Introduction
Burns are the most common form of soft tissue injury
and can result in extensive wound injuries while increasing the
risk of infection, systemic inflammation and sepsis in patients;
moreover, the incidence of severe infection complications after
burns increases mortality by 40% (1,2).
Generally, patients will enter into a high metabolic state
following a burn, with an accelerated metabolic rate; such injury
lasting for several years will result in massive lean muscle loss,
immune damage and delayed wound healing (3). The treatment objective in burn
patients is to prevent infection and optimize recovery function
(4). Although deep burn wounds are
be removed as soon as possible and local antibiotics and dressings
are used in time, the treatment methods of patients vary due to
their different clinical characteristics. In addition, the needs of
patients with burn are specific, while contrary to this, early
treatment is often empirical, which delays effective treatment.
Furthermore, certain patients develop resistance to treatment,
leading to decreased efficacy (5).
Therefore, better indicators are urgently needed to fill the gap of
lack of biological indicators to predict efficacy (6).
Serum hypoxia-inducible factor-1α (HIF-1α), which
plays a role in the process of angiogenesis and healing in
patients, changes with the cell's perception of oxygen, and its
level reflects the cell's oxygen content (7-9).
HIF-1α level is often higher than normal under hypoxic conditions,
and when it rises, it further activates vascular endothelial growth
factor (VEGF) (10,11). VEGF is a more specific angiogenic
factor that promotes the growth of vascular endothelial cells,
which can not only drive but also promote angiogenesis (12). It is also associated with metastasis
and angiogenesis of numerous tumors, and therefore can be used as a
predictive indicator for certain tumors (13,14).
For example, Basagiannis et al (15) showed that VEGF induced VEGFR2
internalization through macrophage phagocytosis, which led to the
activation of the neovascularization signaling pathway driven by
VEGFR2 and angiogenesis. In addition, studies have shown that VEGF
binds to the VEGF receptor on the endothelial cell membrane,
causing autophosphorylation of the receptor, which in turn
activates MAPK and realizing the mitogen characteristics of VEGF,
thereby inducing endothelial cell proliferation (16,17).
However, the expression levels and predictive value of HIF-1α and
VEGF in patients with burns after treatment remain poorly
understood at present.
Therefore, the present study detected the expression
levels of HIF-1α and VEGF in burn patients after treatment, and
observed their predictive value of curative effect, so as to
provide the basis and direction for clinical practice.
Materials and methods
Patients
In total, 84 patients with burns, treated in Jinan
City People's Hospital (Jinan, China) between June 2015 and August
2017, were selected as the study participants, including 48 males
and 36 females, with an average age of 48.3±9.5 years. The present
study was approved by the Ethics Committee of Jinan City People's
Hospital (Jinan, China) and all patients provided signed informed
consent.
Inclusion and exclusion criteria
The inclusion criteria were as follows: i) All
participants presented with mild or moderate burns for the first
time, without prior amputation; ii) participants were willing to
cooperate with the treatment and follow-up; iii) patients had
complete clinical data; and iv) patients had a life expectancy of
>3 months.
The exclusion criteria were patients with: i)
Tumors; ii) acute infectious disease; iii) severe burns iv) liver
or kidney dysfunction; v) complications associated with sepsis; vi)
other severe inflammation; or vii) diabetes.
Reagents and instruments
HIF-1α ELISA detection kit (cat. no. E-EL-H6066) and
VEGF ELISA assay kit (cat. no. E-EL-H1601c) were purchased from
Elabscience Biotechnology Co., Ltd. Moisturizing Burn Cream was
obtained from Mebo Pharmaceutical Co., Ltd.
Treatment efficacy determination
Wound healing rates of >95% was considered as
wound healing. Ineffective treatment efficacy was defined by poor
growth of granulation tissue on the wound surface, with a wound
healing area of <50%. If the wound healing rate was ≥50%, the
therapeutic efficacy was considered to be effective. Wound healing
rate (%) = (total wound area before treatment-total wound area
after treatment)/total wound area before treatment x 100%.
Treatment methods
After the wound was cleaned with Iodophor mixed with
0.9% saline (1:5), the moisture exposed burn ointment (MEBO) was
soaked in sterile gauze and applied on the wound evenly, and the
outer layer was wrapped with medical gauze to ensure full drainage
of the wound exudate. During initial stages of exudate, the
dressing was changed twice a day, and decreased to once a day when
the wound was clean. Patients were treated continuously for 21
days. During the treatment, the wound was kept clean to prevent
infection, and the dressing was changed in time if there was any
abnormality such as the red, swollen or unclean wound.
Sample collection and ELISA test
Aseptic venous blood (5 ml) was collected from
patients at 7 a.m. the next day after admission and at 7 a.m. the
first day following the 21-day treatment regime and placed in a
coagulant tube. Subsequently, the samples were immediately
centrifuged at 3,000 x g at 4˚C for 10 min to separate the serum
and then stored in the refrigerator at -80˚C. ELISA kits (cat. nos.
ab171577 and ab233625; Abcam), was employed to determine HIF-1α and
VEGF levels. The dilution concentrations of VEGF standard samples
were 4,000, 2,000, 1,000, 500, 250, 125, 62.50 and 0 pg/ml, and the
configuration concentrations of HIF-1 were 2,000, 1,000, 500, 250,
125, 62.5, 31.25 and 0 pg/ml. Blank, standard and sample wells to
be tested were set, in which 100 µl sample diluent was added to the
blank wells, 100 µl standard substance was added to the standard
wells and 100 µl sample was added to the sample wells to be tested.
The ELISA plate was coated and incubated at 37˚C for 90 min. After
removing and shaking the liquid in the wells, 100 µl biotinylated
antibody working solution was added into each well, and the ELISA
plate was coated with VEGF and HIF-1 antibody and incubated at 37˚C
for 1 h. Subsequently, the liquid in wells were removed and plates
were washed three times. The liquid was pat dry and 100 µl enzyme
conjugate added to each well and incubated at 37˚C for 30 min after
coating. Following which, the solution was dried, and the plate was
washed five times, followed by the addition of 90 µl chromogenic
reagent and a 15-min incubation in the dark at 37˚C after coating
with enzyme binding buffer. Next, 50 µl termination solution was
added to each well, and the optical density value of each well was
determined at a wavelength of 450 nm within 15 min. The
concentration was of HIF-1α and VEGF in serum was then
calculated.
Outcome measures
The HIF-1α and VEGF levels were measured before and
after treatment. All the patients were grouped according to the
treatment efficacy after treatment: Patients with effective
curative effects were included in the effective group, while
patients with ineffective curative effects were included in the
ineffective group, and their pre-treatment HIF-1 and VEGF levels
were compared. In addition, the predictive value of HIF-1α and VEGF
in therapeutic efficacy was evaluated using an ROC curve, and the
independent risk factors affecting treatment inefficacy were
analyzed via multivariate logistic regression.
Statistical analysis
The data were statistical analyzed using SPSS 20.0
(IBM Corp.), and the required images were plotted using GraphPad
Prism 7 (GraphPad Software, Inc.). The counting data represented by
percentage (%) were compared using the χ2 test. The
Kolmogorov-Smirnov test was employed to analyze the distribution of
the data. The data were expressed as mean ± standard deviation
(SD). All the measurement data conformed to the normal
distribution. Comparisons between the same group before and after
treatment was performed using paired t-test, and those between two
groups were performed using an independent sample Student's t-test,
expressed as t. ROC curves were constructed to evaluate the
predictive value of HIF-1α and VEGF in terms of treatment efficacy.
P<0.05 indicated that there was a statistical difference between
the two groups.
Results
Clinical data
The clinical data of patients were collected,
including sex, age, BMI, burn degree, wound area, treatment
efficacy, residence, smoking history and alcoholism history.
(Table I).
 | Table IClinical data of patients (n=84). |
Table I
Clinical data of patients (n=84).
| Characteristic | Value |
|---|
| Sex, n (%) | |
|
Male | 48 (57.14) |
|
Female | 36 (42.86) |
| Age (years), mean ±
standard deviation | 48.3±9.5 |
| BMI
(kg/m2), mean ± standard deviation | 21.23±2.14 |
| Burn degree, n
(%) | |
|
Mild | 57 (67.86) |
|
Moderate | 27 (32.14) |
| Wound area, n
(%) | |
|
≤15% | 66 (78.57) |
|
>15% | 18 (21.43) |
| Residence, n (%) | |
|
Urban | 72 (85.71) |
|
Rural | 12 (14.29) |
| Treatment efficacy, n
(%) | |
|
Effective | 68 (80.95) |
|
Ineffective | 16 (19.05) |
| Smoking history, n
(%) | |
|
Yes | 21 (25.00) |
|
No | 63 (75.00) |
| Alcoholism history, n
(%) | |
|
Yes | 17 (20.23) |
|
No | 67 (79.76) |
| Type of burn, n
(%) | |
|
Thermal
burn | 58 (69.05) |
|
Electrical
burn | 14 (16.67) |
|
Chemical
burn | 12 (14.28) |
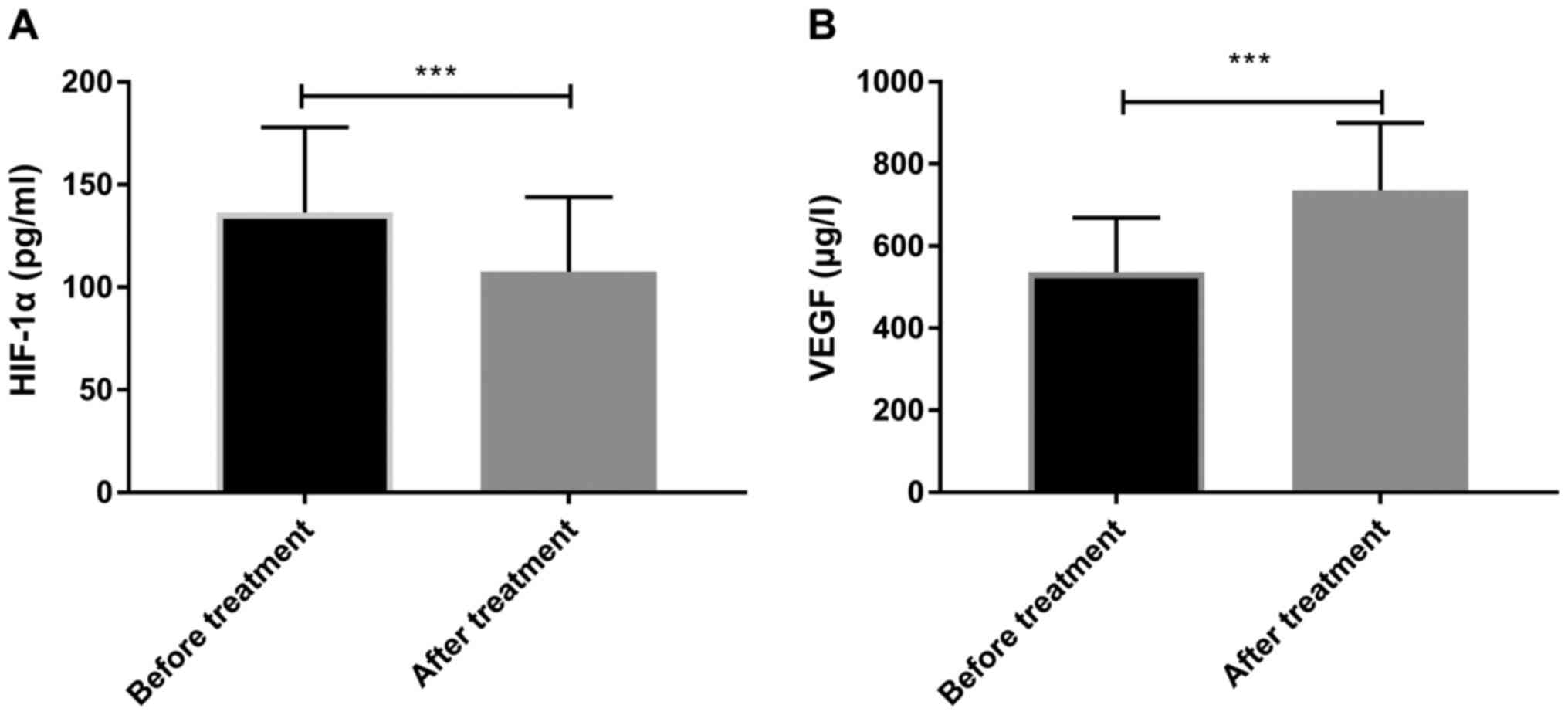
Changes in HIF-1α and VEGF before and
after treatment
By observing the changes of HIF-1α and VEGF before
and after treatment in all patients, it was revealed that serum
HIF-1α levels (107.54±36.38) were significantly lower following
treatment compared with the levels before treatment (136.36±41.54)
(P<0.05), while VEGF (735.26±164.36) was significantly higher
compared with before treatment (536.13±132.36) (P<0.05)
(Fig. 1).
Predictive value of HIF-1α and VEGF
for treatment efficacy
The comparison of HIF-1α and VEGF expression levels
before treatment in patients with effective and ineffective
treatment revealed that patients in the ineffective group had
significantly higher HIF-1α (P<0.05), and significantly lower
VEGF levels than those of patients in the effective group
(P<0.05). The ROC curve exhibited that the AUC of HIF-1α was
0.795, and that of VEGF was 0.826, while the AUC of their joint
detection was 0.847. (Fig. 2 and
Table II)
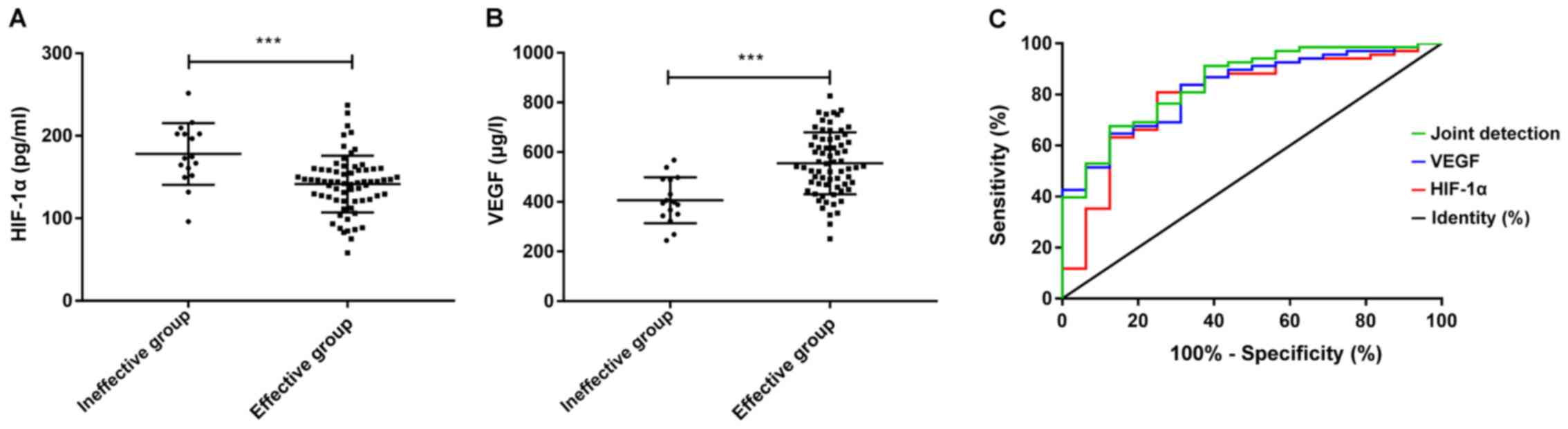 | Figure 2Predictive value of HIF-1α and VEGF
for treatment efficacy. (A) The level of HIF-1α in the ineffective
group was significantly higher compared with the effective group
(t=3.767, P<0.001). (B) VEGF levels in the ineffective group was
significantly lower compared with the effective group (t=4.542,
P<0.001). ***P<0.001. (C) The AUC of HIF-1α for
treatment efficacy was 0.795, and when the cut-off point was
161.757, its optimal specificity and sensitivity were 68.75 and
80.88%, and the Youden index was 49.63%. The AUC of VEGF for
treatment efficacy was 0.826, and when the cut-off point was
437.406, the optimal specificity and sensitivity were 68.75 and
82.35% respectively, and the Youden index was 51.10%. While the AUC
of the joint detection for treatment efficacy was 0.847, and when
the cut-off point was set as 0.847, the optimal specificity and
sensitivity were 87.50 and 66.18% and the Youden index was 53.68%.
HIF, hypoxia-inducible factor; VEGF, vascular endothelial growth
factor; AUC, area under the curve. |
 | Table IIReceiver operating characteristic
data. |
Table II
Receiver operating characteristic
data.
| Parameter | Area under the
curve | 95% CI | Specificity (%) | Sensitivity (%) | Youden index (%) | Cut-off value |
|---|
| HIF-1α | 0.795 | 0.666-0.924 | 68.75 | 80.88 | 49.63 | <161.757 |
| VEGF | 0.826 | 0.725-0.928 | 68.75 | 82.35 | 51.10 | >437.406 |
| Joint detection | 0.847 | 0.746-0.947 | 87.50 | 66.18 | 53.68 | >0.847 |
Univariate analysis of treatment
inefficacy in patients
The clinical data of patients in the effective group
and the ineffective group were collected and analyzed via
univariate analysis. It was revealed that there were no significant
differences in sex, age, BMI, residence, smoking or alcoholism
between the two groups; however, the burn degree, wound area,
HIF-1α level before treatment and VEGF level before treatment
differed significantly between the groups (P<0.05) (Table III).
 | Table IIIUnivariate analysis of treatment
efficacy. |
Table III
Univariate analysis of treatment
efficacy.
| Characteristic | Effective group
(n=68) | Ineffective group
(n=16) |
t/χ2-value | P-value |
|---|
| Sex, n (%) | | | 0.412 | 0.521 |
|
Male | 40 (58.82) | 8 (50.00) | | |
|
Female | 28 (41.18) | 8 (50.00) | | |
| Age (years) | 47.8±8.6 | 49.8±6.2 | 0.876 | 0.383 |
| BMI
(kg/m2) | 21.28±1.65 | 20.81±1.15 | 1.007 | 2.285 |
| Burn degree, n
(%) | | | 8.351 | 0.004 |
|
Mild | 51 (75.00) | 6 (37.50) | | |
|
Moderate | 17 (25.00) | 10 (62.50) | | |
| Wound area, n
(%) | | | 5.849 | 0.016 |
|
≤15% | 57 (83.82) | 9 (56.25) | | |
|
>15% | 11 (16.18) | 7 (43.75) | | |
| Residence, n
(%) | | | 0.322 | 0.571 |
|
Urban | 59 (86.76) | 13 (81.25) | | |
|
Rural | 9 (13.24) | 3 (18.75) | | |
| Smoking history, n
(%) | | | 0.412 | 0.521 |
|
Yes | 16 (23.53) | 5 (31.25) | | |
|
No | 52 (76.47) | 11 (68.75) | | |
| Alcoholism history,
n (%) | | | 0.278 | 0.598 |
|
Yes | 13 (19.12) | 4 (25.00) | | |
|
No | 55 (80.88) | 12 (75.00) | | |
| Serum HIF-1α level
before treatment (pg/ml) | 141.56±34.33 | 178.10±37.39 | 3.767 | <0.001 |
| Serum VEGF level
before treatment (µg/l) | 555.17±124.76 | 406.35±92.44 | 4.482 | <0.001 |
Multivariate analysis of treatment
inefficacy
The indicators with significant differences in the
univariate analysis were included in the assignment (see Table IV for the assignment table), and
multivariate analysis was performed using the logistics regression
equation. The results indicated that inefficacy of treatment was
not associated with wound area, but was associated with burn degree
[odds ratio (OR), 6.026; 95% CI, 3.572-9.247], HIF-1α level before
treatment (OR, 3.475; 95% CI, 1.386-6.834), and VEGF level before
treatment (OR, 3.367; 95% CI, 1.175-8.266) (Table V).
 | Table IVAssignment table. |
Table IV
Assignment table.
| Factors | Assignments |
|---|
| Burn degree | Moderate=1,
mild=0 |
| Wound area | >15%=1,
≤15%=0 |
| HIF-1α level before
treatment | Raw data analysis
for continuous variables |
| VEGF level before
treatment | Raw data analysis
for continuous variables |
| Treatment
efficacy | Ineffective=1,
effective=0 |
 | Table VMultivariate analysis of
survival. |
Table V
Multivariate analysis of
survival.
| | | | | | | 95% CI for Exp
(B) |
|---|
| Factors | B | SE | Wals | Sig. | Exp (B) | Lower | Upper |
|---|
| Burn degree | 1.796 | 0.097 | 8.475 | 0.008 | 6.026 | 3.572 | 9.247 |
| Serum HIF-1α level
before treatment | 1.237 | 0.026 | 5.649 | 0.004 | 3.475 | 1.386 | 6.834 |
| Serum VEGF level
before treatment (µg/l) | 1.245 | 0.118 | 5.287 | 0.003 | 3.367 | 1.175 | 8.266 |
Discussion
Human skin serves immune and metabolic functions,
while maintaining homeostasis in the human body, stabilizing body
temperature and protecting the body from infection. Notably, when
heat causes a large area of skin rupture, the physiological
functions of the skin will change, increasing the risk of wound or
systemic infection (18). HIF-1α
and VEGF are factors associated angiogenesis. Pagani et al
(19) reported that HIF-1α
upregulation significantly enhanced tissue regeneration and
promoted aging skin renewal and wound healing.
In the present study, the changes in serum HIF-1α
and VEGF levels were compared in patients before and after
treatment. It was revealed that following treatment, the HIF-1α
level had significantly decreased, while the VEGF level increased.
The reason behind the elevated expression of VEGF may be that the
patients' skin was in a state of slow healing. However, in recent
years, certain studies have also reported that the increase of VEGF
is not beneficial to all burn patients. For instance, if the VEGF
increases significantly after ocular alkali burn, the promotion of
angiogenesis will result in the neovascularization of the cornea
and damage the patient's vision, in which case anti-VEGF therapy
should be implemented (20). While
HIF-1α is primarily and substantially expressed in skin wounds
under anoxic conditions (21), and
its decreased expression in the current study further suggested
that the hypoxic state of the skin wound was further improved in
the treatment process. Wound growth under hypoxic conditions may
result in excessive growth of fibrous tissue and develop into
scarring. Lei et al (22)
reported that hypoxia-induced HIF-1α expression significantly
inhibited apoptosis and promoted cell proliferation in hypertrophic
scar fibroblasts, but not in normal fibroblasts. Moreover, the
overexpression of HIF-1α can also cause endothelial barrier
dysfunction, which may give rise to decreased vascular permeability
and adversely affect patient recovery (23,24).
Therefore, treatments aim to reduce HIF-1α levels and prevent the
formation of hypertrophic scar after burns (25).
Subsequently, the pre-treatment expression levels of
HIF-1α and VEGF were compared between patients with effective and
ineffective treatment, and it was revealed that the expression of
HIF-1α was significantly higher and VEGF was lower in the
ineffective group compared with the effective group, suggesting
that the levels of HIF-1α and VEGF before treatment may be a
predictor of patients' treatment efficacy. Therefore, the ROC curve
was constructed to test their predictive value. It was revealed
that the AUC of HIF-1α for treatment efficacy was 0.795, and the
optimal specificity and sensitivity were 68.75 and 80.88% when the
cut-off point was 161.757, while the AUC of VEGF was 0.826, and the
optimal specificity and sensitivity were 68.75 and 82.35% when the
cut-off point was 437.406, which also indicated that HIF-1α and
VEGF levels before treatment may predict the efficacy of treatment
in patients. Moreover, differences were identified between the
specificity and sensitivity of the two markers and, therefore, an
assessment of the diagnostic value of measuring the levels of both
markers was performed. The AUC of the joint detection was 0.847,
and the optimal specificity and sensitivity were 87.50 and 66.18%,
respectively, when the cut-off point was 0.847, which was
indicative that the differences were narrowed by joint detection.
Subsequently, a multivariate analysis was performed based on the
clinical data of patients with effective and ineffective treatment.
This revealed a higher HIF-1α level, lower VEGF level and higher
burn degree were independent risk factors for treatment
inefficacy.
However, there are also certain limitations to the
present study. Primarily, the participants enrolled in this study
were all burn patients without any healthy controls selected, which
resulted in the poor understanding of the difference in indicators
between the patients in this study and the normal population.
Secondly, the present study excluded patients with severe burns.
Compared with patients with mild or moderate burns, patients with
severe burns are more prone to infection and shock. Therefore, it
is hoped that patients with severe burns will be studied in the
follow-up research to improve the conclusions of the present study.
Thirdly, it has been reported in previous studies that cytokines
produced as a result of hypoxic conditions in wounds, or
inflammatory cells in excised wounds, can also regulate the
activity of HIF; however, whether this has any impact on the
present results remains unclear (26,27).
Finally, the current study did not further explore the mechanisms
underlying the influence of HIF-1α and VEGF on burn patients, which
represents a target of future research.
Taken together, HIF-1α level will decrease and VEGF
expression will increase in burn patients after treatment, and
HIF-1α and VEGF levels before treatment may be of predictive value
for treatment efficacy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL conceived the study. LL and XC collected and
analyzed data. LG helped perform statistical analysis. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The current study was approved by the Ethics
Committee of Jinan City People's Hospital, China. Patients who
participated in this research, signed the informed consent and had
complete clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rowan MP, Cancio LC, Elster EA, Burmeister
DM, Rose LF, Natesan S, Chan RK, Christy RJ and Chung KK: Burn
wound healing and treatment: Review and advancements. Crit Care.
19(243)2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kraft R, Herndon DN, Finnerty CC, Cox RA,
Song J and Jeschke MG: Predictive value of IL-8 for sepsis and
severe infections after burn injury - A clinical. study. Shock.
43:222–227. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Clark A, Imran J, Madni T and Wolf SE:
Nutrition and metabolism in burn patients. Burns Trauma.
5(11)2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Oryan A, Alemzadeh E and Moshiri A: Burn
wound healing: Present concepts, treatment strategies and future.
directions. J Wound Care. 26:5–19. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hidalgo F, Mas D, Rubio M and
Garcia-Hierro P: Infections in critically ill burn patients. Med
Intensiva. 40:179–185. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Saaiq M, Ahmad S and Zaib MS: Burn wound
infections and antibiotic susceptibility patterns at Pakistan
institute of medical sciences, Islamabad, Pakistan. World J Plast
Surg. 4:9–15. 2015.PubMed/NCBI
|
|
7
|
Park SY, Lee SW, Kim HY, Lee WS, Hong KW
and Kim CD: HMGB1 induces angiogenesis in rheumatoid arthritis via
HIF-1α activation. Eur J Immunol. 45:1216–1227. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kim Y, Nam HJ, Lee J, Park DY, Kim C, Yu
YS, Kim D, Park SW, Bhin J, Hwang D, et al: Methylation-dependent
regulation of HIF-1α stability restricts retinal and tumour
angiogenesis. Nat Commun. 7(10347)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
MacLauchlan SC, Calabro NE, Huang Y,
Krishna M, Bancroft T, Sharma T, Yu J, Sessa WC, Giordano F and
Kyriakides TR: HIF-1α represses the expression of the angiogenesis
inhibitor thrombospondin-2. Matrix Biol. 65:45–58. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang X, Liu L, Wei X, Tan YS Tong L,
Chang R, Ghanamah MS, Reinblatt M, Marti GP, Harmon JW and Semenza
GL: Impaired angiogenesis and mobilization of circulating
angiogenic cells in HIF-1alpha heterozygous-null mice after burn
wounding. Wound Repair Regen. 18:193–201. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Banyard DA, Adnani BO, Melkumyan S,
Araniego CA and Widgerow AD: Endothelial progenitor cells and burn
injury-exploring the relationship. Burns Trauma.
4(4)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rumney RMH, Tozzi G, Kao A, Lanham SA,
Kanczler JM, Kao AP, Thiagarajan L, Dixon JE, Tozzi G and Oreffo
ROC: In vivo delivery of VEGF RNA and protein to increase
osteogenesis and intraosseous angiogenesis. Sci Rep.
9(17745)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Feng Q, Zhang C, Lum D, Druso JE, Blank B,
Wilson KF, Welm A, Antonyak MA and Cerione RA: A class of
extracellular vesicles from breast cancer cells activates VEGF
receptors and tumour angiogenesis. Nat Commun.
8(14450)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Morland C, Andersson KA, Haugen ØP, Hadzic
A, Kleppa L, Gille A, Rinholm JE, Palibrk V, Diget EH, Kennedy LH,
et al: Exercise induces cerebral VEGF and angiogenesis via the
lactate receptor HCAR1. Nat Commun. 8(15557)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Basagiannis D, Zografou S, Murphy C,
Fotsis T, Morbidelli L, Ziche M, Bleck C, Mercer J and
Christoforidis S: VEGF induces signalling and angiogenesis by
directing VEGFR2 internalisation through macropinocytosis. J Cell
Sci. 129:4091–4104. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Grad S, Ertel W, Keel M, Infanger M,
Vonderschmitt DJ and Maly FE: Strongly enhanced serum levels of
vascular endothelial growth factor (VEGF) after poly-trauma and
burn. Clin Chem Lab Med. 36:379–383. 1998.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hwang C, Ucer S, Sorkin M, Loder S, Chung
MT, Pagani C, Li J, Priest C, Breuler C, Vasquez K, et al:
Identifying the role of and treatment targeting bone progenitor
cell VEGF secretion on the niche supporting traumatic heterotopic
ossification. Plast Reconstr Surg Glob Open. 6:21–22. 2018.
|
|
18
|
Church D, Elsayed S, Reid O, Winston B and
Lindsay R: Burn wound infections. Clin Microbiol Rev. 19:403–434.
2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pagani A, Aitzetmüller MM, Brett EA, König
V, Wenny R, Thor D, Radtke C, Huemer GM, Machens HG and Duscher D:
Skin rejuvenation through HIF-1α modulation. Plast Reconstr Surg.
141:600e–607e. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chen L, Zhong J, Li S, Li W, Wang B, Deng
Y and Yuan J: The long-term effect of tacrolimus on alkali
burn-induced corneal neovascularization and inflammation surpasses
that of anti-vascular endothelial growth factor. Drug Des Devel
Ther. 12:2959–2969. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ciarlillo D, Celeste C, Carmeliet P,
Boerboom D and Theoret C: A hypoxia response element in the Vegfa
promoter is required for basal Vegfa expression in skin and for
optimal granulation tissue formation during wound healing in mice.
PLoS One. 12(e0180586)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lei R, Li J, Liu F, Li W, Zhang S, Wang Y,
Chu X and Xu J: HIF-1α promotes the keloid development through the
activation of TGF-β/Smad and TLR4/MyD88/NF-κB pathways. Cell Cycle.
18:3239–3250. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Qi H, Wang P, Liu C, Li M, Wang S, Huang Y
and Wang F: Involvement of HIF-1α in MLCK-dependent endothelial
barrier dysfunction in hypoxia. Cell Physiol Biochem. 27:251–262.
2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tian KY, Liu XJ, Xu JD, Deng LJ and Wang
G: Propofol inhibits burn injury-induced hyperpermeability through
an apoptotic signal pathway in microvascular endothelial cells.
Braz J Med Biol Res. 48:401–407. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wu X, Li J, Yang X, Bai X, Shi J, Gao J,
Li Y, Han S, Zhang Y, Han F, et al: miR-155 inhibits the formation
of hypertrophic scar fibroblasts by targeting HIF-1α via PI3K/AKT
pathway. J Mol Histol. 49:377–387. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Albina JE and Reichner JS: Oxygen and the
regulation of gene expression in wounds. Wound Repair Regen.
11:445–451. 2003.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Haroon ZA, Raleigh JA, Greenberg CS and
Dewhirst MW: Early wound healing exhibits cytokine surge without
evidence of hypoxia. Ann Surg. 231:137–147. 2000.PubMed/NCBI View Article : Google Scholar
|