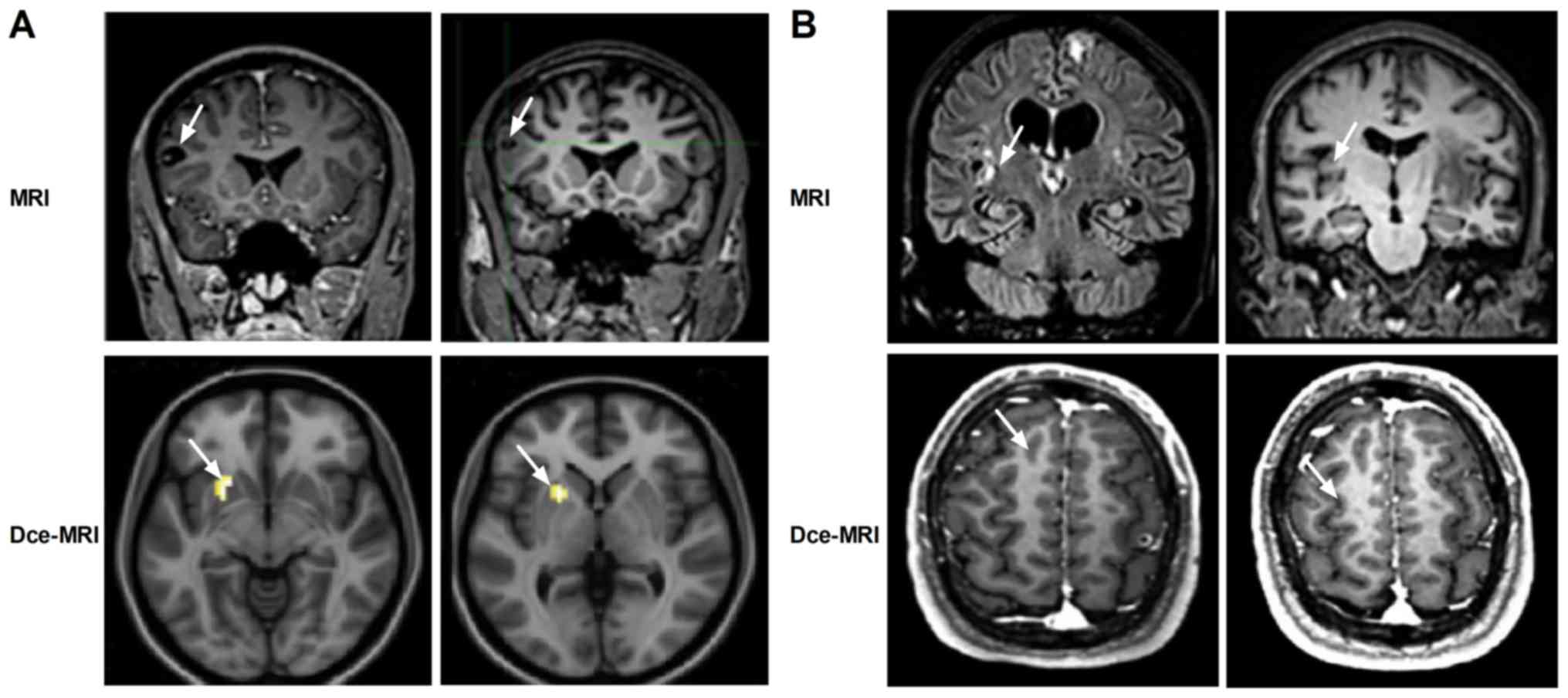
Dce-MRI revealed markedly clearer morphometric and
textural features of the lesions compared with MRI in patients with
epilepsy (Fig. 2). The morphometric
analysis showed that the epileptic lesions were predominantly
localized in the parietal, temporal, frontal/temporal, anterior
temporal or frontal lobes in patients with epilepsy, where the
shape of the parietal lobes was atrophic. Textural analysis
revealed increasing variations in spatial arrangements in the
hippocampal region, indicative of progressive microstructural
heterogeneity. The magnitudes of epileptic injuries in patients
with epilepsy in the MRI and Dce-MRI groups are shown in Table II. The grading system for epilepsy
was determined using the criteria from the American Academy of
Neurology Practice Parameter (27).
These data suggest that the ability of Dce-MRI to identify lesions
in patients with epilepsy is more efficient compared with that of
MRI.
 | Table IIMagnitude of epileptic injuries in
epileptic patients in the MRI and Dce-MRI groups. |
Table II
Magnitude of epileptic injuries in
epileptic patients in the MRI and Dce-MRI groups.
| Magnitude of
epileptic injury | MRI | Dce-MRI |
|---|
| Grade I | 51 (36.7) | 55 (39.2) |
| Grade II | 55 (39.6) | 51 (36.3) |
| Grade III | 18 (12.5) | 22 (15.8) |
| Grade IV | 16 (11.2) | 12 (8.7) |
Diagnostic efficacy of Dce-MRI for
focal cortical dysplasia in patients with epilepsy
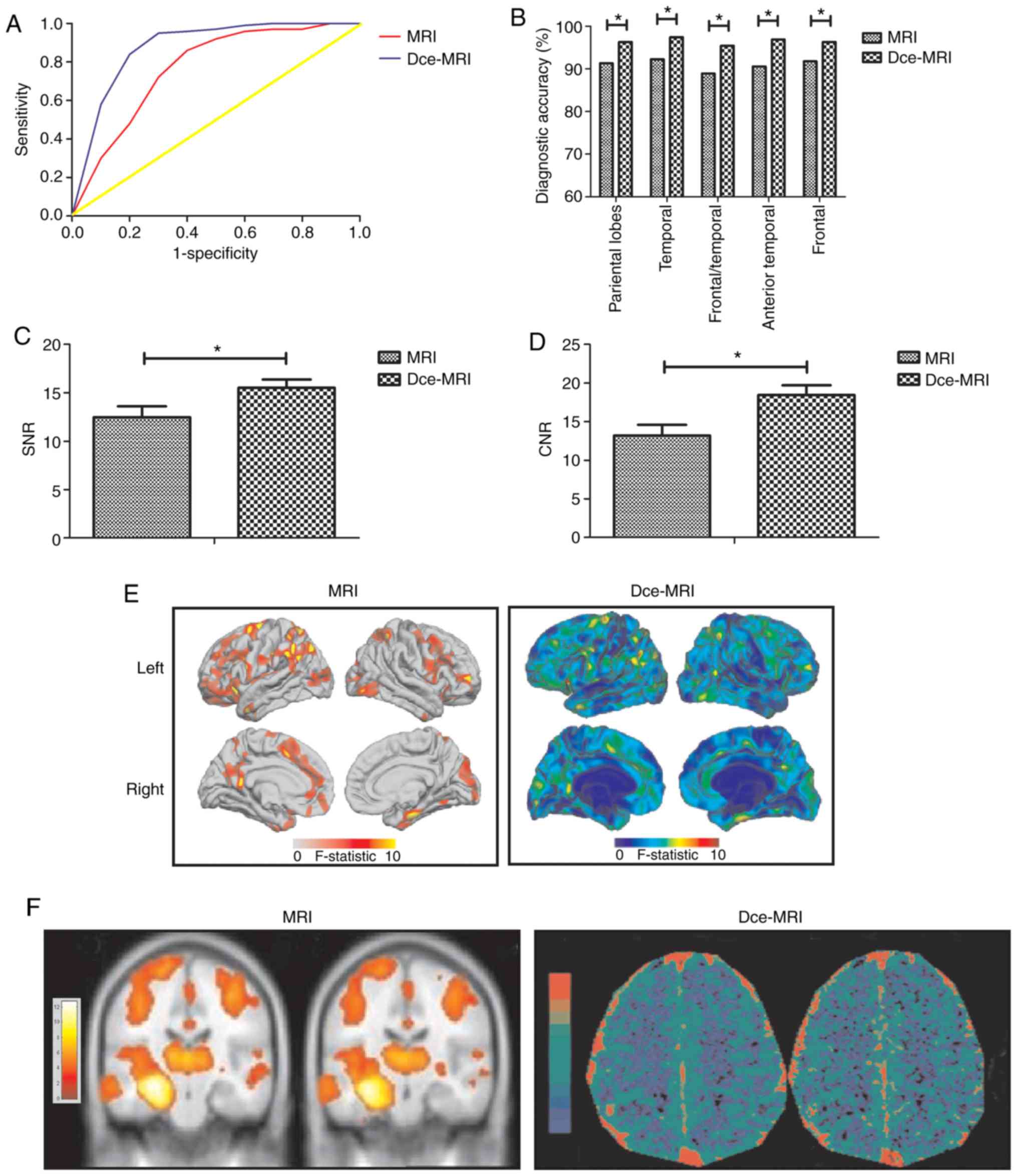
To verify the diagnostic efficacy of Dce-MRI, ROC
analysis was performed to compare the diagnostic efficacy of
Dce-MRI and MRI for focal cortical dysplasia in patients with
epilepsy (Fig. 3A). Dce-MRI
exhibited higher diagnostic accuracy [area under the curve (AUC),
0.910; 95% CI, 0.868-0.946] compared with MRI (AUC, 0.852; 95% CI,
0.810-0.906). Using the cutoff value of 1.764 for Dce-MRI, the
optimal sensitivity and specificity were calculated to be 93.2 and
94.5% respectively. In addition, Dce-MRI also demonstrated higher
accuracy compared with MRI for the identification of cortical
lesions and the successful mapping of images to the suspected zone
of seizure onset (Fig. 3B). In
addition, Dce-MRI increased diagnostic quality by significantly
increasing the SNR and CNR, and revealed the areas of GWMC and
epileptogenic lesions more clearly compared with MRI (Fig. 3C-F). These data suggest that Dce-MRI
is a reliable method for the diagnosis of epileptic lesions in
patients with epilepsy.
Analysis of patients with active
lesions using Dce-MRI
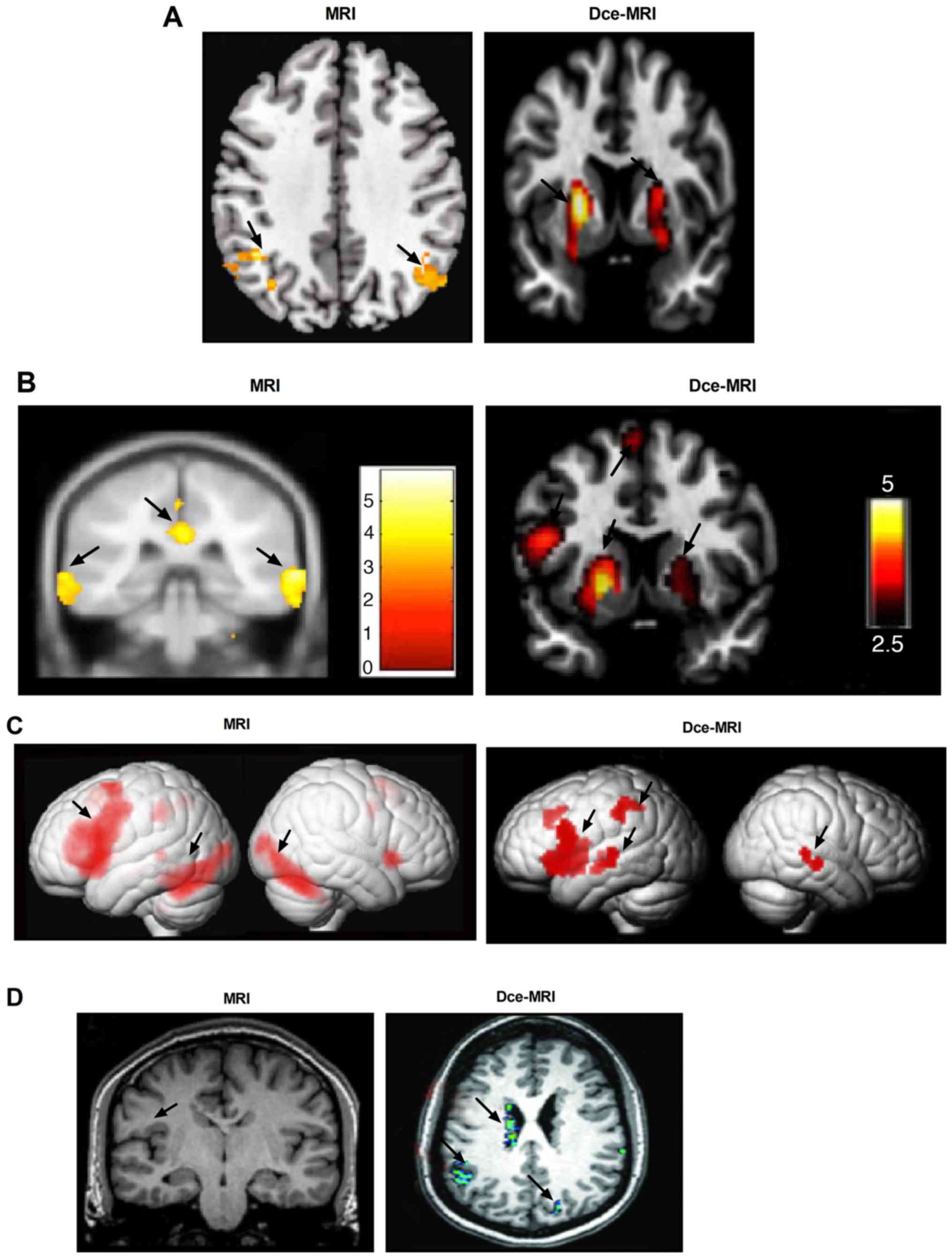
Compared with patients imaged using MRI, those
imaged using Dce-MRI demonstrated increased activity in the left
inferior frontal gyrus (Fig. 4A).
Dce-MRI revealed higher numbers of active epileptic lesions in the
whole brain compared with MRI (Fig.
4B). The outcomes demonstrated that brain activities associated
with epilepsy were completely and clearly displayed by Dce-MRI
compared with MRI (Fig. 4C).
Dce-MRI presented with higher degrees of accuracy in locating
epileptic lesions compared with MRI, supported by observations that
the image quality of Dce-MRI was adequate for the detection of the
epileptogenic lesions of periventricular nodular heterotopia (PNH;
Fig. 4D). These observations
suggest that Dce-MRI can evaluate the location and characteristics
of epileptic pathology in patients with epilepsy.
Discussion
The pathophysiology of epilepsy includes the
simultaneous presence of hippocampal sclerosis and its
comorbidities associated with psychiatric and cognitive
complications (28). Clinically,
MRI is routinely performed to diagnose and assess non-lesional
cingulate epilepsy (19,29,30).
Dce-MRI examination has previously demonstrated considerable
accuracy in the preoperative localization of epileptic foci,
suggesting that Dce-MRI is suitable for use as a basic reference
for the diagnosis of patients with epilepsy (20). In the present study, the diagnostic
efficacy and potency of the Dce-MRI platform was comprehensively
investigated, and the results demonstrated that Dce-MRI can be
applied to identify and locate pathological lesions in cases of
epilepsy.
Focal cortical dysplasia is the major
histopathological type among patients with epilepsy who require
surgical resection, and previous data suggest that the MRI
diagnostic platform provides essential information for the
detection of focal cortical dysplasia (31). Morphometric analysis is based on the
most common features of focal cortical dysplasia, including
cortical thickness and the blurring of the grey-white matter
interface (32). The application of
structural MRI emphasizes the unique role of this non-invasive
technique, which enables the location of focal regions that are
potentially associated with epilepsy and the designation of
management strategies for patients with epilepsy (33). In the present study, the locations
of epileptic lesions in patients were clearly identified and
analyzed using Dce-MRI. Compared with MRI, images obtained by
Dce-MRI exhibited higher resolution, which clearly displayed the
minimal abnormal lesions in the brain, clarified the primary
localization of epileptic foci and detected the abnormal structures
in the brain (20). Additional data
obtained, including the morphometric/textural lesions and signal
intensity, showed that the epileptic lesions were primarily located
in the parietal lobes of patients with epilepsy where the shape was
abnormal. It is therefore of importance to recognize that epileptic
features and outcomes as in the present study suggest the improved
sensitivity of identifying an abnormality using Dce-MRI.
Measurements from MRI images can be applied for the
identification of epileptic regions and size estimation for
clinical reference (34-36).
Resting functional MRI data analysis using temporal clustering is
an efficient method, which can assist clinical practice in
characterizing the epileptogenic network in patients with epilepsy
(37). In the present study,
Dce-MRI exhibited higher diagnostic sensitivity and sensitivity for
epilepsy compared with MRI. In addition, Dce-MRI demonstrated
higher accuracy in identifying cortical lesions compared with MRI,
since the imaging findings co-localized to the diagnosed zone of
suspected seizure onset, the SNR and CNR were increased in images
of the hippocampus, and the areas of the GWMC and epileptogenic
lesions were increased. Reorganization of the brain may occur in
patients with epilepsy prior to treatment and/or surgery;
therefore, leaving more regions of the brain and GWMC intact
provides a foundation for functional recovery following diagnosis.
Additionally, patients diagnosed with epilepsy using Dce-MRI
presented with higher activity on the left inferior frontal gyrus,
higher numbers of active lesions and PNH compared with those
diagnosed using MRI. These findings suggest that epileptic features
in the brain can be readily detected using Dce-MRI.
Limitations associated with the present study
concern the study population, follow-up after diagnosis and the
representativeness of the results. The sample size of the cases of
epilepsy investigated was relatively small, meaning that further
studies using larger sample sizes would be required to determine
the diagnostic efficacy and recognized standardization of Dce-MRI
procedures in patients with epilepsy. In addition, the present
study did not include long-term follow-up data following epilepsy
diagnosis. The present study design also did not include a
comparison between MRI and Dce-MRI on a single patient, rendering
impossible the establishment of intraclass correlation
coefficients, which could be used to estimate the degree to which
both imaging methods match the parameters in patients with
epilepsy. In the future, multi-center studies should be conducted,
which should include feasibility of outcomes analyses, in addition
to analyzing further pathological details in a larger cohort to
obtain data that can adequately represent the general population of
patients with epilepsy.
In conclusion, diagnostic data obtained using
Dce-MRI in the present study suggest that Dce-MRI is a promising
diagnostic approach for minimizing cognitive morbidity associated
with open surgical resection for treating epilepsy. Dce-MRI can
detect epileptic lesions, aid in the identification of pathological
features and locate focal cortical dysplasia. By identifying the
potential regions of suspected epileptic activity in the brain
using Dce-MRI, clinicians can design strategies of therapeutic
intervention using data obtained from Dce-MRI.
Acknowledgements
Not applicable.
Funding
The present study was supported by Project of
Sichuan Science and Technology Department (grant no.
2018JY0604).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DF, GJ and WP performed the experiments. WJ analyzed
the data. JR designed the study and wrote the manuscript. All
authors read and approved this article.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
The General Hospital of Western Theater Command (approval no.
55942116.5.0000.5404). Signed and written informed consent was
provided by all participants.
Patient consent for publication
Patients consented to the publication of their MRI
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Veersema TJ, Swampillai B, Ferrier CH, van
Eijsden P, Gosselaar PH, van Rijen PC, Spliet WGM, Mühlebner A,
Aronica E and Braun KPJ: Long-term seizure outcome after epilepsy
surgery in patients with mild malformation of cortical development
and focal cortical dysplasia. Epilepsia Open. 4:170–175.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Auvin S, Wirrell E, Donald KA, Berl M,
Hartmann H, Valente KD, Van Bogaert P, Cross JH, Osawa M, Kanemura
H, et al: Systematic review of the screening, diagnosis, and
management of ADHD in children with epilepsy Consensus paper of the
task force on comorbidities of the ILAE pediatric commission.
Epilepsia. 59:1867–1880. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Vaughan KA, Lopez Ramos C, Buch VP, Mekary
RA, Amundson JR, Shah M, Rattani A, Dewan MC and Park KB: An
estimation of global volume of surgically treatable epilepsy based
on a systematic review and meta-analysis of epilepsy. J Neurosurg
1-15: 2018.
|
|
4
|
Verche E, San Luis C and Hernandez S:
Neuropsychology of frontal lobe epilepsy in children and adults:
Systematic review and meta-analysis. Epilepsy Behav. 88:15–20.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kwok SC: Website review: Paediatric
epilepsy network of new south Wales. J Paediatrics Child Health.
54(1045)2018.
|
|
6
|
Zhao H, Lin G, Shi M, Gao J, Wang Y, Wang
H, Sun H and Cao Y: The mechanism of neurogenic pulmonary edema in
epilepsy. J Physiol Sci. 64:65–72. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tian XB, Li RC, Bu HL, Liu C, Liu TT,
Xiang HB and Lu CJ: The mechanism of electroacupuncture for
predicting the efficacy of deep brain stimulation in
pharmacoresistant epilepsy may be involved in the melanocortinergic
signal. Epilepsy Behav. 29:594–596. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cho CH: New mechanism for glutamate
hypothesis in epilepsy. Front Cell Neurosci. 7(127)2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Middlebrooks EH, Grewal SS, Stead M,
Lundstrom BN, Worrell GA and Van Gompel JJ: Differences in
functional connectivity profiles as a predictor of response to
anterior thalamic nucleus deep brain stimulation for epilepsy: A
hypothesis for the mechanism of action and a potential biomarker
for outcomes. Neurosurg Focus. 45(E7)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Beckonert NM, Opitz T, Pitsch J, Soares da
Silva P and Beck H: Polyamine modulation of anticonvulsant drug
response: A potential mechanism contributing to pharmacoresistance
in chronic epilepsy. J Neurosci. 38:5596–5605. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Viswanatha GL, Venkataranganna MV, Prasad
NBL and Godavarthi A: Achyranthes aspera attenuates epilepsy in
experimental animals: Possible involvement of GABAergic mechanism.
Metab Brain Dis. 32:867–879. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
St Louis EK and Cascino GD: Diagnosis of
epilepsy and related episodic disorders. Continuum (Minneap Minn).
22:15–37. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Helbig KL, Farwell Hagman KD, Shinde DN,
Mroske C, Powis Z, Li S, Tang S and Helbig I: Diagnostic exome
sequencing provides a molecular diagnosis for a significant
proportion of patients with epilepsy. Genet Med. 18:898–905.
2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Carmichael DW, Thornton JS, Rodionov R,
Thornton R, McEvoy A, Allen PJ and Lemieux L: Safety of localizing
epilepsy monitoring intracranial electroencephalograph electrodes
using MRI: Radiofrequency-induced heating. J Magn Reson Imaging.
28:1233–1244. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Brown MG, Drees C, Nagae LM, Thompson JA,
Ojemann S and Abosch A: Curative and palliative MRI-guided laser
ablation for drug-resistant epilepsy. J Neurol Neurosurg
Psychiatry. 89:425–433. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Drane DL: MRI-Guided stereotactic laser
ablation for epilepsy surgery: Promising preliminary results for
cognitive outcome. Epilepsy Res. 142:170–175. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Delev D, Quesada CM, Grote A, Boström JP,
Elger C, Vatter H and Surges R: A multimodal concept for invasive
diagnostics and surgery based on neuronavigated voxel-based
morphometric MRI postprocessing data in previously nonlesional
epilepsy. J Neurosurg. 128:1178–1186. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fredriksen JR, Carr CM, Koeller KK,
Verdoorn JT, Gadoth A, Pittock SJ and Kotsenas AL: MRI findings in
glutamic acid decarboxylase associated autoimmune epilepsy.
Neuroradiology. 60:239–245. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ruber T, David B and Elger CE: MRI in
epilepsy: Clinical standard and evolution. Curr Opin Neurol.
31:223–231. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang GB, Long W, Li XD, Xu GY and Lu JX:
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI)
combined with positron emission tomography-computed tomography
(PET-CT) and video-electroencephalography (VEEG) have excellent
diagnostic value in preoperative localization of epileptic foci in
children with epilepsy. Med Sci Monit. 23:1–10. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
de Oliveira MS, Betting LE, Mory SB,
Cendes F and Castellano G: Texture analysis of magnetic resonance
images of patients with juvenile myoclonic epilepsy. Epilepsy
Behav. 27:22–28. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kulaseharan S, Aminpour A, Ebrahimi M and
Widjaja E: Identifying lesions in paediatric epilepsy using
morphometric and textural analysis of magnetic resonance images.
Neuroimage Clin. 21(101663)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Iachinski RE, de Meneses MS, Simão CA, da
Rocha SF, de Oliveira Braga F and Kowacs PA: Patient satisfaction
with temporal lobectomy/selective amygdalohippocampectomy for
temporal lobe epilepsy and its relationship with Engel
classification and the side of lobectomy. Epilepsy Behav.
31:377–380. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chen L, Ye Y, Chen H, Chen S, Jiang J, Dan
G and Huang B: Dynamic contrast-enhanced magnetic resonance imaging
for differentiating between primary tumor, metastatic node and
normal tissue in head and neck cancer. Curr Med Imaging Rev.
14:416–421. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Boling W: Diagnosis and surgical treatment
of epilepsy. Brain Sci. 8(E115)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Rösch J, Hamer HM, Mennecke A, Kasper K,
Engelhorn T, Doerfler A and Graf W: 3T-MRI in patients with
pharmacoresistant epilepsy and a vagus nerve stimulator: A pilot
study. Epilepsy Res. 110:62–70. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Harden C, Tomson T, Gloss D, Buchhalter J,
Cross JH, Donner E, French JA, Gil-Nagel A, Hesdorffer DC, Smithson
WH, et al: Practice guideline summary: Sudden unexpected death in
epilepsy incidence rates and risk factors: Report of the guideline
development, dissemination, and implementation subcommittee of the
American academy of neurology and the American epilepsy society.
Neurology. 88:1674–1680. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mehvari Habibabadi J, Badihian S, Tabrizi
N, Manouchehri N, Zare M, Basiratnia R, Barekatain M, Moein H,
Mehvari Habibabadi A, Moein P and Gookizadeh P: Evaluation of dual
pathology among drug-resistant epileptic patients with hippocampal
sclerosis. Neurol Sci. 40:495–502. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang S, Jin B, Aung T, Katagiri M, Jones
SE, Krishnan B, Gonzalez-Martinez JA, Prayson RA, Najm IM,
Alexopoulos AV, et al: Application of MRI post-processing in
presurgical evaluation of non-lesional cingulate epilepsy. Front
Neurol. 9(1013)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Colon AJ, Osch MJPV, Buijs M, Grond JVD,
Hillebrand A, Schijns O, Wagner GJ, Ossenblok P, Hofman P, Buchem
MAV and Boon P: MEG-guided analysis of 7T-MRI in patients with
epilepsy. Seizure. 60:29–38. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Jin B, Krishnan B, Adler S, Wagstyl K, Hu
W, Jones S, Najm I, Alexopoulos A, Zhang K, Zhang J, et al:
Automated detection of focal cortical dysplasia type II with
surface-based magnetic resonance imaging postprocessing and machine
learning. Epilepsia. 59:982–992. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Alshafai L, Ochi A, Go C, McCoy B, Hawkins
C, Otsubo H, Snead OC, Rutka J and Widjaja E: Clinical, EEG, MRI,
MEG, and surgical outcomes of pediatric epilepsy with astrocytic
inclusions versus focal cortical dysplasia. Epilepsia.
55:1568–1575. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Najafi MR, Malekian M, Akbari M and Najafi
MA: Magnetic resonance imaging and electroencephalography findings
in a sample of Iranian patients with epilepsy. J Res Med Sci.
23(106)2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Torres CV, Pastor J, Garcia-Navarrete E,
Pulido-Rivas P and Sola RG: Classification of structural lesions in
magnetic resonance imaging. Surgical implications in drug-resistant
epilepsy patients. Rev Neurol. 61:241–248. 2015.PubMed/NCBI(In English, Spanish).
|
|
35
|
Peng SJ, Harnod T, Tsai JZ, Ker MD, Chiou
JC, Chiueh H, Wu CY and Hsin YL: Evaluation of subcortical grey
matter abnormalities in patients with MRI-negative cortical
epilepsy determined through structural and tensor magnetic
resonance imaging. BMC Neurol. 14(104)2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
van Rooijen BD, Backes WH, Schijns OE,
Colon A and Hofman PA: Brain imaging in chronic epilepsy patients
after depth electrode (stereoelectroencephalography) implantation:
Magnetic resonance imaging or computed tomography? Neurosurgery.
73:543–549. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Pizarro R, Nair V, Meier T, Holdsworth R,
Tunnell E, Rutecki P, Sillay K, Meyerand ME and Prabhakaran V:
Delineating potential epileptogenic areas utilizing resting
functional magnetic resonance imaging (fMRI) in epilepsy patients.
Neurocase. 22:362–368. 2016.PubMed/NCBI View Article : Google Scholar
|