Introduction
Acute transverse myelitis (ATM) is a myelopathy
characterized by acute or subacute movement, sensory, and autonomic
neurospinal dysfunction, with one to four new cases per million
people per year (1). The
pathological and physiological mechanisms of ATM have not yet been
elucidated, but it may be caused by ascending and descending spinal
tracts of specific levels of bone marrow (2). Symptoms and signs of motor, sensory
and autonomic nerve dysfunction in ATM patients may occur
simultaneously, mainly depending on the level of the spinal cord
involved (3). One-third of patients
with ATM can fully recover, another third have moderate residual
neurological deficits, and the remaining third show clinically
severe residual neurological deficits. ATM patients with poor
prognosis have spinal shock and high disability (4-6).
Currently, the first-line therapy for clinical
treatment of ATM is glucocorticoid treatment, such as dexamethasone
and methylprednisolone. Traditional dexamethasone treatment has
slower onset, longer treatment time and more adverse reactions,
often leaving serious neurological dysfunction (7,8).
However, methylprednisolone can produce non-specific
immunosuppressive responses to the nerve center, play a role in
anti-inflammatory and anti-edema, improve the blood circulation of
nerves, and reduce spinal cord inflammation (9). γ-globulin is an IgG obtained from
thousands of human plasma, which can rapidly supplement antibodies
in patients, inhibit the mononuclear phagocytic system, and it has
good efficacy in both autoimmune and inflammatory diseases
(10). At present, the pathogenesis
of ATM has not been clearly clarified, and immune dysfunction may
be one of its pathogenesis (11).
Although there have been many studies on the application of
glucocorticoids and γ-globulin in ATM (12-14),
there are few studies on effects of γ-globulin combined with
dexamethasone and methylprednisolone, respectively, on immune
function and quality of life.
In this study, γ-globulin was combined with
dexamethasone or methylprednisolone to treat patients with ATM.
Clinical efficacy of the two treatment schemes and their effects on
immune function and quality of life were compared.
Patients and methods
General data
A retrospective analysis of medical records from 136
ATM patients admitted to the hospital from July 2014 to September
2017 was performed. Patients treated with dexamethasone combined
with γ-globulin were enrolled in group A (66 cases), and those
treated with methylprednisolone combined with γ-globulin were
included in group B (70 cases). In group A, there were 40 males and
26 females, aged 24 to 49 years, with a mean age of 34.7±5.9; in
group B, there were 39 males and 31 females, aged 23 to 50 years,
with a mean age of 35.9±6.4. All patients were informed and signed
an informed consent. This study was approved by the Ethics
Committee of Linzi District People's Hospital (Zibo, China).
Inclusion and exclusion criteria
Inclusion criteria were as follows: patients
complied with the diagnostic criteria of ATM in the International
Collaboration Group of Acute Myelitis (TMCWG) (15); the protein of the lumbar puncture
cerebrospinal fluid increased, and the diagnosis was confirmed by
imaging MRI; patients bilaterally asymmetric, with symmetric
nervous system signs and symptoms; the level of muscular strength
of double lower limbs of people was from 0 to 3, with sensory plane
barriers and sphincter dysfunction; patients with complete clinical
data; patients with drug contraindications in this treatment, and
anti-inflammatory, immunosuppressive drugs used in the past month.
Exclusion criteria were as follows: Patients with myelitis caused
by vascular, metabolic, radioactive and oppressive factors;
patients with other neurological deficits, peripheral nerve damage,
central nervous system diseases, severe liver and kidney
dysfunction, severe infections, malignant tumors, systemic immune
diseases, cardiovascular and cerebrovascular disease, hypertension,
diabetes and inflammation; patients with cognitive dysfunction and
mental illness.
Treatment methods
Altogether 0.3-0.5 mg/(kg•day) of dexamethasone
(Shandong Chenxin Pharmaceutical Co., Ltd., batch no. H37021969)
was given by intravenous drip to patients in group A; at the same
time, 200-400 mg/(kg•day) of γ-globulin (Beijing Tiantan Biological
Products Co., Ltd., batch no. S20023026) was given by intravenous
drip; after continuous use for five to seven days, prednisone
tablets (Zhejiang Xianyi Pharmaceutical Co., Ltd., batch no.
H33021207) were administered orally once a day, with an oral dose
of 60 mg and a reduction of 5 mg per three days. Patients in group
B were given high-dose methylprednisolone (Belgium PFIZER SA, batch
no. H20130302) 1,000 mg mixed with 500 ml of 10% glucose injection
combined with intravenous drip; 200-400 mg/(kg•day) of γ-globulin
was given by intravenous drip at the same time, and then changed to
take prednisone tablets orally. The usage and dosage were
consistent with those in group A. During the treatment, antacids,
antibiotics, and neurotrophins were normally used, and other
immunosuppressants were prohibited. Both groups were treated for
four weeks.
Efficacy evaluation and observation
indicators
Indicators of spinal cord function were observed,
including time of sensory recovery, self-walking, improving muscle
strength at two levels and urination recovery. Efficacy of patients
was assessed four weeks after treatment. Efficacy of the two groups
was evaluated according to the recovery of sensory disturbance,
dyskinesia, and autonomic nerve dysfunction, as shown in Table I. Quality of life of patients after
admission and at two months was assessed in conjunction with the
Quality of Life Scale (SF-36) developed by the American Institute
of Medicine (16). The assessment
included general health (GH), physical function (PF), role physical
(RP), body pain (BP), social function (SF), role emotional (RE),
Mental Health (MH) and vitality (VT) eight dimensions, and each
dimension was divided into 100 points; the higher the score, the
better the quality of life.
 | Table ICriteria of clinical efficacy. |
Table I
Criteria of clinical efficacy.
| Efficacy | Sensory
disturbance | Dyskinesia | Autonomic nerve
dysfunction |
|---|
| Recovery | Back to normal | Normal walking
ability | Normal excretion
function |
| Significantly | Subtle
abnormalities | Able to walk by
holding | Basically normal
excretion |
| effective | | on to something | function with subtle
barriers |
| Effective | Some
abnormalities | Unable to walk, but
muscle function has improved | Unable to relieve
oneself, but bladder function has improved |
| Ineffective | Clinical symptoms and
signs were not changed or even deteriorated |
Detection of T-lymphocyte subsets
Ratios of CD3+, CD4+ and
CD8+ as well as CD4+/CD8+ in
peripheral blood were measured by Attune NxT flow cytometry
(Shanghai Thermo Fisher Technology Co., Ltd.) on admission and
after treatment. In total, 100 µl of anticoagulated blood was
placed in the TruCOUNT tube, and then 20 µl of fluorescein
isothiocyanate (FITC) fluorescently labeled monoclonal antibody
CD3-PC5, CD4-PE and CD8-ECD (Shanghai Kemin Biotechnology Co.,
Ltd., item no. 6607010, DXT-130-109-451, 737659) were added to the
tube. Then mixed evenly, and placed at room temperature for 20 min;
500 µl of hemolysin was added to lyse the cells, and the solution
was placed at room temperature for 15 min; 500 µl PBS buffer
solution was added; mixed well, and then placed at room temperature
for 10 min. Samples were tested on a flow cytometer, and data of
CD3+, CD4+, CD8+ and
CD4+/CD8+ were read.
Statistical analysis
SPSS 22.0 (Beijing Boao Yijie Technology Co., Ltd.)
was used for the statistical analysis of the data; GraphPad 7 was
used to draw the illustrations; normal distribution data were
represented as mean ± standard deviation (SD); independent sample
t-test was used for comparison between groups, and paired t-test
was used for comparison of the same group before and after
treatment; the counting data were represented by [n(%)] and those
between groups were tested by the Chi-square. When the theoretical
frequency in the Chi-square test was less than five, continuous
correction Chi-square test was adopted. P<0.05 was considered
statistically significant.
Results
General data of the two groups
There was no significant difference in general
clinical data including sex, body mass index (BMI), course of
disease, age, history of upper respiratory tract, history of
drinking, history of smoking, location of spinal lesions,
cerebrospinal fluid pressure, protein level of cerebrospinal fluid,
quadriplegia, paraplegia, or place of residence between group A and
B (P>0.05) (Table II).
 | Table IIGeneral data of group A and group B [n
(%), mean ± SD]. |
Table II
General data of group A and group B [n
(%), mean ± SD].
| Category | Group A (n=66) | Group B (n=70) | t/χ2
value | P-value |
|---|
| Sex | | | 0.334 | 0.563 |
|
Male | 40 (60.61) | 39 (55.71) | | |
|
Female | 26 (39.39) | 31 (44.29) | | |
| BMI
(kg/m2) | 22.75±2.68 | 22.57±2.76 | 0.386 | 0.700 |
| Course of disease
(days) | 6.6±1.3 | 6.4±1.2 | 0.933 | 0.352 |
| Age (years) | 34.7±5.9 | 35.9±6.4 | 1.135 | 0.258 |
| History of upper
respiratory tract | | | 0.053 | 0.818 |
|
Yes | 27 (40.91) | 30 (42.86) | | |
|
No | 39 (59.09) | 40 (57.14) | | |
| History of
drinking | | | 0.293 | 0.588 |
|
Yes | 29 (43.94) | 34 (48.57) | | |
|
No | 37 (56.06) | 36 (51.43) | | |
| History of
smoking | | | 0.576 | 0.448 |
|
Yes | 25 (37.88) | 31 (44.29) | | |
|
No | 41 (62.12) | 39 (55.71) | | |
| Location of spinal
lesions | | | 0.724 | 0.867 |
|
Limited to
the cervical spine | 13 (19.70) | 14 (20.00) | | |
|
Limited to
the chest spine | 35 (53.03) | 40 (57.14) | | |
|
Union of
chest and neck | 14 (21.21) | 11 (15.72) | | |
|
Lumbar
spinal cord involved | 4 (6.06) | 5 (7.14) | | |
| Cerebrospinal fluid
pressure (mmH2O) | 157.39±46.58 | 162.37±49.75 | 0.602 | 0.548 |
| Protein level of
cerebrospinal fluid (g/l) | 0.41±0.22 | 0.43±0.24 | 0.506 | 0.614 |
| Quadriplegia | | | 0.033 | 0.855 |
|
Yes | 17 (25.76) | 19 (27.14) | | |
|
No | 49 (74.24) | 51 (72.86) | | |
| Paraplegia | | | 0.319 | 0.572 |
|
Yes | 39 (59.09) | 38 (54.29) | | |
|
No | 27 (40.91) | 32 (45.71) | | |
| Place of
residence | | | 0.102 | 0.749 |
|
City | 46 (69.70) | 47 (67.14) | | |
|
Country | 20 (30.30) | 23 (32.86) | | |
Spinal cord function indicators after
treatment in the two groups
Time of sensory recovery, self-walking, improving
muscle strength at two levels and urination recovery after
treatment were significantly shorter in group B than those in group
A (P<0.001) (Table III).
 | Table IIIComparison of spinal cord function
indicators after treatment in group A and group B (mean ± SD,
days). |
Table III
Comparison of spinal cord function
indicators after treatment in group A and group B (mean ± SD,
days).
| Indicators | Group A (n=66) | Group B (n=70) | t value | P-value |
|---|
| Sensory recovery
time | 8.61±3.52 | 5.94±2.28 | 5.280 | <0.001 |
| Self-walking
time | 21.73±8.75 | 17.32±5.39 | 3.561 | <0.001 |
| Time of improving
muscle strength at two levels | 13.49±4.68 | 9.45±3.52 | 5.710 | <0.001 |
| Urination recovery
time | 11.36±3.74 | 7.97±2.86 | 5.958 | <0.001 |
Effects of treatment in the two
groups
After treatment, there were 14 cases (21.21%)
recovered, 31 cases (46.97%) significantly effective, 10 cases
(15.15%) effective, 11 cases (16.67%) ineffective in group A, and
the effective rate was 83.33%; there were 21 cases (30.00%)
recovered, 30 cases (42.86%) significantly effective, 15 cases
(21.43%) effective, and 4 cases (5.71%) ineffective in group B, and
the effective rate was 94.29%. Effective rates of treatment in
group B were significantly higher than those in group A
(P<0.05). More details were shown in Table IV.
 | Table IVComparison of effective rates between
group A and group B [n (%)]. |
Table IV
Comparison of effective rates between
group A and group B [n (%)].
| Groups | n | Recovery | Significantly
effective | Effective | Ineffective | Effective rate
(%) |
|---|
| Group A | 66 | 14 (21.21) | 31 (46.97) | 10 (15.15) | 11 (16.67) | 83.33 |
| Group B | 70 | 21 (30.00) | 30 (42.86) | 15 (21.43) | 4 (5.71) | 94.29 |
| χ2
value | - | 1.373 | 0.232 | 0.892 | 4.153 | 4.153 |
| P-value | - | 0.241 | 0.630 | 0.345 | 0.042 | 0.042 |
Incidence rates of adverse reactions
in the two groups
During treatment, there were six cases with nausea
and vomiting (9.09%), three cases with rash (4.55%), four cases
with bellyache (6.06%), five cases with elevated blood glucose
(7.58%) in group A, and the incidence rate of adverse reactions was
27.27%. During treatment, there were four cases (5.71%) with nausea
and vomiting, one case (1.43%) with bellyache, four cases (5.71%)
with elevated blood glucose in group B, and the incidence rate of
adverse reactions were 12.86%. Incidence rate of adverse reactions
in group B was significantly lower than that in group A (P<0.05)
(Table V).
 | Table VIncidence rate of adverse reactions
in group A and group B [n (%)]. |
Table V
Incidence rate of adverse reactions
in group A and group B [n (%)].
| Groups | n | Nausea and
vomiting | Rash | Bellyache | Elevated blood
glucose | Incidence rate
(%) |
|---|
| Group A | 66 | 6 (9.09) | 3 (4.55) | 4 (6.06) | 5 (7.58) | 27.27 |
| Group B | 70 | 4 (5.71) | 0 (0.00) | 1 (1.43) | 4 (5.71) | 12.86 |
| χ2
value | - | 0.569 | 1.488 | 0.958 | 0.190 | 4.437 |
| P-value | - | 0.451 | 0.223 | 0.328 | 0.662 | 0.035 |
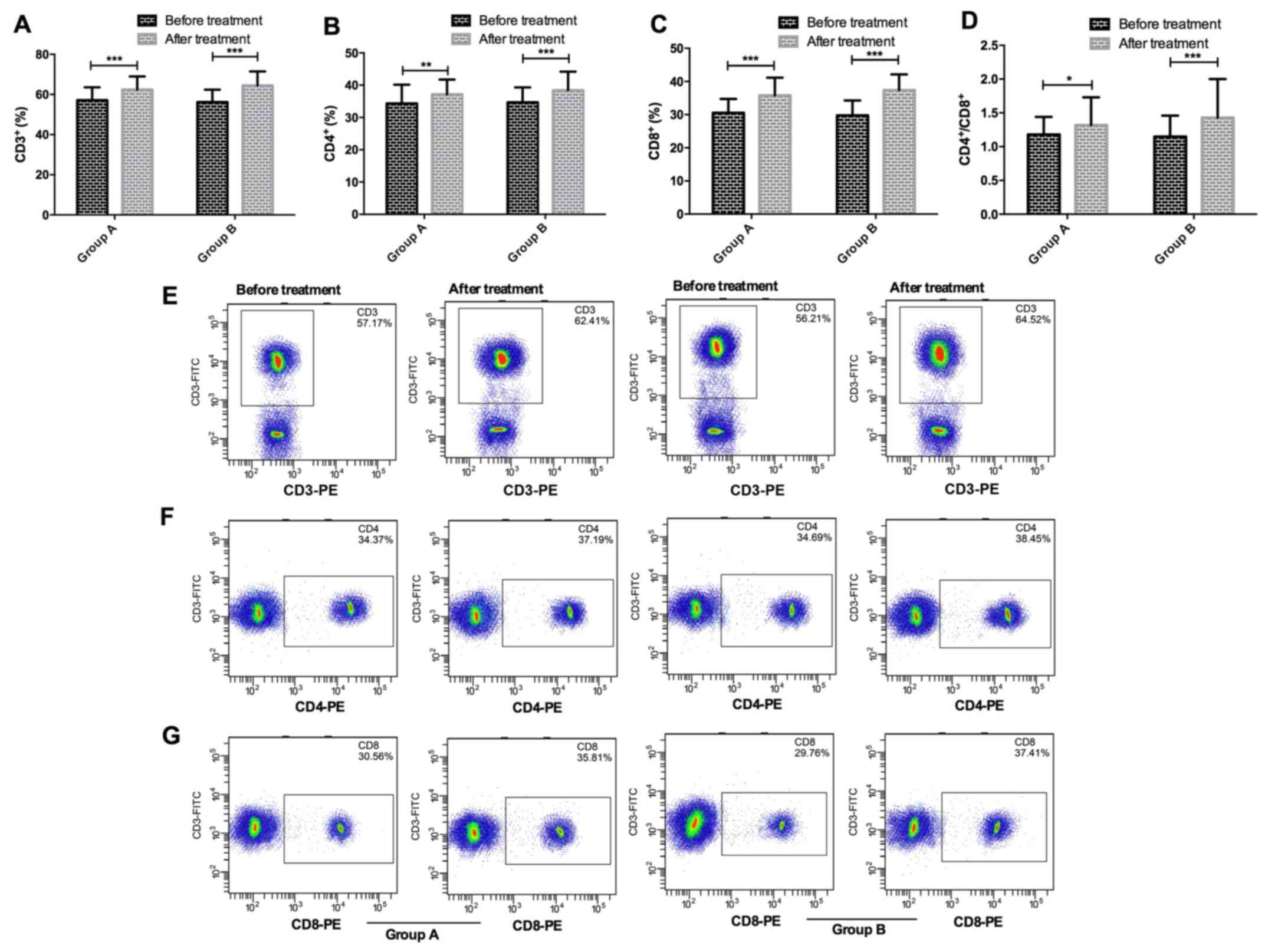
Results of T-lymphocyte subsets in
peripheral blood in the two groups
There were no significant differences in ratios of
CD3+, CD4+ and CD8+ cells or
CD4+/CD8+ in peripheral blood between group A
and group B before or after treatment (P>0.05); ratios of
CD3+, CD4+ and CD8+ cells and
CD4+/CD8+ in peripheral blood between group A
and group B after treatment were significantly higher than those
before treatment (P<0.05) (Fig.
1).
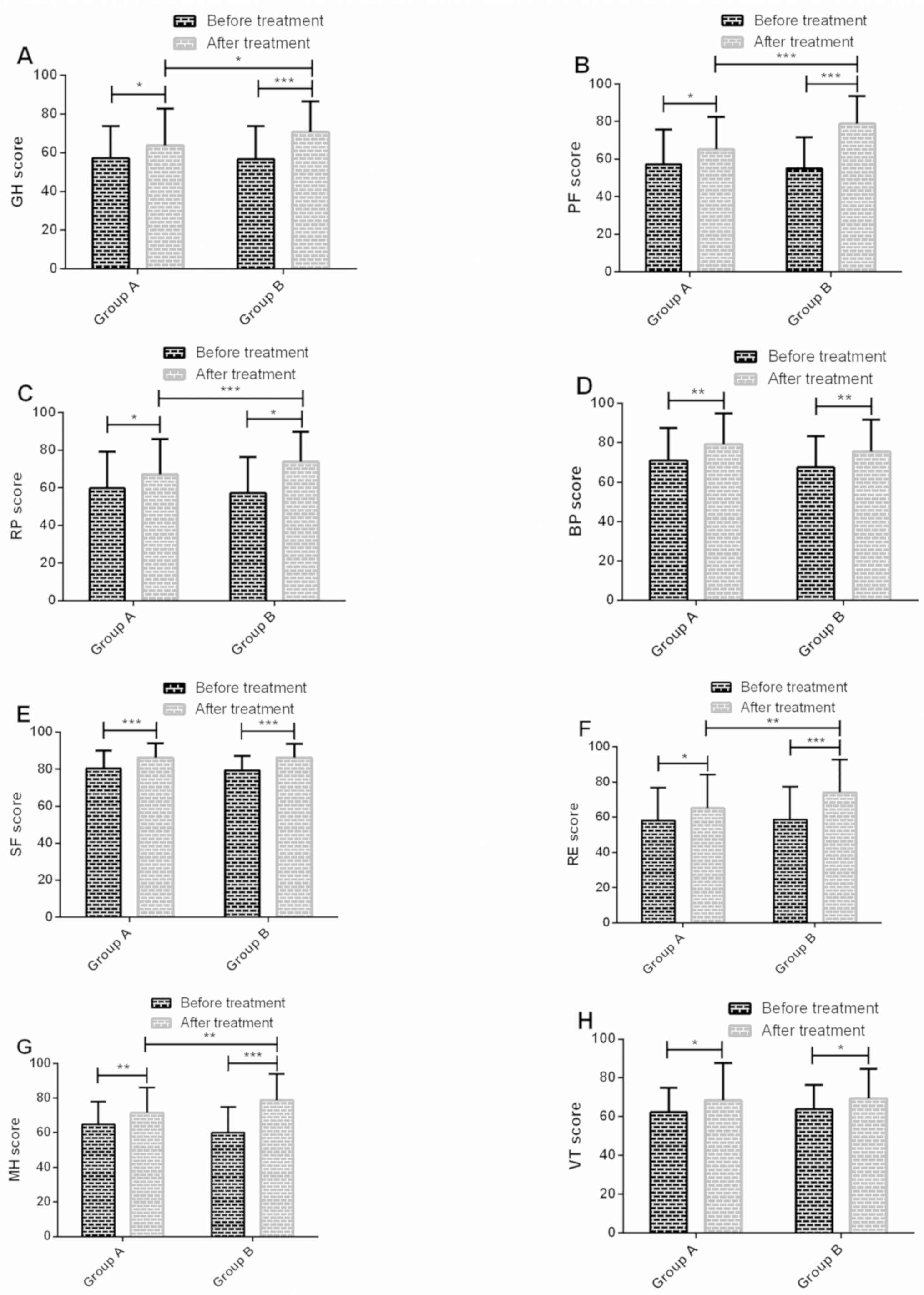
Comparison of results of quality of
life before and after treatment in the two groups
There were no significant differences in scores of
GH, PF, RP, BP, SF, RE, MH and VT between group A and group B
before treatment (P>0.05); scores of GH, PF, RP, BP, SF, RE, MH
and VT in group A and group B after treatment were significantly
higher than those before treatment (P<0.05); scores of GH, PF,
RP, RE and MH in group B after treatment were significantly higher
than those in group A (P<0.05) (Fig.
2).
Discussion
The onset and progress of ATM are relatively rapid.
If it is not treated in time, functional disorders in sensory,
motor and autonomic nerves may occur, and even death in severe
cases (17,18). In our study, the combination of
γ-globulin and methylprednisolone in the treatment of ATM has
better clinical symptoms, which can effectively improve the
clinical symptoms of patients with fewer adverse reactions, and
improve their immune function and quality of life.
Clinically, γ-globulin is mostly used for the
treatment of infectious diseases, and can deliver antibodies to
patients, so that they can achieve immune protection from low
immunity or no immunity, and promote their recovery (19). Dexamethasone is a commonly used
glucocorticoid therapy drug for ATM, with anti-allergic and
anti-toxic effects. However, its onset time is relatively slow, and
some patients may suffer from dizziness, limb movement disorder,
mental retardation and other complications (20). Methylprednisolone is a synthetic
glucocorticoid, which can promote the synthesis of a variety of
enzyme proteins, thus playing an anti-inflammatory and
immunosuppressive role and promoting the recovery of nerve function
(21). In recent years, there have
been many studies on the application of methylprednisolone used in
ATM patients. Defresne et al (22) reported that effects of high-dose
methylprednisolone were better than not using hormone or low-dose
methylprednisolone in the treatment of children with severe ATM;
time of bladder function recovery, walking and muscle strength of
children was significantly improved. Kovacs et al (23) verified that methylprednisolone
combined with cyclophosphamide in the treatment of ATM patients was
significantly better than cyclophosphamide alone. In this study,
time of recovery, self-walking, improving muscle strength at two
levels and urination recovery of group B was significantly shorter
than that of group A, and effective rates of group B were
significantly higher than those of group A. Thus indicating that
γ-globulin combined with methylprednisolone was effective in the
treatment of ATM, which could promote the recovery of spinal cord
function with fewer adverse reactions. Further research found that
the incidence rate of adverse reactions in group B was
significantly lower than that in group A, indicating that there
were fewer adverse reactions in combination of the two. Although
some adverse reactions occurred during the treatment, all patients
recovered completely without special treatment, so this is in line
with expectations. Pavlou et al (24) confirmed that a patient with ATM was
treated with high-dose methylprednisolone, and the condition was
not effectively controlled. There were still aggravated limb
paralysis and respiratory muscles paralysis, followed by
intravenous γ-globulin. Patients' neurological function began to
recover with no apparent complications, which was similar to our
study. Changes in T-lymphocyte subsets reflect the status of immune
system function, and they are classified into CD3+,
CD4+ and CD8+ cells according to their
functions and surface markers (25), in which CD3+ is
positively correlated with T-lymphocyte immune function, while the
increase and decrease of ratios of CD4+ and
CD8+ can cause the ratios to become imbalanced, which
leads to abnormal immune function (26). The present study showed that ratios
of CD3+, CD4+, CD8+ and
CD4+/CD8+ in peripheral blood of group A and
group B were significantly higher than those before treatment, but
there were no significant differences between the groups.
γ-globulin combined with dexamethasone and methylprednisolone,
respectively, can improve the immune status of patients and improve
their immunity. Larroche et al (27) reported that γ-globulin was an
important drug for the treatment of various autoimmune and
neurological diseases, and one of its mechanisms was to
immunomodulate patients. It may be that γ-globulin exerts enhancing
immunity (28), while
glucocorticoid drugs can alleviate the damage of peroxide to spinal
cord tissue (29). Combination of
these two drugs can eliminate the abnormal immune function of ATM
patients, reduce the damage of immune responses to spinal cord,
thereby promoting the recovery of spinal cord function. Gelfand
(30) reported that γ-globulin
could be used as clinical immunomodulatory therapy to treat a
variety of immune diseases, such as Guillain-Barré syndrome,
myasthenia gravis, Kawasaki disease, and in kidney transplant.
However, γ-globulin is a mixed blood product, which is relatively
expensive. Therefore, the application of γ-globulin may be
accompanied by cost-effectiveness considerations. Finally, SF-36
was adopted to assess patients' quality of life. The results
revealed that scores of GH, PF, RP, BP, SF, RE, MH and VT in group
A and group B after treatment were significantly higher than those
before treatment, and scores of GH, PF, RP, RE and MH after
treatment in group B were significantly higher than those in group
A, which indicated that γ-globulin combined with methylprednisolone
also had a certain improvement effect on patients' quality of life.
This may be because the γ-globulin combined with methylprednisolone
has better efficacy and can improve the clinical symptoms and signs
of patients, thereby improving their quality of life.
This study confirmed the clinical efficacy of
γ-globulin combined with methylprednisolone in the treatment of
ATM.However, the long-term quality of life of ATM patients was not
observed and the influencing factors of the efficacy remain to be
analyzed.
In conclusion, γ-globulin combined with
methylprednisolone in the treatment of ATM patients has definite
clinical efficacy and fewer adverse reactions, which can improve
their immune function and quality of life.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PW conceived the study and wrote the manuscript. SZ
analyzed and interpreted the patient general data. HLv and GQ
performed flow cytometry. XZ, HLi and LZ were responsible for the
analysis of the observation indicators. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Linzi District People's Hospital (Zibo, China). Patients who
participated in this study had complete clinical data. Signed
informed consents were obtained from the patients and/or
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
He H, Jin L, Ju M, Tu G and Luo Z: Acute
transverse myelitis of the cervical spine secondary to psoas
abscess. BMC Infect Dis. 16(579)2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Frohman EM and Wingerchuk DM: Clinical
practice. Transverse myelitis. N Engl J Med. 363:564–572.
2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
The Lancet Infectious Diseases:
Enterovirus D68: The unexpected guest. Lancet Infect Dis 14: 1023,
2014.
|
|
4
|
Holm-Hansen CC, Midgley SE and Fischer TK:
Global emergence of enterovirus D68: A systematic review. Lancet
Infect Dis. 16:e64–e75. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Huh Y, Park EJ, Jung JW, Oh S and Choi SC:
Clinical insights for early detection of acute transverse myelitis
in the emergency department. Clin Exp Emerg Med. 2:44–50.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chen L, Li J, Guo Z, Liao S and Jiang L:
Prognostic indicators of acute transverse myelitis in 39 children.
Pediatr Neurol. 49:397–400. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kuo SC, Cho WH, Shih HI and Tu YF:
Idiopathic acute transverse myelitis in children: A retrospective
series. Neuropediatrics. 46:307–312. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li J, Zhou J and Zhan QS: Comparative
observation of Methylprednisolone and gamma globulin in the
treatment of acute myelitis. Chin J Biochem Pharm. 37:163–164.
2017.
|
|
9
|
Nelson GR, Bonkowsky JL, Doll E, Green M,
Hedlund GL, Moore KR and Bale JF Jr: Recognition and management of
acute flaccid myelitis in children. Pediatr Neurol. 55:17–21.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Branch DR: Serologic problems associated
with administration of intravenous immune globulin (IVIg).
Immunohematology. 35:13–15. 2019.PubMed/NCBI
|
|
11
|
Greenberg BM and Frohman EM:
Immune-mediated myelopathies. Continuum (Minneap Minn). 21 (1
Spinal Cord Disorders):121–131. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Patel V, Griffith NC, Blackwood E, Dias M
and Cordato DJ: Spectrum disorder of neuromyelitis optica in a
patient presenting with intractable vomiting and hiccups,
transverse myelitis and acute encephalopathy. J Clin Neurosci.
19:1576–1578. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hurlbert RJ: Methylprednisolone for the
treatment of acute spinal cord injury: Point. Neurosurgery. 61
(Suppl 1):32–35. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Torisu H, Kira R, Ishizaki Y, Sanefuji M,
Yamaguchi Y, Yasumoto S, Murakami Y, Shimono M, Nagamitsu S,
Masuzaki M, et al: Clinical study of childhood acute disseminated
encephalomyelitis, multiple sclerosis, and acute transverse
myelitis in Fukuoka Prefecture, Japan. Brain Dev. 32:454–462.
2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
West TW: Transverse myelitis - a review of
the presentation, diagnosis, and initial management. Discov Med.
16:167–177. 2013.PubMed/NCBI
|
|
16
|
Yarlas A, Bayliss M, Cappelleri JC, Maher
S, Bushmakin AG, Chen LA, Manuchehri A and Healey P: Psychometric
validation of the SF-36® Health Survey in ulcerative
colitis: Results from a systematic literature review. Qual Life
Res. 27:273–290. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Annunziata P, Masi G, Cioni C, Gastaldi M,
Marchioni E, D'amico E, Patti F, Laroni A, Mancardi G, Vitetta F,
et al: Clinical, laboratory features, and prognostic factors in
adult acute transverse myelitis: An Italian multicenter study.
Neurol Sci. 40:1383–1391. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
West TW, Hess C and Cree BAC: Acute
transverse myelitis: Demyelinating, inflammatory, and infectious
myelopathies. Semin Neurol. 32:97–113. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Dhandha MM, Siegfried EC and Knutsen AP:
Treatment of selective antibody deficiency with IVIG resulting in
decreased frequency of streptococcal infection and improvement of
guttate psoriasis. Dermatol Online J. 23(23)2017.PubMed/NCBI
|
|
20
|
Tomietto P, D'Agostini S, Annese V, De
Vita S and Ferraccioli G: Mycophenolate mofetil and intravenous
dexamethasone in the treatment of persistent lupus myelitis. J
Rheumatol. 34:588–591. 2007.PubMed/NCBI
|
|
21
|
Costa DD, Beghi E, Carignano P, Pagliacci
C, Faccioli F, Pupillo E, Messina P, Gorio A and Redaelli T:
Tolerability and efficacy of erythropoietin (EPO) treatment in
traumatic spinal cord injury: A preliminary randomized comparative
trial vs. methylprednisolone (MP). Neurol Sci. 36:1567–1574.
2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Defresne P, Meyer L, Tardieu M, Scalais E,
Nuttin C, De Bont B, Loftus G, Landrieu P, Kadhim H and Sébire G:
Efficacy of high dose steroid therapy in children with severe acute
transverse myelitis. J Neurol Neurosurg Psychiatry. 71:272–274.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kovacs B, Lafferty TL, Brent LH and
DeHoratius RJ: Transverse myelopathy in systemic lupus
erythematosus: An analysis of 14 cases and review of the
literature. Ann Rheum Dis. 59:120–124. 2000.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Pavlou E, Gkampeta A, Kouskouras K,
Evangeliou A and Athanasiadou-Piperopoulou F: Idiopathic acute
transverse myelitis: Complete recovery after intravenous
immunoglobulin. Hippokratia. 16:283–285. 2012.PubMed/NCBI
|
|
25
|
den Otter I, Willems LN, van Schadewijk A,
van Wijngaarden S, Janssen K, de Jeu RC, Sont JK, Sterk PJ and
Hiemstra PS: Lung function decline in asthma patients with elevated
bronchial CD8, CD4 and CD3 cells. Eur Respir J. 48:393–402.
2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhao Z, Liao H and Ju Y: Effect of
compound Kushen injection on T-cell subgroups and natural killer
cells in patients with locally advanced non-small-cell lung cancer
treated with concomitant radiochemotherapy. J Tradit Chin Med.
36:14–18. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Larroche C, Chanseaud Y, Garcia de la
Pena-Lefebvre P and Mouthon L: Mechanisms of intravenous
immunoglobulin action in the treatment of autoimmune disorders.
BioDrugs. 16:47–55. 2002.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ballow M: The IgG molecule as a biological
immune response modifier: Mechanisms of action of intravenous
immune serum globulin in autoimmune and inflammatory disorders. J
Allergy Clin Immunol. 127:315–323; quiz 324-325. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Esposito E, Bruscoli S, Mazzon E,
Paterniti I, Coppo M, Velardi E, Cuzzocrea S and Riccardi C:
Glucocorticoid-induced leucine zipper (GILZ) over-expression in T
lymphocytes inhibits inflammation and tissue damage in spinal cord
injury. Neurotherapeutics. 9:210–225. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gelfand EW: Intravenous immune globulin in
autoimmune and inflammatory diseases. N Engl J Med. 367:2015–2025.
2012.PubMed/NCBI View Article : Google Scholar
|