Introduction
Obesity is associated with excessive accumulation of
adipose tissue, increased risk of diabetes, hypertension,
cardiovascular diseases, and depression, and causes enormous
economic and social burden (1).
Although the molecular mechanisms that link obesity with a spectrum
of metabolic and cardiovascular defects are not well understood,
previous studies have indicated that endoplasmic reticulum (ER)
stress and inflammation are stimulated in obesity (2,3). There
are two major responses to ER stress: the unfolded protein response
(UPR) and the ER-associated protein degradation (ERAD) pathway
(4). PKR-like endoplasmic reticulum
kinase (PERK)-eukaryotic initiation factor (eIF2), activating
transcription factor 6 (ATF6), and inositol requiring enzyme 1
(IRE1)-X-box binding protein (XBP1) are the three main signaling
factors involved in the activation of the UPR pathway, and mutation
or knockout of these genes results in insulin resistance in mouse
models (5,6). In the ERAD pathway, synoviolin
(SYVN1), a mammalian homolog of yeast Hrd1p/Der3p, plays an
important role as an E3 ubiquitin ligase to promote the degradation
of misfolded proteins (7-9).
SYVN1 was identified from the cDNA of rheumatoid
synovial cells and is overexpressed in synovial tissue and PBMCs
from rheumatoid arthritis patients (7,10). We
demonstrated that the expression of SYVN1 is
transcriptionally regulated by pro-inflammatory cytokines such as
tumor necrosis factor α (TNFα), interleukin (IL)-1, and IL-6
(11,12) via Ets transcription factors,
GA-binding protein (GABP) α, GABP β (13) and interleukin enhancer binding
protein 3 (ILF-3) (14), and ER
stress via ATF6 and XBP1(15).
Recently, we found that SYVN1 deficiency resulted in weight loss
and a reduced accumulation of white adipose tissue in WT mice and
genetically obese mice (ob/ob and
db/db) (16). SYVN1
negatively regulated peroxisome proliferator-activated receptor
coactivator (PGC)-1β, a thermogenic coactivator via its
ubiquitination. In adipose tissue, there were significantly more
mitochondria, a higher mitochondrial respiration level, and an
increased basal energy expenditure. Furthermore, the selective
SYVN1 inhibitor LS-102 prevented obesity and accumulation of fat in
mice (16). Thus, SYVN1 is a
potential therapeutic target in obesity treatment. Although high
expression of Syvn1 has been found in the white adipose
tissue of genetically obese mice (ob/ob and
db/db), the expression of SYVN1 in humans has
not been elucidated.
We hypothesize that individuals with
obesity/overweight will demonstrate increased expression of
SYVN1 compared to healthy controls. In this study, we
examine the differences in the expression levels of ER stress- and
inflammation-associated genes in the circulating leukocytes of
Japanese volunteers with a BMI <25.0 compared to those with a
BMI ≥25.0.
Materials and methods
Volunteers
The present study was approved by ethics committee
of Tokyo Medical University (no. 2728, 2729) and written informed
consent was obtained from all subjects. The patient was recruited
from March to June in 2015 at a hospital in Kochi prefecture in
Japan. A total of 35 volunteers of Japanese origin participated in
this study. Twenty four subject (68.5%) were women and 11 (31.4%)
were men (mean age 39.0 years). We excluded patients who had a
history of rheumatoid arthritis (RA) (n=3).
Cell separation
Blood samples of 10 ml were obtained and PBMCs were
isolated by Ficoll gradient (GE Healthcare Bio-Sciences AB,
Uppsala, Sweden) (17). Briefly,
blood samples were centrifuged for 10 min at 2800 rpm, and blood
cell pellets were resuspended with PBS-. 10 ml of
Percoll-paque (d=1.077 g/ml) was slowly added, and then tube was
centrifuged for 30 min at 1600 rpm. PBMCs were collected and washed
with RPMI 1640 medium containing 1% FBS.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA from PBMCs was purified using ISOGEN
(Nippon Gene, Tokyo, Japan) according to the manufacturer's
instructions, and reverse transcribed using ReverTra Ace with
random primers (Toyobo, Osaka, Japan). RT-qPCR was performed using
a LightCycler 480 Probes Master (Roche Diagnostics, Mannheim,
Germany). Expression levels were determined relative to that of
ribosomal protein large P0 (RPLP0) and the mean of the
control group (BMI <25.0) set to 1. Primers and probes used in
this study are shown in Table
I.
 | Table IPrimer sequences and probes. |
Table I
Primer sequences and probes.
| Gene |
Directiona | Primer
sequence | Probeb |
|---|
| SYVN1 | F |
ccagtacctcaccgtgctg | #16 |
| | R |
tctgagctagggatgctggt | |
| RPLPO | F |
gcagaaggggagacacattt | #62 |
| | R |
tgtggtcttgttatgggtggt | |
| IL1 | F |
ggttgagtttaagccaatcca | #6 |
| | R |
tgctgacctaggcttgatga | |
| TNF | F |
cagcctcttctccttcctgat | #29 |
| | R |
gccagagggctgattagaga | |
| IL6 | F |
caggagcccagctatgaact | #7 |
| | R |
gaaggcagcaggcaacac | |
| ATF6 | F |
gcagaaggggagacacattt | #62 |
| | R |
tgtggtcttgttatgggtggt | |
| XBP1 | F |
ggagttaagacagcgcttgg | #37 |
| | R |
cactggcctcacttcattcc | |
| eIF2 | F |
gaagctaagaaagctgcaaagc | #43 |
| | R |
cagtgtttcgtggtgtgctc | |
| IRE-1 | F |
gaagcatgtgctcaaacacc | #50 |
| | R |
tctgtcgctcacgtcctg | |
PCR arrays
Five hundred nanograms of total RNA was reverse
transcribed using ReverTra Ace with random primers (Toyobo, Osaka,
Japan) and subsequently loaded onto either an Inflammatory
Cytokines and Receptors (for measurement of inflammatory cytokine
genes) or an Unfolded Protein Response (for measurement of RT
stress genes) RT2 Profiler PCR Array, according to the
manufacturer's instructions (Qiagen). Fold change was calculated by
determining the ratio of mRNA levels to control values using the Δ
threshold cycle (Ct) method (2−ΔΔCt). All data were
normalized to RPL13a or RPLP0 mRNA. PCR conditions
were: 10 min at 95˚C, followed by 45 cycles of 15 sec at 95˚C and 1
min at 60˚C.
Statistical analysis
All the values reported are expressed as mean ± SE
and were analyzed using Excel Statistics 2012 (SSRI Japan Co.,
Ltd., Tokyo). Differences between volunteers with a BMI ≥25.0 and
volunteers with a BMI <25.0 were calculated using an unpaired
Student's t-test. The Mann-Whitney U test was used in the
differences of ratio of women in donors. For all statistical tests,
P<0.05 was considered to indicate a statistically significant
difference.
Results
Characteristics of donors
The main characteristics of the 35 volunteers are
shown in detail in Table II. Of a
total of 35 volunteers, 24 (68.5%) were women and 11 (31.4%) were
men. The mean age of volunteers was 39.0 years. Our study found
25.7% (9 donors) had a BMI ≥25.0 and 74.3% (26 donors) had a normal
BMI of <25.0. Of the 9 donors with a BMI ≥25.0, two thirds (n=6)
were classed as over weight (25.0 ≤≤BMI <30.0) and the other
third (n=3) were classed as obese (BMI ≥≥30.0).
 | Table IICharacteristics of donors. |
Table II
Characteristics of donors.
| Variable | All donors | Donor (BMI <25
kg/m2) (n=26) | Donor (BMI ≥25
kg/m2) (n=9) | P-values |
|---|
| Age | 39.0±2.0 | 38.0±2.4 | 42.1±3.4 | 0.37 |
| Women (%) | 24 (68.5) | 17(65) | 7(78) | 0.50 |
| Weight (kg) | 59.4±2.1 | 53.9±1.4 | 75.0±4.0 |
2.7x10-07 |
| Height (m) | 1.6±0.01 | 1.6±0.02 | 1.6±0.02 | 0.29 |
| BMI | 23.3±0.9 | 20.9±0.4 | 30.2±1.8 |
1.6x10-08 |
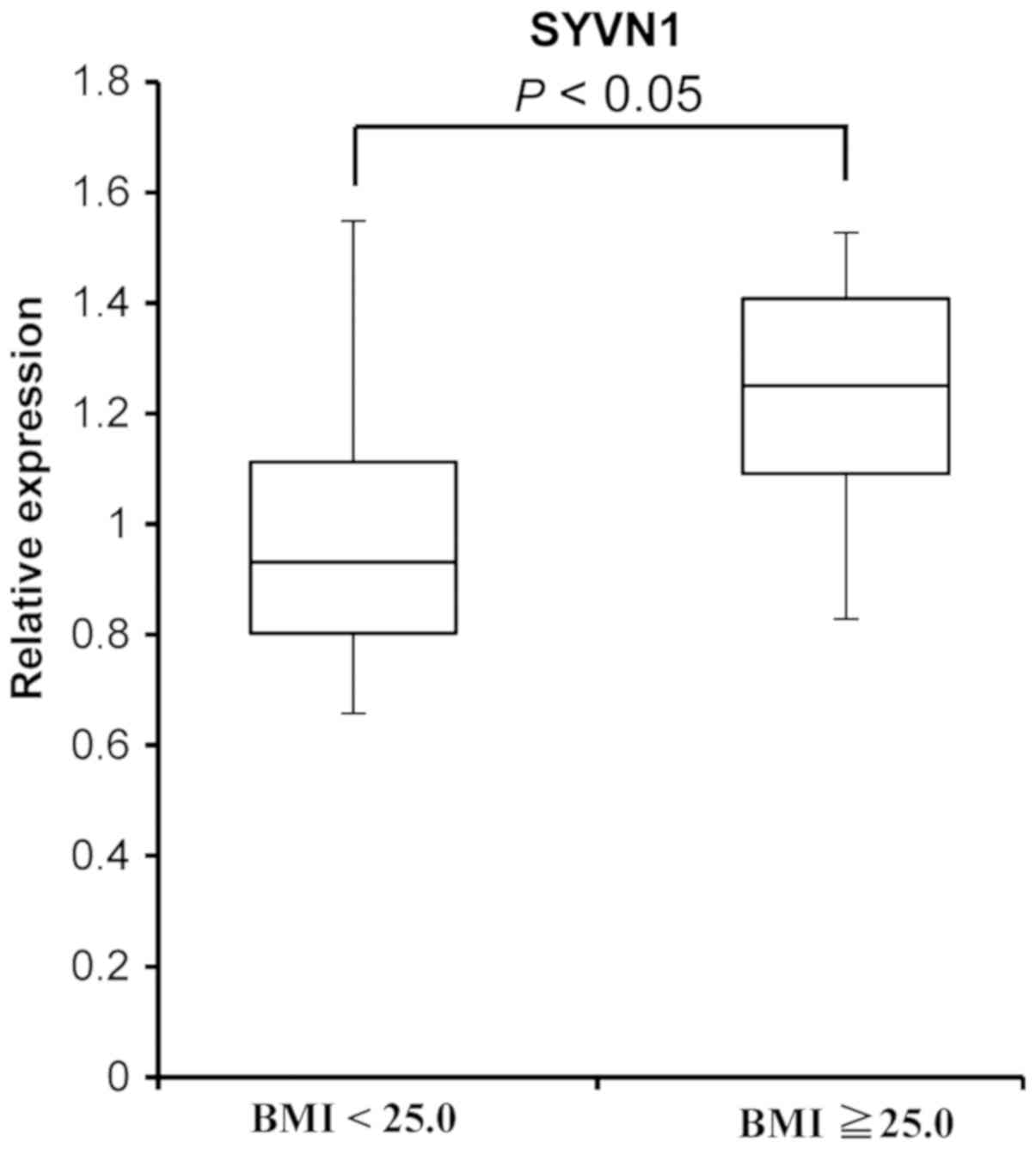
Expression of SYVN1
To investigate the level of SYVN1 mRNA in
PBMCs from volunteers with a BMI ≥25.0 compared with the expression
in PBMCs from volunteers with a BMI <25.0, we performed a
RT-qPCR assay (Fig. 1).
SYVN1 mRNA expression was 1.2-fold higher in PBMCs from
volunteers with a BMI ≥25.0 than in PBMCs from volunteers with a
BMI <25.0 (P=0.023, n=35).
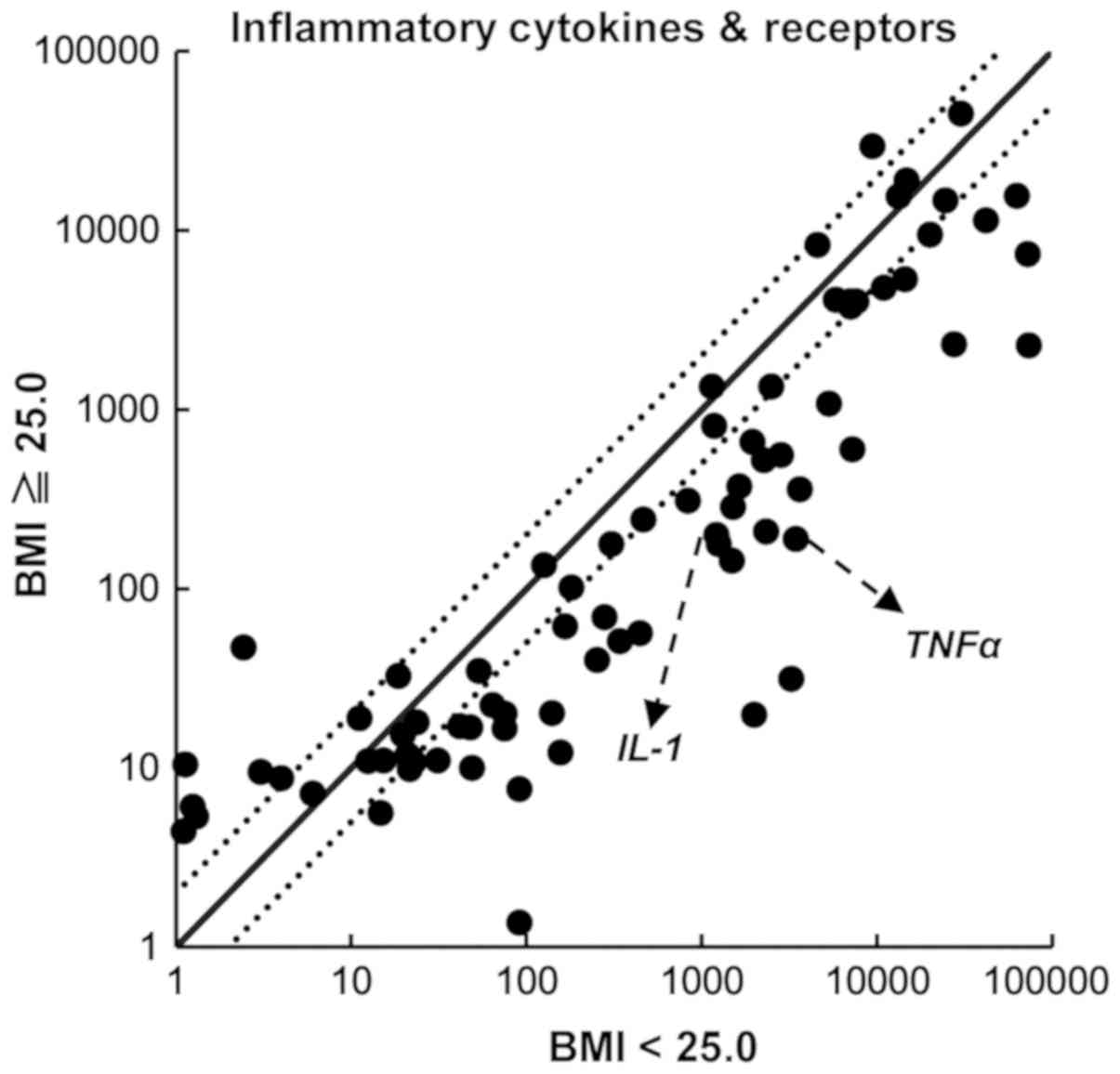
Expression of inflammatory cytokine
genes
Ours and other previous studies indicate that the
expression of SYVN1 is mainly regulated by inflammatory
cytokine signals and ER stress signals (11,12,15).
Therefore, we investigated the upstream signals of SYVN1
expression. To examine the expression of inflammatory cytokines and
their receptors, we performed a PCR array using an Inflammatory
Cytokines and Receptors RT2 Profiler PCR Array System.
We compared the expression of 84 inflammatory chemokines,
cytokines, and their receptor genes. As shown in Fig. 2, the expression of TNFα and
IL-1 was lower in PBMCs from volunteers with a BMI ≥25.0
than in those with a BMI <25.0. To confirm the results of the
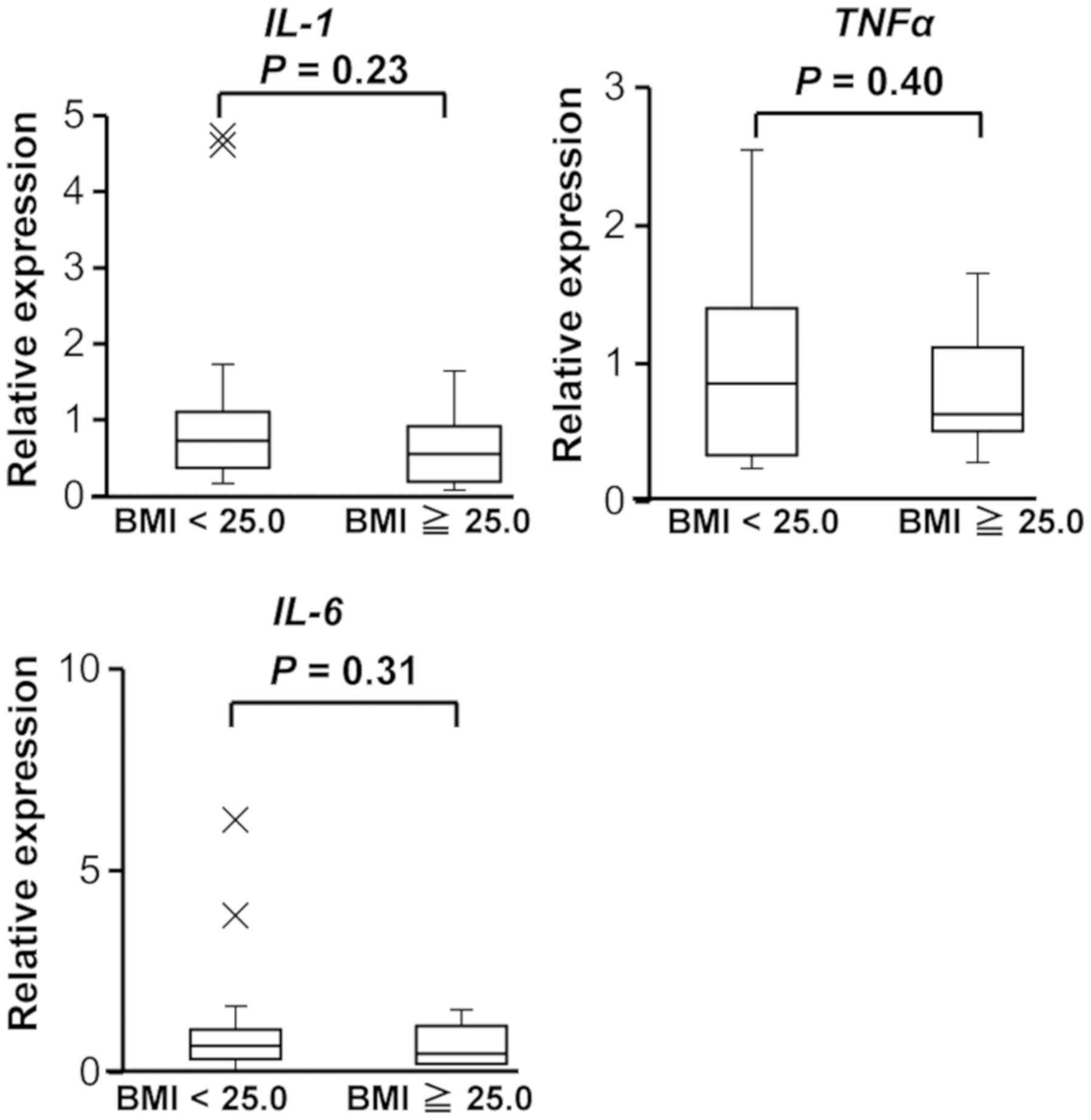
PCR array, we performed an RT-qPCR assay. In addition, we examined
the expression of IL-6, because IL-6 regulates the expression of
SYVN1(12). As shown in Fig. 3, the expression of TNFα,
IL-1, and IL-6 did not significantly differ between
the two groups.
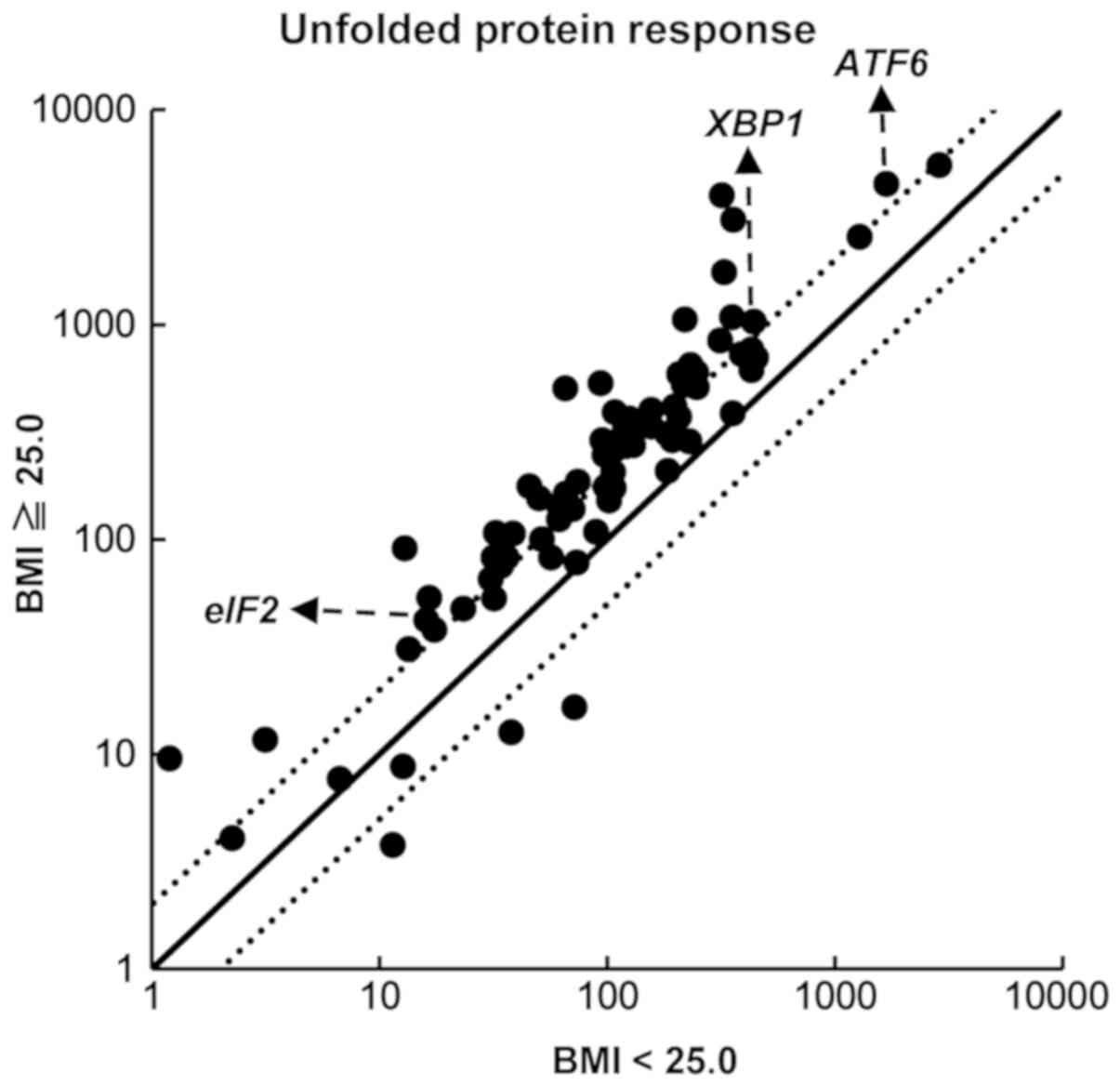
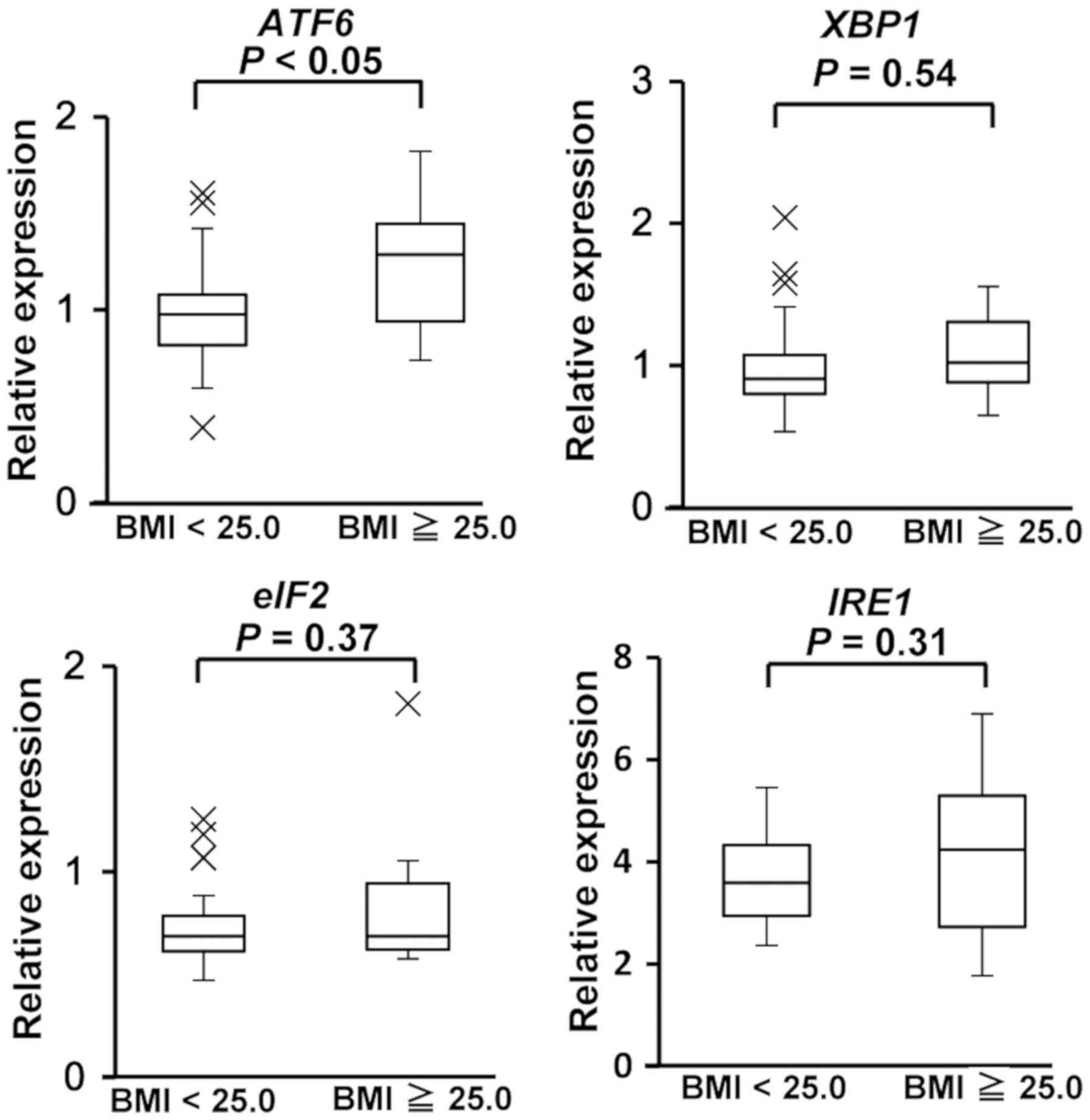
Expression of ER stress genes
We next examined the expression of ER stress signals
using an Unfolded Protein Response RT2 Profiler PCR
Array system. The expression of ER stress-responsive genes, such as
ATF6, XBP1, and eIF2a, were higher in PBMCs
from volunteers with a BMI ≥25.0 compared with the expression in
PBMCs from volunteers with a BMI <25.0 (Fig. 4). To confirm the results of the PCR
array, we performed a RT-qPCR assay to investigate the expression
of ATF6, XBP1, IRE1, and eIF2a. We
investigated the expression of IRE1, because IRE1 is
important factor to regulate activity of XBP1(18). We found that ATF6 mRNA
expression was significantly higher in PBMCs from volunteers with a
BMI ≥25.0 than BMI <25.0 (P=0.046) (Fig. 5). There were no statistically
significant differences in the expression of XBP1,
IRE1, and eIF2a between volunteers with a BMI ≥25.0
and those with a BMI <25.0.
Discussion
In the present study, we investigated the expression
of SYVN1 in PBMCs from obese/overweight Japanese volunteers.
We found that the level of SYVN1 expression was higher in
PBMCs from volunteers with a BMI ≥25.0 than those with a BMI
<25.0. Previous studies, including our own, have revealed that
SYVN1 is transcriptionally regulated by inflammatory
cytokines including TNFα, IL-1, and IL-6, and
by ER stress. In the current study, the expression of TNFα,
IL-1, and IL-6 was not significantly different
between PBMCs from donors with a BMI ≥25.0 and those with a BMI
<25.0 (Fig. 3). In addition,
there were no statistically significant differences in the
expression of the ER stress-responsive genes XBP1,
IRE1, and eIF2a (Fig.
5). However, ATF6 mRNA expression was significantly
higher in PBMCs from volunteers with a BMI ≥25.0 than those with a
BMI <25.0 (Fig. 5). Taken
together, these results suggest that the ATF6-SYVN1
axis may be activated in obese/overweight donors.
Previous studies have suggested that increased ER
stress is associated with metabolic syndrome and type 2 diabetes.
Increased levels of ER stress markers have been found in liver and
adipose tissue from genetically obese mice (ob/ob, db/db) and high
fat diet-induced obese mice models (19,20).
The expression of ER stress markers is also increased in adipose
tissue and endothelial cells from obese humans (21,22)
and in PBMCs from patients with metabolic syndrome and type 2
diabetes (23,24). In our study, the expression of
SYVN1 and ATF6 was significantly increased in PBMCs
from volunteers with a BMI ≥25.0 than those with a BMI <25.0,
whereas the expression of the ER stress markers XBP1,
IRE1, and eIF2a were not (Fig. 5). Most studies on humans have
examined patients with chronic conditions, whereas we investigated
donors with a BMI ≥25.0, classed as obese/overweight. Therefore,
the different expression patterns of ER stress markers might be
dependent on whether patients have a chronic condition.
SYVN1 is associated with both RA and
overweight/obesity. In recent years, it has become clear that
obesity is a risk factor for the onset of RA and to biologics
(25,26). We previously demonstrated that
SYVN1 was present in the cDNA of rheumatoid synovial cells
and that overexpression of Syvn1 in transgenic mice leads to
advanced arthropathy via reduction of apoptosis in synoviocytes
(7). Toh et al (10) demonstrated that the expression of
SYVN1 is also increased in human PBMCs from RA patients
compared with those from controls. In addition, the expression of
SYVN1 was reduced in responders, but not nonresponders, of
infliximab treatment, suggesting that the expression of
SYVN1 in PBMCs could be marker for nonresponders of
infliximab treatment. In the present study, we found that elevated
levels of SYVN1 were present in circulating PBMCs from volunteers
with a BMI ≥25.0. We previously demonstrated that high expression
of Syvn1 was observed in obese mice (db/db and
ob/ob mice), whereas conditional knockout mice of
Syvn1 revealed that loss of Syvn1 expression induced
reduced body weight. In addition, treatment with the SYVN1
inhibitor LS-102 attenuated weight gain in C57BL/6J and
db/db mice. These studies suggested that the expression
level of SYVN1 in PBMCs could be marker of
overweight/obesity and that SYVN1 has a pivotal role in
overweight/obesity and RA, and it is possible that SYVN1 is
involved in onset of RA and chronic inflammation due to obesity,
and also sensitivity to treatment.
Taken together, in the present study we provide
evidence that the expression of SYVN1 was higher in
circulating PBMCs from volunteers with a BMI ≥25.0 compared to
those with a BMI of <25.0. The small sample number and the low
proportion of obese patients are limitations of our study. Further
analysis on a large scale, with more participants, will be needed
to determine whether the expression of SYVN1 has the
potential to become a biomarker and will also help contribute to
the understanding of the physiological significance of SYVN1
regulation.
Acknowledgements
The authors thank Mr. S. Shibata (Tokyo Medical
University) for technical assistance. The authors also thank all
members of Dr. Nakajima's laboratory and Dr. Khin Thuzar Wynn
(Yangon Speciality Hospital).
Funding
The present study was supported in part by the Japan
Society for the Promotion of Science KAKENHI (grant nos. 23659176,
26670479, 26461478 and 16H05157). This study was also supported in
part by funds provided through a MEXT-Supported program of the
Strategic Research Foundation at Private Universities (S1411011,
2014-2018) from the Ministry of Education, Culture, Sports, Science
and Technology of Japan. This work was funded in part by grants
from the Naito Foundation, Natural Science Scholarship
Daiichi-Sankyo Foundation of Life Science, Mitsubishi, Tanabe
Pharma Corporation, Bureau of Social Welfare and Public Health,
Academic contribution of Pfizer, Eisai, Santen Pharmaceutical,
Abbvie, Takeda Science Foundation, AstraZeneca (R&D Grant
2013), and ONO Medical Research Foundation and Industry-University
Cooperation (BioMimetics Sympathies Inc.).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HF, SA, NY and TN conceived the project and designed
the experiments. HF, SA, IM and TN performed experiments and
analyzed data. HF and TN wrote the manuscript. All authors
discussed the results and commented on the manuscript.
Ethics approval and consent to
participate
All human experimental protocols in this study (no.
2728, 2729) were approved by the Ethics Review Committee of Tokyo
Medical University. Written informed consent was obtained from all
participants prior to collection of joint tissue samples.
Patient consent for publication
Consent for publication was obtained from all
participants.
Competing interests
The authors and employees of BioMimetics Sympathies
Inc. have declared that they have no competing interests.
References
|
1
|
Wickelgren I: Obesity: How big a problem?
Science. 280:1364–1367. 1998.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Roden M and Bernroider E: Hepatic glucose
metabolism in humans-its role in health and disease. Best Pract Res
Clin Endocrinol Metab. 17:365–383. 2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hotamisligil GS: Role of endoplasmic
reticulum stress and c-Jun NH2-terminal kinase pathways in
inflammation and origin of obesity and diabetes. Diabetes. 54
(Suppl 2):S73–S78. 2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tsai YC and Weissman AM: The unfolded
protein response, degradation from endoplasmic reticulum and
cancer. Genes Cancer. 1:764–778. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cnop M, Foufelle F and Velloso LA:
Endoplasmic reticulum stress, obesity and diabetes. Trends Mol Med.
18:59–68. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang S and Kaufman RJ: The impact of the
unfolded protein response on human disease. J Cell Biol.
197:857–867. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Amano T, Yamasaki S, Yagishita N,
Tsuchimochi K, Shin H, Kawahara K, Aratani S, Fujita H, Zhang L,
Ikeda R, et al: Synoviolin/Hrd1, an E3 ubiquitin ligase, as a novel
pathogenic factor for arthropathy. Genes Dev. 17:2436–2449.
2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bernasconi R, Galli C, Calanca V, Nakajima
T and Molinari M: Stringent requirement for HRD1, SEL1L, and
OS-9/XTP3-B for disposal of ERAD-LS substrates. J Cell Biol.
188:223–235. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yagishita N, Yamasaki S, Nishioka K and
Nakajima T: Synoviolin, protein folding and the maintenance of
joint homeostasis. Nat Clin Pract Rheumatol. 4:91–97.
2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Toh ML, Marotte H, Blond JL, Jhumka U,
Eljaafari A, Mougin B and Miossec P: Overexpression of synoviolin
in peripheral blood and synoviocytes from rheumatoid arthritis
patients and continued elevation in nonresponders to infliximab
treatment. Arthritis Rheum. 54:2109–2118. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gao B, Calhoun K and Fang D: The
proinflammatory cytokines IL-1beta and TNF-alpha induce the
expression of Synoviolin, an E3 ubiquitin ligase, in mouse synovial
fibroblasts via the Erk1/2-ETS1 pathway. Arthritis Res Ther.
8(R172)2006.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Xu YM, Wang HJ, Chen F, Guo WH, Wang YY,
Li HY, Tang JH, Ding Y, Shen YC, Li M, et al: HRD1 suppresses the
growth and metastasis of breast cancer cells by promoting IGF-1R
degradation. Oncotarget. 6:42854–42867. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tsuchimochi K, Yagishita N, Yamasaki S,
Amano T, Kato Y, Kawahara K, Aratani S, Fujita H, Ji F, Sugiura A,
Izumi T, et al: Identification of a crucial site for synoviolin
expression. Mol Cell Biol. 25:7344–7356. 2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Izumi T, Fujii R, Izumi T, Nakazawa M,
Yagishita N, Tsuchimochi K, Yamano Y, Sato T, Fujita H, Aratani S,
et al: Activation of synoviolin promoter in rheumatoid synovial
cells by a novel transcription complex of interleukin enhancer
binding factor 3 and GA binding protein alpha. Arthritis Rheum.
60:63–72. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kaneko M, Yasui S, Niinuma Y, Arai K,
Omura T, Okuma Y and Nomura Y: A different pathway in the
endoplasmic reticulum stress-induced expression of human HRD1 and
SEL1 genes. FEBS Lett. 581:5355–5360. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fujita H, Yagishita N, Aratani S,
Saito-Fujita T, Morota S, Yamano Y, Hansson MJ, Inazu M, Kokuba H,
Sudo K, et al: The E3 ligase synoviolin controls body weight and
mitochondrial biogenesis through negative regulation of PGC-1β.
EMBO J. 34:1042–1055. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ulmer AJ, Scholz W, Ernst M, Brandt E and
Flad HD: Isolation and subfractionation of human peripheral blood
mononuclear cells (PBMC) by density gradient centrifugation on
Percoll. Immunobiology. 166:238–250. 1984.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yoshida H, Matsui T, Yamamoto A, Okada T
and Mori K: XBP1 mRNA is induced by ATF6 and spliced by IRE1 in
response to ER stress to produce a highly active transcription
factor. Cell. 107:881–891. 2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nakatani Y, Kaneto H, Kawamori D,
Yoshiuchi K, Hatazaki M, Matsuoka TA, Ozawa K, Ogawa S, Hori M,
Yamasaki Y and Matsuhisa M: Involvement of endoplasmic reticulum
stress in insulin resistance and diabetes. J Biol Chem.
280:847–851. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi
NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH and Hotamisligil
GS: Endoplasmic reticulum stress links obesity, insulin action, and
type 2 diabetes. Science. 306:457–461. 2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kaplon RE, Chung E, Reese L, Cox-York K,
Seals DR and Gentile CL: Activation of the unfolded protein
response in vascular endothelial cells of nondiabetic obese adults.
J Clin Endocrinol Metab. 98:E1505–E1509. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sharma NK, Das SK, Mondal AK, Hackney OG,
Chu WS, Kern PA, Rasouli N, Spencer HJ, Yao-Borengasser A and
Elbein SC: Endoplasmic reticulum stress markers are associated with
obesity in nondiabetic subjects. J Clin Endocrinol Metab.
93:4532–4541. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Komura T, Sakai Y, Honda M, Takamura T,
Matsushima K and Kaneko S: CD14+ monocytes are vulnerable and
functionally impaired under endoplasmic reticulum stress in
patients with type 2 diabetes. Diabetes. 59:634–643.
2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sage AT, Holtby-Ottenhof S, Shi Y,
Damjanovic S, Sharma AM and Werstuck GH: Metabolic syndrome and
acute hyperglycemia are associated with endoplasmic reticulum
stress in human mononuclear cells. Obesity (Silver Spring).
20:748–755. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Qin B, Yang Y, Yang Z and Zhong R:
Response to: 'Body mass index and the risk of rheumatoid arthritis:
A systematic review and dose-response meta-analysis'-authors'reply.
Arthritis Res Ther. 17(86)2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ljung L and Rantapää-Dahlqvist S:
Abdominal obesity, gender and the risk of rheumatoid arthritis-a
nested case-control study. Arthritis Res Ther.
18(277)2016.PubMed/NCBI View Article : Google Scholar
|