Introduction
Oesophageal squamous cell carcinoma (ESCC) is a
major type of oesophageal cancer and is one of the most common
types of cancer, which causes a large number of cancer-related
deaths worldwide (1-3).
In recent decades, considerable attention has been paid to
treatment innovation through surgical resection in combination with
chemo- or radiotherapy; however, the overall survival time of
patients with ESCC remains poor due to the high recurrence and
metastasis rates (4,5). Thus, it is of great importance to
explore the regulatory mechanisms underlying ESCC progression to
guide the development of novel diagnostic and therapeutic
strategies for ESCC (6-9).
N6-methyladenosine (m6A) is the most abundant type
of methylation, and it serves a crucial role in RNA stability,
localization, splicing and translation (10,11).
The target genes of m6A are associated with cellular proliferation,
organization and transport, as well as cancer-related signalling
pathways (10).
Methyltransferase-like 3 (METTL3) is the 70-kDa subunit of MT-A,
which is part of N6-adenosine-methyltransferase;
N6-adenosine-methyltransferase has been implicated in the
post-transcriptional methylation of internal adenosine residues in
eukaryotic mRNAs, and thus forms m6A (12-14).
It has been widely reported that METTL3 is frequently increased and
has an oncogenic role in common types of human cancer, including
gastric (15), lung (16), bladder (17), ovarian (18), pancreatic cancer (19), melanoma (20) and breast cancer (21). For example, METTL3 is overexpressed
in breast cancer and promotes breast cancer progression through
inhibiting the tumour suppressor, let-7g (21). Taketo et al reported that
METTL3 promoted chemoresistance and radioresistance in pancreatic
cancer cells (19). In contrast, a
recent study demonstrated that METTL3 functioned as a tumour
suppressor in renal cell carcinoma; METTL3 inhibited renal cell
carcinoma proliferation, cell cycle progression, migration and
invasion (22). However, to the
best of our knowledge, no previous study has focused on the
expression pattern and function of METTL3 in ESCC. In the present
study, the mRNA and protein expression levels of METTL3 in ESCC
were investigated and the clinical significance of METTL3
expression in ESCC was explored. Subsequently, the function of
METTL3 in regulating the malignant phenotypes of ESCC cell lines
in vitro, and the molecular mechanism behind METTL3 in ESCC
were investigated. The findings of this study may provide novel
therapeutic strategies for the treatment of ESCC.
Materials and methods
Patient studies
A total of 53 ESCC and paired adjacent tissues were
collected from 53 patients with ESCC who underwent resection at The
Second Xiangya Hospital of Central South University, China between
March 2012 and May 2013. These patients included 30 men and 23
women between 42 and 78 years old. The inclusion criterion was that
all patients exhibited primary ESCC, and the exclusion criterion
was patients that had received chemotherapy or radiotherapy before
surgery. The tissues were stored at -80˚C until required for
further experimentation. The follow-up time was 5 years following
surgery.
Cell culture and reagents
Normal oesophageal epithelial cell line (HET-1A) and
four ESCC cell lines (TE-9, Eca-109, KYSE150 and EC9706) were
purchased from the Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences. Cells were cultured in DMEM (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.), and incubated in a humidified
atmosphere at 37˚C and 5% CO2.
Cell transfection
Lipofectamine® 2000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) was used for cell transfection,
according to the manufacturer's protocol. TE-9 and Eca-109 cells
(5x105 cells/ml) in the logarithmic growth phase were
transfected with 100 nM negative control (NC) small interfering RNA
(siRNA) or 100 nM METTL3-specific siRNAs (Shanghai GenePharma Co.,
Ltd.) at 37˚C. Following transfection for 48 h, METTL3 expression
levels were evaluated using reverse transcription-quantitative PCR
(RT-qPCR).
RT-qPCR
Total RNA was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA was
reversed transcribed into cDNA using a High-Capacity cDNA Reverse
Transcription kit (Thermo Fisher Scientific, Inc.). The RT
conditions were 16˚C for 30 min, followed by 42˚C for 30 min and
85˚C for 5 min. qPCR was subsequently performed using the
All-in-One qPCR mix (Thermo Fisher Scientific, Inc.). The following
thermocycling conditions were used for qPCR: 95˚C for 3 min,
followed by 40 cycles of 95˚C for 15 sec and 60˚C for 30 sec. The
following primer pairs were used for the qPCR: METTL3, forward
5'-TTGTCTCCAACCTTCCGTAGT-3', reverse 5'-CCAGATCAGAGAGGTGGTGTAG-3';
and GAPDH, forward 5'-CTGGGCTACACTGAGCACC-3' and reverse
5'-AAGTGGTCGTTGAGGGCAATG-3'. Relative METTL3 mRNA expression levels
were quantified using the 2-ΔΔCq method (23) and normalized to the internal
reference gene GAPDH.
Western blotting
Total protein was extracted using RIPA buffer
(Thermo Fisher Scientific, Inc.). Protein concentrations were
determined using a bicinchoninic acid assay kit (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Proteins (50 µg/lane) were separated via SDS-PAGE on a 12% gel and
the separated proteins were subsequently transferred onto a PVDF
membrane (Roche Diagnostics) and blocked overnight at 4˚C with 5%
milk. The membranes were incubated with primary antibodies against
METTL3 (1:500; cat. no. ab66660; Abcam), Bax (1:250; cat. no.
ab182733; Abcam), caspase-3 (1:300; cat. no. ab13847; Abcam), Bcl-2
(1:300; cat. no. ab32124; Abcam), PI3K (1:500; cat. no. ab40755;
Abcam), phosphorylated (p)-PI3K (1:200; cat. no. ab182651; Abcam),
AKT (1:500; cat. no. ab8805; Abcam), p-AKT (1:500; cat. no. ab8933;
Abcam) and GAPDH (1:500; cat. no. ab9485; Abcam) for 4 h at room
temperature. Following the primary antibody incubation, membranes
were incubated with a horseradish peroxidase-conjugated secondary
antibody (1:10,000; cat. no. ab6721; Abcam) for 1 h at room
temperature. Protein bands were visualized using Pierce ECL Western
Blotting substrate (Thermo Fisher Scientific, Inc.) and protein
expression was semi-quantified using ImageJ software (version 1.46;
National Institutes of Health) with GAPDH as the loading
control.
Cell viability assay
A total of 5x103 cells/well of
transfected TE-9 and Eca-109 cells were seeded into 96-well plates
and cultured in a humidified incubator containing 5% CO2
for 0, 24, 48 or 72 h. CCK-8 solution (10 µl; Thermo Fisher
Scientific, Inc.) was added to each well, and after incubation at
37˚C for 2 h, the absorbance was measured at 450 nm using a
microplate reader (Bio-Rad Laboratories, Inc.).
Colony formation assay
A total of 1x103 transfected TE-9 and
Eca-109 cells/well were seeded into 6-well plates and cultured in
an incubator containing 5% CO2 at 37˚C for 14 days.
Subsequently, cells were fixed with 75% ethanol at room temperature
for 1 h and stained with 0.1% crystal violet (Sigma-Aldrich; Merck
KGaA) at room temperature for 5 min. The colonies were visualised
and counted using a light microscope (magnification, x10).
Flow cytometric analysis of
apoptosis
Transfected TE-9 and Eca-109 cells (1x106
cells/ml) were fixed in 75% ethanol at 4˚C for 3 h and subsequently
washed with PBS three times. The cells were stained with Annexin
V-FITC and propidium iodide using the Annexin V-FITC Apoptosis
Detection kit I (BD Biosciences), according to the manufacturer's
protocol. Apoptotic cells were subsequently analysed using a
FACScan flow cytometer (BD Biosciences) and BD Accuri™ C6 software
(version 1.0; BD Biosciences).
Would healing assay
Transfected TE-9 and Eca-109 cells were plated at a
density of 5x105 cells/well were seeded in 12-well
plates and cultured to ≥95% confluence. A 200-µl sterile pipette
tip was used to generate the wounds. The cells were washed with
Dulbecco's PBS and DMEM (Gibco; Thermo Fisher Scientific, Inc.) was
subsequently added. The cells were visualized using a light
microscope (magnification, x40) at 0 h and after 24 h incubation at
37˚C. The width of the wounds at 0 and 24 h were determined using
ImageJ software (version 1.5; National Institutes of Health).
Matrigel invasion assay
Cell invasion was determined using Matrigel-coated
Transwell chambers with an 8-µm pore size membrane (BD
Biosciences). In brief, transfected TE-9 and Eca-109 cells
(1x105 cells/well) and 300 µl serum-free DMEM (Gibco;
Thermo Fisher Scientific, Inc.) were plated in the upper chamber.
DMEM (500 ml) supplemented with 10% FBS was plated in the lower
chamber. After 24 h at 37˚C, the invading cells on the lower
surface of the chamber were stained with 0.1% crystal violet at
room temperature (Thermo Fisher Scientific, Inc.) for 5 min and
subsequently washed with PBS (Thermo Fisher Scientific, Inc.).
Stained cells were counted using a light microscope (magnification,
x200).
Statistical analysis
All experiments were repeated at least three times.
All data are expressed as the mean ± SD. Statistical analysis was
performed using SPSS 19.0 software (IBM Corp.). Differences between
groups were determined using Student's t-test or one-way ANOVA
followed by Tukey's post hoc test. A χ2 test was used to
analyse the clinical significance of METTL3 expression in ESCC, and
Kaplan-Meier analysis and log-rank test were applied for survival
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Overexpression of METTL3 promotes ESCC
progression
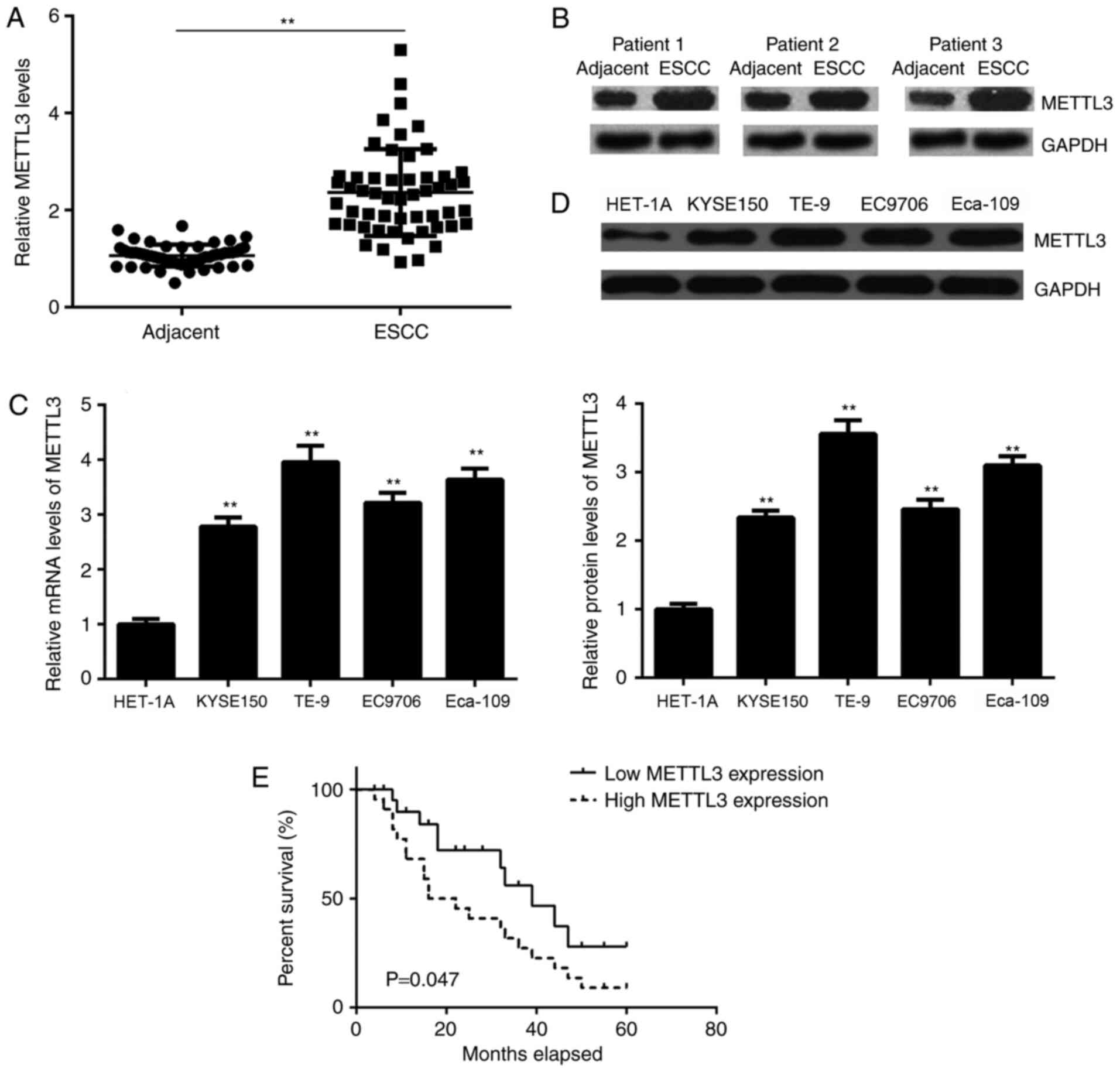
The expression levels of METTL3 were determined
using RT-qPCR and western blotting in ESCC and adjacent non-tumour
tissues. The mRNA expression levels of METTL3 were significantly
increased in ESCC tissues compared with adjacent non-tumour tissues
(Fig. 1A). Similar results were
obtained by western blotting; with METTL3 protein levels increased
in ESCC tissues (Fig. 1B).
Consistent with the clinical data, the METTL3 mRNA and protein
expression levels were significantly higher in ESCC cell lines
compared with the normal oesophageal cell line HET-1A (Fig. 1C and D). To study the clinical significance of
METTL3 expression in ESCC, the patients with ESCC were divided into
high and low METTL3 expression groups based on the median mRNA
expression value (2.58) of METTL3. A χ2 test reported
that high METTL3 expression was significantly associated with
advanced clinical stage and metastasis of ESCC (Table I). Kaplan-Meier analysis indicated
that patient survival was worse in those patients with high METTL3
levels compared with patients with low METTL3 levels (Fig. 1E). These findings suggested that the
upregulation of METTL3 promoted tumour progression and predicted a
poor prognosis in ESCC.
 | Table IAssociation between METTL3 expression
and clinicopathological features in patients with oesophageal
squamous cell carcinoma. |
Table I
Association between METTL3 expression
and clinicopathological features in patients with oesophageal
squamous cell carcinoma.
| | METTL3
expression | |
|---|
| Variables | Cases | Low (n=28) | High (n=25) | χ2 | P-value |
|---|
| Age (years) | | | | 2.369 | 0.124 |
|
<65 | 25 | 16 | 9 | | |
|
≥65 | 28 | 12 | 16 | | |
| Sex | | | | 0.408 | 0.523 |
|
Male | 30 | 17 | 13 | | |
|
Female | 23 | 11 | 12 | | |
| Tumor location | | | | 0.821 | 0.365 |
|
Upper,
middle | 31 | 18 | 13 | | |
|
Lower | 22 | 10 | 12 | | |
| Lymph node
metastasis | | | | 8.355 | 0.004b |
|
Negative | 34 | 23 | 11 | | |
|
Positive | 19 | 5 | 14 | | |
| TNM stage | | | | 6.718 | 0.010a |
|
I+II | 33 | 22 | 11 | | |
|
III+IV | 20 | 6 | 14 | | |
Silencing METTL3 gene expression
inhibits ESCC cell viability and colony formation, while inducing
cellular apoptosis
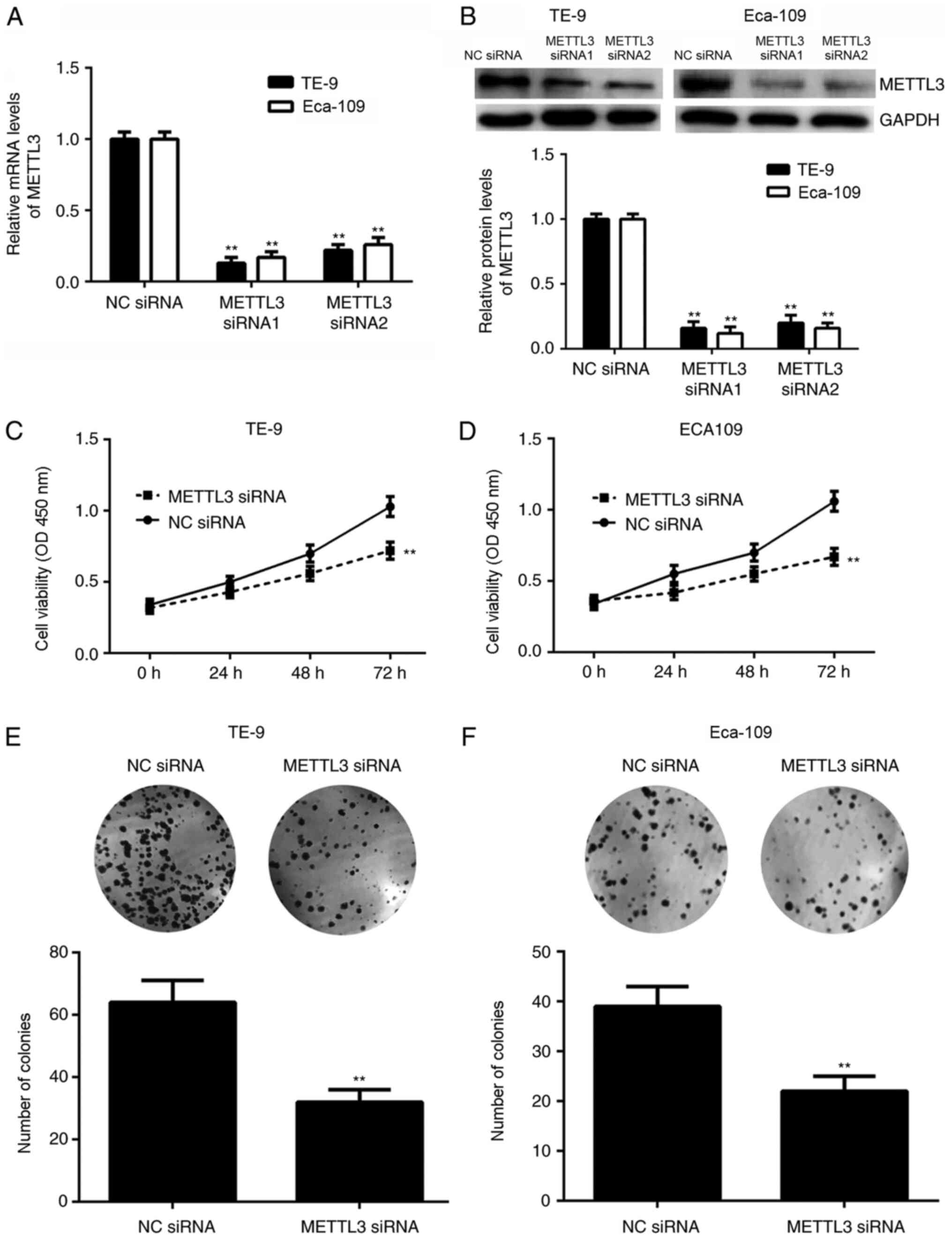
The role of METTL3 in regulating the malignant
phenotypes of ESCC cells was studied. TE-9 and Eca-109 cells were
transfected with two METTL3 siRNAs to knockdown the expression of
METTL3. Transfection with METTL3 siRNAs significantly decreased
METTL3 mRNA and protein expression levels compared with the cells
transfected with NC siRNA in both cell lines (Fig. 2A and B). METTL3 siRNA1 demonstrated a more
potent suppressive effect over mRNA and protein expression and was
thus selected as the siRNA of choice for further experiments. CCK-8
assays indicated that gene silencing of METTL3 expression
significantly suppressed the viability of TE-9 and Eca-109 cells at
72 h compared with NC siRNA-transfected cells (Fig. 2C and D). In addition, the number of colonies
formed of TE-9 and Eca-109 cells was significantly reduced in the
presence of METTL3 siRNA compared with NC siRNA (Fig. 2E and F).
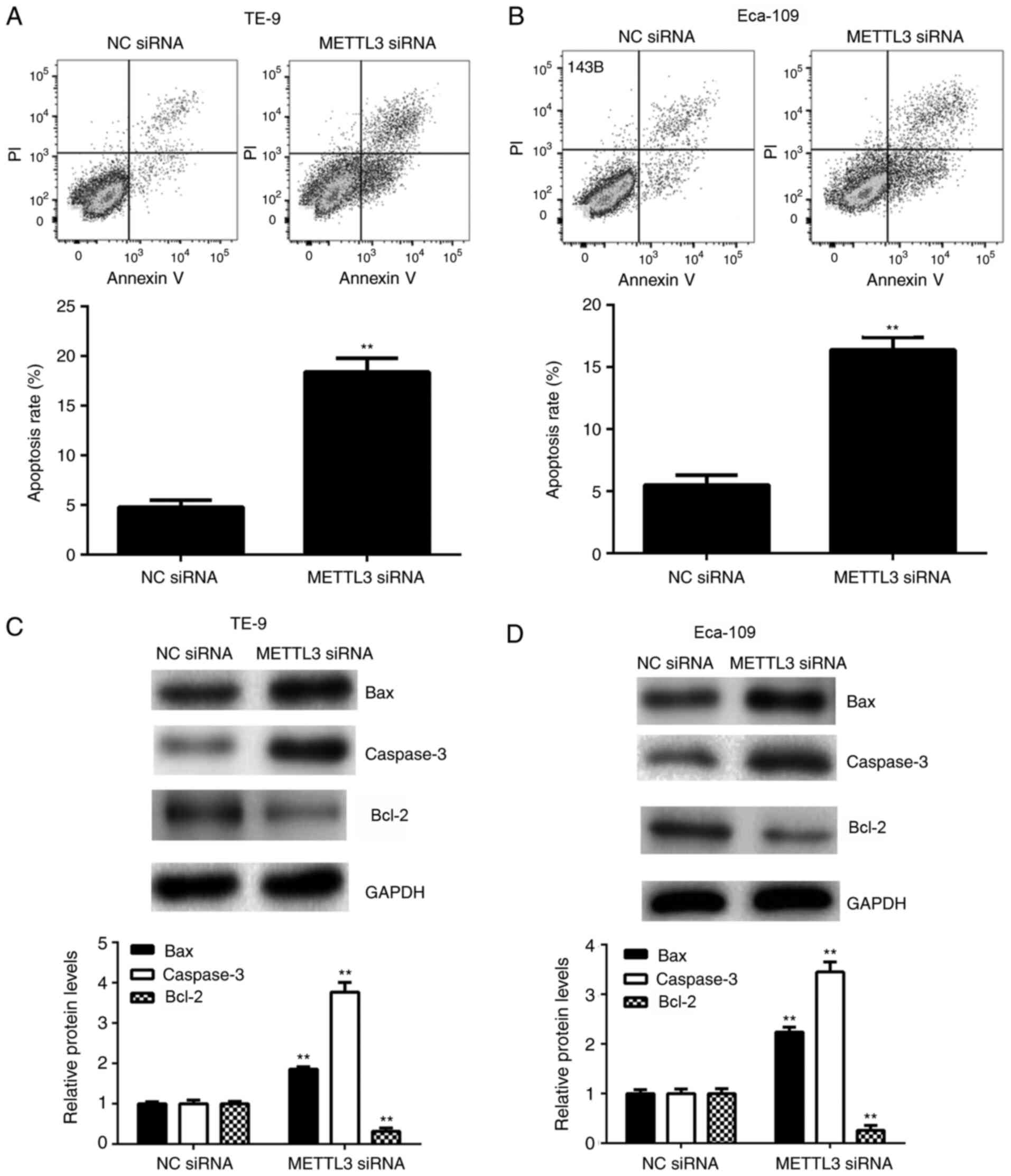
It was hypothesized that apoptosis may be involved
in METTL3-mediated ESCC cell viability. Therefore, the effects of
METTL3 inhibition on ESCC cell apoptosis were examined. The
apoptotic rate of ESCC cells was significantly enhanced following
METTL3 knockdown with siRNA compared with the NC siRNA transfected
cells in both cell lines (Fig. 3A
and B), indicating that silencing
METTL3 gene expression induced ESCC cell apoptosis. In addition,
silencing METTL3 gene expression significantly increased the
protein levels of pro-apoptotic Bax and caspase-3 and decreased
those of anti-apoptotic Bcl-2 in TE-9 and Eca-109 cells compared
with the controls (Fig. 3C and
D).
METTL3 knockdown with siRNA suppresses
ESCC cellular migration and invasion
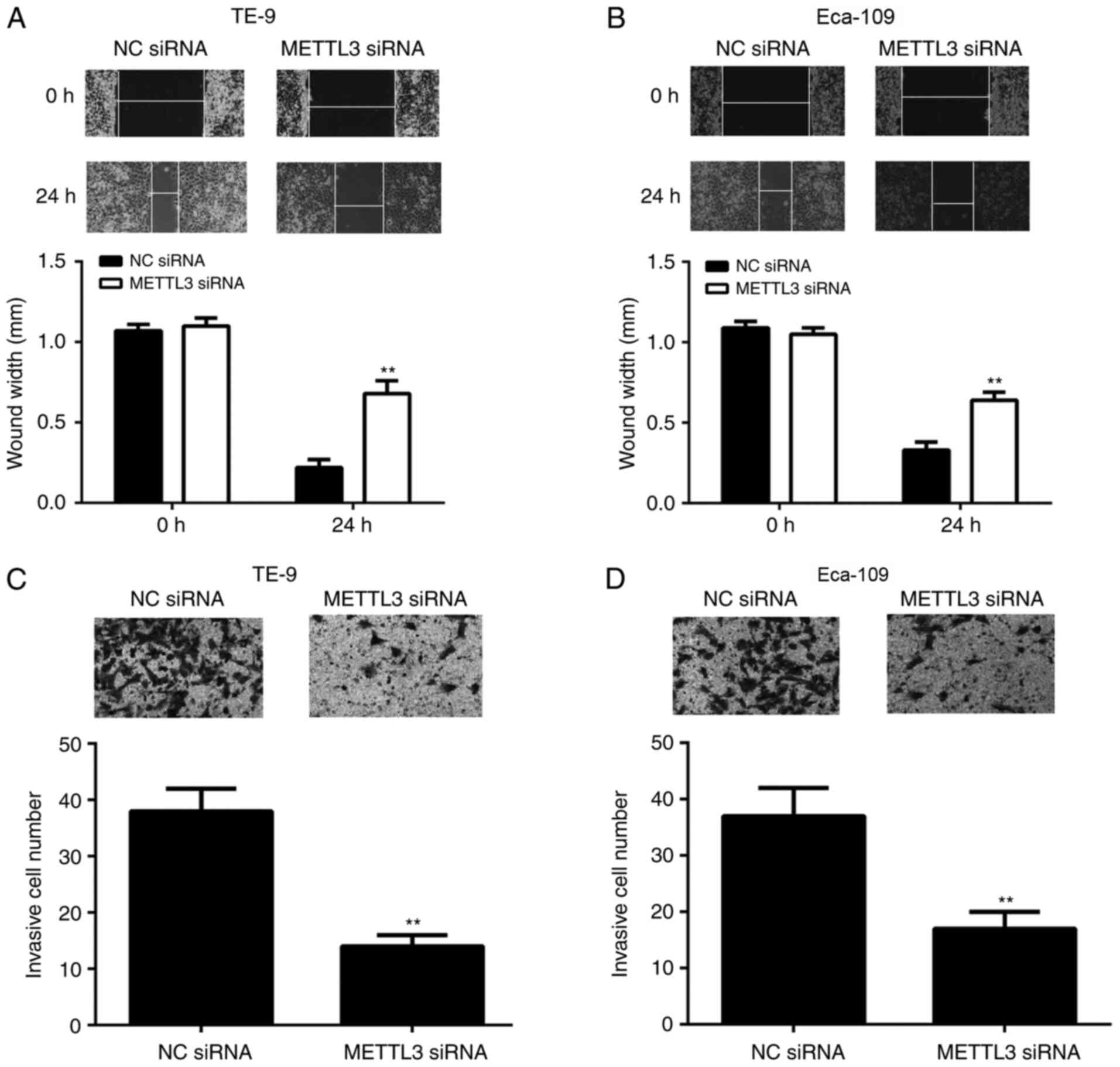
To further study the function of METTL3 in ESCC
metastasis, wound healing and Matrigel Transwell assays were used
to assess cellular migration and invasion following METTL3
knockdown with siRNA. As shown in Fig.
4A and B, the migratory
capacity of TE-9 and Eca-109 cells were significantly inhibited
following siRNA METTL3 knockdown compared with the NC
siRNA-transfected cells in both cell lines. Similarly, following
knockdown of METTL3 expression by siRNA, the invasion of TE-9 and
Eca-109 cells was also significantly repressed (Fig. 4C and D). These findings suggested that the
genetic knockdown of METTL3 with siRNA may inhibit ESCC
metastasis.
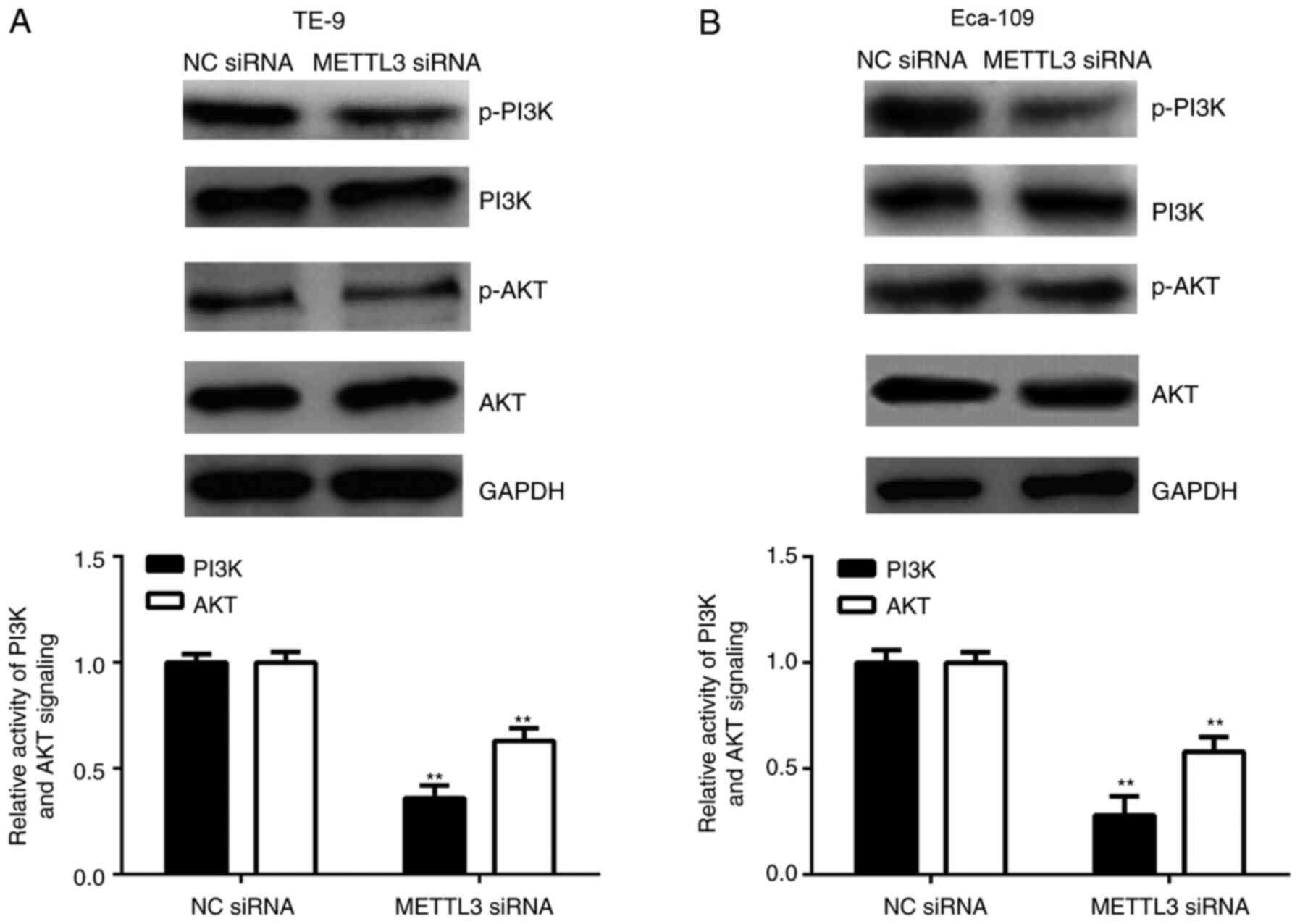
METTL3 inhibition decreases PI3K/AKT
signalling pathway activity
The effects of METTL3 knockdown with siRNA on the
activity of the PI3K/AKT signalling pathway in ESCC cells was
investigated; the PI3K/AKT signalling pathway serves a crucial role
in cancer cell growth and metastasis (22). In Fig.
5, the quantitative analysis refers to the ratio between total
and phosphorylated protein levels. Our data indicated that the
phosphorylated protein levels of PI3K and AKT were significantly
reduced following transfection with METTL3 siRNA compared with NC
siRNA in TE-9 and Eca-109 cells, indicating that METTL3 inhibition
decreased the PI3K/AKT signalling pathway activity (Fig. 5A and B).
Discussion
The expression pattern and function of METTL3 in
ESCC has previously not been reported. The present study
demonstrated that METTL3 was significantly upregulated in ESCC,
which was associated with ESCC progression and poorer prognoses,
and that the genetic knockdown of METTL3 with siRNA inhibited ESCC
cell viability, colony formation, cellular migration and invasion,
induced apoptosis, and decreased PI3K/AKT signalling.
The upregulation of, and the oncogenic function of
METTL3 has been reported in various types of human cancer (16-18).
For example, METTL3 is upregulated in bladder cancer tissues, and
promotes bladder cancer progression via regulation of AF4/FR2
family member 4/NF-κB/MYC signaling (17). METTL3 is upregulated in ovarian
carcinoma; it promotes the malignant phenotype of ovarian cancer
cells and increases tumour formation in nude mice (18). Regarding the underlying molecular
mechanism, a previous study reported that METTL3 promoted mRNA
translation of multiple oncogenes, such as the epidermal growth
factor receptor and the Hippo pathway effector TAZ, through
recruiting translation initiation factors in human cancer cells
(11). In addition, Choe et
al (24) identified a direct
interaction between METTL3 and eukaryotic translation initiation
factor 3 subunit H and revealed that this interaction served a
crucial role in translation, densely packed polyribosome formation
and oncogenic transformation. However, the exact role of METTL3 in
ESCC remains to be elucidated. In the present study, it was
demonstrated that the mRNA and protein expression levels of METTL3
were significantly increased in tumour tissues and cell lines
compared with adjacent normal tissues and the normal oesophageal
epithelial cell line, HET-1A. It was further observed that METTL3
upregulation was associated with advanced TNM stage, metastasis and
poorer prognoses in ESCC. These findings suggested that
upregulation of METTL3 may contribute to the malignant progression
of ESCC, and that METTL3 expression may be used as a predictor for
worse outcomes of patients with ESCC.
To further clarify the exact role of METTL3 in ESCC,
TE-9 and Eca-109 cell lines were selected for further in
vitro experiments owing to their high METTL3 expression levels.
As METTL3 is upregulated in ESCC cells, TE-9 and Eca-109 cells were
transfected with METTL3-specific siRNAs to knockdown its
expression. Downregulating METTL3 significantly inhibited ESCC cell
viability and colony formation. Similarly, Lin et al
(15) reported that METTL3 promoted
the proliferation of gastric cancer cells. In addition, knockdown
of METTL3 drastically reduced bladder cancer cell proliferation and
survival in vitro and tumorigenicity in vivo
(17). Overexpression of METTL3
significantly promoted ovarian cancer cell proliferation in
vitro as well as tumor formation in nude mice (18). Therefore, the present findings
suggested that METTL3 plays a promoting role in ESCC growth. As
reduced cell viability may be due to increased cellular apoptosis,
the effects of METTL3 downregulation on ESCC apoptosis were
studied. The data demonstrated that apoptosis of TE-9 and Eca-109
cells was significantly higher following METTL3 gene knockdown.
These findings suggested that METTL3 may inhibit cell apoptosis.
Similarly, knockdown of METTL3 also induced breast cancer cell
apoptosis (21). To further confirm
this, the expression levels of several key factors associated with
cell apoptosis, including pro-apoptotic Bax and caspase-3 and
anti-apoptotic Bcl2 (25,26) were evaluated. METTL3 inhibition
upregulated Bax and caspase-3, and downregulated Bcl-2 in ESCC
cells, consistent with the apoptosis assay results. Cancer cell
migration and invasion are two key processes of tumour metastasis
(27,28); since METTL3 upregulation was
associated with ESCC progression in the clinical samples, the
function of METTL3 in regulating ESCC cell migration and invasion
was also studied in vitro. Gene silencing of METTL3
significantly inhibited the migratory and invasive ability of ESCC
cells, suggesting that METTL3 had a promoting role in ESCC
migration and invasion in vitro.
It has been well established that the PI3K/AKT
signalling pathway serves a crucial role in most aspects of tumour
growth and metastasis, and the inactivation of this signalling
pathway could effectively inhibit the malignant phenotypes of ESCC
cells (29,30). Wang et al (29) reported that the long non-coding RNA,
growth-arrest specific 5, suppressed ESCC cell viability and
migration through inactivation of the PI3K/AKT signalling pathway.
He et al (31) demonstrated
that circRNA VRK serine/threonine kinase 1 inhibited ESCC
progression and radioresistance through regulating the expression
of microRNA-624-3p, in addition to the activity of the PI3K/AKT
signalling pathway. The present study reported that METTL3
knockdown significantly reduced the phosphorylation levels of PI3K
and AKT, indicating that the PI3K/AKT signalling pathway was
inactivated. Similarly, knockdown of METTL3 inhibited the
expression and phosphorylation of proteins involved in the PI3K
signaling pathway in lung cancer cells (16). These findings suggested that the
PI3K/AKT signalling pathway may serve an important role in
METTL3-mediated effects in ESCC cells. One limitation of this study
is that the clinical sample number was small and more patients
should be included in future studies. Moreover, the relationship
between METTL3 and AKT should be studied using clinical samples in
future studies.
In conclusion, it was demonstrated that upregulation
of METTL3 promoted ESCC progression and that inhibition of METTL3
significantly suppressed the malignant phenotype of ESCC cells, at
least in part, through downregulating PI3K/AKT signalling. This
study may help elucidate the molecular mechanism underlying ESCC
progression.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WH designed the study, wrote and revised the
manuscript. WL, HL, CZ, MZ and BZ performed all the experiments and
the statistical analysis. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Second Xiangya Hospital (Changsha, China). Written informed consent
was obtained from all patients.
Patient consent for publication
All participants provided written informed consent
for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhang J, Yu H, Zhang Y, Zhang X, Zheng G,
Gao Y, Wang C and Zhou L: A functional TNFAIP2 3'-UTR rs8126
genetic polymorphism contributes to risk of esophageal squamous
cell carcinoma. PLoS One. 9(e109318)2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Toh Y, Egashira A and Yamamoto M:
Epigenetic alterations and their clinical implications in
esophageal squamous cell carcinoma. Gen Thorac Cardiovasc Surg.
61:262–269. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wang M, Smith JS and Wei WQ: Tissue
protein biomarker candidates to predict progression of esophageal
squamous cell carcinoma and precancerous lesions. Ann N Y Acad Sci.
1434:59–69. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hua Y, Zhao K, Tao G, Dai C and Su Y:
miR-25 promotes metastasis via targeting FBXW7 in esophageal
squamous cell carcinoma. Oncol Rep. 38:3030–3038. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Du YY, Zhao LM, Chen L, Sang MX, Li J, Ma
M and Liu JF: The tumor-suppressive function of miR-1 by targeting
LASP1 and TAGLN2 in esophageal squamous cell carcinoma. J
Gastroenterol Hepatol. 31:384–393. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhu Y, Ma Y, Peng H, Gong L, Xiao M, Xiang
L, He D and Cao K: MiR-130b promotes the progression of oesophageal
squamous cell carcinoma by targeting SASH1. J Cell Mol Med.
23:93–103. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yu X, Li W, Xia Z, Xie L, Ma X, Liang Q,
Liu L, Wang J, Zhou X, Yang Y and Liu H: Targeting MCL-1 sensitizes
human esophageal squamous cell carcinoma cells to cisplatin-induced
apoptosis. BMC Cancer. 17(449)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang SY, Zhang SW, Fan XN, Meng J, Chen
Y, Gao SJ and Huang Y: Global analysis of N6-methyladenosine
functions and its disease association using deep learning and
network-based methods. PLoS Comput Biol.
15(e1006663)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lin S, Choe J, Du P, Triboulet R and
Gregory RI: The m(6)A methyltransferase METTL3 promotes translation
in human cancer cells. Mol Cell. 62:335–345. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang
L, Jia G, Yu M, Lu Z, Deng X, et al: A METTL3-METTL14 complex
mediates mammalian nuclear RNA N-6-adenosine methylation. Nat Chem
Biol. 10:93–95. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ping XL, Sun BF, Wang L, Xiao W, Yang X,
Wang WJ, Adhikari S, Shi Y, Lv Y, Chen YS, et al: Mammalian WTAP is
a regulatory subunit of the RNA N6-methyladenosine
methyltransferase. Cell Res. 24:177–189. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Schwartz S, Mumbach MR, Jovanovic M, Wang
T, Maciag K, Bushkin GG, Mertins P, Ter-Ovanesyan D, Habib N,
Cacchiarelli D, et al: Perturbation of m6A writers reveals two
distinct classes of mRNA methylation at internal and 5' sites. Cell
Rep. 8:284–296. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lin S, Liu J, Jiang W, Wang P, Sun C, Wang
X, Chen Y and Wang H: METTL3 promotes the viability and mobility of
gastric cancer cells. Open Med (Wars). 14:25–31. 2019.
|
|
16
|
Wei W, Huo B and Shi X: miR-600 inhibits
lung cancer via downregulating the expression of METTL3. Cancer
Manag Res. 11:1177–1187. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cheng M, Sheng L, Gao Q, Xiong Q, Zhang H,
Wu M, Liang Y, Zhu F, Zhang Y, Zhang X, et al: The m6A
methyltransferase METTL3 promotes bladder cancer progression via
AFF4/NF-κB/MYC signaling network. Oncogene. 38:3667–3680.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hua W, Zhao Y, Jin X, Yu D, He J, Xie D
and Duan P: METTL3 promotes ovarian carcinoma growth and invasion
through the regulation of AXL translation and epithelial to
mesenchymal transition. Gynecol Oncol. 151:356–365. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Taketo K, Konno M, Asai A, Koseki J,
Toratani M, Satoh T, Doki Y, Mori M, Ishii H and Ogawa K: The
epitranscriptome m6A writer METTL3 promotes chemo- and
radioresistance in pancreatic cancer cells. Int J Oncol.
52:621–629. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Dahal U, Kang L and Gupta M: RNA m6A
methyltransferase METTL3 regulates invasiveness of melanoma cells
by matrix metallopeptidase 2. Melanoma Res. 29:382–389.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cai X, Wang X, Cao C, Gao Y, Zhang S, Yang
Z, Liu Y, Zhang X, Zhang W and Ye L: HBXIP-elevated
methyltransferase METTL3 promotes the progression of breast cancer
via inhibiting tumor suppressor let-7g. Cancer Lett. 415:11–19.
2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li X, Tang J, Huang W, Wang F, Li P, Qin
C, Qin Z, Zou Q, Wei J, Hua L, et al: The M6A methyltransferase
METTL3: Acting as a tumor suppressor in renal cell carcinoma.
Oncotarget. 8:96103–96116. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Choe J, Lin S, Zhang W, Liu Q, Wang L,
Ramirez-Moya J, Du P, Kim W, Tang S, Sliz P, et al: mRNA
circularization by METTL3-eIF3h enhances translation and promotes
oncogenesis. Nature. 561:556–560. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lin H, Wang Z, Shen J, Xu J and Li H:
Intravenous anesthetic ketamine attenuates complete Freund's
adjuvant-induced arthritis in rats via modulation of MAPKs/NF-κB.
Inflamm Res. 68:147–155. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ouyang G, Xiong L, Liu Z, Lam B, Bui B, Ma
L, Chen X, Zhou P, Wang K, Zhang Z, et al: Inhibition of autophagy
potentiates the apoptosis-inducing effects of photodynamic therapy
on human colon cancer cells. Photodiagnosis Photodyn Ther.
21:396–403. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhou K, Zhang C, Yao H, Zhang X, Zhou Y,
Che Y and Huang Y: Knockdown of long non-coding RNA NEAT1 inhibits
glioma cell migration and invasion via modulation of SOX2 targeted
by miR-132. Mol Cancer. 17(105)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li T, Zhou W, Li Y, Gan Y, Peng Y, Xiao Q,
Ouyang C, Wu A, Zhang S, Liu J, et al: MiR-4524b-5p/WTX/β-catenin
axis functions as a regulator of metastasis in cervical cancer.
PLoS One. 14(e0214822)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang G, Sun J, Zhao H and Li H: Long
non-coding RNA (lncRNA) growth arrest specific 5 (gas5) suppresses
oesophageal squamous cell carcinoma cell proliferation and
migration by inactivating phosphatidylinositol 3-kinase
(PI3K)/AKT/mammalian target of rapamycin (mTOR) signaling pathway.
Med Sci Monit. 24:7689–7696. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
He B, Peng F, Li W and Jiang Y:
Interaction of lncRNA-MALAT1 and miR-124 regulates HBx-induced
cancer stem cell properties in HepG2 through PI3K/Akt signaling. J
Cell Biochem. 120:2908–2918. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
He Y, Mingyan E, Wang C, Liu G, Shi M and
Liu S: CircVRK1 regulates tumor progression and radioresistance in
esophageal squamous cell carcinoma by regulating
miR-624-3p/PTEN/PI3K/AKT signaling pathway. Int J Biol Macromol.
125:116–123. 2019.PubMed/NCBI View Article : Google Scholar
|