Introduction
Moyamoya disease (MMD) is an idiopathic chronic
disease characterized by progressive steno-occlusive alteration of
the internal carotid artery terminal and the beginning of the
anterior cerebral artery and middle cerebral artery (1). Dural arteriovenous fistula (DAVF) is
an uncommon vascular malformation that is characterized by abnormal
connections between meningeal arteries and dural venous sinuses,
meningeal veins or cortical veins (2).
MMD is currently considered a genetic disease
(1). For DAVF, progressive stenosis
or thrombosis of the dural venous sinus are thought to have a
pivotal role in the genesis of DAVF (2). In general, there is no relationship
between MMD and DAVF; however, in extremely rare circumstances,
patients with MMD have been reported to have concurrent DAVF
(3,4). In the present study, another case of
MMD concurrent with DAVF was reported. In addition, a literature
review of the reported cases was also performed to further expound
this rare scenario.
Case report
A 47-year-old male was admitted was admitted to The
First Hospital of Jilin University (Changchun, China) on Sep 27th
2015 due to sudden onset of headache. The patient was generally
healthy and denied a history of hypertension, diabetes or any other
chronic diseases. Physical examination was unremarkable except for
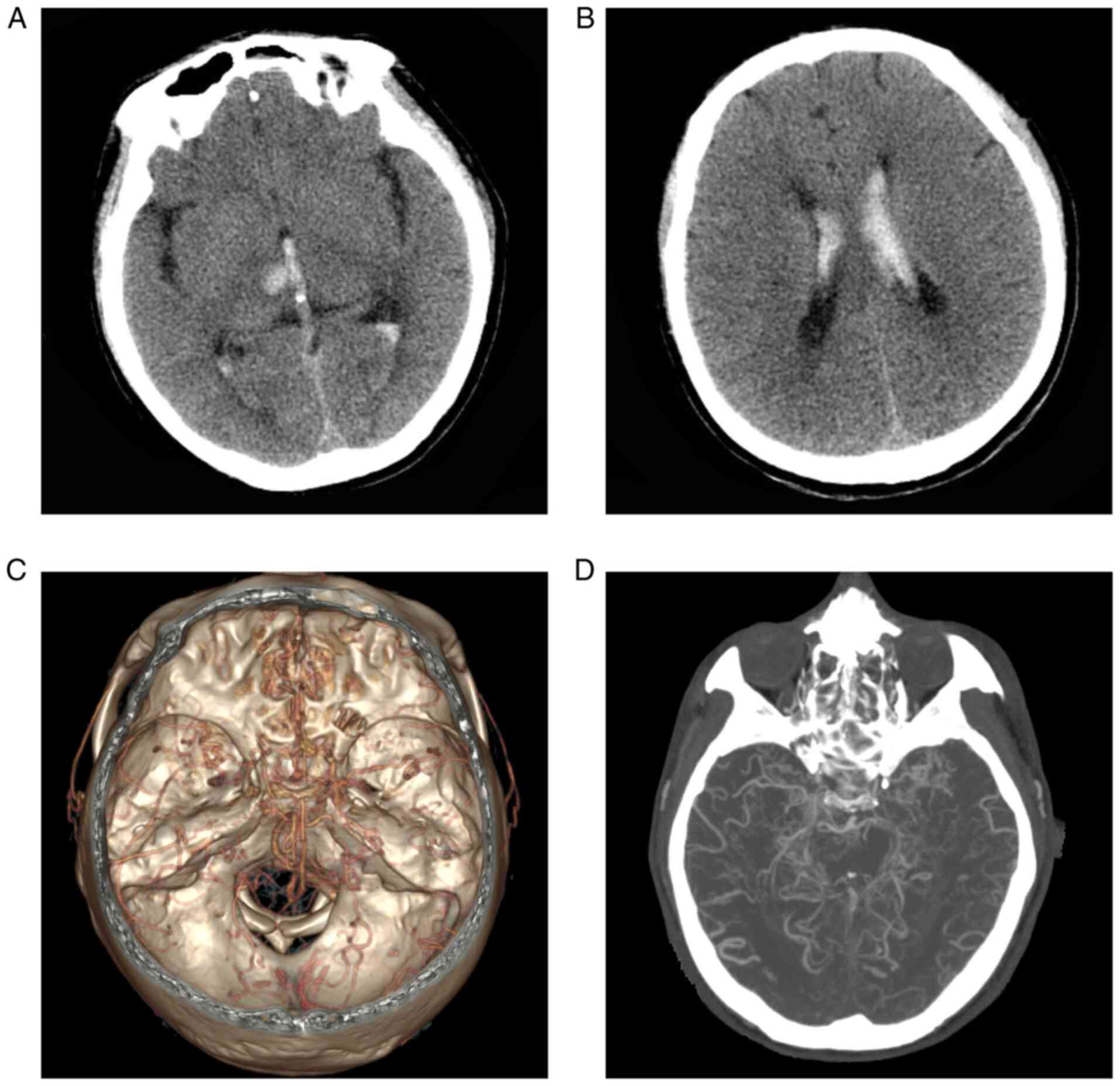
mild neck stiffness. Head CT indicated hemorrhage of the right
thalamus with ventricular extension (Fig. 1A and B). CT angiography (CTA) revealed that the
normal vasculature in the anterior and posterior circulation
disappeared and was replaced by moyamoya-like vessels (Fig. 1C and D). A diagnosis of hemorrhagic MMD was
made. The patient received conservative management, including
analgesic (flurbiprofen, 50 mg/bid), antemetic (tropisetron
hydrochloride, 5 mg/bid) and fluid infusion (normal saline and 5%
glucose solution) and was discharged 3 days later.
After three months, the patient was readmitted due
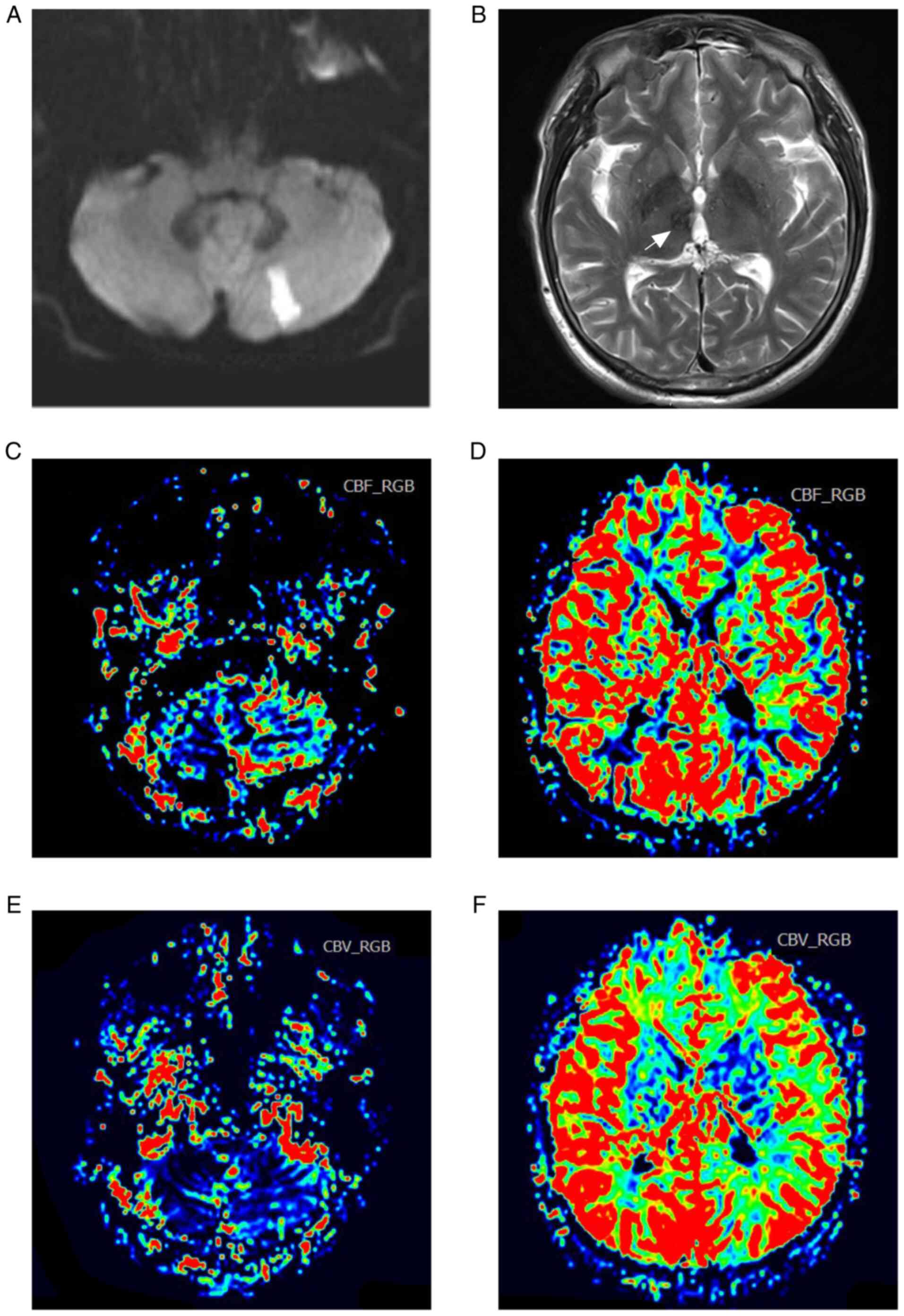
to dizziness and gait disturbance. Diffusion-weighted MRI indicated
acute infarction in the left cerebellar hemisphere (Fig. 2A) and encephalomalacia in the right
thalamus (Fig. 2B). The cerebral
blood volume (Fig. 2C and D) and cerebral blood flow (Fig. 2E and F) maps on perfusion-weighted MRI suggested
relatively normal blood perfusion in the bilateral hemispheres.
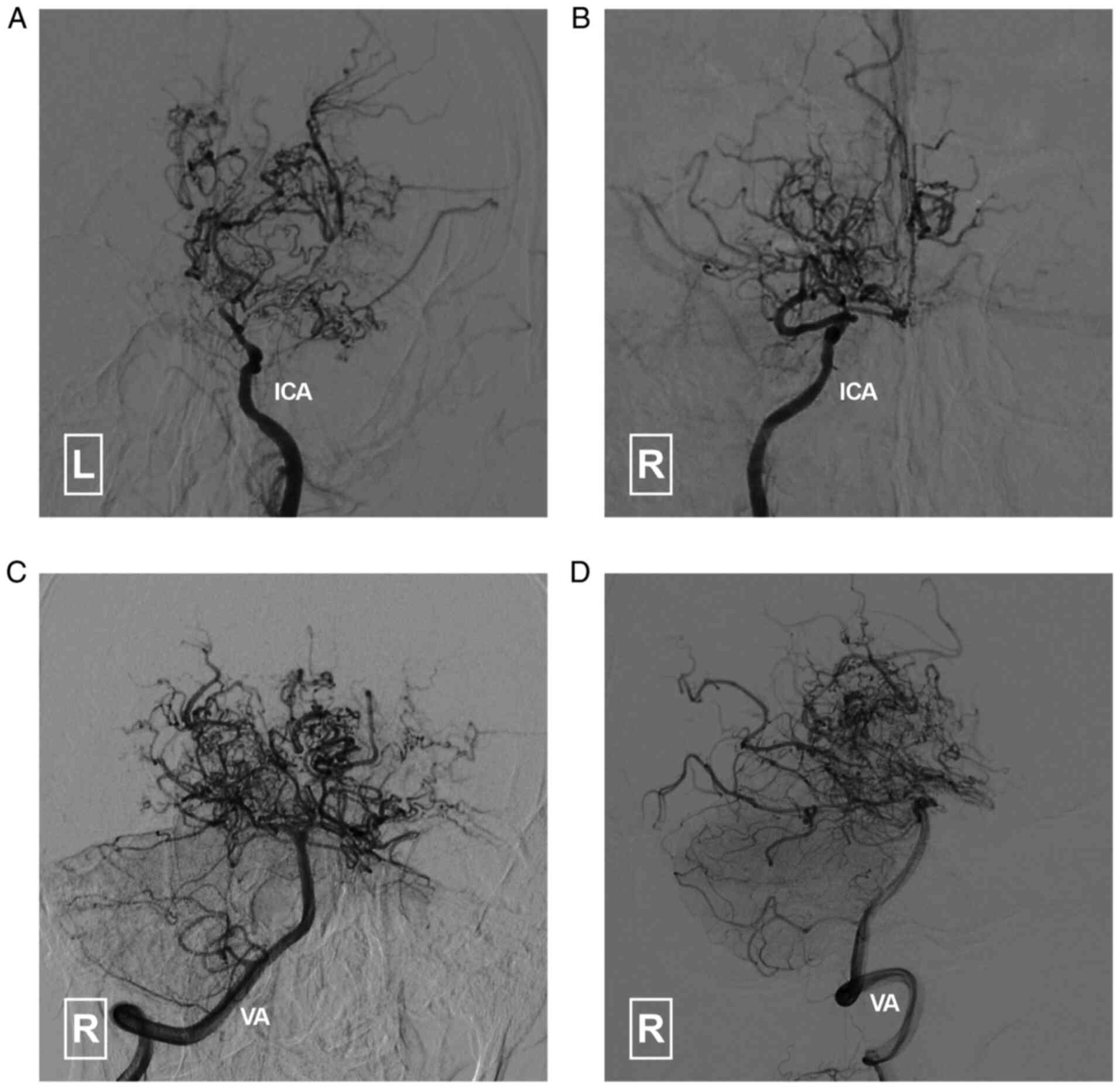
Conventional angiography of the internal carotid
arteries and vertebral arteries (Fig.
3) confirmed the findings from previous CTA. An angiogram of
the external carotid arteries indicated that the middle meningeal
artery (MMA) and occipital artery (OA) had formed efficient
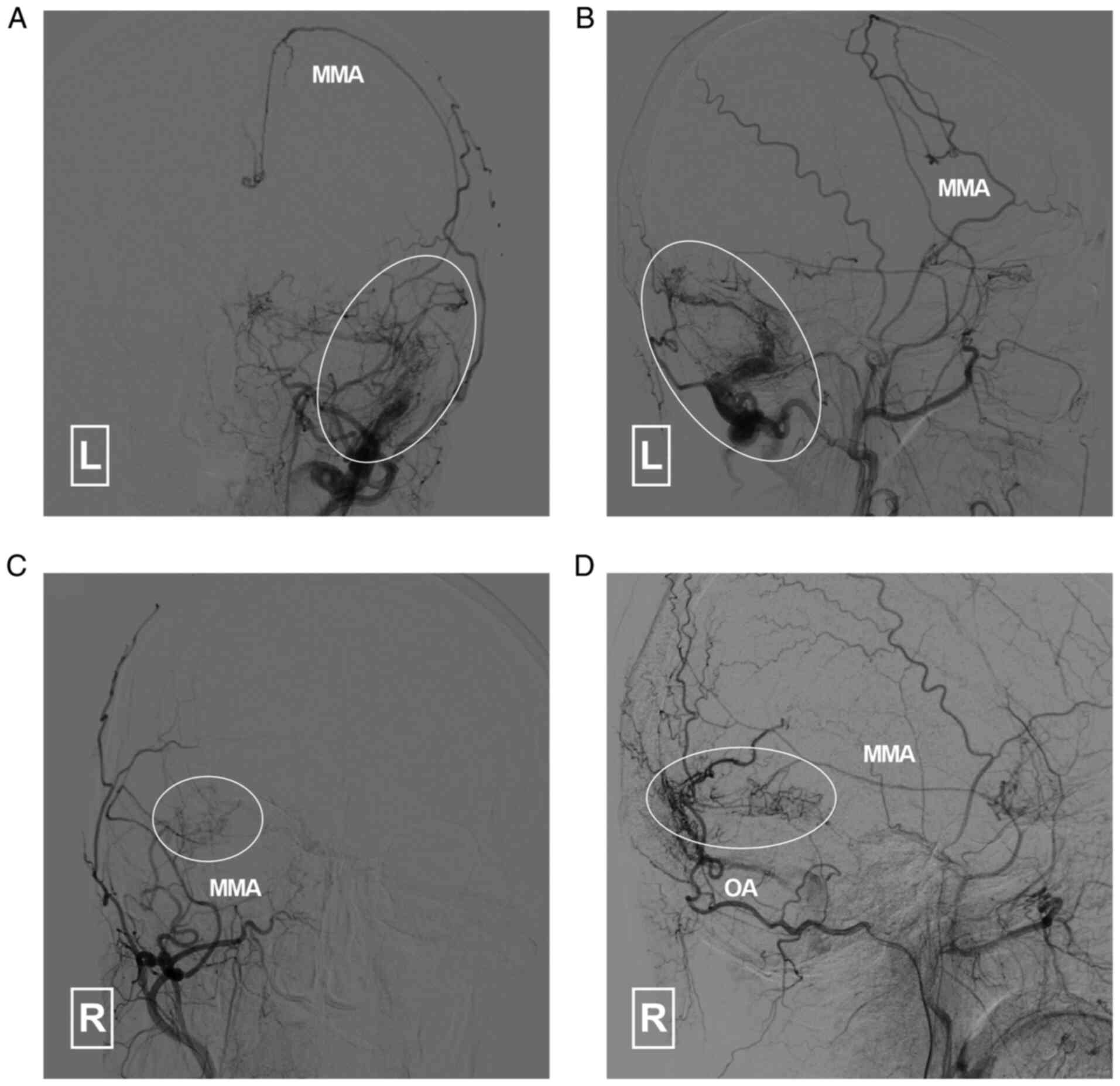
collaterals with the brain vasculature (Fig. 4). A DAVF was also noted. The DAVF
(Cognard classification Ⅰ) was fed by the left MMA, OA and
posterior meningeal artery (PMA). It drained into the
transverse-sigmoid sinus and occipital sinus (Fig. 5).
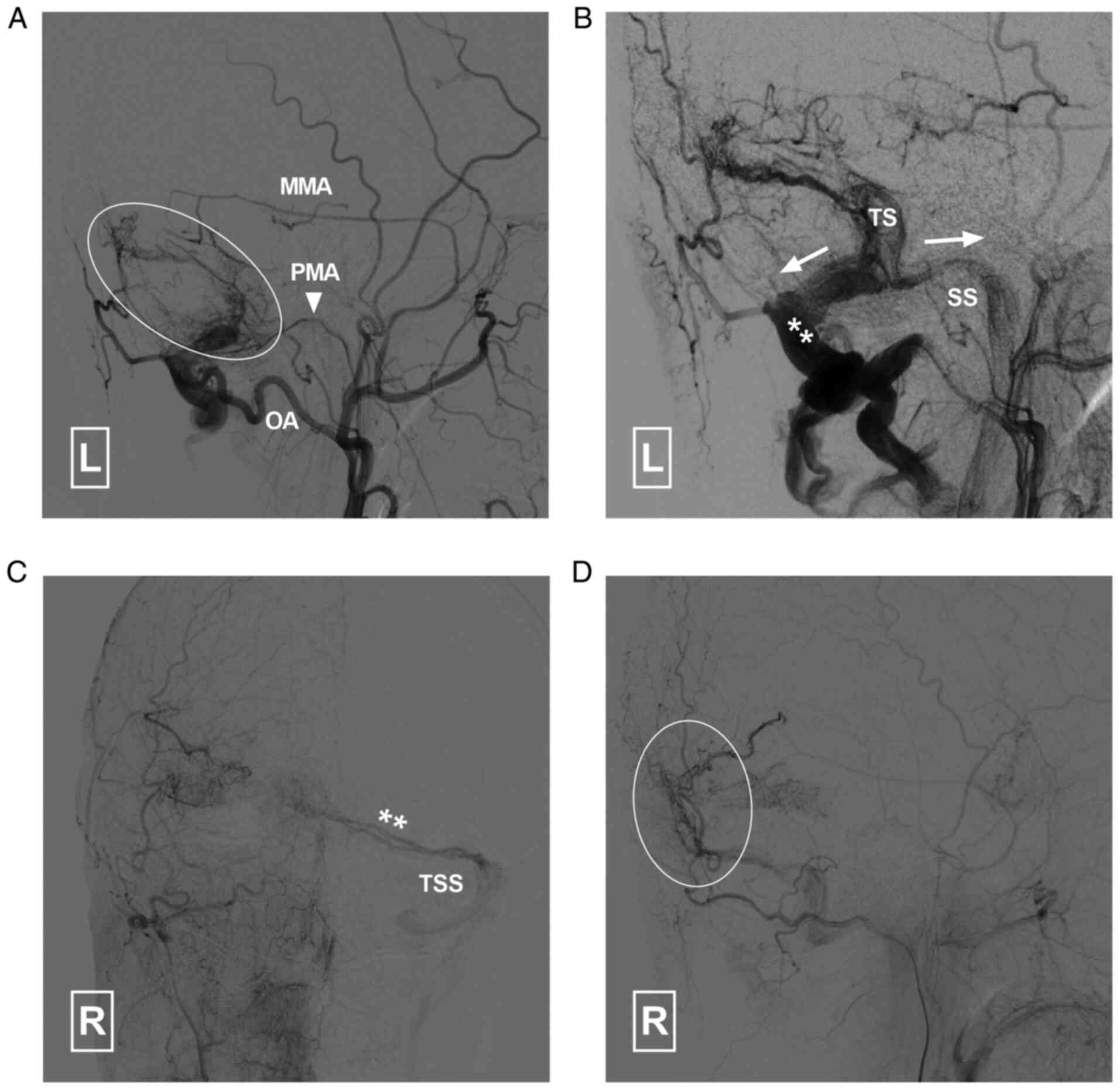 | Figure 5Angiogram of the left ECA in (A)
arterial and (B) capillary phases indicates that the DAVF is
supplied by the left MMA, PMA (arrowhead) and OA. It drained (white
arrows) to the TS, SS and occipital sinus. Angiogram in (C)
anteroposterior and (D) lateral views of the right ECA indicates
that the DAVF (double asterisk and circle) is also supplied by the
right ECA and drains to the left TSS. DAVF, dural arteriovenous
fistula; ECA, external carotid artery; MMA, middle meningeal
artery; PMA, posterior meningeal artery; SS, sigmoid sinus; TS,
transverse sinus; TSS, transverse-sigmoid sinus; L, left; R,
right. |
As no retrograde blood flow or cortical venous
drainage was noted, conservative management and follow-up were
proposed for the DAVF. Oral aspirin (100 mg, QD) was prescribed.
The patient was discharged 1 month later with no neurological
deficit. He was stable and lived independently during a 4-year
follow-up. However, the patient refused re-examination of the
digital subtraction (DSA) and MR perfusion due to economic factors
during the follow-up period. It was not possible to produce any
further radiological evidence for the evaluation of the development
of MMD and DAVF.
Discussion
MMD is an idiopathic steno-occlusive disease that
mainly affects the anterior circulation. In a small proportion of
patients, the posterior circulation may also be involved (1). As a consequence of an insufficient
blood supply across the involved brain tissue, collateral vessels
may arise from the cranial base perforators, which leads to the
characteristic moyamoya-like vasculature in the cranial base
(5).
According to previous studies, MMD may coexist with
intracranial aneurysms, brain arteriovenous malformation (BAVM) and
primitive carotid-basilar anastomosis (6). In extremely rare circumstances,
transdural collaterals may anastomose directly with intracranial
venous structures, leading to the formation of DAVFs (3,4). In a
literature review of studies published in the English language,
only 6 cases of AVF concurrent with MMD or moyamoya syndrome (MMS)
were identified (Table I) (3,4,7-10).
Among the 7 reported cases (including the case of the present
study), 6 were DAVFs and 1 was a pial AVF. A total of 5 patients
had concurrent MMD and 2 patients had concurrent MMS.
 | Table IClinical data of the patients with
MMD/MMS and concurrent DAVFs reported in the literature. |
Table I
Clinical data of the patients with
MMD/MMS and concurrent DAVFs reported in the literature.
| First
author/year | Age/sex | Presenting
symptom | Imaging findings on
admission | Concurrent
diseases | MMD or MMS | Type of AVF | Detection of MMD/MMS
and AVF | Side of AVF | Supplying arteries of
AVF | Draining veins of
AVF | Cognard
classification | Treatment for
MMD/MMS | Treatment for
AVF | Outcome (mRS) | (Refs.) |
|---|
| Killory/2008 | 44/M | Headache and
tinnitus | IVH | NA/NM | MMD | DAVF | Simultaneously | R | OA, MMA, PAA,
APhA | TS | Ⅰ | STA-MCA bypass | TAE, TVE | 0 | (3) |
| Zaletel/2011 | 71/M | Urinary retention,
cognitive decline | SAH | Hypertension | MMS | DAVF | Simultaneously | R | OA | TS | Ⅰ | No | Conservative | 4 | (7) |
| Hanaoka/2011 | 45/F | Hemiparesis | CI | NA/NM | MMD | DAVF | Delayed | L | NA/NM | T-SS | ⅡB | STA-MCA bypass | TVE | NA/NM | (4) |
| Feroze/2015 | 51/F | Transient aphasia,
progressive extremity weakness, headache | NA/NM | NA/NM | MMD | Pial AVF | Delayed | Bilateral | Branch of ICA | Vein of Trolard | NA/NM | STA-MCA bypass | Conservative | 0 | (8) |
| Liu/2016 | 52/F | Proptosis and
chemosis of right eye, tinnitus | NA/NM | NA/NM | MMD | CDAVF | Simultaneously | R | MHT | SOV, IPS | NA/NM | No | TVE via direct
cannulation of SOV | 0 | (9) |
| Koduri/2019 | 14/F | Tonic-clonic seizure,
facial droop, hemibody weakness | CI | Down syndrome,
hypothyroidism | MMS | DAVF | Delayed | Bilateral | Right MMA, Left MMA,
OA, and muscular branches of VA | Left SS, Right
SS | Left Ⅰ, Right Ⅰ | Bilateral pial
synangiosis | Conservative | NA/NM | (10) |
| Present case | 47/M | Dizziness and gait
disturbance | Cerebellar
infarction | No | MMD | DAVF | Delayed | Left | MMA, OA, PMA | Left T-SS, OS | Ⅰ | No | Conservative | 1 | / |
As a result of its rarity and the lack of research,
the mechanisms underlying DAVF formation during MMD progression
have remained elusive. It requires to be further investigated
whether an association exists between DAVF and MMD. The specific
location of a DAVF concurrent with MMD also remains to be studied.
In clinical practice, progressive stenosis or occlusion of the
dural venous sinus have a pivotal role in the formation of DAVF
(2). Trauma, craniotomy, infection
and venous sinus thrombosis are also responsible for a small
proportion of DAVFs (11).
However, none of the previous case studies have
reported thrombosis or stenosis of the venous system. A total of 3
patients developed AVF (1 case of pial AVF and 2 of DAVF) after
extracranial-to-intracranial vascular bypass (3,4,8). No
other risk factors were identified. However, concurrent AVF is an
acquired disease rather than a congenital anomaly. This deduction
is based on the following evidence. First, 4 of the reported AVFs
were identified in a delayed fashion during the imaging follow-up
of MMD or MMS (4,8,10).
Furthermore, delayed development of BAVM has also been reported in
patients with MMD or MMS (12,13).
As the BAVM shares a similar vasculature with AVF, it may share a
similar pathogenic mechanism with AVF in patients with MMD or
MMS.
According to the limited evidence, the ischemic
environment and consequent angiogenesis have been suggested to have
pivotal roles in the formation of AVFs. The levels of proangiogenic
factors such as basic fibroblast growth factor and vascular
endothelial growth factor are both elevated in the dura of patients
with MMD or DAVF (3). For these
patients, progressive arterial occlusion both in the anterior and
posterior circulation may create a robust ischemic environment for
collateral formation from the external carotid and vertebral
arteries. A DAVF may form during this angiogenic process (14).
The management of MMD-associated AVF depends on its
clinical presentation and invasiveness. Among the cases retrieved
in the present literature review, 3 patients underwent successful
transvenous and/or transarterial embolization of the AVFs; 2 of
these patients were treated for persistent tinnitus or eye symptoms
and 1 patient was treated for the presence of cortical venous
drainage (3,4,9). A
total of 4 patients were managed conservatively (7,8,10).
For the patient of the present study, a close
follow-up strategy was also adopted. This conservative strategy was
selected for the following reasons. First, the DAVF was
incidentally detected and the patient was asymptomatic. The patient
was first admitted to our hospital for thalamus hemorrhage and
re-admitted for cerebellar infarction 3 months later. The
hemorrhage and infarction have nothing to do with the DAVF. It may
be proposed that both the hemorrhage and infarction resulted from
the MMD, based on the following reasons: i) This patient has no
history of hypertension, which is the most common cause of cerebral
hemorrhage in the thalamus or basal ganglion; ii) DAVF is a
superficial cerebrovascular disease and cannot lead to deep
parenchymal hemorrhage such as thalamus hemorrhage in this patient;
iii) patients with MMD frequently develop slim and fragile vessels
across the brain surface and paraventricular area and these fragile
vessels and microaneurysms in the fragile vessels are prone to
bleed (15); iv) catheter angiogram
did not reveal any arteriovenous malformation or aneurysm around
the hemorrhagic thalamus; v) the cerebellum is supplied by the
three paired cerebellar arteries (superficial cerebellar artery,
anterior inferior cerebellar artery and posterior inferior
cerebellar artery). However, the DAVF in this patient was supplied
by the MMA, OA and PMA. Hence, the DAVF was not responsible for the
cerebellar infarction.
Furthermore, no cortical venous drainage was
identified on angiogram. For patients with DAVF, cortical venous
drainage is considered a risk factor of future hemorrhage. Those
patients without cortical venous drainage would have a relatively
benign natural course (2).
In addition, the perfusion-weighted MRI indicated
relatively normal blood perfusion in the bilateral hemispheres,
i.e. no severe blood insufficiency was noted across the bilateral
hemispheres, which may have been compensated by the collaterals
during the progression of the disease.
Finally, there were multiple feeders supplying the
DAVF. Embolization of the feeders may pose a risk of compromising
the transdural collaterals. Furthermore, evidence that bypass
surgery is superior to medical therapy is not convincing in adult
patients at present (16). Hence,
for asymptomatic DAVFs or those without cerebral venous drainage,
close follow-up is a reasonable option. However, for those patients
concurrent with high-grade DAVFs, aggressive management through the
endovascular route or open surgery is recommended to avoid future
catastrophic intracranial hemorrhage. However, re-examination of
the DSA and MR perfusion was refused by the patient of the present
study during follow-up. The radiological progression of the MMD and
DAVF remained undetermined for this patient, which limits the
universality of the conservative treatment for this patient.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
KH and YZ designed the study and drafted the
manuscript. XC, KX and JY collected and analyzed of the clinical
data. JY critically revised the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Ethics approval is not required for case reports at
our institution. Informed consent for participation in the study or
use of the medical data was obtained from the patient.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this manuscript and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bang OY, Chung JW, Kim DH, Won HH, Yeon
JY, Ki CS, Shin HJ, Kim JS, Hong SC, Kim DK and Koizumi A: Moyamoya
disease and spectrums of RNF213 vasculopathy. Transl Stroke Res.
11:580–589. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Reynolds MR, Lanzino G and Zipfel GJ:
Intracranial dural arteriovenous fistulae. Stroke. 48:1424–1431.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Killory BD, Gonzalez LF, Wait SD, Ponce
FA, Albuquerque FC and Spetzler RF: Simultaneous unilateral
moyamoya disease and ipsilateral dural arteriovenous fistula: Case
report. Neurosurgery. 62:E1375–E1376; discussion E1376.
2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hanaoka M, Matsubara S, Satoh K and
Nagahiro S: Dural arteriovenous fistulae after cerebral infarction:
Report of two cases. Neurosurgery. 68:E575–E579; discussion E580.
2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Liu ZW, Han C, Zhao F, Qiao PG, Wang H,
Bao XY, Zhang ZS, Yang WZ, Li DS and Duan L: Collateral circulation
in moyamoya disease: A new grading system. Stroke. 50:2708–2715.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hou K, Ji T, Guo Y, Xu K and Yu J: The
coexistence of persistent primitive trigeminal artery, moyamoya
disease, and multiple intracranial aneurysms: A case report and
literature review. World Neurosurg: Jan 23, 2019 (Epub ahead of
print).
|
|
7
|
Zaletel M, Surlan-Popović K, Pretnar-Oblak
J and Zvan B: Moyamoya syndrome with arteriovenous dural fistula
after head trauma. Acta Clin Croat. 50:115–120. 2011.PubMed/NCBI
|
|
8
|
Feroze AH, Kushkuley J, Choudhri O, Heit
JJ, Steinberg GK and Do HM: Development of arteriovenous fistula
after revascularization bypass for moyamoya disease: Case report.
Neurosurgery. 11 (Suppl 2):E202–E206. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu P, Xu Y, Lv X, Ge H, Lv M and Li Y:
Progression of unilateral moyamoya disease resulted in spontaneous
occlusion of ipsilateral cavernous dural arteriovenous fistula:
Case report. Interv Neuroradiol. 22:362–364. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Koduri S, Wilkinson DA, Griauzde JM,
Gemmete JJ and Maher CO: Development of bilateral dural
arteriovenous fistulae following pial synangiosis for moyamoya
syndrome: Case report. J Neurosurg Pediatr. 24:9–13.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Guo Y and Yu J, Zhao Y and Yu J: Progress
in research on intracranial multiple dural arteriovenous fistulas.
Biomed Rep. 8:17–25. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
O'Shaughnessy BA, DiPatri AJ Jr, Parkinson
RJ and Batjer HH: Development of a de novo cerebral arteriovenous
malformation in a child with sickle cell disease and moyamoya
arteriopathy. Case report. J Neurosurg. 102 (Suppl 2):S238–S243.
2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Schmit BP, Burrows PE, Kuban K, Goumnerova
L and Scott RM: Acquired cerebral arteriovenous malformation in a
child with moyamoya disease. Case report. J Neurosurg. 84:677–680.
1996.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hou K, Ji T, Guo Y, Xu B, Xu K and Yu J:
Current status of endovascular treatment for dural arteriovenous
fistulas in the superior sagittal sinus region: A systematic review
of the literature. World Neurosurg. 122:133–143. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lu J, Li Z, Zhao Y, Chen X, Shi G and Zhao
J: Hemorrhagic transformation in ischemic moyamoya disease:
Clinical characteristics, radiological features, and outcomes.
Front Neurol. 11(517)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Moussouttas M and Rybinnik I: A critical
appraisal of bypass surgery in moyamoya disease. Ther Adv Neurol
Disord. 13(1756286420921092)2020.PubMed/NCBI View Article : Google Scholar
|