Introduction
Renal cell carcinoma (RCC) is the most common type
of kidney malignancy (1,2). About 190,000 new cases are diagnosed,
and over 91,000 RCC patients were related with death in 2003
worldwide, with the incidence of RCC increasing by ~2% every 10
years (3). The morbidity and
mortality associated with RCC are also typically increased in males
compared with females (4,5). The high rates of morbidity and
mortality as a result of RCC are mainly due to RCC metastasis
(6). Although improvements have
been made in cancer therapy, RCC remains relatively insensitive to
conventional therapeutic interventions (7), as metastasis is a common feature of
late-stage RCC. Therefore, metastatic tumor cells further aggravate
the condition of patients with RCC. Effective treatment for
metastatic RCC that are available remain insufficient, leading to
poor prognoses (8). Molecular
targeted therapy against cancer has garnered significant attention
worldwide, where research effort has been focused on the
exploration and development of targeted therapy against RCC.
Therefore, the identification of a potential target that can
interfere with RCC progression is of utmost importance.
Ras protein activator like 2 (RASAL2) is a Ras
GTPase-activating protein. RASAL2 catalyzes GTP to GDP, which
inactivates Ras. Dysregulation of the Ras signaling pathway is
frequently observed in cancer cells (5). RASAL2 expression has also been
previously revealed to be aberrantly altered in a number of
different tumors (9-14),
where it appears to serve as a tumor suppressor gene (5,15,16).
Although RASAL2 has been reported to inhibit epithelial-mesenchymal
transition (EMT), a process which serves important roles in cancer
cell metastasis (17,18), the functional profile of RASAL2 in
RCC remains poorly understood. Therefore, in the present study,
RASAL2 expression and function was investigated in RCC.
Ras-MAPK signaling pathway dysfunction, particularly
those of ERK1/2 and p38 MAPK, is frequently observed in human
malignancies (19-21).
In particular, a number of important cellular processes, including
cell growth and apoptosis, are associated with the ERK1/2 and p38
MAPK signaling pathways (22,23).
The MAPK signaling pathway has been reported to regulate EMT by
modulating the expression of Sox2 (24-27),
a transcription factor that is involved in the regulation of cancer
cell physiology.
In the present study, the hypothesis that RASAL2 may
regulate RCC progression by modulating the Sox2/MAPK signaling
pathway was explored. The results from the present study may
provide novel insights into the mechanism underlying
RASAL2-mediated regulation of RCC progression, which can be a
potential therapeutic target in RCC therapy.
Materials and methods
Tissue collection
The experiment protocol on human tissues was
approved by the Ethics Committee of the Shaanxi Friendship
Hospital. Tumor tissues and the matched para-carcinoma tissues from
the same patient were collected from 21 patients with RCC (Average
age: 64.5±11.3 years; male/female: 13/8) who were admitted to the
Shaanxi Friendship Hospital between August 2016 and November 2017.
Inclusion criteria included the pathological diagnosis was clearly
RCC; successful surgical treatment; and recurrence or metastasis of
RCC was the immediate cause of death. Exclusion criteria included
unclear pathological diagnosis; multiple basic diseases; no
surgical treatment; and patients with fatal cardiovascular or
cerebrovascular diseases or accidental death.
The patients enrolled were pathologically diagnosed
with RCC and signed informed consent forms. According to the World
Health Organization grading system (28), RCC tissues were divided into the
following three groups in the present study: i) High-grade, ii)
middle-grade; and iii) low-grade.
Cell culture
ACHN cells, a human renal carcinoma cell line, was
purchased from the American Type Culture Collection (ATCC). Cells
were cultured routinely in Eagle's Minimum Essential Medium (EMEM;
ATCC) containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
and 10% penicillin/streptomycin, at 37˚C with 5%
CO2.
Cell transfection
Cells were seeded into 6-well plates at a density of
1.0x104 cells/well. Following starving overnight without
serum, cells were transfected with the 1 µg control vector
(pcDNA3.1), 1 µg RASAL2 overexpression plasmid (pcDNA3.1-RASAL2), 1
µg short hairpin (sh) RASAL2 plasmid (pGPU6-RASAL2; targeting
RASAL2 sequence, 5'-CCCTCGTGTTCTTGCTGATAT-3') οr 1 µg shRASAL2 or 1
µg scramble plasmid (negative control shRNA plasmid). All plasmids
were purchased from Shanghai GenePharma Co., Ltd.. Transfection
reactions were performed using Lipofectamine® 3000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. All cells were collected and subjected to
further experiments 24 h following transfection.
Cell counting Kit-8 (CCK-8) assay
Cells were seeded into 96-well plates at a density
of 1x102 cells/well prior to transfection using the
protocol aforementioned. After 24 h, 10 µl CCK-8 reagent (Beyotime
Institute of Biotechnology) were added into each well. The cells
were then incubated at 37˚C for a further 4 h. Absorbance values
were measured in each well at 450 nm using a microplate reader
(Thermo Fisher Scientific, Inc.), which were then correlated to
cell proliferation activity. Cell proliferation activity (%)=(OD
value in the experimental group-OD value in the blank group)/(OD
value in the control group-OD value in the blank group) x100%.
5-bromo-2'-deoxyuridine (BrdU)
staining
Cells (2x104 cells) from each group were
inoculated on cell culture slides and incubated at 37˚C for 72 h,
followed by being labeled with 10 µmol/l BrdU solution
(Sigma-Aldrich; Merck KGaA; cat. no. B5002) at 37˚C for 48 h. After
washing with PBS, the cells were fixed with 4% paraformaldehyde at
room temperature for 30 min. After washing with PBS, the cells were
treated with 2 mol/l HCl at 37˚C for 5 min, neutralized with 0.1
mol/l sodium tetraborate at room temperature for 10 min and then
washed with 0.2% triton X-100 at room temperature for 5 min. After
sealing with 3% BSA (Thermo Fisher Scientific, Inc.; cat. no.
BP9704-100) at room temperature for 1 h, Brdu antibodies (1:200;
Abbiotec, Inc.; cat. no. 251163) was applied at 4˚C overnight.
After washing with PBS, cells were incubated with sheep anti-mouse
IgG/Alexa Fluor594 (1:100, ProteinTech Group, Inc.; cat. no.
SA00006-3) at room temperature in darkness for 1 h. After washing
with PBS, 1 mg/ml DAPI (Invitrogen, Thermo Fisher Scientific, Inc.;
cat. no. D1306) was added to the cells at room temperature for 10
min. The BrdU positive cells were observed under a fluorescence
microscope (Leica FW 4500 B microscope; Leica Microsystems GmbH),
magnification x100.
Transwell assays
Cell invasion and migration were evaluated using
Transwell assays. the upper chamber (BD Biosciences) were precoated
with or without Matrigel (cat. no. M8370; Beijing Solarbio Science
and Technology Co., Ltd.) for 30 mins at 37˚C. Chambers that were
coated with Matrigel were used for invasion assays whilst those
without were used for migration assays. Briefly, the transfected
RCC cells with serum-free medium were seeded into the upper chamber
(BD Biosciences) at a density of 1.0x105 cells/ml. The
lower chamber was added with medium supplemented with 10% FBS.
Cells that invaded or migrated through the membrane were
subsequently stained with 500 µl 0.1% crystal violet at 37˚C for 30
min after incubation for 36 h. The cells were observed using a
light microscope (magnification, x100). Five different fields were
observed and photographed. The relative cell migration and invasion
rates were counted through the number of the migrated or invaded
cells/the number of the inoculated cells in the same field.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from tissues and cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocols. cDNA was obtained
from 1 µg total RNA using GoScript™ Reverse
Transcription kit (Promega Corporation) according to manufacturer's
protocol, using the following temperature setting: 25˚C for 5 min,
at 42˚C for 60 min and 70˚C for 15 min. Subsequent qPCR
amplification was performed using Fast SYBR™ Green
Master Mix (Invitrogen; Thermo Fisher Scientific, Inc.) according
to manufacturer's protocol. The following thermocycling conditions
were used: Initial denaturation at 95˚C for 20 sec, followed by 40
cycles at denature at 95˚C for 3 sec, and annealing/extension at
60˚C for 30 sec. The sequences of primers used were as follows:
RASAL2 forward, 5'-TCCCTCGTGTTCTTGCTGAT-3' and reverse:
5'-GTCTGTGTTGTCCTGGCTTG-3'; GAPDH forward,
5'-TGCACCACCAACTGCTTAGC-3' and reverse,
5'-GGCATGGACTGTGGTCATGAG-3'. Relative RASAL2 expression was
normalized to that of GAPDH and calculated using the
2-ΔΔCq method (12).
Western blotting
Total proteins were extracted from tissues and cells
using Total Protein Extraction kit (Merck Millipore; cat. no.
XY-AP200A). Protein concentration was measured using the
Bicinchoninic Acid protein assay kit (Shanghai Qcbio Science &
Technologies Co., Ltd.). A total of 1 µg total protein were loaded
per well and separated by 10% SDS-PAGE, proteins were transferred
onto PVDF membranes (EMD Millipore). The membranes were then
blocked using 5% bovine serum albumin (Gibco; Thermo Fisher
Scientific, Inc.) for 1 h at 37˚C. Following incubation with
primary antibodies at 4˚C overnight, the membranes were then
treated with corresponding horseradish peroxidase-conjugated
secondary antibodies (1:5,000; cat. nos., ab205719 and ab205719;
Abcam) at room temperature for 1 h. ECL Plus reagent (Beyotime
Institute of Biotechnology) was used to visualize the membranes,
following which Quantity One software (version 4.62; Bio-Rad
Laboratories, Inc.) was used for densitometric analysis according
to the manufacturer's protocols. The following primary antibodies
were used: Anti-RASAL2 antibody (cat. no. ab121578; 1:100; Abcam),
anti-SOX2 (cat. no. ab97959; 1:200; Abcam), anti-phosphorylated
(p)-ERK1/2 (cat. no. ab201015; 1:400; Abcam), anti-ERK1/2 (cat. no.
ab54230; 1:1,000; Abcam), anti-p38 (cat. no. ab27986; 1:1,000;
Abcam), anti-p-p38 (cat. no. ab47363; 1:2,000; Abcam), anti-MMP-2
(cat. no. ab97779; 1:2,000; Abcam), anti-MMP-9 (cat. no. ab38898;
1:2,000; Abcam), anti-TIMP1 (cat. no. ab61224; 1:1,000; Abcam) and
anti-GAPDH (cat. no. ab9485; 1:2,500; Abcam).
Immunohistochemical (IHC)
staining
The tumor and para-carcinoma normal tissues were
fixed using 4% paraformaldehyde overnight at 4˚C, embedded in
paraffin and sliced into sections of 3-4 µm thickness. RCC was
divided into low-grade and high-grade according to previously
published criteria (29). Slides
were subsequently dewaxed with dimethylbenzene, hydrated with
dimethylbenzene and treated with an alcohol gradient (100, 95, 80
and 75%). After washing, the antigen retrieval was performed by
heating the sections at 95˚C in sodium citrate buffer (pH 6.0; 10
mM; cat. no. 25229-1; Wuhan Sanying Biotechnology). The
cooled sections were then incubated in 3% hydrogen peroxide
(H2O2) solution for 10 min at room
temperature. Following blocking with 10% normal goat serum (Thermo
Fisher Scientific, Inc.) at 37˚C for 30 min, slides were incubated
with anti-RASAL2 antibody (1:50; cat. no. ab216127; Abcam) at 4˚C
overnight. The slides were then incubated with biotin-labeled
secondary antibody (cat. no. A0279; 1:200; Beyotime Institute of
Biotechnology) at room temperature for 1 h. Slides were first
treated with horseradish peroxidase-linked streptavidin (1:500,
Vector Laboratories; cat. no. SA-5014) at room temperature for 30
min, following which they were incubated with 3'3'-diaminobenzidine
(DAB) for 5 min at room temperature. The sections were then stained
with hematoxylin at room temperature for 2 min. Sections were
observed using a light microscope (magnification x100). The results
were assessed by two senior pathologists. RASAL2 was positive with
brown-yellow staining of nucleus/cytoplasm. According to the
percentage of positive cells: 1 point: 1-10%; 2 points: 10-50%; 3
points: 50-80%; 4 points: >80%.
Immunofluorescence (IF) staining
Cells (2x105 cells/ml) were fixed with 4%
paraformaldehyde at room temperature for 15 min and treated with
0.5% Triton X-100 for 20 min at room temperature. Following
blocking with 10% normal goat serum (Thermo Fisher Scientific,
Inc.) at 37˚C for 30 min, slides were incubated with the
anti-RASAL2 primary antibody (1:100; cat. no. ab121578; Abcam) at
4˚C overnight. The slides were then incubated with Alexa
Fluor® 594-conjugated secondary antibody (1:200; cat.
no. ab150120; Abcam) for 1 h at room temperature, following which
nuclei were stained using DAPI (1:10; cat. no. D1306; Invitrogen;
Thermo Fisher Scientific, Inc.) at room temperature for 5 mins and
the slides were observed using a fluorescence microscope (Leica FW
4500 B microscope; Leica Microsystems GmbH).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism software version 6.0 (GraphPad Software, Inc.). Data are
presented the mean ± SD from at least three replicates of the
experiment, where comparisons were calculated using one-way ANOVA
followed by Tukey's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
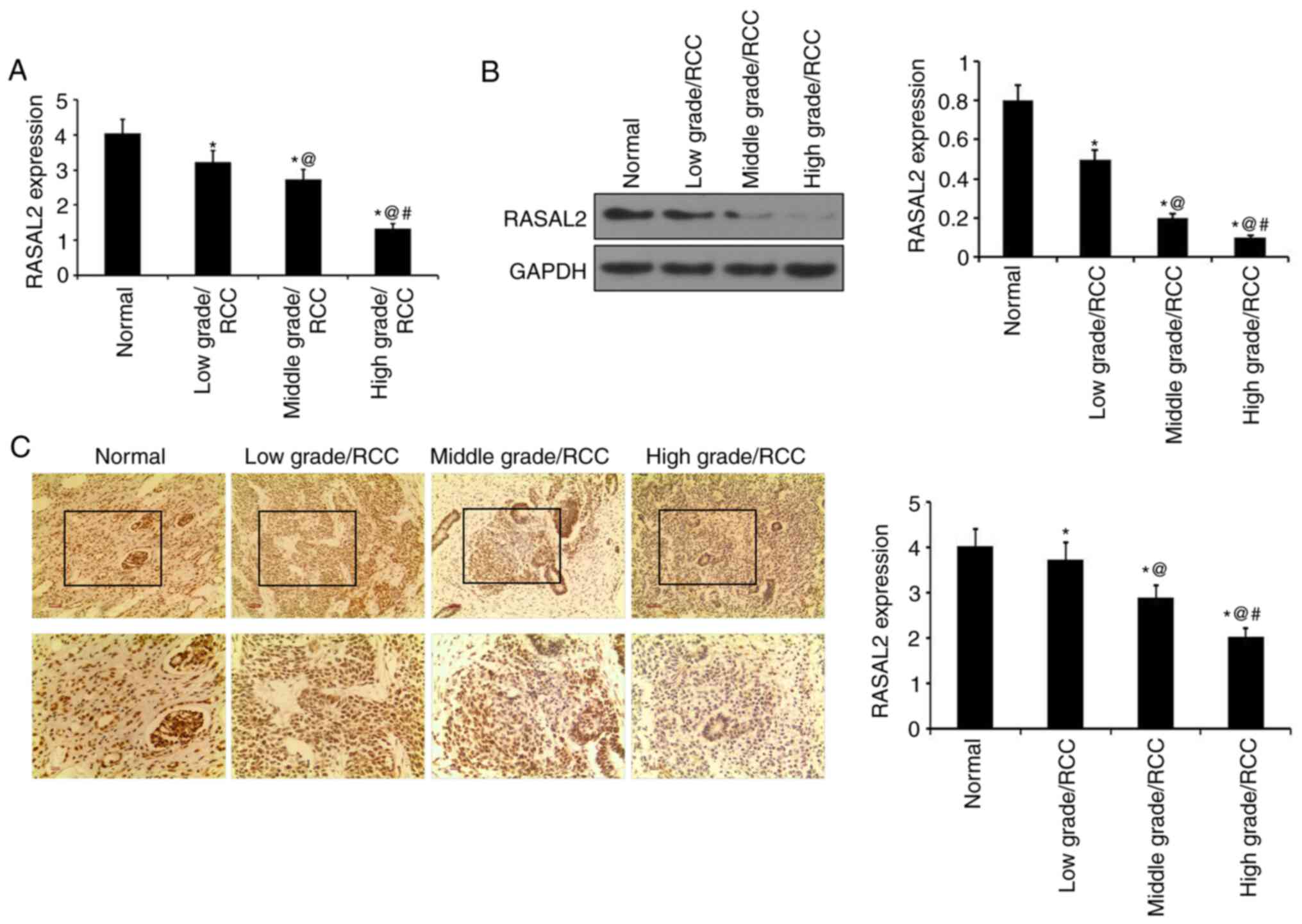
RASAL2 expression is decreased in RCC
tissues
RT-qPCR and western blotting were performed to
measure RASAL2 mRNA and protein expression levels in RCC tissues.
RASAL2 expression was revealed to be significantly decreased in RCC
tissues at mRNA and protein levels compared with adjacent normal
tissues (Fig. 1A and B). Specifically, RASAL2 expression was
significantly decreased in high-grade RCC tissues compared with the
middle-grade samples, whilst RASAL2 expression was in turn
significantly decreased in middle-grade RCC tissues compared those
in low-grade RCC tissues (P<0.05; Fig. 1A and B). In IHC staining, the brown staining
represented RASAL2 staining. And it was discovered that RASAL2
expression was significantly decreased in RCC tissues compared with
in normal tissues. RASAL2 expression was significantly decreased in
high-grade RCC tissues compared with middle-grade RCC tissues,
whilst RASAL2 expression in middle-grade RCC tissues was
significantly decreased compared with low-grade RCC tissues
(P<0.05; Fig. 1C). Therefore,
these results suggest that RASAL2 was downregulated in RCC tissues
compared with corresponding normal tissues, where its expression
level is negatively associated with histological tumor grade.
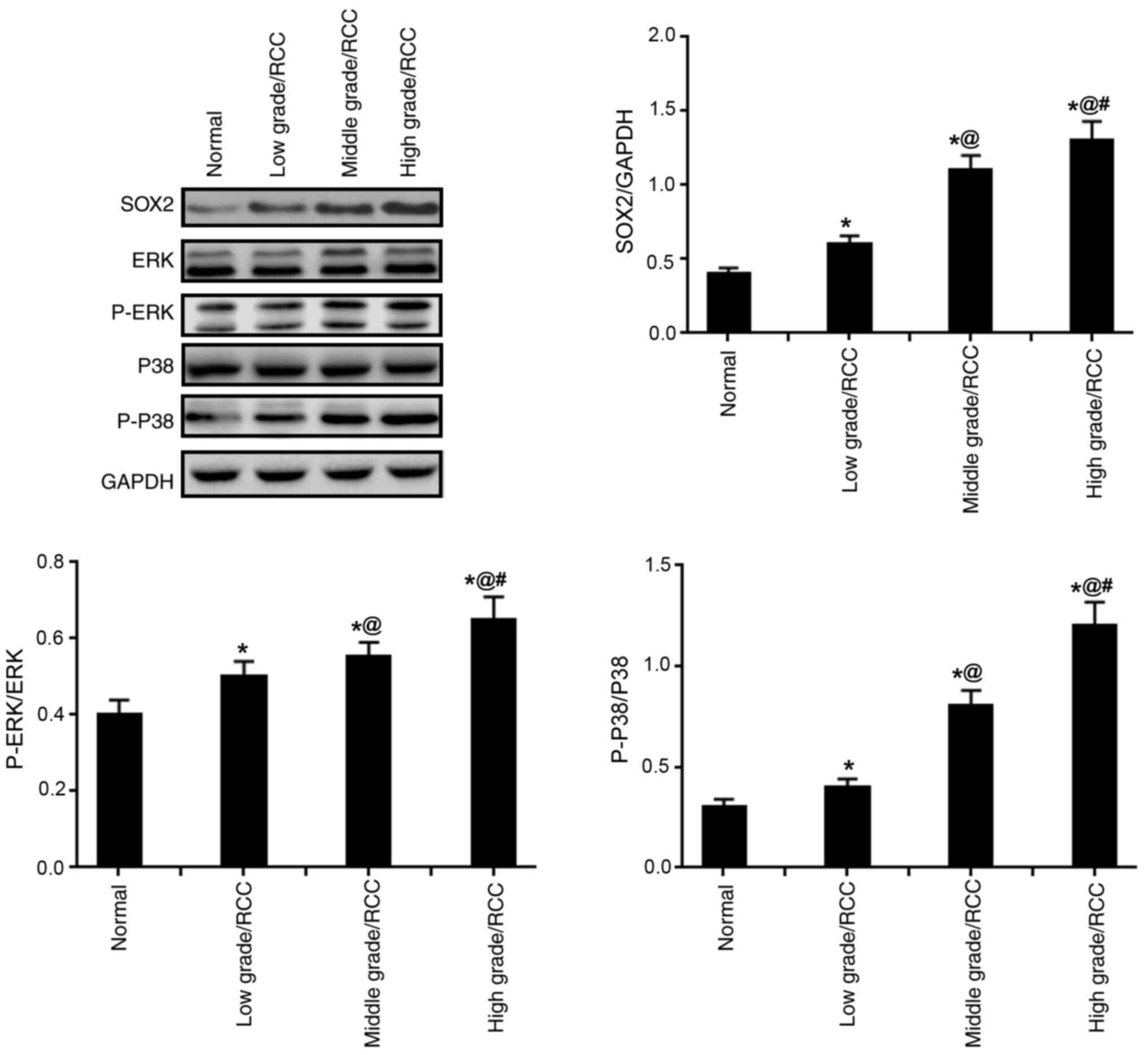
SOX2 expression, and ERK and p38 MAPK
activation levels are increased in RCC tissues
The expression levels of SOX2, p-ERK/ERK and
p-p38/p38 were identified to be significantly increased in RCC
tissues compared with those in normal tissues; a trend that was
observed to be positively associated with tumor grade, suggesting
that SOX2 and the phosphorylated ERK and P38 were significantly
enhanced in RCC tissues (P<0.05; Fig. 2).
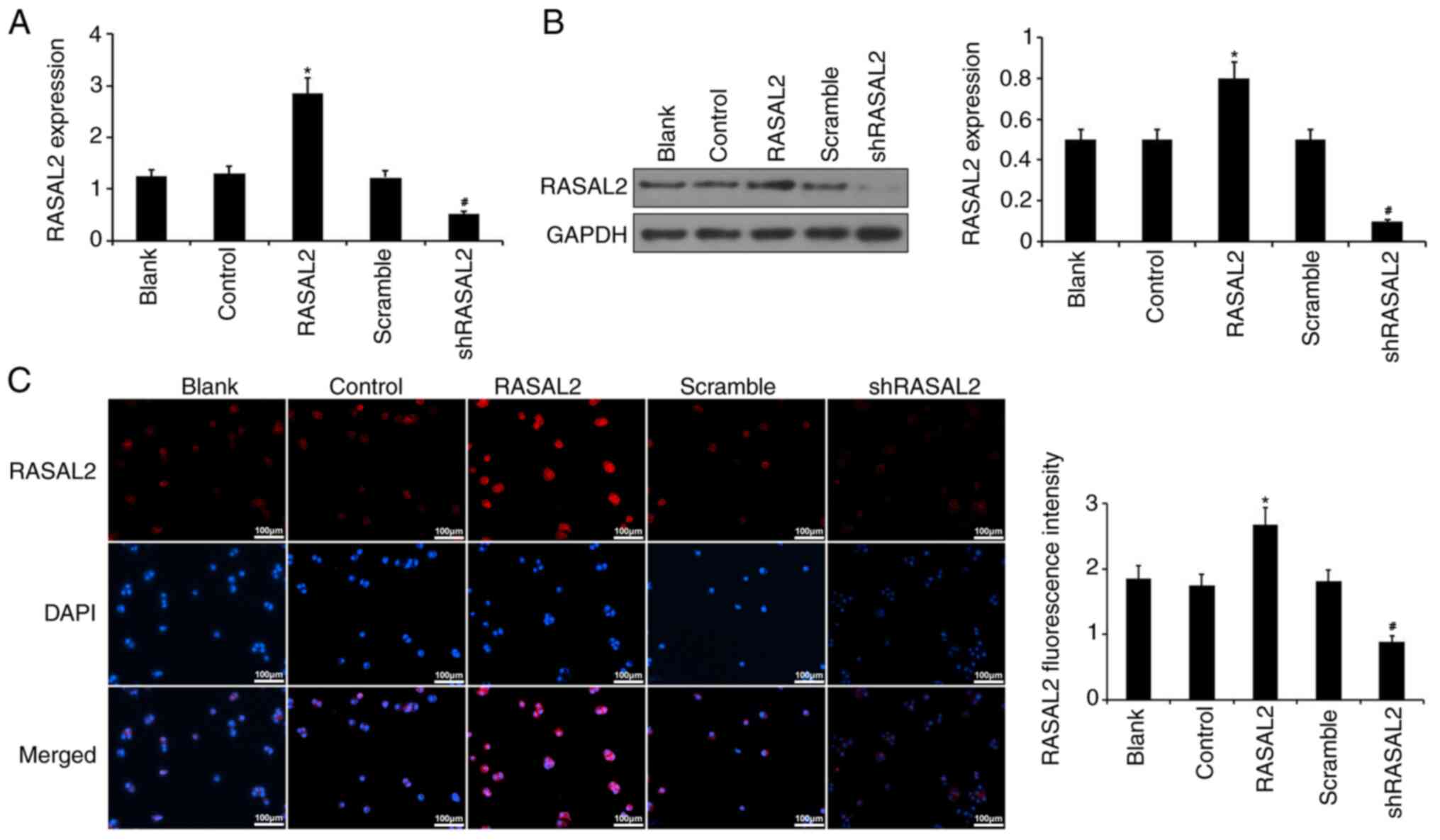
Overexpression and knockdown of RASAL2
expression in ACHN cells
To investigate the potential underlying mechanism
and function of RASAL2 in RCC, ACHN cells were transfected with
plasmids encoding either the RASAL2 protein or RASAL2 shRNA to
overexpress or silence RASAL2 expression, respectively. Data from
RT-qPCR, western blotting and IF staining demonstrated that RASAL2
expression was significantly increased in cells transfected with
RASAL2 plasmids compared with those transfected with corresponding
control, whilst RASAL2 expression was significantly lower in cells
transfected with shRASAL2 compared with those transfected with the
scramble shRNA (P<0.05; Fig. 3).
Therefore, these observations suggest that the manipulation of
RASAL2 expression ACHN cells was successful.
Effect of RASAL2 expression on RCC
cell viability, invasion and migration
The effects of RASAL2 overexpression and knockdown
on RCC cell viability, invasion and migration were subsequently
evaluated on ACHN cells. As shown in Fig. 4A, compared with the control group,
overexpression of RASAL2 significantly decreased cell viability
(P<0.05), and the cell viability was significantly increased in
RASAL2 knockdown group relative to scramble group (P<0.05). In
addition, the migration and invasion abilities of ACHN cells were
significantly decreased in RASAL2 overexpression group with respect
to the control group; by contrast, the migration and invasive
capacities of ACHN cells were significantly increased in the RASAL2
knockdown group versus that in the scramble group (P<0.05;
Fig. 4B-D). Moreover, the results
of BrdU staining also disclosed that overexpression of RASAL2
prominently suppressed the proliferation of RCC cells, and
knockdown of RASAL2 had a significant promoting effect on the RCC
cell proliferation (P<0.05; Fig.
4E).
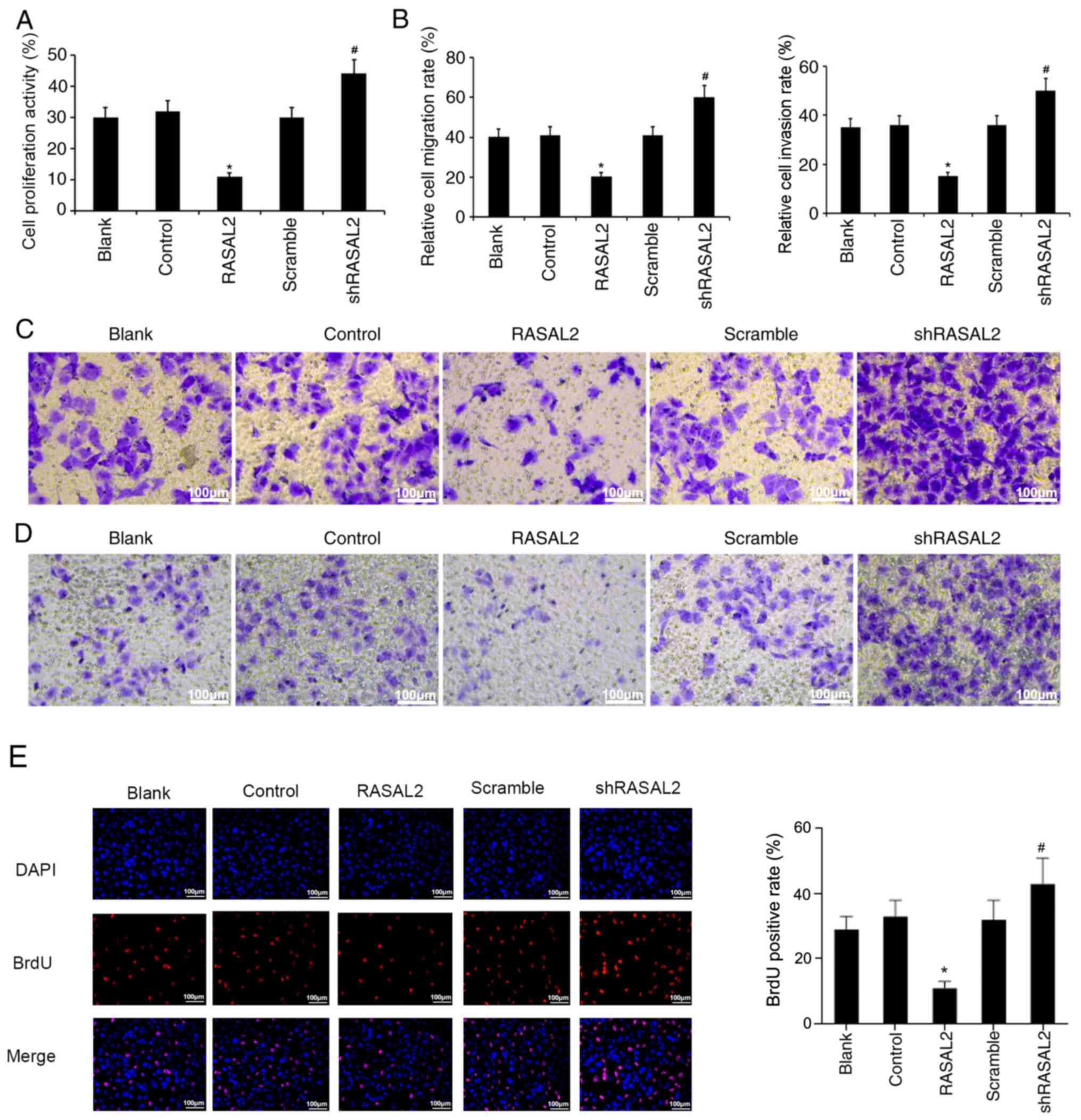 | Figure 4Effect of manipulating RASAL2
expression on RCC cell viability, migration and invasion. (A)
Following RASAL2 overexpression or knockdown in ACHN cells, cell
viability was measured using Cell Counting Kit-8 assay. (B-D) ACHN
cell migration and invasion ability was evaluated using Transwell
assays following RASAL2 overexpression or knockdown. Magnification,
x200; scale bars=50 µm. Relative cell migration and invasion rates
were calculated depending on the number of cells in different
fields. (E) BrdU staining was conducted to evaluate the
proliferation of RCC cells after RASAL2 overexpression or
knockdown. Magnification, x100; scale bars=100 µm
*P<0.05 vs. Control group. #P<0.05 vs.
scramble group. OD, optical density; Blank, un-transfected control;
Control, control pcDNA.31 plasmid; sh, short hairpin RNA; scramble,
scrambled shRNA; RASAL2, ras protein activator like 2; shRASAL2,
RASAL2 shRNA. |
Effect of RASAL2 expression on MMP-2,
MMP-9 and TIMP-1 in RCC cells
Subsequently, the possible regulatory effects of
RASAL2 overexpression or knockdown on the MMP-2, MMP-9 and TIMP-1
expressions in RCC were further investigated. The results of
western blot assay exhibited that relative to the control group,
RASAL2 overexpression significantly downregulated MMP-2 and MMP-9
expressions, and significantly upregulated TIMP-1 expression in
ACHN cells. By contrast, compared with the scramble group, RASAL2
knockdown significantly increased MMP-2 and MMP-9 expressions, and
markedly decreased TIMP-1 expression in ACHN cells (P<0.05;
Fig. 5).
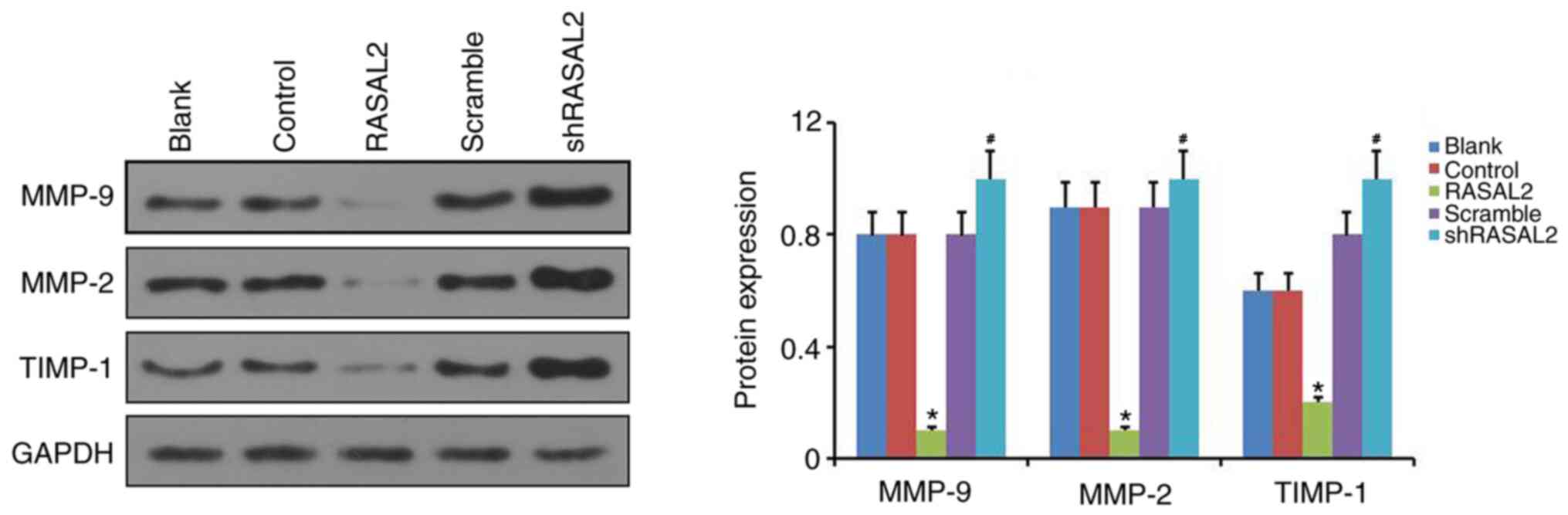 | Figure 5Effect of manipulating RASAL2
expression on MMP-2, MMP-9 and TIMP-1 expression in RCC cells.
Protein expression of MMP-2, MMP-9 and TIMP-1 was assessed by
western blotting in ACHN cells following RASAL2 overexpression or
knockdown. GAPDH was used as the loading control.
*P<0.05 vs. Control group. #P<0.05 vs.
scramble group. Blank, un-transfected control; Control, control
pcDNA.31 plasmid; sh, short hairpin RNA; scramble, scrambled shRNA;
RASAL2, ras protein activator like 2; shRASAL2, RASAL2 shRNA; MMP,
matrix metalloproteinase; TIMP-1, tissue inhibitor of
metalloproteinases 1. |
Effect of RASAL2 expression on SOX2
expression and the activation of ERK and p38 MAPK in RCC cells
Subsequently, western blot assays were used to
evaluate the effects of RASAL2 overexpression and silencing on SOX2
expression and the activations of ERK and p38 MAPK in ACHN cells.
It was discovered that the levels of SOX2, p-ERK/ERK and p-p38/p38
were significantly downregulated in ACHN cells transfected with
RASAL2 plasmid compared with that cells transfected with
corresponding control plasmid. Meanwhile, compared with ACHN cells
transfected with the scramble, silence of RASAL2 exhibited
significant promoting effects on the expressions of SOX2, p-ERK/ERK
and p-p38/p38 in ACHN cells (P<0.05; Fig. 6). Taken together, these results
suggest that RASAL2 overexpression suppressed the activation of
SOX2/ERK/p38 MAPK signaling pathway, and RASAL2 knockdown
potentiated the SOX2/ERK/p38 MAPK signaling pathway in RCC
cells.
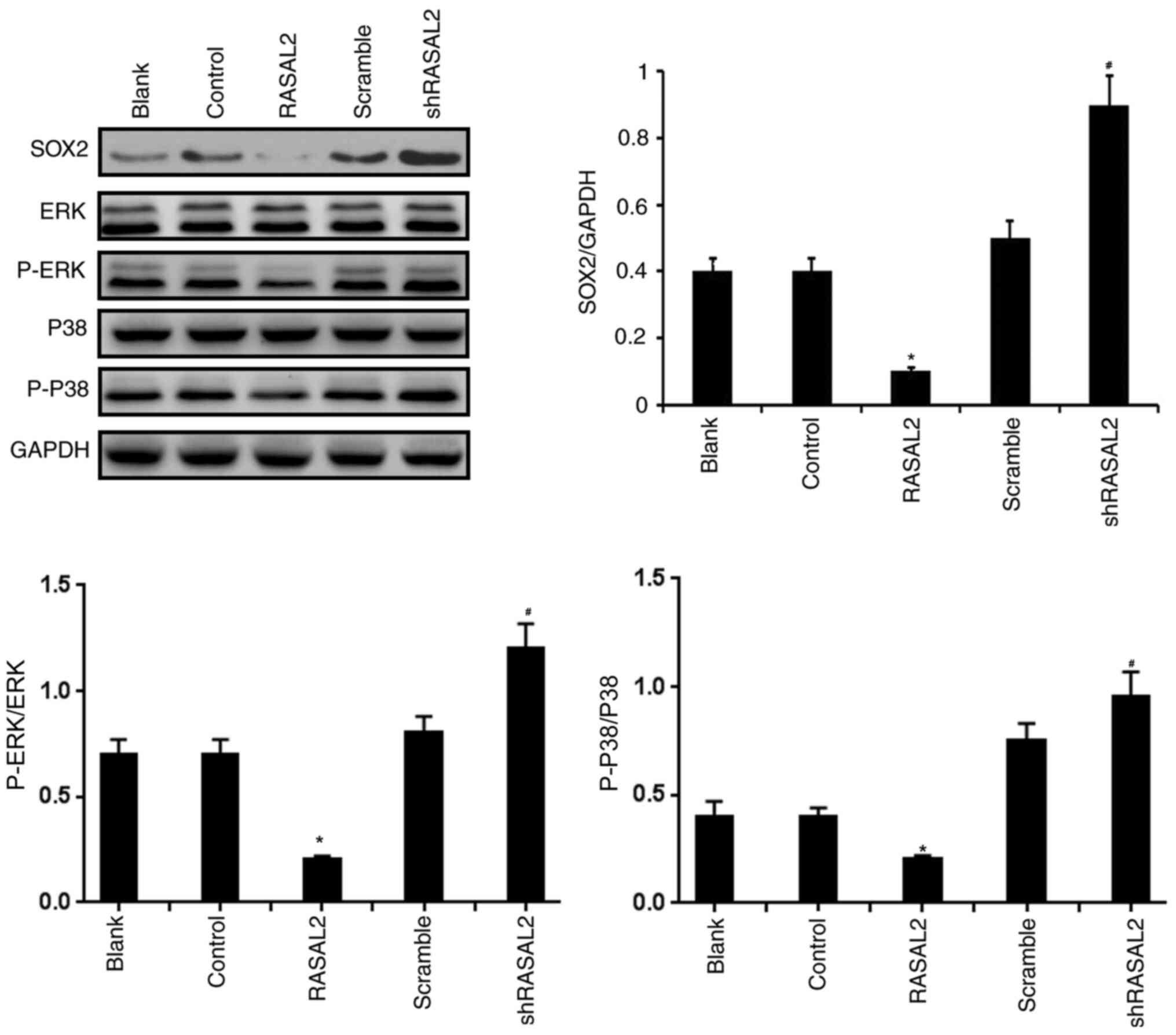 | Figure 6Effect of manipulating RASAL2
expression on SOX2 expression, ERK phosphorylation and p38 MAPK
phosphorylation in RCC cells. SOX2, ERK, p38 MAPK expression, in
addition to ERK and p38 MAPK phosphorylation, were assessed in ACHN
cells by western blotting following RASAL2 overexpression or
knockdown. GAPDH was used as loading control. *P<0.05
vs. Control group. #P<0.05 vs. scramble group. Blank,
un-transfected control; Control, control pcDNA.31 plasmid; sh,
short hairpin RNA; scramble, scrambled shRNA; RASAL2, ras protein
activator like 2; shRASAL2, RASAL2 shRNA; p-, phosphorylated. |
Discussion
The clinical prognosis of patients with metastatic
RCC is poor, where the numbers of therapeutic methods that are
effective in prolonging the survival time of patients with RCC
remain insufficient (30,31). Therefore, it is of importance to
elucidate the specific mechanism and function underlying the
pathophysiology of RCC. The present study demonstrated that RASAL2
exerted inhibitory effects on RCC by inhibiting cell migration and
invasion through regulation of the SOX2/MAPK signaling pathway.
RASAL2 has been previously demonstrated to be a Ras
GAP tumor suppressor in gastric cancer (32). In the present study, RASAL2
expression was identified to be decreased in RCC tissues,
particularly in high-grade RCC. This suggests that the inhibition
of RASAL2 may contribute to the progression of RCC. Consistent with
this notion, RASAL2 suppression promoted tumorigenesis in breast
cancer in a previous study (5) In
addition, the expression levels of RASAL2 were identified to be
inversely associated with pathological grade in triple-negative or
estrogen receptor-negative breast tumors (11). In the present study, the role of
RASAL2 in RCC was investigated in vitro, where the
overexpression and suppression of RASAL2 expression were
successfully performed in ACHN cells, a model RCC cell line.
Subsequently, the effect of RASAL2 on RCC cell viability was
investigated, as excessive cell proliferation is one of the primary
hallmarks of cancer physiology (33). ACHN cell viability was revealed to
be inhibited by RASAL2 overexpression but was increased by RASAL2
knockdown. Additionally, invasion and migration levels were
decreased by RASAL2 overexpression but enhanced by RASAL2 knockdown
in ACHN cells, suggesting that decreased RASAL2 expression promotes
the invasive capabilities of RCC. Supporting this, a previous study
has reported that RASAL2 downregulation in ovarian cancer cells
increased EMT and metastasis (34).
During the EMT process, epithelial cells lose their polarity and
transform into mesenchymal cells, where they acquire migratory and
invasive phenotypes (35). During
this process, matrix metalloproteinases (MMPs) such as MMP-2/9 and
tissue inhibitor of metalloproteinases 1 (TIMPs) such as TIMP-1,
their corresponding endogenous inhibitors, exert pivotal roles
(36,37). Therefore, the effects of RASAL2
which support the observations from the invasion assay were
subsequently investigated. MMP-2/9 expression was demonstrated to
be decreased by RASAL2 overexpression, whilst it was increased by
RASAL2 knockdown. By contrast, the opposite trend was observed in
TIMP-1 expression compared with that of MMP2/9 following the
manipulation of RASAL2 expression. Altogether, these results
suggest that RASAL2 served as an inhibitory factor in RCC by
suppressing invasion, consistent with results identified in
previous studies on RASAL2(14).
However, as previous reports have also previously demonstrated that
RASAL2 serves as an oncogene in other types of cancer (16,38),
the role of RASAL2 is most likely to be dependent on the type of
malignancy, where its physiological role remains controversial.
Interaction between growth factors and their
corresponding receptors is essential for the initiation of
signaling cascades through activation of downstream signaling
pathways, with the MAPK pathway one of the most widely studied
(39). MAPK is of great
significance in signal transduction (40) and can result in a variety of effects
on cellular processes (41). The
MAPK signaling family includes ERK1/2, JNK1/2 and p38 MAPK
(40). Previous studies have
demonstrated that the activation status of ERK1/2 and p38 were
associated with metastatic tumors (42-44),
including that of RCC (45-47).
As SOX2 has also been reported to associate with ERK1/2 and p38
signaling in bladder cancer (48),
the involvement of the SOX2/ERK/p38 pathway on RCC was evaluated.
SOX2 expression, in addition to the activation of ERK1/2 and p38
MAPK, were identified to be elevated in RCC tissues.
RASAL2 is involved in a number of cancer types
through the SOX2/MAPK signaling pathway (17,49).
Therefore, in the present study it was hypothesized that a
potential association between RASAL2 and SOX2/ERK/p38 signaling may
exist during RCC onset. To study the mechanism underlying the
effects of RASAL2 on RCC, the activity of the SOX2/ERK/p38
signaling pathway was subsequently determined in vitro
following manipulation of RASAL2 expression. ERK and p38 MAPK
activation were found to be decreased and increased by RASAL2
overexpression and knockdown in ACHN cells, respectively. According
to previous studies, the inhibition of ERK1/2 and p38 MAPK
signaling may prove to be beneficial to the inhibition of prostate
cancer progression (50,51). In addition, it has also been
previously reported that SOX2 bridges RASAL2 to the MAPK pathway in
the regulation of EMT in bladder cancer cells (7,8). In
the present study, RASAL2 overexpression increased the expression
of SOX2, whilst its RASAL2 knockdown decreased SOX2 expression,
suggesting that SOX2 may function as a bridge between RASAL2 and
the ERK/p38 MAPK signaling pathway. Intracellular mechanisms
underlying tumor pathophysiology are highly complex. Although the
involvement of ERK1/2 and p38 MAPK in cell invasion has been
reported in prostate cancer (51),
the role of ERK1/2 and p38 MAPK in RCC cells has yet to be fully
elucidated. Therefore, the use of ERK1/2 or p38 MAPK inhibitors may
be useful to illustrate the role of ERK1/2 and p38 MAPK further in
RCC in future studies, which serve as a limitation to the present
study. In addition, the effect of RASAL2 on the SOX2/ERK/p38 MAPK
signaling pathway should be determined in vivo in future
studies, to confirm the role of RASAL2 in RCC.
Taken together, the present study demonstrated that
RASAL2 overexpression can inhibit RCC cell viability, migration and
invasion. In addition, these results support the notion that RASAL2
can serve as a potential therapeutic target for RCC.
Acknowledgements
Not applicable.
Funding
Not funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SW and QW conceptualized and designed the present
study. XH and SH performed the experiments and contributed to data
collection. SW and CL performed the statistical analysis and
drafted the manuscript. QW examined and corrected the manuscript.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The experiment protocol on human tissue was approved
by the Ethics Committee of the Shaanxi Friendship Hospital. The
patients enrolled signed the informed consent form.
Patient consent for publication
The patients enrolled signed the informed consent
form.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Matalliotakis M, Velegrakis A, Laliotis A,
Niraki E and Trivli A: Exacerbation of neurofibromatosis and
adverse pregnancy outcome. A case report and review of the
literature. Clin Exp Obstet Gynecol. 45:126–128. 2018.
|
|
2
|
Stühler V and Bedke J: Overview of
treatment of localized and metastatic renal cell carcinoma (RCC).
MMW Fortschr Med. 160:45–51. 2018.(In German). PubMed/NCBI View Article : Google Scholar
|
|
3
|
Osawa T, Takeuchi A, Kojima T, Shinohara
N, Eto M and Nishiyama H: Overview of current and future systemic
therapy for metastatic renal cell carcinoma. Jpn J Clin Oncol.
49:395–403. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cohen HT and McGovern FJ: Renal-cell
carcinoma. N Engl J Med. 353:2477–2490. 2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
McLaughlin SK, Olsen SN, Dake B, De Raedt
T, Lim E, Bronson RT, Beroukhim R, Polyak K, Brown M, Kuperwasser C
and Cichowski K: The RasGAP gene, RASAL2, is a tumor and metastasis
suppressor. Cancer Cell. 24:365–378. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Curtis SA, Cohen JV and Kluger HM:
Evolving immunotherapy approaches for renal cell carcinoma. Curr
Oncol Rep. 18(57)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liu M, Wu H, Shangguan D, Jiang Y, Li X,
Liu S, Zhou B, Yin T and Gong Z: Immunomodulatory therapies for
renal cell carcinoma. Protein Pept Lett. 25:534–547.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Abel EJ and Wood CG: Cytoreductive
nephrectomy for metastatic RCC in the era of targeted therapy. Nat
Rev Urol. 6:375–383. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Matheu A, Collado M, Wise C, Manterola L,
Cekaite L, Tye AJ, Canamero M, Bujanda L, Schedl A, Cheah KS, et
al: Oncogenicity of the developmental transcription factor Sox9.
Cancer Res. 72:1301–1315. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Malki S, Bibeau F, Notarnicola C, Roques
S, Berta P, Poulat F and Boizet-Bonhoure B: Expression and
biological role of the prostaglandin D synthase/SOX9 pathway in
human ovarian cancer cells. Cancer Lett. 255:182–193.
2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lu X, Wan F, Zhang H, Shi G and Ye D:
ITGA2B and ITGA8 are predictive of prognosis in clear cell renal
cell carcinoma patients. Tumour Biol. 37:253–262. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Büttner F, Winter S, Rausch S, Reustle A,
Kruck S, Junker K, Stenzl A, Agaimy A, Hartmann A, Bedke J, et al:
Survival prediction of clear cell renal cell carcinoma based on
gene expression similarity to the proximal tubule of the nephron.
Eur Urol. 68:1016–1020. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hui K, Yue Y, Wu S, Gu Y, Guan B, Wang X,
Hsieh JT, Chang LS, He D and Wu K: The expression and function of
RASAL2 in renal cell carcinoma angiogenesis. Cell Death Dis.
9(881)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang Z, Wang J, Su Y and Zeng Z: RASAL2
inhibited the proliferation and metastasis capability of
nasopharyngeal carcinoma. Int J Clin Exp Med. 8:18765–18771.
2015.PubMed/NCBI
|
|
16
|
Li N and Li S: RASAL2 promotes lung cancer
metastasis through epithelial-mesenchymal transition. Biochem
Biophys Res Commun. 455:358–362. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hui K, Gao Y, Huang J, Xu S, Wang B, Zeng
J, Fan J, Wang X, Yue Y, Wu S, et al: RASAL2, a RAS
GTPase-activating protein, inhibits stemness and
epithelial-mesenchymal transition via MAPK/SOX2 pathway in bladder
cancer. Cell Death Dis. 8(e2600)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Micalizzi DS and Ford HL:
Epithelial-mesenchymal transition in development and cancer. Future
Oncol. 5:1129–1143. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Santarpia L, Lippman SM and El-Naggar AK:
Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy.
Expert Opin Ther Targets. 16:103–119. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kohno M and Pouyssegur J: Targeting the
ERK signaling pathway in cancer therapy. Ann Med. 38:200–211.
2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bradham C and McClay DR: p38 MAPK in
development and cancer. Cell Cycle. 5:824–828. 2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chuang SM, Wang IC and Yang JL: Roles of
JNK, p38 and ERK mitogen-activated protein kinases in the growth
inhibition and apoptosis induced by cadmium. Carcinogenesis.
21:1423–1432. 2000.PubMed/NCBI
|
|
23
|
Wakeman TP, Wyczechowska D and Xu B:
Involvement of the p38 MAP kinase in Cr(VI)-induced growth arrest
and apoptosis. Mol Cell Biochem. 279:69–73. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Otsubo T, Akiyama Y, Hashimoto Y, Shimada
S, Goto K and Yuasa Y: MicroRNA-126 inhibits SOX2 expression and
contributes to gastric carcinogenesis. PLoS One.
6(e16617)2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chen Y, Huang Y, Huang Y, Chen J, Wang S
and Zhou J: The prognostic value of SOX2 expression in non-small
cell lung cancer: A meta-analysis. PLoS One.
8(e71140)2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Thu KL, Becker-Santos DD, Radulovich N,
Pikor LA, Lam WL and Tsao MS: SOX15 and other SOX family members
are important mediators of tumorigenesis in multiple cancer types.
Oncoscience. 1:326–335. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Huang W, Chen Z, Shang X, Tian D, Wang D,
Wu K, Fan D and Xia L: Sox12, a direct target of FoxQ1, promotes
hepatocellular carcinoma metastasis through up-regulating Twist1
and FGFBP1. Hepatology. 61:1920–1933. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kanamaru H, Akino H, Suzuki Y, Noriki S
and Okada K: Prognostic value of nuclear area index in combination
with the world health organization grading system for patients with
renal cell carcinoma. Urology. 57:257–261. 2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sun M, Abdollah F, Bianchi M, Trinh QD,
Jeldres C, Tian Z, Shariat SF, Widmer H, Zorn K, Menon M, et al: A
stage-for-stage and grade-for-grade analysis of cancer-specific
mortality rates in renal cell carcinoma according to age: A
competing-risks regression analysis. Eur Urol. 60:1152–1159.
2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lalani AA, McGregor BA, Albiges L,
Choueiri TK, Motzer R, Powles T, Wood C and Bex A: Systemic
treatment of metastatic clear cell renal cell carcinoma in 2018:
Current paradigms, use of immunotherapy, and future directions. Eur
Urol. 75:100–110. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zarrabi K, Fang C and Wu S: New treatment
options for metastatic renal cell carcinoma with prior
anti-angiogenesis therapy. J Hematol Oncol. 10(38)2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang S, Tie J, Wang R, Hu F, Gao L, Wang
W, Wang L, Li Z, Hu S, Tang S, et al: SOX2, a predictor of survival
in gastric cancer, inhibits cell proliferation and metastasis by
regulating PTEN. Cancer Lett. 358:210–219. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Goodlad R: Workshop report: Increased cell
proliferation as a cause of human cancer. Food Cancer Prev.
300–301. 2005.
|
|
34
|
Huang Y, Zhao M, Xu H, Wang K, Fu Z, Jiang
Y and Yao Z: RASAL2 down-regulation in ovarian cancer promotes
epithelial-mesenchymal transition and metastasis. Oncotarget.
5:6734–6745. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Orlichenko LS and Radisky DC: Matrix
metalloproteinases stimulate epithelial-mesenchymal transition
during tumor development. Clin Exp Metastasis. 25:593–600.
2008.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bourboulia D and Stetler-Stevenson WG:
Matrix metalloproteinases (MMPs) and tissue inhibitors of
metalloproteinases (TIMPs): Positive and negative regulators in
tumor cell adhesion. Semin Cancer Biol. 20:161–168. 2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Feng M, Bao Y, Li Z, Li J, Gong M, Lam S,
Wang J, Marzese DM, Donovan N, Tan EY, et al: RASAL2 activates RAC1
to promote triple-negative breast cancer progression. J Clin
Invest. 124:5291–5304. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Sebolt-Leopold JS and Herrera R: Targeting
the mitogen-activated protein kinase cascade to treat cancer. Nat
Rev Cancer. 4:937–947. 2004.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinases. Science. 298:1911–1912. 2002.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Sun Y, Liu WZ, Liu T, Feng X, Yang N and
Zhou HF: Signaling pathway of MAPK/ERK in cell proliferation,
differentiation, migration, senescence and apoptosis. J Recept
Signal Transduct Res. 35:600–604. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Suthiphongchai T, Promyart P, Virochrut S,
Tohtong R and Wilairat P: Involvement of ERK1/2 in invasiveness and
metastatic development of rat prostatic adenocarcinoma. Oncol Res.
13:253–259. 2003.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ge X, Fu YM and Meadows GG: U0126, a
mitogen-activated protein kinase kinase inhibitor, inhibits the
invasion of human A375 melanoma cells. Cancer Lett. 179:133–140.
2002.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Graf K, Xi XP, Yang D, Fleck E, Hsueh WA
and Law RE: Mitogen-activated protein kinase activation is involved
in platelet-derived growth factor-directed migration by vascular
smooth muscle cells. Hypertension. 29:334–339. 1997.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Serrano-Oviedo L, Ortega-Muelas M,
García-Cano J, Valero ML, Cimas FJ, Pascual-Serra R,
Fernandez-Aroca DM, Roche O, Ruiz-Hidalgo MJ, Belandia B, et al:
Autophagic cell death associated to Sorafenib in renal cell
carcinoma is mediated through Akt inhibition in an ERK1/2
independent fashion. PLoS One. 13(e0200878)2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Li JK, Chen C, Liu JY, Shi JZ, Liu SP, Liu
B, Wu DS, Fang ZY, Bao Y, Jiang MM, et al: Long noncoding RNA
MRCCAT1 promotes metastasis of clear cell renal cell carcinoma via
inhibiting NPR3 and activating p38-MAPK signaling. Mol Cancer.
16(111)2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Wu K, Hu L and Hou J: Selective
suppression of Notch1 inhibits proliferation of renal cell
carcinoma cells through JNK/p38 pathway. Oncol Rep. 35:2795–2800.
2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Uchiyama A, Nayak S, Graf R, Cross M,
Hasneen K, Gutkind JS, Brooks SR and Morasso MI: SOX2 epidermal
overexpression promotes cutaneous wound healing via activation of
EGFR/MEK/ERK signaling mediated by EGFR ligands. J Invest Dermatol.
139:1809–1820.e8. 2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zhou B, Zhu W, Jiang X and Ren C: RASAL2
plays inconsistent roles in different cancers. Front Oncol.
9(1235)2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Uzgare AR, Kaplan PJ and Greenberg NM:
Differential expression and/or activation of P38MAPK, erk1/2, and
jnk during the initiation and progression of prostate cancer 1 1
Prostate 2003;55: 128-39. Urol Oncol. 22:82–83. 2004.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Chen L, He HY, Li HM, Zheng J, Heng WJ,
You JF and Fang WG: ERK1/2 and p38 pathways are required for P2Y
receptor-mediated prostate cancer invasion. Cancer Lett.
215:239–247. 2004.PubMed/NCBI View Article : Google Scholar
|