Introduction
Bronchopneumonia is a common infectious disease
among infants, and has a very high incidence in children (1). Mycoplasma pneumoniae, together
with bacteria and viruses, is the most important cause of
bronchopneumonia and there are also infections caused by both
bacteria and viruses (2). The main
effect of bacterial pneumonia is pulmonary parenchymal damage,
whereas the main effect of viral pneumonia is interstitial
involvement (2). The obstruction of
ventilation is caused by the thickening of the respiratory membrane
and the obstruction of the lower respiratory tract caused by
pulmonary inflammation. The main signs are respiratory, such as
fine rales, fever, cough, asthma and lung pain (3).
Oral antibiotics constitute the basic clinical
treatment for bronchopneumonia. However, antibiotics cause great
damage to the nervous system, blood system and liver function
(4). In the present study, aerosol
inhalation was used for the treatment of bronchopneumonia in order
to convert liquid medicine into water mist through ultrasonic
electronic high-frequency vibration. This method is convenient for
children for active respiratory inhalation and as the drug directly
targets lesions, and is also effective for expectoration, cough
relief, anti-inflammation and spasmolysis (5). Intravenous injection of ambroxol
hydrochloride is a common treatment for bronchopneumonia in adults
(6). However, children often have
fear of oral and injectable drugs, which affects treatment
compliance and coordination, and requires long-term
hospitalization, increasing the risk of iatrogenic infections
(7). Therefore, in recent years,
aerosol inhalation has been included in the treatment of pediatric
bronchopneumonia, effectively reducing side effects and ensuring
children's safety (8).
In clinical practice, commonly used drugs for
bronchopneumonia are ambroxol hydrochloride and
N-acetylcysteine, which are antitussive drugs, although
N-acetylcysteine is an amino acid with strong mucus
dissolution. In the present study, the efficacy of ambroxol
hydrochloride and N-acetylcysteine aerosol inhalation were
compared in the treatment of children with bronchial pneumonia, as
well as their effect on immune function and prognosis.
Patients and methods
General data
A total of 120 children with bronchial pneumonia
admitted to The Affiliated Yantai Yuhuangding Hospital of Qingdao
University (Yantai, China) from July 2015 to August 2018 were
enrolled in the present study. Among them, 58 children treated with
N-acetylcysteine were selected as the experimental group,
and 62 children treated with ambroxol hydrochloride were selected
as the control group. There were 38 boys and 20 girls in the
experimental group, 1-7 years of age, with median age of 4.6±1.57
years; 39 boys and 23 girls in the control group, 1-7 years of age,
with median age of 4.5±1.57 years. There was no significant
difference in the general characteristics of the selected children,
indicating that the two groups were comparable (P>0.05; Table I).
 | Table IPatient general characteristics [n
(%), mean ± SD]. |
Table I
Patient general characteristics [n
(%), mean ± SD].
| Factors | Experimental group
(n=58) | Control group
(n=62) | t/χ2 | P-value |
|---|
| Sex | | | 0.089 | 0.765 |
|
Male | 38 (65.52) | 39 (62.90) | | |
|
Female | 20 (34.48) | 23 (37.10) | | |
| Age (years) | 4.6±1.57 | 4.5±1.57 | 0.349 | 0.728 |
| WBC
(x109/l) | 17.68±2.45 | 16.37±2.97 | 1.844 | 0.068 |
| CRP (mg/l) | 29.38±5.62 | 28.46±6.03 | 0.863 | 0.390 |
| Pulmonary
auscultation | | | | |
|
Pulmonary
rales | 46 (79.31) | 43 (69.35) | 1.550 | 0.213 |
|
Tubular
sound | 12 (20.69) | 19 (30.65) | | |
| Fever | | | | |
|
Yes | 51 (87.93) | 55 (88.71) | 0.018 | 0.894 |
|
No | 7 (12.07) | 7 (11.29) | | |
| Cough | | | | |
|
Yes | 56 (96.55) | 61 (98.39) | 0.417 | 0.520 |
|
No | 2 (3.45) | 1 (1.61) | | |
| Asthma | | | | |
|
Yes | 47 (81.03) | 50 (80.65) | 0.0001 | 0.976 |
|
No | 11 (18.97) | 12 (19.35) | | |
| Hospitalization time
(months) | 1.96±2.34 | 2.16±0.05 | 0.498 | 0.619 |
Inclusion and exclusion criteria
Inclusion criteria: i) Children that did not resist
the treatment. The children's immediate family members and/or
guardians consented to their participation in the study by signing
an informed consent form; ii) diagnosis was confirmed by chest
X-ray and blood routine examination, referring to clinical
diagnostic criteria for infants' bronchopneumonia (9), and iii) children with normal liver and
kidney function and no history of allergy.
Exclusion criteria: i) Children with other diseases
affecting survival, such as tumor and immune system diseases; ii)
children that resisted the treatment, and iii) children with other
serious medical conditions. The study was approved by the Ethics
Committee of The Affiliated Yantai Yuhuangding Hospital of Qingdao
University and all immediate family members and/or guardians of the
children that participated in the study were informed and signed an
informed consent form.
Methods
Patients in both the experimental and control group
were treated with oxygen, anti-infection and fluid replenishment
therapy [anti-infection therapy can improve clinical symptoms to a
certain extent, thus, the two groups were treated with the same
anti-infection therapy and were given penicillin 200,000-400,000
U/(kg×day) for 3 days]. The patients in the control group received
ambroxol hydrochloride (Ambroxol™, specifications: 30 mg x20
tablets, batch no. 20130507; Shanghai Boehringer Ingelheim
Pharmaceutical Co., Ltd.). One tablet of ambroxol was added into 3
ml of 0.9% sodium chloride, and the solution was inserted into the
mask of atomizer which was connected to the oxygen device. After
the oxygen flow rate was adjusted to 7 l/min, atomization
inhalation was carried out: 2 ml/time, 2 times/day, with 1 week for
a course of treatment, for a total of two courses. The patients in
the experimental group were treated with N-acetylcysteine
(Fulushi™, specifications: 600 mg x4 tablets, batch no. 20131016;
Zambon Company S.p.A.). A total of 3 ml of N-acetylcysteine
were added into ~3 mg of 10% sodium chloride, and the solution was
inserted into a separate mask atomizer which was connected to an
oxygen device. After the oxygen flow rate was adjusted to 7 l/min,
atomization inhalation was performed: 3 ml/time, 2 times/day, 1
week for a course of treatment, for a total of two courses.
Observation indicators
Effective rate of treatments
To compare the effective rate of the clinical
treatments, the following evaluation criteria of the therapeutic
effects were used: Significantly effective treatment, all clinical
symptoms disappeared and clinical signs basically disappeared after
treatment; Effective treatment, the clinical symptoms almost
disappeared and the clinical signs significantly improved after
treatment; Ineffective treatment, there was no difference between
the indicators before and after treatment, and there was no
improvement or aggravation of the clinical symptoms and signs after
treatment. Total effective rate=[(no. of significantly effective
cases + no. of effective cases)/no. of total cases] x100%.
Symptoms disappearance time
The disappearance time of symptoms, such as fever,
cough, lung rale, asthma and lung X-ray shadow was recorded in the
two groups, and the efficacy was evaluated according to the
standard literature (4).
Detection of immune function
A total of 4 ml of fasting venous blood were
collected from the patients of the two groups before and after
treatment, and were divided into two samples of 2 ml each. The
samples were placed into an anticoagulation tube, and after
centrifugation at 3,000 x g for 15 min at 4˚C, serum humoral and
cellular immune functions were detected. The humoral immune
function was detected by the immunorapid ejection turbidimetric
method (10) and the related
observation indicators immunoglobulin A (IgA), immunoglobulin G
(IgG), immunoglobulin M (IgM), complement C3 and C4 were
investigated. Flow cytometry was used for the detection of cellular
immune function, and the related indicators CD3, CD4, CD8 and
CD4/CD8 were detected and analyzed. The specific detection process
was as follows: Anticoagulation treatment was first performed on
the whole blood, and then 100 µl of whole blood were added into the
tube. A total of 20 µl of CD4-FITC (1:500, cat. no. 561005), 10 µl
of CD8-PE (1:500, cat. no. 560959) and 10 µl of CD3-PerCP (1:500,
cat. no. 552851) monoclonal fluorescent antibodies (BD Biosciences)
were added. The tube was placed in the dark for 15 min at room
temperature, and 2 ml of hemolysin were added to dissolve the red
blood cells. Each sample was mixed and left at room temperature for
10 min, and then centrifuged at 1,500 x g at 4˚C for 10 min. The
supernatant was discarded and PBS buffer containing 0.1%
NaN3 was added. After centrifugation at 1,500 x g at 4˚C
for 10 min, the supernatant was removed. Next, the cells were
resuspended and FACSCalibur flow cytometer (BD Biosciences) was
used for the detection. CellQuest software (BD Biosciences) was
used for analysis.
Quality of life
For the comparison of the quality of life of the
patients in the two groups after 3 months of treatment, the Short
Form (SF)-36 health survey (11)
was used for evaluation, with 100 points in each scale (the higher
the score, the higher the quality of life).
Adverse reactions
The adverse reactions of the two groups were
recorded and compared, including vomiting, abdominal pain, rash,
diarrhea and nausea.
Statistical analysis
Experimental data were analyzed by SPSS v20.0
statistical software (Shanghai Yuchuang Network Technology Co.,
Ltd.). The enumeration data were compared by χ2 test,
whereas the measurement data were expressed as the mean ± standard
deviation and t-test was used for their comparison between two
groups. Paired t-test was used for the comparison of data at
different time points. ANOVA and Least Significant Difference post
hoc test were used for the comparison of data between multiple
groups. The figures were generated using the GraphPad Prism 6
software (Emerald Biotech Co, Ltd.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Treatment efficacy
The total effective rate in the experimental group
was 94.83%, which was higher than that in the control group
(82.26%). The difference was statistically significant (P<0.05;
Table II).
 | Table IIComparison of treatment efficacy
between the two groups [n (%)]. |
Table II
Comparison of treatment efficacy
between the two groups [n (%)].
| Group | No. of cases | Significantly
effective | Effective | Ineffective | Total efficacy |
|---|
| Experimental
group | 58 | 35 (60.34) | 20 (34.48) | 3 (5.17) | 55 (94.83) |
| Control group | 62 | 30 (48.39) | 21 (33.87) | 11 (17.74) | 51 (82.26) |
| χ2 | | 1.726 | 0.005 | 4.594 | 4.594 |
| P-value | | 0.189 | 0.944 | 0.032 | <0.05 |
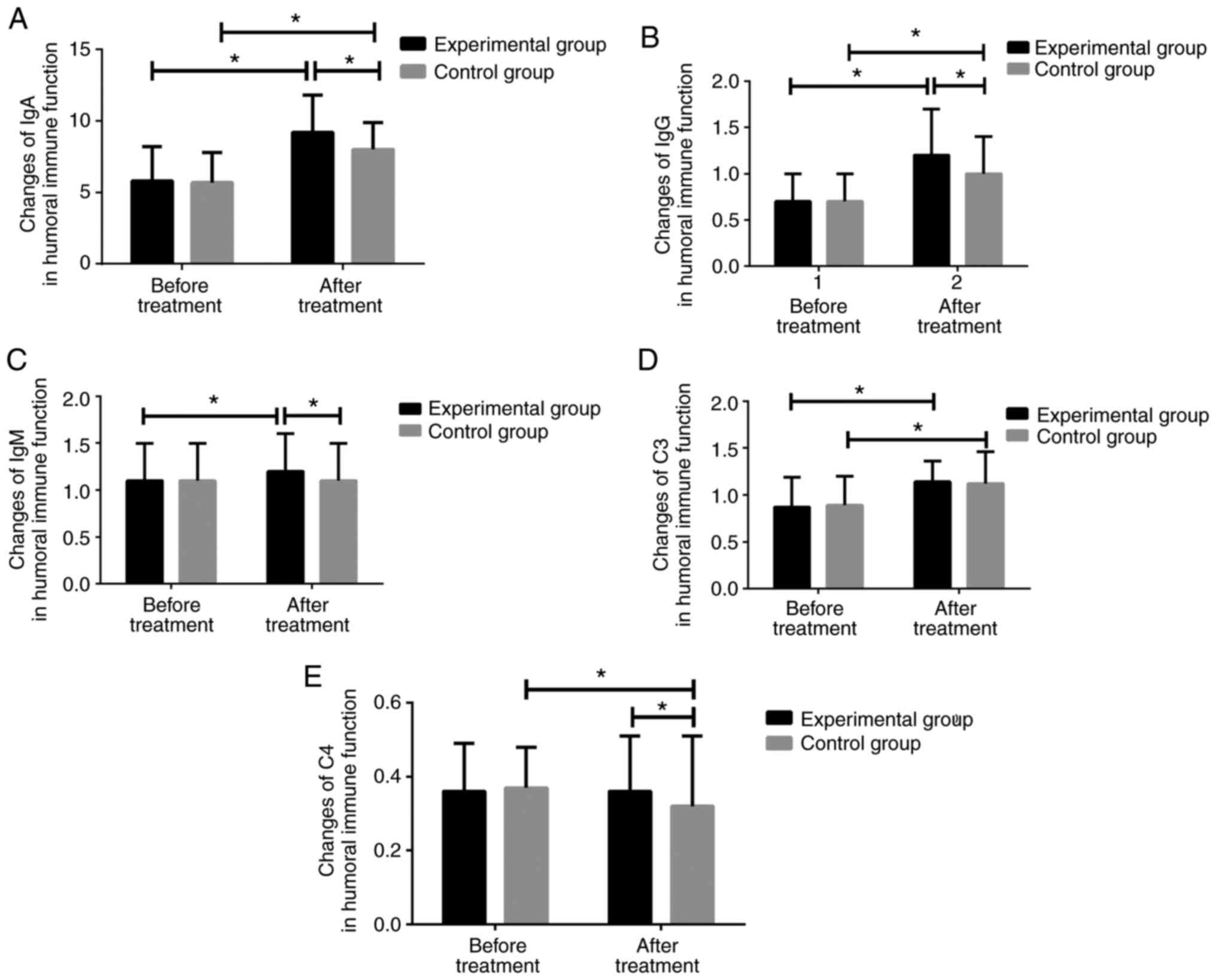
Changes in the immune function of
children in the two groups
There was no significant difference in the humoral
or cellular immune function indexes between the two groups before
treatment (P>0.05). After treatment, IgA, IgG and complement C3
were significantly higher in both groups, compared with those
before treatment (P<0.01). The experimental group had
significantly higher IgM level after treatment compared with that
before treatment; however, there was no significant difference
between the IgM levels before and after treatment in the control
group (P>0.05). In addition, there was no significant difference
in complement C4 before and after treatment in the experimental
group (P>0.05), whereas the complement C4 in the control group
was significantly lower after treatment than that before treatment.
However, there was no significant difference in C3 levels between
the two groups after treatment (P>0.05; Fig. 1).
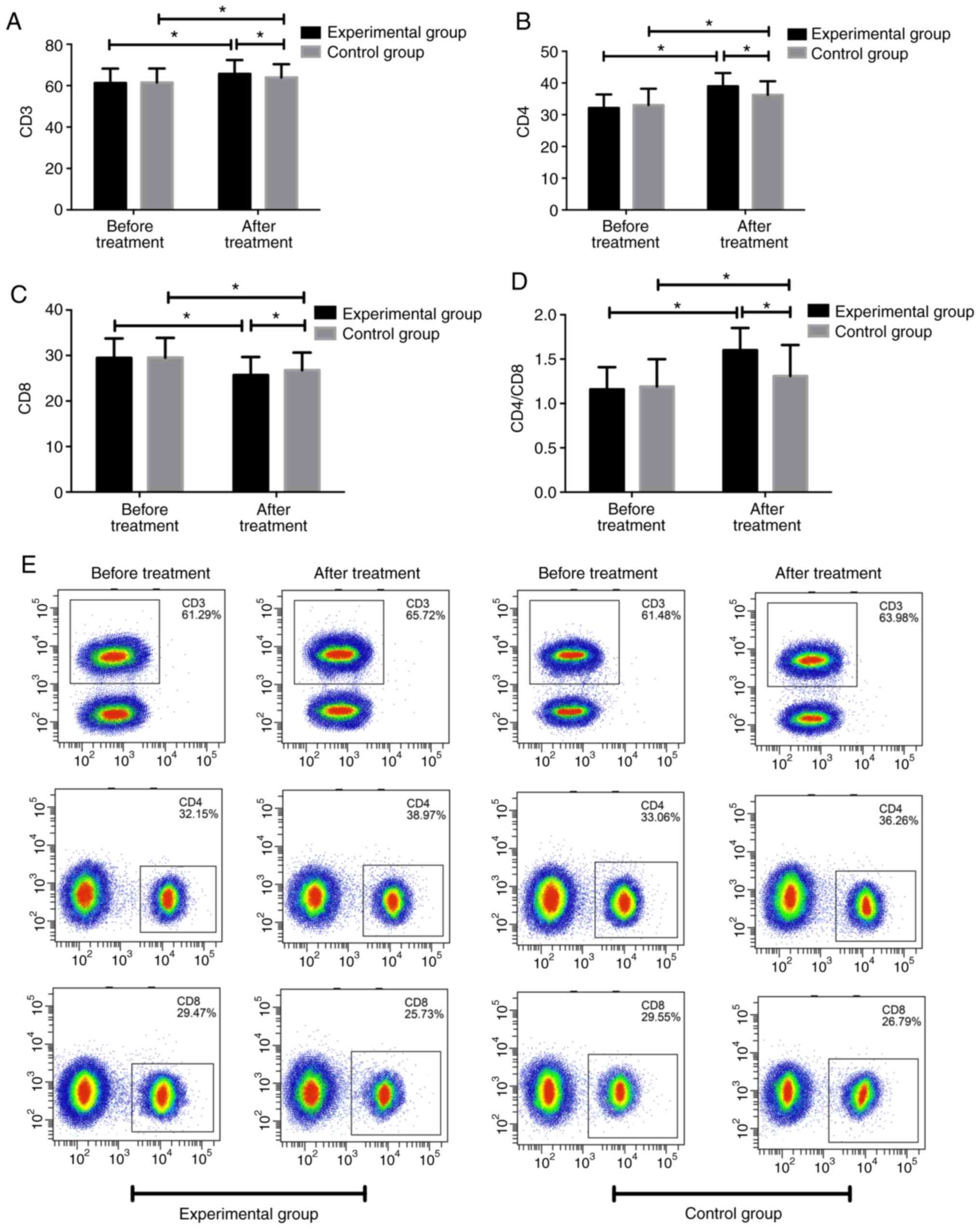
Moreover, there was no significant difference in
CD3, CD4, CD8 and CD4/CD8 between the two groups before treatment.
After treatment, CD3, CD4, and CD4/CD8 were significantly increased
in both groups, compared with those before treatment; however the
CD8 levels were significantly decreased in both groups (P<0.05).
The levels of CD3, CD4, CD4/CD8 after treatment were significantly
higher in the experimental group compared with those in the control
group (P<0.05), whereas CD8 was significantly lower in the
experimental group than that in the control group (P<0.05;
Fig. 2).
Comparison of symptom disappearance
time between the two groups
The disappearance time of symptoms such as fever,
cough, asthma, shadow of the lung X-ray, time of a cough
disappearing and lung rales in the experimental group were
significantly shorter than those in the control group (P<0.001
or <0.05; Table III).
 | Table IIIComparison of symptoms disappearance
time between the two groups (mean ± SD, days). |
Table III
Comparison of symptoms disappearance
time between the two groups (mean ± SD, days).
| Group | No. of cases | Fever | Cough | Asthma | Lung X-ray
shadow | Lung rales | Time of a cough
disappearing |
|---|
| Experimental
group | 58 | 2.1±0.5 | 4.2±0.3 | 3.7±0.6 | 5.7±0.4 | 4.12±1.23 | 4.31±1.46 |
| Control group | 62 | 2.6±0.4 | 5.5±0.3 | 5.2±0.5 | 6.1±0.5 | 6.87±1.69 | 6.84±1.42 |
| t | | 6.068 | 17.12 | 14.91 | 4.818 | 10.13 | 9.621 |
| P-value | | <0.001 | <0.001 | <0.001 | <0.001 | <0.05 | <0.05 |
Comparison of adverse reactions
between the two groups of patients
After statistical analysis, the total number of
patients with adverse reactions in the experimental group of
patients, treated with N-acetylcysteine, was significantly
lower than that of the control group of patients, treated with
bromate hydrochloride (P<0.05; Table IV).
 | Table IVComparison of adverse reactions after
treatment between the two groups [n (%)]. |
Table IV
Comparison of adverse reactions after
treatment between the two groups [n (%)].
| Group | No. of cases | Vomit | Abdominal pain | Erythema | Diarrhea | Nausea | Total |
|---|
| Experimental
group | 58 | 1 (1.72) | 1 (1.71) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 2 (3.45) |
| Control group | 62 | 2 (3.23) | 2 (3.23) | 1 (1.61) | 1 (1.61) | 2 (3.23) | 8 (12.90) |
| χ2 | | 0.277 | 0.277 | 0.943 | 0.943 | 1.903 | 4.409 |
| P-value | | 0.599 | 0.599 | 0.331 | 0.331 | 0.168 | 0.037 |
Comparison of quality of life between
the two groups after 3 months of treatment
The quality of life of patients in the experimental
and control group after 3 months of treatment was investigated,
including physiological functioning, somatic pain and general
health status. The scores of physiological functioning, somatic
pain, general health status and vitality of the experimental group
were significantly higher than those of the control group
(P<0.001). However, there was no significant difference in
social role functioning, emotional role functioning and mental
health between the two groups (P>0.05; Table V).
 | Table VComparison of quality of life between
the two groups after 3 months of treatment (mean ± SD). |
Table V
Comparison of quality of life between
the two groups after 3 months of treatment (mean ± SD).
| Factors | Experimental group
(n=58) | Control group
(n=62) | t | P-value |
|---|
| Physiological
functioning | 81.36±10.96 | 69.29±11.25 | 5.947 | <0.001 |
| Somatic pain | 88.05±10.49 | 69.23±12.54 | 8.885 | <0.001 |
| General health
status | 83.66±9.81 | 71.07±12.14 | 6.223 | <0.001 |
| Vitality | 83.14±10.26 | 73.79±13.79 | 4.191 | <0.001 |
| Social role
functioning | 63.21±10.67 | 63.54±11.37 | 0.164 | 0.8703 |
| Emotional role
functioning | 72.63±12.09 | 69.98±12.04 | 0.232 | 1.202 |
| Mental health | 83.62±10.48 | 81.18±10.46 | 1.276 | 0.2045 |
Discussion
In recent years, with the opening of policies, as
the birth rate has increased, the incidence of bronchopneumonia in
children, which is mainly related to the immature lung development
and immune system (low defense ability of the body), is higher than
the incidence of neonatal diarrhea. When the cough frequency caused
by pneumonia is too high, patients experience pain in the bilateral
costal cartilage, accompanied by the occurrence of choking and
other conditions. In infants, the bronchial tissue support and
rebound elasticity are relatively weak, and the ciliary movement
ability is not strong, thus, the secretions in the respiratory
tract cannot be discharged in time. Another important reason for
obstructing the airway is that the airway smooth muscle is weak,
and cough response is insufficient, further reducing secretion
excretion (12). As a result, the
course of disease in some children is prolonged, and even secondary
complications, such as respiratory failure, heart failure or toxic
intestinal paralysis pose serious threats to life. At present, the
clinical treatment of bronchopneumonia in infants mainly relies on
anti-infection therapies and elimination of inflammation and the
curative effect is still good, although the course of treatment
required for the improvement of symptoms is long. Therefore, the
focus of clinical research is to effectively shorten the duration
of symptoms and improve the overall curative effect of treatment
(13).
The main purpose of the present study was to compare
the clinical efficacy and effect on prognosis of ambroxol
hydrochloride and N-acetylcysteine treatments in children
with bronchopneumonia. The results showed that the clinical symptom
relief time in children who received N-acetylcysteine
aerosol inhalation treatment was significantly shorter than that of
the control group, and the total clinical effective rate was
94.83%, which was higher than that of the control group (82.26%)
(P<0.05). This result is consistent with the study of
Trastotenojo et al (14),
who demonstrated the distinct advantages of nebulized inhalation
therapy with N-acetylcysteine. N-acetylcysteine is a
derivative of acetyl group of cysteine and has a free radical
scavenging effect. Because of its strong mucilage dissolving
effect, N-acetylcysteine is a solution with good solubility
which can be used as an expectorant in clinic (15). In-depth research has also revealed
that: i) N-acetylcysteine is an antioxidant, which can
effectively reduce reactive oxygen species and inhibit the activity
of nuclear factor in disease-causing cells in vivo (16); and ii) the fiber in the concentrated
sputum is cut off by the sulfhydryl group contained in the
N-acetylcysteine molecule, so that the sputum is easily
liquefied and removed from the body (15). The experimental results showed that
all immune factors were significantly improved after treatment
except for C3 and CD8. Hermeyer et al (17) pointed out that the key link in the
occurrence and development of bronchial pneumonia was inflammatory
reaction. With the aggregation of granulocytes into the
inflammatory area and the release of a large number of free
radicals, lipid peroxidation of the cell membrane occurs, leading
to cell autolysis and tissue necrosis. N-acetylcysteine has
good cell membrane penetration after entering the cell, and
therefore plays an antioxidant role and doesn't cause aggravation
or tissue cell damage. In clinical practice, ambroxol hydrochloride
atomization inhalation has a good clinical effect in the adjuvant
treatment of pediatric bronchopneumonia, indicating that
atomization inhalation has a good effect in drug delivery. In the
present study, the results showed that, compared with hydrochloric
acid, N-acetylcysteine atomization inhalation effects are
more significant. In order to further explore the curative effect
of N-acetylcysteine atomization inhalation therapy the cell
and humoral immunity function in the two groups of children were
investigated. The results showed that the immune function of the
two groups of children was obviously affected after treatment, with
the immune function showing a certain degree of recovery, which was
more apparent in the patients of the experimental group (18). The reason for the failure of the
bronchopneumonia treatment is that the main antibody against
infection is significantly reduced during the anti-inflammatory
reaction and the incomplete development of the immune function in
children makes it impossible to be replenished in time. In the
present study the SF-36 scoring system was also used to measure the
quality of life of the two groups of patients after treatment,
including the assessment of physiological functioning, somatic
pain, general health status, vitality, social functioning,
emotional functioning and mental health. The results revealed that
the patients treated with N-acetylcysteine had significantly
higher scores than the patients treated with ambroxol
hydrochloride. N-acetylcysteine dissolves sputum, reduces
sputum adhesion force, increases the cilia movement and inhibits
the growth of pathogenic bacteria, reducing the local inflammation
reaction, thus, impairing the immunity system, so that the body's
humoral immunity gradually returns to normal. In the present study,
the number of patients selected was small, and the experimental
results are therefore limited. The reliability of the presented
data needs to be further verified by future studies with a greater
sample size.
In summary, the present study showed that the total
effective rate, cellular immune function, humoral immune function
and the quality of life in the two groups of children were
significantly improved after the different treatments except for
C4. Inhalation of N-acetylcysteine can effectively improve
the clinical symptoms and signs, as well as the immune function of
children with bronchial pneumonia, and the curative effect is
remarkable, suggesting that N-acetylcysteine treatment is
worthy of use in clinic.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL wrote the manuscript, analyzed and interpreted
the patient data. WW designed the study and performed the flow
cytometry and immunoturbidimetric assay. XG was responsible for the
analysis and interpretation of the data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Affiliated Yantai Yuhuangding Hospital of Qingdao University
(Yantai, China). Patients who participated in this research had
complete clinical data. Signed written informed consents were
obtained from all children's immediate family members and/or
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dhanapal CK, Mathew J, Madhusudhan S,
Manavalan R and Roy V: A Hospital Based study on some
Clinico-epidemiological aspect of bronchopneumonia among infants
and young children. Int J Chem Sci, 2009 doi:
10.1234/jgpt.v1i1.37.
|
|
2
|
Zhang XL, Ji W, Ji ZH, Ding YF, Zhu H, Yan
YD, Huang YP, He YX, Ye JX and Ji XQ: Epidemiological study on
respiratory syncytial virus and its bronchopneumonia among children
in Suzhou. Zhonghua Yu Fang Yi Xue Za Zhi. 41:371–374. 2007.(In
Chinese). PubMed/NCBI
|
|
3
|
Chang CC, Cheng AC and Chang AB: Over the
counter (OTC) medications to reduce cough as an adjunct to
antibiotics for acute pneumonia in children and adults. Cochrane
Database Syst Rev. 10(CD006088)2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Che XY: Effect of aerosol inhalation of
ipratropium bromide in combined with budesonide and terbutaline on
the cytokines in children with bronchopneumonia. J Hainan Med Univ.
22:65–68. 2016.
|
|
5
|
Chunyun Z: Atomization-based
inhalation-induced cough reflex in treating chronic obstructive
pulmonary disease. J Trop Med, 2010.
|
|
6
|
Zhou WF, Chen Q, Jin MF, Ji ZH, Zhang MZ,
Li HM, Liu FJ and Ji W: The diagnostic accuracy of high-mobility
group box 1 protein and twelve other markers in discriminating
bacterial, viral and co-infected bronchial pneumonia in Han
children. Microbiol Immunol. 55:279–288. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pairet M, Engelmann P, Von Nicolai H,
Champeroux P, Richard S, Rauber G and Engelhardt G: Ambroxol
improves the Broncho-spasmolytic activity of clenbuterol in the
guinea-pig. J Pharm Pharmacol. 49:184–186. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Behrendt G, Baer K, Buffo A, Curtis MA,
Faull RL, Rees MI, Götz M and Dimou L: Dynamic changes in myelin
aberrations and oligodendrocyte generation in chronic amyloidosis
in mice and men. Glia. 61:273–286. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li YQ, Yuan W and Zhang SL: Clinical and
experimental study of xiao er ke cuan ling oral liquid in the
treatment of infantile bronchopneumonia. Zhongguo Zhong XI Yi Jie
He Za Zhi. 12:719–721, 737, 708. 1992.(In Chinese). PubMed/NCBI
|
|
10
|
Chen X, Liu Z, Ge X, Luo X, Huang S, Zhou
Y, Li D, Cheng H, Li L, Huang L, et al: Associations between
manganese exposure and multiple immunological parameters in
manganese-exposed workers healthy cohort. J Trace Elem Med Biol.
59(126454)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Olivieri A, Carli GFD, Panebianco R, et
al: The use of SF-36 questionnaire for the evaluation of quality of
life of patients with chronic bronchitis undergoing to antibiotic
prophylaxis of acute exacerbations. Qual Life Res. 3:88–89.
1994.
|
|
12
|
Mosbah AA, Abdellatif NA, Sorour EI and
Awadallah MF: Serum Sp-D levels as a biomarker of lung injury in
Children suffering of bronchopneumonia. J Egypt Soc Parasitol.
42:25–32. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Miyata T, Ishii T, Sugiyama N, Okano Y,
Nishi N, Takahama K, Ogasawara S, Oda Y, Yokoyama K, Murata Y, et
al: Effect of N-acetylneuraminic acid on respiratory tract
secretion and inflammation in the bronchitic rabbit. Arch Int
Pharmacodyn Ther. 304:277–289. 1990.PubMed/NCBI
|
|
14
|
Trastotenojo MS, Harsoyo N, Sachro AD,
Soemantri AG and Said HW: Use of acetyl cysteine in respiratory
tract disease in children. Paediatr Indones. 24:1–10.
1984.PubMed/NCBI
|
|
15
|
Zafarullah M, Li WQ, Sylvester J and Ahmad
M: Molecular mechanisms of N-acetylcysteine actions. Cell MolLife
Sci. 60:6–20. 2003.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bernard GR, Wheeler AP, Arons MM, Morris
PE, Paz HL, Russell JA and Wright PE: A trial of antioxidants
N-acetylcysteine and Procysteine in ARDS. Chest. 112:164–172.
1997.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hermeyer K, Buchenau I, Thomasmeyer A,
Baum B, Spergser J, Rosengarten R and Hewicker-Trautwein M: Chronic
pneumonia in calves after experimental infection with Mycoplasma
bovis strain 1067: Characterization of lung pathology, persistence
of variable surface protein antigens and local immune response.
Acta Vet Scand. 54(9)2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rong-Xin J, Yi Y and Pediatrics DO:
Comparison of curative effects of N-acetylcysteine and ambroxol
hydrochloride in treatment of children with bronchial pneumonia and
the effect on immune function and clinical symptoms. Maternal Child
Health Care China, 2018.
|