Introduction
Cardiac arrest (CA) for >5 min often leads to
irreversible brain damage (1).
Implementing effective brain resuscitation to save ischemic neurons
and accelerate the repair of damaged nerve tissue to maintain
normal nerve function has become a global challenge (2,3), but
the study of drug-induced cerebral resuscitation has progressed
slowly (4,5). Although hypothermia therapy has
certain neuroprotective effects, it remains difficult to reverse
neurological damage following CA/cardiopulmonary resuscitation
(CPR) (6). Currently, there is no
effective treatment for improving neurological function after
CA/CPR, although numerous clinical and basic studies have been
performed to identify effective therapies to improve the quality of
cerebral resuscitation (7).
Oxidative stress injury serves a key role in
cerebral ischemia. The abrupt cessation of blood flow often leads
to cerebral ischemia and hypoxia followed by CA, and ATP, an
oxygen-dependent membrane ion transporter, stops functioning when
ischemia and hypoxia occur (8).
Subsequently, calcium floods into cells and promotes the apoptosis
of brain cells (8). In addition to
excitotoxicity, the excessive stimulation of neurotransmitters can
also lead to neuronal damage (9).
Moreover, reperfusion injury following CPR may induce oxidative
stress and promote the formation of free radicals and reactive
oxygen species (ROS), which can react with numerous macromolecules
and destroy intracellular macromolecules, including DNA, proteins
and lipids (10,11).
Human urine-derived stem cells (hUSCs) have been
reported to be promising candidates for tissue engineering
therapies due to their expansion efficiency and multilineage
differentiation properties, as well as their capacity to secrete
vasoactive peptides that regulate the function of vessels (12). The advantages of the transplantation
of hUSCs, which have become an ideal adult stem cell system,
include the non-invasiveness of their collection and their wide
range of sources (13). For
example, hUSCs can be harvested from voided urine through a simple,
and low-cost procedure. Additionally, hUSCs have been revealed to
be involved in the repair and reconstruction of bone, skin, the
intestinal tract and the urinary tract (12). Previous studies have shown that the
transplantation of hUSCs can improve the function of the kidney and
bladder in pathological model rats (14,15).
Another study revealed that hUSCs can promote vascular endothelial
growth factor (VEGF) expression in collagen hydrogels during
myogenesis and innervation following subcutaneous implantation in
nude mice (16). In addition, hUSC
transplantation is beneficial for wound recovery in diabetic
patients (17). Therefore, hUSCs
are considered promising multipotent stem cells.
To the best of our knowledge, no studies of the
protective effects of hUSCs on neurological function after CA/CPR
have been previously reported. Thus, the aim of the present study
was to investigate the effects of hUSCs on the recovery of
neurological function in rats after CA/CPR. The current study
evaluated the hypothesis that hUSC transplantation can effectively
improve the neurological function of rats following CA/CPR, which
may benefit patients with CA in the future.
Materials and methods
Ethical approval
This study was approved by the Ethics Committee of
The Affiliated Suzhou Hospital of Nanjing Medical University. All
urine donors provided informed written consent before giving urine
samples. Voided urine samples (80-400 ml) from four healthy men
(age, 25-33 years) were collected between June and December 2018 at
The Affiliated Suzhou Hospital of Nanjing Medical University. All
animal experiments were conducted according to the National
Institutes of Health guidelines (18).
Cell isolation, culture and
identification
Volumes of 300 ml urine, either pooled from multiple
donors or from a single donor if the sample volume was low, were
centrifuged at 500 x g for 5 min at room temperature and then at
2,000 x g for 10 min at 4˚C. The cell pellets were then suspended
in mixed medium composed of embryo fibroblast medium (EFM; Gibco;
Thermo Fisher Scientific, Inc.) and keratinocyte serum-free medium
(KSFM; Gibco; Thermo Fisher Scientific, Inc.; EFM-KSFM, 1:1 ratio)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.).
The cells were cultured at 37˚C in a humidified atmosphere with 5%
CO2 in 24-well plates (~105/well) for 3-5
days, at which point hUSC clones appeared. When the cells reached
60-70% confluence, they were transferred to 6-well plates. The
cells adhered to the bottom of the flask, and cell colonies formed
(passage 1). At passage, hUSCs were seeded in a 6-well tissue
culture plate at a density of 103 cells/cm2.
The majority of these cells were adherent to the plate, and they
displayed a mixture of morphologies.
The neuronal differentiation potential of hUSCs was
evaluated using immunofluorescence staining for neurofilament
protein-200 (NF200) and glial fibrillary acidic protein (GFAP) on
day 14. Briefly, cells (5x104) were seeded in 24-well
plates and washed with PBS. Cells were then fixed with 4%
paraformaldehyde for 20 min at room temperature and blocked with
0.3% Triton X-100 in 0.5% BSA (Beyotime Institute of Biotechnology)
for 1 h. Primary antibodies for NF200 (cat. no. sc-32729; 1:300;
Santa Cruz Biotechnologies Inc.), GFAP (cat. no. sc-33673; 1:500;
Santa Cruz Biotechnologies Inc.) were added and incubated overnight
at 4˚C. The Alexa Fluor® 488-labeled secondary IgG
antibody (cat. no. ab150157; 1:200; Abcam) was added for 3 h at
4˚C, and the cells were then incubated with DAPI (Beyotime
Institute of Biotechnology) for a further 45 min at 4˚C. Cells were
observed using a fluorescence microscope (Carl Zeiss AG). The
expression levels of surface marker proteins, including CD29 (cat.
no. ab134179), CD90 (cat. no. ab23894), CD44 (cat. no. ab6124),
CD105 (cat. no. ab44967), CD73 (cat. no. ab202122), CD224 (cat. no.
ab55138), CD146 (cat. no. ab75769), CD34 (cat. no. ab81289) and
human leukocyte antigen-DR isotype (HLA-DR; cat. no. ab92511), on
hUSCs were detected using flow cytometry (Beckman Coulter, Inc.).
All flow cytometry antibodies were obtained from Abcam and the
dilution of all antibodies was 1:100.
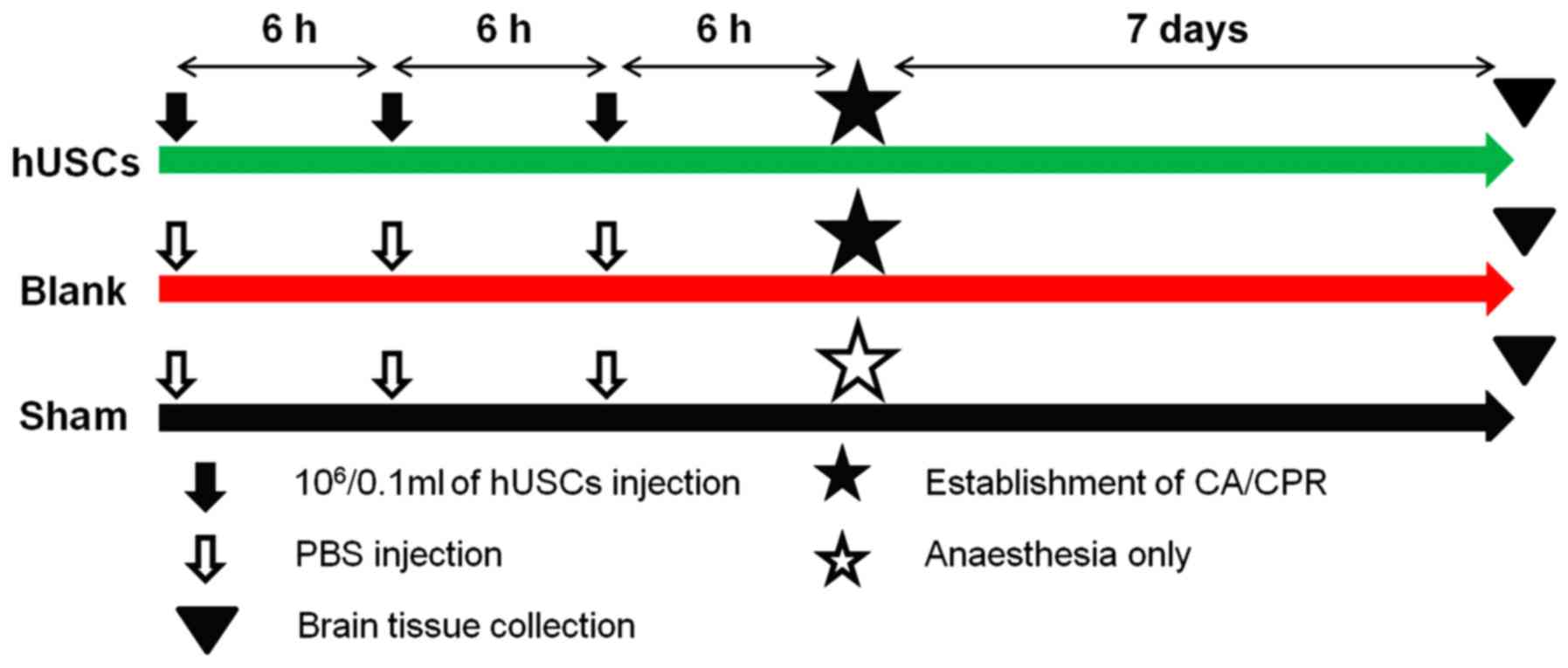
Experimental groups
Male Sprague-Dawley rats were purchased from Zhao
Yan New Drug Research Center. Rats were housed in microisolator
cages with sterile bedding and water and food was available ad
libitum. The rats were maintained in an environment of 24-26˚C,
at a humidity of 50-60% on 12 h light/dark cycles. Healthy male
specific pathogen free Sprague-Dawley rats (n=36; age, 6-12 weeks;
weight, 200-300 g) were randomly divided into three groups: Sham
(no CA/CPR model established; n=12), blank (CA/CPR model
established; n=12) and hUSCs (CA/CPR model established and cells
added; n=12) groups. In the hUSCs group, 5x106/0.1 ml
hUSCs labeled with PKH26 (Merck KGaA) at room temperature for 3
min, according to the manufacturer's instructions, were
administered three times (with an interval of 6 h between each
administration) via caudal vein injection 1 day before the CA/CPR
model was established. Equivalent volumes of PBS to the volumes of
cells were injected into the sham group and blank group (Fig. 1). Prior to injection the rats were
anesthetized using pentobarbital sodium via intraperitoneal
injection at a dose of 40 mg/kg. The animals were allowed to
recover from anesthesia and returned to their cages for 7 days.
Rat model of CA/CPR
The rat CA/CPR model were established based on
previously published studies (19,20).
Rats were anesthetized using pentobarbital sodium via
intraperitoneal injection at a dose of 40 mg/kg. The rats were then
fixed on the operating Table in the supine position, and limb leads
were connected for electrocardiographic monitoring. The 24 G venous
catheters were placed in the right femoral artery and left femoral
vein, which were connected to the Powerlab system (ADInstuments Pty
Ltd.), used to monitor blood pressure, and an infusion device (RWD
Life Science Co., Ltd.), respectively. A 16 G venous indwelling
soft cannula was inserted via the mouth and connected to a small
animal ventilator (RWD Life Science Co., Ltd.) for mechanical
ventilation. The respiratory rate was adjusted to 80 times/min, the
tidal volume was 6 ml/kg and the fraction of inspired oxygen was
21%, and the parameters were kept stable for 10 min. Changes in
blood pressure and electrocardiographic changes were closely
observed after the clipping of the tracheal tube at the end of
expiration. Systolic blood pressure (SBP) <25 mmHg was used as
the criterion for CA.
Chest compression was performed at 200 times per min
according to the rhythm of a metronome. The compression depth was
maintained at 1/3 of the diameter of the thorax of the rats.
Adrenaline (0.04 mg/kg; Shanghai Harvest Pharmaceutical, Co., Ltd.)
and 1 ml Wanwen (hydroxyethyl starch 130/0.4 sodium chloride
injection; cat. no. H2012043 Fresenius Kabi Deutschland GmbH) were
injected via the femoral vein, and then blood pressure and heart
rate were measured, and electrocardiograms were recorded.
Restoration of spontaneous circulation was defined as an increase
in mean arterial pressure >60 mmHg lasting ≥10 min following
spontaneous rhythm (21). If
spontaneous rhythm did not appear after 3 min of chest compressions
or if the SBP fell <60 mmHg, rescue was abandoned, and
resuscitation was considered unsuccessful. Surviving rats continued
to receive ventilator support, and mechanical ventilation was
stopped after spontaneous breathing recovered.
During the operation, rectal temperature was
monitored using a temperature feedback system and maintained at
37.0±0.5˚C. All rats were subcutaneously injected with 2 ml 10%
glucose every 6 h after the operation until the animals could drink
water and eat independently, and the rats were placed in a separate
cage with food at a constant temperature of 23˚C. The rats were
euthanized using cervical dislocation at 7 days after surgery under
deep anesthesia. No signs of pain or distress were observed
throughout the whole process. Mortality was confirmed by physical
signs of apnea, CA and absence of brain stem reflexes.
Immunofluorescence histochemistry
The hippocampus and temporal cortex were removed
quickly after the rats were euthanized. Each region was identified
and punched under a stereomicroscope. The hippocampus and temporal
cortex were dissected from brain slices. Immunofluorescence
histochemistry was performed as previously described (22). Briefly, after perfusion with 4% PFA
at room temperature overnight, dehydration through graded sucrose
(20 and 30%) and embedding in optimal cutting temperature compound
(Sakura Finetek USA, Inc.), the tissue was cut at 5 µm by freezing
microtome (model, CM 1850; Leica Microsystems GmbH) for
immunofluorescence staining. Transverse spinal sections were cut
using a cryostat and collected in 0.01 M PBS, pH 7.3. The tissues
were incubated with 0.3% Triton X-100 at room temperature for 30
min and then with rabbit anti-rat NF200 (cat. no. sc-32729; 1:300),
GFAP (cat. no. sc-33673; 1:500), brain-derived neurotrophic factor
(BDNF; cat. no. sc-65514; 1:500) and VEGF (cat. no. sc-7269; 1:200)
primary antibodies (all from Santa Cruz Biotechnology, Inc.)
overnight at 4˚C. Then, the slices were washed twice with PBS and
incubated with secondary antibodies (rabbit anti-goat IgG; cat. no.
ZF-0314; 1:100; Zhongshanjinqiao, Inc.) for 60 min at room
temperature. The slices were observed under a fluorescent
microscope (Carl Zeiss AG).
Analysis of brain water content
Resected brain tissues were wiped clean with filter
paper before being weighed, to obtain the wet weight. The dry-wet
ratio was used to analyze brain edema. The brain tissues were dried
at 70˚C overnight to determine the dry weight. Brain water content
(%)=(wet weight-dry weight)/wet weight x100%.
Serum S100 calcium binding protein B
(S100B) analysis
In total, 1 ml fresh blood was extracted from the
tail vein of each rat. The fresh blood was centrifuged at 1,000 x g
for 10 min at room temperature after heparin anticoagulation, and
the supernatant collected as serum for follow-up experiments.
According to the instructions of a commercial ELISA kit (cat. no.
ab234573; Abcam) the absorbance (optical density) was measured at
450 nm using a microplate reader, and the concentration of S100B
was calculated according to a standard curve.
Western blotting
The total protein expression of cleaved caspase-3
(C-caspase-3; 1:1,000; cat. no. 9661s; Cell Signaling Technology,
Inc.), Bax (1:1,000; cat. no. 2772s; Cell Signaling Technology,
Inc.) and B-cell lymphoma 2 (Bcl-2; 1:1,000; cat.no. 59348; Abcam)
and GAPDH (1:1,000; cat. no. AF1186; Beyotime Institute of
Biotechnology) in tumor tissues was analyzed by western
blotting. Western blotting was performed as previously
described (23). Briefly, after
quantification with a BCA kit (cat. no. P0012S, Beyotime Institute
of Biotechnology), 20 µg of protein samples were resolved by 10%
SDS-PAGE and transferred to nitrocellulose membranes. The
nitrocellulose membrane was then blocked with 5% nonfat milk for 1
h at room temperature. After washing, the nitrocellulose membrane
was incubated with C-caspase-3 (1:1,000; cat. no. 9661s; Cell
Signaling Technology), Bax (1:1,000; cat. no. 2772s; Cell Signaling
Technology), BCL-2 (1:1,000; cat. no. 59348, Abcam, Cambridge, UK)
and GAPDH antibody (1:1,000; cat. no. AF1186; Beyotime Institute of
Biotechnology, China) overnight at 4˚C. After incubation with the
secondary antibody (1:2,000; cat. no. A0181; Beyotime Institute of
Biotechnology) for 2 h at room temperature, the membranes were then
visualized using an ECL kit (Tanon Science and Technology Co.,
Ltd.) on the Tanon Imaging System.
Neural deficit scores (NDS)
Neurological functions were assessed by three
investigators blinded to the treatment using an established NDS
scale (24), which evaluated
general behavior, neurological function, sensory function, motor
function and coordination. NDS of the surviving rats was assessed
at days 1, 3 and 7 after CA/CPR. On this scale, normal rats have an
NDS of 80, and 0 indicates death.
Statistical analysis
The SPSS 19.0 statistical package (IBM Corp.) was
used for statistical analysis. All data were obtained from at least
3 independent experiments. Data are presented as the mean ± SD. A
one-way ANOVA was performed to analyze multiple group comparisons
of quantitative data. Bonferroni post hoc test was used after the
one-way ANOVA. P<0.05 was considered to indicate a statistically
significant difference.
Results
Characterization of hUSCs
Immunofluorescence staining demonstrated that hUSCs
expressed NF200 and GFAP following neurogenesis induction in
vitro, which suggested that hUSCs had the potential for
multidirectional differentiation (Fig.
2A). The immunophenotypes of hUSCs were determined using flow
cytometry. It was found that the surface markers CD29, CD90, CD44,
CD105, CD73, CD224, CD146, CD34 and HLA-DR were expressed on 97.6,
84.1, 76.1, 81.7, 96.9, 62.8, 73.5, 0.45 and 0.74% of hUSCs,
respectively (Fig. 2B).
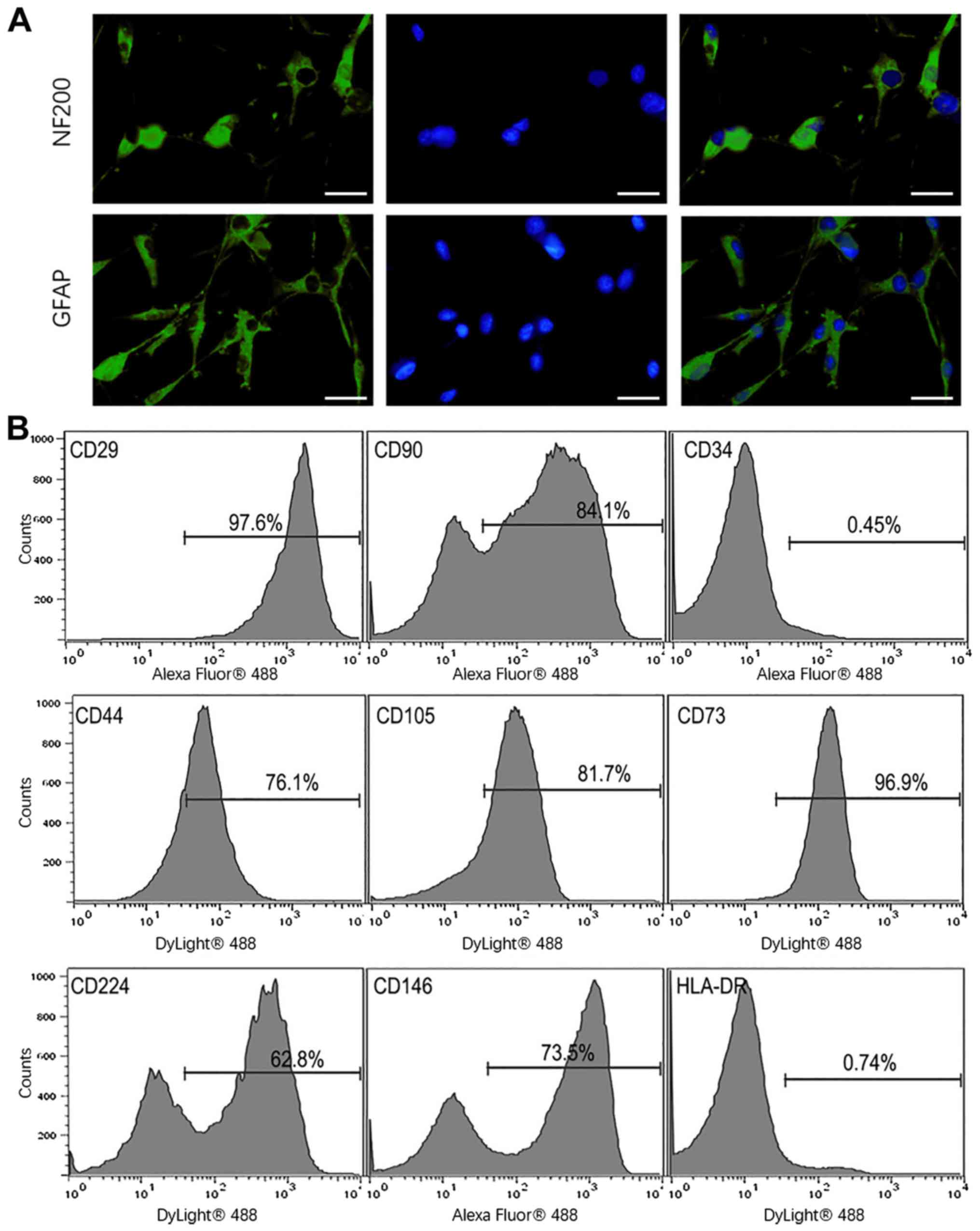 | Figure 2Identification of hUSCs. (A) NF200
and GFAP immunofluorescence of hUSC. Magnification, x100; scale
bar, 50 µm. Increased protein expression levels of NF200 and GFAP
were observed after neurogenesis induction. (B) Phenotypic
expression of hUSCs was evaluated using flow cytometry. hUSCs had
upregulated CD29, CD90, CD44, CD105, CD73, CD224 and CD146, and
expressed low levels CD34 and HLA-DR. hUSC, human urine-derived
stem cells; HLA-DR, human leukocyte antigen-DR isotype; GFAP, glial
fibrillary acidic protein; NF200, neurofilament protein-200. |
Prognosis of the rats and the
distribution of hUSCs after transplantation
In two rats, one from the hUSCs group, one from the
blank group and none from the sham group, CPR failed. The remaining
rats survived and met the experimental criteria.
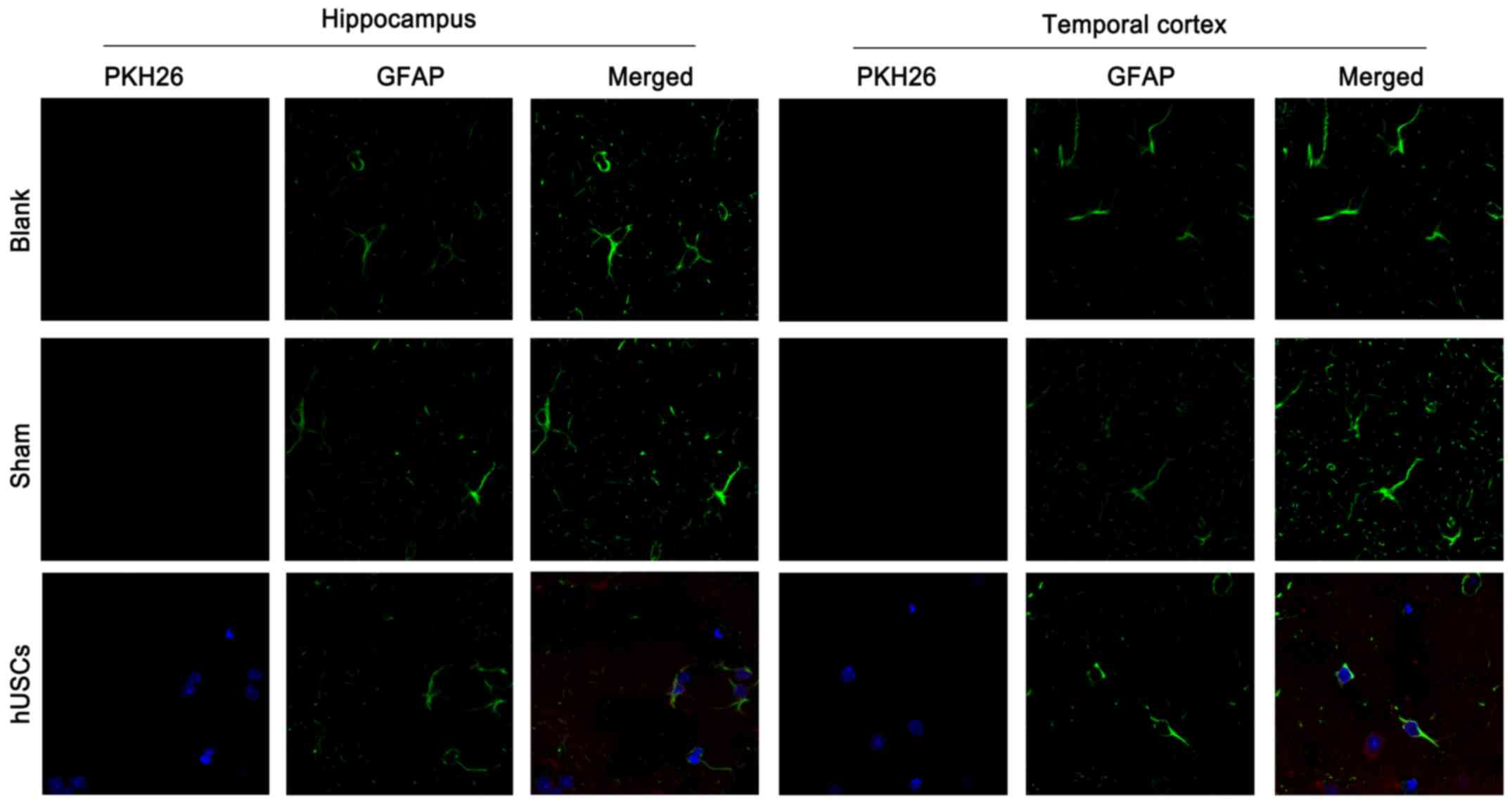
To observe the distribution of cells in the brain,
hUSCs were labeled with PKH26 before transplantation. High
expression of PKH26 was identified in the hippocampus and temporal
cortex of rats after pretreatment with hUSCs in comparison with
sham group or blank group, indicating that hUSCs aggregated and
were distributed in brain tissue following CA/CPR (Fig. 3).
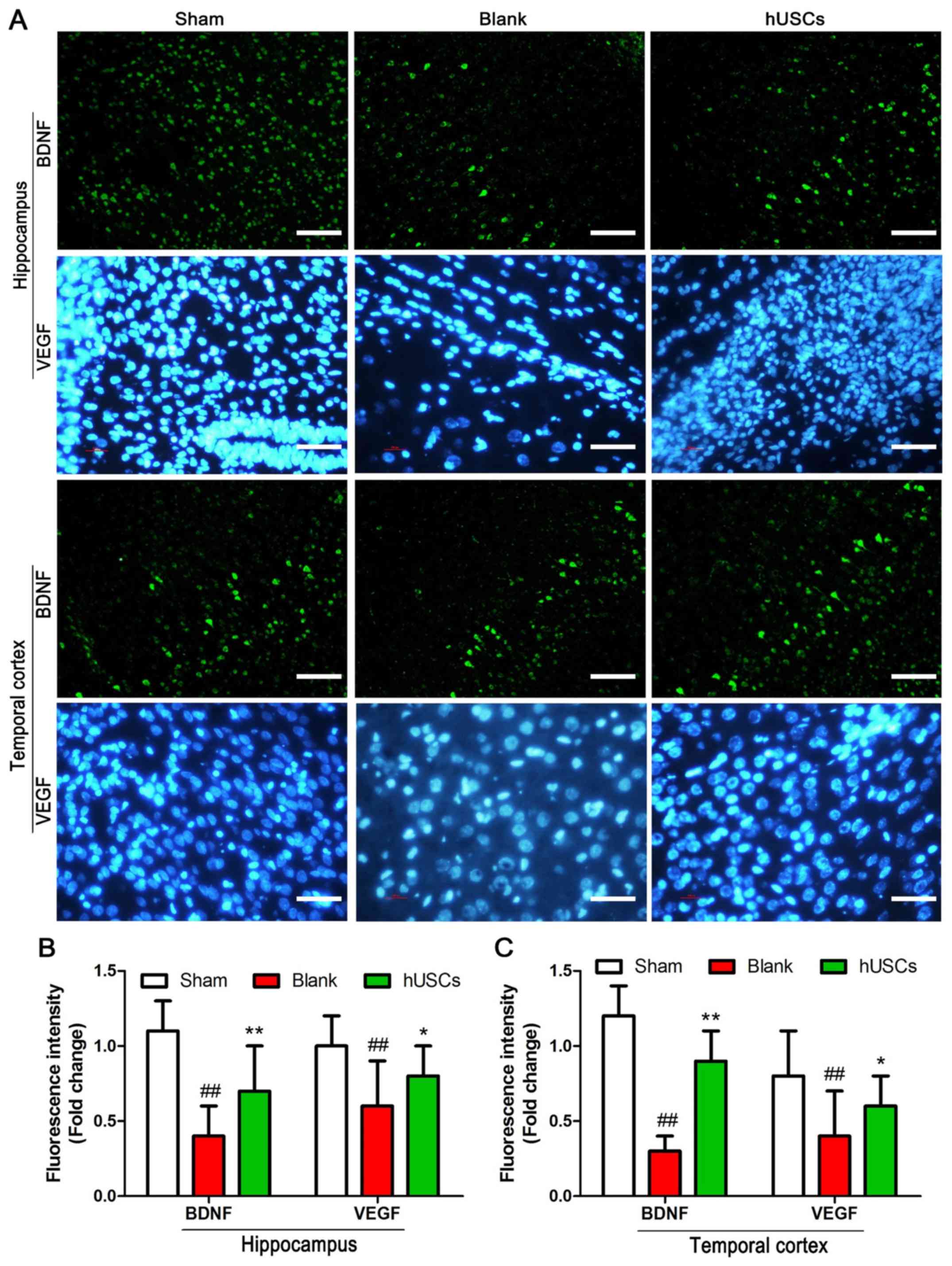
BDNF and VEGF expression levels after
hUSC transplantation
To evaluate the expression levels of BDNF and VEGF
in brain tissue following hUSC transplantation, an
immunofluorescence assay was performed. The results demonstrated
that the expression levels of BDNF and VEGF in both the hippocampus
and temporal cortex were significantly decreased after CA/CPR.
Moreover, pretreatment with hUSC transplantation significantly
increased the expression levels of BDNF and VEGF in brain tissue
after CA/CPR in comparison with blank group (Fig. 4).
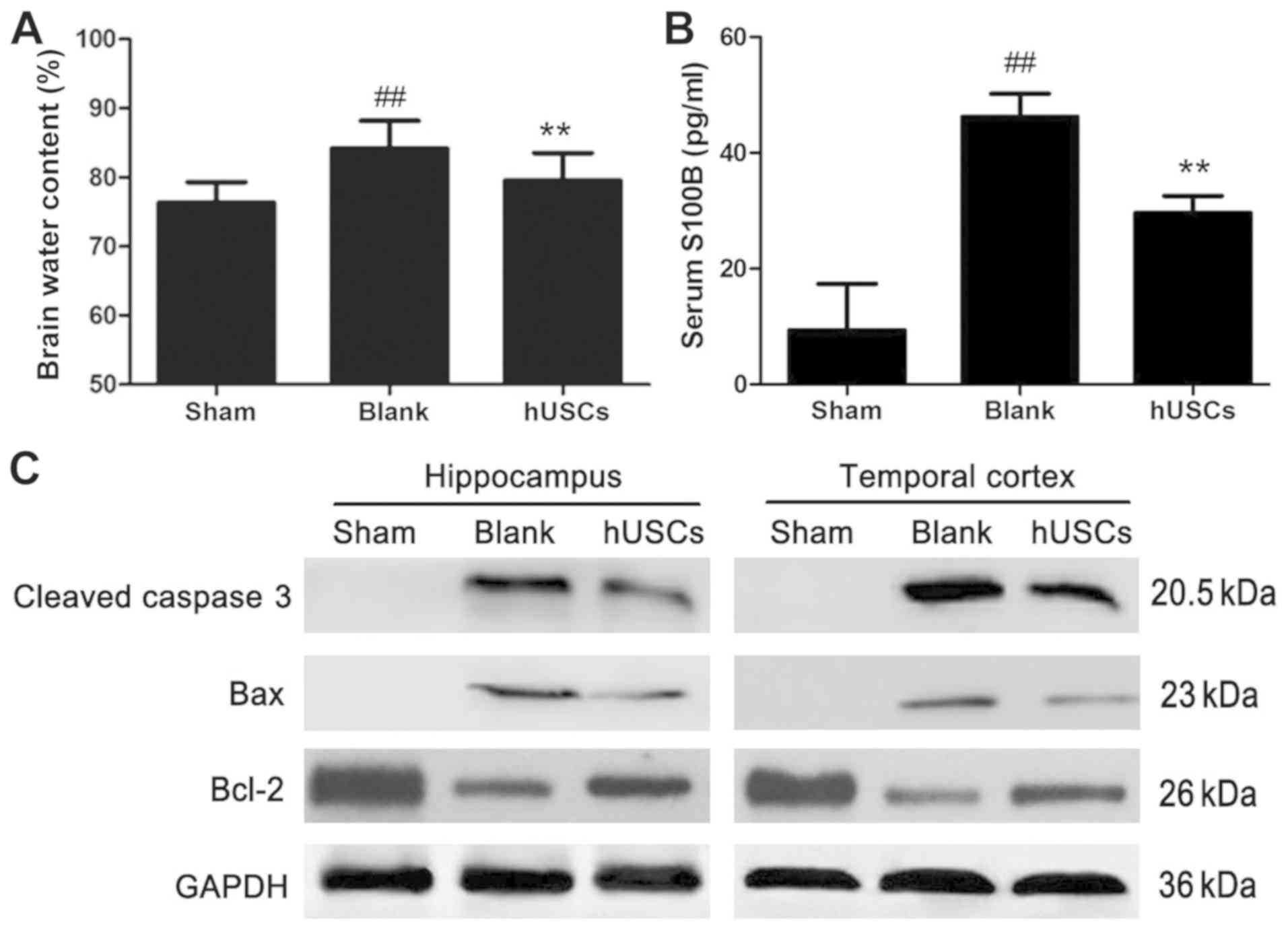
Evaluation of brain tissue injury
after hUSC transplantation
To assess the degree of recovery from brain injury
after hUSC transplantation, brain water content, serum S100B levels
and apoptosis were evaluated. These experiments demonstrated that
brain water content and serum S100B levels were significantly
increased after CA/CPR, and that these increases were significantly
reversed by hUSC transplantation. In addition, western blot results
show that the expression of Bax was augmented, accompanied by the
downregulation of Bcl-2 and C-caspase-3, in the hUSC
transplantation group compared to the blank group. These data
indicate that hUSC pretreatment notably inhibited apoptotic
(Fig. 5).
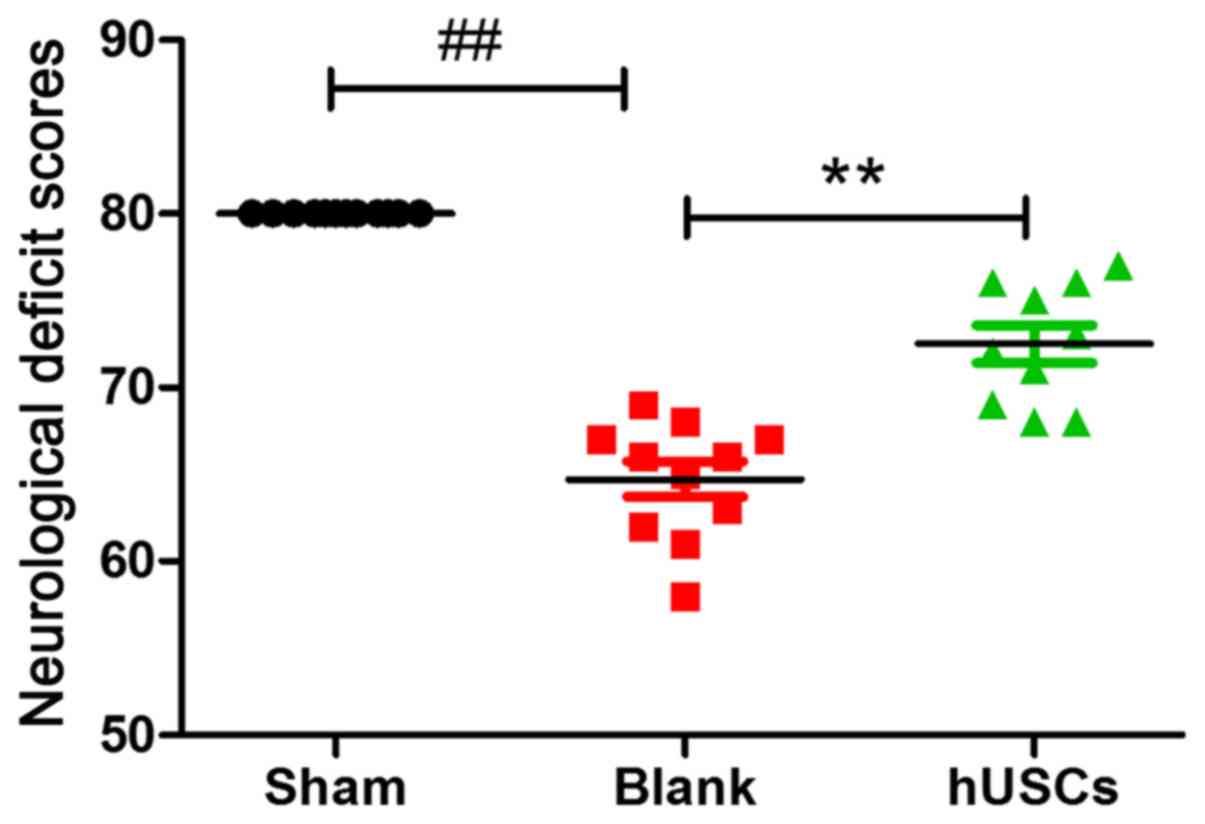
NDS after hUSC transplantation
To examine neurological function after hUSC
transplantation, NDS were evaluated. The results suggested that NDS
were significantly decreased after CA/CPR, and pretreatment with
hUSCs significantly increased neurological function following
CA/CPR (Fig. 6).
Discussion
Currently, stem cell therapy is considered one of
the most promising treatments for numerous refractory diseases, and
it has been revealed to exert neuroprotective effects in several
models of neurotrauma and degenerative neuropathies (25,26).
Intravenous stem cell infusion can effectively improve the
prognosis of patients and is associated with the inhibition of
apoptosis (27). Previous studies
have reported that mesenchymal stem cells (MSCs) can significantly
reduce global cerebral ischemia-reperfusion injury (GCIRI),
providing a new prospective strategy for brain resuscitation
(28). MSCs can be induced to
differentiate into neuronal precursor cells or neuron-like cells
in vitro and in vivo (29). Animal experiments on focal cerebral
ischemia have shown that MSCs can successfully accumulate around
the injured brain area, eventually rescuing injured neurons,
inducing nerve regeneration and promoting functional recovery after
MSC transplantation (30).
Moreover, the neuroprotective effects of MSC transplantation can be
achieved directly via the secretion of neuroprotective compounds or
indirectly via the regulation of immune factors, the promotion of
angiogenesis or the activation of the endogenous neural stem cell
response (31).
hUSCs have been reported to contribute to the repair
of nerve injury. For instance, Zhang et al (32) revealed that hUSCs can be induced to
differentiate into neuronal cells, and that this is a feasible and
suitable approach for neurological disease modeling. Furthermore,
Guan et al (33) observed
that hUSCs can differentiate into neuron-like cells in the rat
brain, and suggested that hUSCs are a promising cell source for
tissue engineering and regenerative medicine. The present study
demonstrated that the pretreatment of a rat model of CA/CPR with
hUSCs significantly improved neurological function following
CA/CPR. Immunofluorescence assays identified that hUSCs aggregated
in the hippocampus and temporal cortex of rats, as well as promoted
the expression levels of BDNF and VEGF in both areas. Further
experiments indicated that brain edema and serum S100B levels were
significantly increased after CA/CPR and that pretreatment with
hUSCs significantly reversed this trend.
ROS are a class of metabolic substances important
for the maintenance of human life. For example, ROS reflect the
oxidative stress status of the body, participate in the regulation
of human physiology and pathology and maintain the homeostasis of
cells (34). Increasing levels of
ROS result in damage to DNA, the destruction of the endothelium of
tubules and apoptosis (35). To
assess the effect of hUSCs on apoptosis in the brain, apoptotic
proteins, such as caspase 3 and Bax/Bcl-2, were detected using
western blotting. It was found that hUSC pretreatment notably
inhibited neuronal apoptosis in both the hippocampus and temporal
cortex following CA/CPR.
Considering the potent neuroprotective properties of
hUSCs reported in previous studies (36,37),
it was hypothesized that an improved neurological outcome may be
achieved via earlier hUSC transplantation after CA/CPR. In the
current study, rats received three injections of hUSCs (6 h between
injections) 1 day before CA/CPR was established. Brain tissues,
especially the hippocampal and temporal cortex tissues, are
considered potential targets for drug therapy (38). In the current study, PKN26-labeled
hUSCs were injected via the caudal vein, and it was identified that
a sufficient number of hUSCs aggregated in the hippocampus and
temporal cortex, suggesting that hUSCs effectively reached brain
tissues by passing through the blood-brain barrier (BBB) (39). The present results indicated that
hUSCs, similar to MSCs, can cross the BBB and prevent BBB
disruption and endothelial damage, which are initiated in the early
phase of GCIRI (40,41). Thus, the current study provides
preclinical experimental data for the future clinical application
of hUSCs in patients with CA. While the hUSC platform remains in
its infancy, this technology is expected to be further developed
and adapted by further research. Moreover, future studies should
focus on the directed differentiation and efficiency of hUSCs in
brain tissues.
There are certain limitations to the current study.
First, as immunosuppressant drugs were not used, immune rejection
may have occurred in this rat model, and subsequent studies should
further analyze and confirm this possibility. Secondly, the
directed differentiation and efficiency of hUSCs in brain tissues
should be further studied in vivo and in vitro.
In conclusion, to the best of our knowledge, the
current study was the first to demonstrate that hUSC
transplantation can effectively improve the neurological function
of rats following CA/CPR, possibly by promoting the expression
levels of BDNF and VEGF and inhibiting brain edema.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the
Scientific Research Topics of Jiangsu Provincial Health Commission
(grant no. H2018027) and the Suzhou Minsheng Science and Technology
Project (grant nos. SS2019068 and SYS201730).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QL conceived and designed the study; CP was the
principal experimenter and the author of the manuscript; XZ
assisted the experiment and provided data analysis; QC designed the
study and revising this manuscript critically for important
intellectual content; LW conducted a literature search and
interpreted the data. LW, CP and XZ coordinated with the clinical
laboratory for sample collection. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
The Affiliated Suzhou Hospital of Nanjing Medical University. All
urine donors gave informed written consent before providing urine
samples.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Reis C, Akyol O, Araujo C, Huang L,
Enkhjargal B, Malaguit J, Gospodarev V and Zhang JH:
Pathophysiology and the monitoring methods for cardiac arrest
associated brain injury. Int J Mol Sci. 18(129)2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Elmer J and Callaway CW: The brain after
cardiac arrest. Semin Neurol. 37:19–24. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sekhon MS and Griesdale DE: Individualized
perfusion targets in hypoxic ischemic brain injury after cardiac
arrest. Crit Care. 21(259)2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mulder M and Geocadin RG: Will the promise
of drug-induced therapeutic hypothermia be fulfilled? Crit Care
Med. 42:221–223. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Liakopoulos OJ, Hristov N, Buckberg GD,
Triana J, Trummer G and Allen BS: Resuscitation after prolonged
cardiac arrest: Effects of cardiopulmonary bypass and
sodium-hydrogen exchange inhibition on myocardial and neurological
recovery. Eur J Cardiothorac Surg. 40:978–984. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pasquier M, Hugli O, Paal P, Darocha T,
Blancher M, Husby P, Silfvast T, Carron PN and Rousson V:
Hypothermia outcome prediction after extracorporeal life support
for hypothermic cardiac arrest patients: The HOPE score.
Resuscitation. 126:58–64. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Metrailler-Mermoud J, Hugli O, Carron PN,
Kottmann A, Frochaux V, Zen-Ruffinen G and Pasquier M: Avalanche
victims in cardiac arrest are unlikely to survive despite adherence
to medical guidelines. Resuscitation. 141:35–43. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jentzer JC, Clements CM, Wright RS, White
RD and Jaffe AS: Improving survival from cardiac arrest: A review
of contemporary practice and challenges. Ann Emerg Med. 68:678–689.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li M, Song W, Ouyang YH, Wu DH, Zhang J,
Wang LX and Li J: Clinical evaluation of active abdominal lifting
and compression CPR in patients with cardiac arrest. Am J Emerg
Med. 35:1892–1894. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Singh SK, Kumar R and Koonwar S:
Epidemiology and outcome of pediatric in-hospital cardiopulmonary
resuscitation in Northern India. J Pediatr Intensive Care. 2:55–61.
2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hackenhaar FS, Medeiros TM, Heemann FM,
Behling CS, Putti JS, Mahl CD, Verona C, da Silva AC, Guerra MC,
Gonçalves CA, et al: Therapeutic hypothermia reduces oxidative
damage and alters antioxidant defenses after cardiac arrest. Oxid
Med Cell Longev. 2017(8704352)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chen L, Li L, Xing F, Peng J, Peng K, Wang
Y and Xiang Z: Human urine-derived stem cells: Potential for
cell-based therapy of cartilage defects. Stem Cells Int.
2018(4686259)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhao T, Luo D, Sun Y, Niu X, Wang Y, Wang
C and Jia W: Human urine-derived stem cells play a novel role in
the treatment of STZ-induced diabetic mice. J Mol Histol.
49:419–428. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yang H, Chen B, Deng J, Zhuang G, Wu S,
Liu G, Deng C, Yang G, Qiu X, Wei P, et al: Characterization of
rabbit urine-derived stem cells for potential application in lower
urinary tract tissue regeneration. Cell Tissue Res. 374:303–315.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li J, Luo H, Dong X, Liu Q, Wu C, Zhang T,
Hu X, Zhang Y, Song B and Li L: Therapeutic effect of urine-derived
stem cells for protamine/lipopolysaccharide-induced interstitial
cystitis in a rat model. Stem Cell Res Ther. 8(107)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Liu G, Wang X, Sun X, Deng C, Atala A and
Zhang Y: The effect of urine-derived stem cells expressing VEGF
loaded in collagen hydrogels on myogenesis and innervation
following after subcutaneous implantation in nude mice.
Biomaterials. 34:8617–8629. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen CY, Rao SS, Ren L, Hu XK, Tan YJ, Hu
Y, Luo J, Liu YW, Yin H, Huang J, et al: Exosomal DMBT1 from human
urine-derived stem cells facilitates diabetic wound repair by
promoting angiogenesis. Theranostics. 8:1607–1623. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Iwamuro H, Tachibana Y, Ugawa Y, Saito N
and Nambu A: Information processing from the motor cortices to the
subthalamic nucleus and globus pallidus and their somatotopic
organizations revealed electrophysiologically in monkeys. Eur J
Neurosci. 46:2684–2701. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hai K, Chen G, Gou X, Jiang H, Gong D,
Cheng Y, Gong C, Li X, Liu Y, Li H, et al: Monoacylglycerol lipase
inactivation by using URB602 mitigates myocardial damage in a rat
model of cardiac arrest. Crit Care Med. 47:e144–e151.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Huang Y, Gao X, Zhou X, Xie B, Zhang Y,
Zhu J and Zhu S: Mitophagy in the hippocampus is excessive
activated after cardiac arrest and cardiopulmonary resuscitation.
Neurochem Res. 45:322–330. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yang L, Wang J, Deng Y, Gong C, Li Q, Chen
Q, Li H, Jiang C, Zhou R, Hai K, et al: Melatonin improves
neurological outcomes and preserves hippocampal mitochondrial
function in a rat model of cardiac arrest. PLoS One.
13(e0207098)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sun X, Zheng W, Qian C, Wu Q, Hao Y and Lu
G: Focal adhesion kinase promotes BMP2-induced osteogenic
differentiation of human urinary stem cells via AMPK and Wnt
signaling pathways. J Cell Physiol. 235:4954–4964. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chen Q, Xia R, Zheng W, Zhang L, Li P, Sun
X and Shi J: Metronomic paclitaxel improves the efficacy of PD-1
monoclonal antibodies in breast cancer by transforming the tumor
immune microenvironment. Am J Transl Res. 12:519–530.
2020.PubMed/NCBI
|
|
24
|
Gong B, Dong Y, He C, Jiang W, Shan Y,
Zhou BY and Li W: Intravenous transplants of human adipose-derived
stem cell protect the rat brain from ischemia-induced damage. J
Stroke Cerebrovasc Dis. 28:595–603. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mills RJ, Titmarsh DM, Koenig X, Parker
BL, Ryall JG, Quaife-Ryan GA, Voges HK, Hodson MP, Ferguson C,
Drowley L, et al: Functional screening in human cardiac organoids
reveals a metabolic mechanism for cardiomyocyte cell cycle arrest.
Proc Natl Acad Sci USA. 114:E8372–E8381. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bao Z, Han Z, Zhang B, Yu Y, Xu Z, Ma W,
Ding F, Zhang L, Yu M, Liu S, et al: Arsenic trioxide blocked
proliferation and cardiomyocyte differentiation of human induced
pluripotent stem cells: Implication in cardiac developmental
toxicity. Toxicol Lett. 309:51–58. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Darkazalli A, Vied C, Badger CD and
Levenson CW: Human mesenchymal stem cell treatment normalizes
cortical gene expression after traumatic brain injury. J
Neurotrauma. 34:204–212. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ahn JH, Chen BH, Park JH, Shin BN, Lee TK,
Cho JH, Lee JC, Park JR, Yang SR, Ryoo S, et al: Early IV-injected
human dermis-derived mesenchymal stem cells after transient global
cerebral ischemia do not pass through damaged blood-brain barrier.
J Tissue Eng Regen Med. 12:1646–1657. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Satija NK, Singh VK, Verma YK, Gupta P,
Sharma S, Afrin F, Sharma M, Sharma P, Tripathi RP and Gurudutta
GU: Mesenchymal stem cell-based therapy: A new paradigm in
regenerative medicine. J Cell Mol Med. 13:4385–4402.
2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ko HR, Ahn SY, Chang YS, Hwang I, Yun T,
Sung DK, Sung SI, Park WS and Ahn JY: Human UCB-MSCs treatment upon
intraventricular hemorrhage contributes to attenuate hippocampal
neuron loss and circuit damage through BDNF-CREB signaling. Stem
Cell Res Ther. 9(326)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hawkins KE, Corcelli M, Dowding K, Ranzoni
AM, Vlahova F, Hau KL, Hunjan A, Peebles D, Gressens P, Hagberg H,
et al: Embryonic stem cell-derived mesenchymal stem cells (MSCs)
have a superior neuroprotective capacity over fetal MSCs in the
hypoxic-ischemic mouse brain. Stem Cells Transl Med. 7:439–449.
2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang SZ, Ma LX, Qian WJ, Li HF, Wang ZF,
Wang HX and Wu ZY: Modeling neurological disease by rapid
conversion of human urine cells into functional neurons. Stem Cells
Int. 2016(2452985)2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Guan JJ, Niu X, Gong FX, Hu B, Guo SC, Lou
YL, Zhang CQ, Deng ZF and Wang Y: Biological characteristics of
human-urine-derived stem cells: Potential for cell-based therapy in
neurology. Tissue Eng Part A. 20:1794–1806. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Shokoohi M, Olad Saheb Madarek E, Khaki A,
Shoorei H, Khaki AA, Soltani M and Ainehchi N: Investigating the
effects of onion juice on male fertility factors and pregnancy rate
after testicular torsion/detorsion by intrauterine insemination
method. Int J Women's Health Reprod Sci. 6:499–505. 2018.
|
|
35
|
Ameli M, Hashemi MS, Moghimian M and
Shokoohi M: Protective effect of tadalafil and verapamil on
testicular function and oxidative stress after torsion/detorsion in
adult male rat. Andrologia. 50(e13068)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li G, Xie B, He L, Zhou T, Gao G, Liu S,
Pan G, Ge J, Peng F and Zhong X: Generation of retinal organoids
with mature rods and cones from urine-derived human induced
pluripotent stem cells. Stem Cells Int.
2018(4968658)2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yi H, Xie B, Liu B, Wang X, Xu L, Liu J,
Li M, Zhong X and Peng F: Derivation and identification of motor
neurons from human urine-derived induced pluripotent stem cells.
Stem Cells Int. 2018(3628578)2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wahul AB, Joshi PC, Kumar A and
Chakravarty S: Transient global cerebral ischemia differentially
affects cortex, striatum and hippocampus in bilateral common
carotid arterial occlusion (BCCAo) mouse model. J Chem Neuroanat.
92:1–15. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hu J, Yu Q, Xie L and Zhu H: Targeting the
blood-spinal cord barrier: A therapeutic approach to spinal cord
protection against ischemia-reperfusion injury. Life Sci. 158:1–6.
2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Garbuzova-Davis S, Haller E, Tajiri N,
Thomson A, Barretta J, Williams SN, Haim ED, Qin H, Frisina-Deyo A,
Abraham JV, et al: Blood-spinal cord barrier alterations in
subacute and chronic stages of a rat model of focal cerebral
ischemia. J Neuropathol Exp Neurol. 75:673–688. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lee JY, Lee HE, Kang SR, Choi HY, Ryu JH
and Yune TY: Fluoxetine inhibits transient global ischemia-induced
hippocampal neuronal death and memory impairment by preventing
blood-brain barrier disruption. Neuropharmacology. 79:161–171.
2014.PubMed/NCBI View Article : Google Scholar
|