Introduction
Cervical cancer is the 4th most common cancer type
worldwide and is a prevalent type of malignant tumor in the female
reproductive system (1). There are
~500,000 new cases annually worldwide, where the incidence is
rising annually with diagnosis being common among younger women
(2,3). Surgery plus radiotherapy can
significantly improve the treatment effect of early-stage cancer,
but for the late stage, the treatment effect of patients with
relapse and metastasis remains at the bottleneck stage, and the
1-year survival rate of patients with late-stage cancer is <20%
(4,5). Therefore, it is important to identify
the molecular pathogenesis of cervical cancer, in order to develop
complementary or substitute treatment methods, as well as more
effective strategies to prevent cervical cancer, which will be of
great theoretical and practical significance to improve the quality
of life of patients with this disease worldwide.
Currently, comprehensive treatments, including
surgery, lack a breakthrough in the curative effect of advanced and
recurrent cervical cancer, and thus researchers have investigated
traditional Chinese medicine (6,7).
Arsenic is a traditional Chinese medicine that has three inorganic
forms: Red arsenic (As4S4), yellow arsenic
(As2S3) and white arsenic
[As2O3; arsenic trioxide (ATO)], which are
produced by oxidation of arsenic at high temperatures (8). In the mid and late 20th century,
Chinese scholars first identified that ATO had significant clinical
efficacy for the treatment of acute promyelocytic leukemia, and it
is now used in clinical practice (9,10).
Recently, additional research has examined solid tumors outside the
blood system, and observed a good treatment efficacy in response to
ATO (11). Previous studies have
reported that ATO exerts antitumor effects on various solid tumors,
including liver, breast, osteosarcoma and ovarian cancer, and its
possible mechanism of action has also been investigated (12-14).
However, its efficacy against cervical cancer has yet to be
elucidated, where the underlying mechanism of action remain
unknown.
Reactive oxygen species (ROS) is a general term for
several active substances formed by oxygen, and the metabolism of
ROS is closely associated with numerous metabolic regulatory
activities, such as glucose metabolism, in the human body (15,16).
The vast majority of oxygen accepts four electrons to combine with
H+ to form water, while some oxygen molecules produce
ROS in a single-valent reduction form (17). ROS are important signaling molecules
that not only regulate the structure and function of blood vessels,
but also serve an important role in regulating cell proliferation,
migration and differentiation by effecting the cellular proteins,
lipids and nucleic acids (18-20).
Previous studies have revealed that
hypoxia-inducible factor-1α (HIF-1α) is an important factor in
promoting cell proliferation, invasion and metastasis of malignant
tumors (21,22). Moreover, ATO can suppress HIF-1α
production to serve an antitumor function in lung carcinoma and
breast cancer cells (23,24). However, to the best of our
knowledge, the relationship of ATO and HIF-1α has been rarely
studied in cervical cancer.
The aim of the present study was to identify a novel
strategy for the prevention and treatment of cervical cancer. In
the present study, the effect of different times and concentrations
of ATO treatment on cervical cancer was investigated. SiHa cells
were used to examine the IC50 of ATO in cervical cancer
cells, as well as to assess the effects of ATO on cell
proliferation and invasion. Furthermore, ROS and HIF-1α production
were detected to elucidate the mechanism of ATO.
Materials and methods
Materials
The SiHa cervical cancer cell line and 293T cells
were purchased from Shanghai Bioengineering Co., Ltd. FBS and
RPMI-1640 medium were purchased from Gibco (Thermo Fisher
Scientific, Inc.), while the BeyoFast™ SYBR-Green quantitative PCR
premix Mix kit, Hoechst 33258 Staining kit (cat. no. C0003), RIPA
lysis buffer (cat. no. P0013B), bicinchoninic acid (BCA) protein
quantitative kit and MTT kit were all purchased from Beyotime
Institute of Biotechnology. PVDF 45-µm non-sterile 50/pk membranes
were purchased from EMD Millipore. ATO (white) was purchased from
Sigma-Aldrich (Merck KGaA) and the Annexin V-FITC/PI
double-staining kit (cat. no. 40302ES20) was purchased from
Shanghai Yeasen Biotechnology Co., Ltd.
Cell culture and treatment
ATO powder (0.1 g) was dissolved in 10 ml sodium
hydroxide (5 mol/l) before the pH of the solution was adjusted to
neutral with hydrogen chloride. ATO was wrapped in foil and kept at
-4˚C. Before it was used in cell culture, ATO solution was diluted
to the appropriate concentrations (0, 5, 10, 15 and 20 µM) with PBS
and filtered with a 0.22-µm filter. SiHa cells were cultured in
RPMI-1640 medium containing 10% FBS at 5% CO2, 37˚C and
saturated humidity, and the medium was changed once daily. After
the cells entered the logarithmic long-term phase of growth, they
were digested using 0.25% pancreatin at 37˚C for 3 min. The cells
were inoculated into 96-well (5x104 cells/well), 24-well
(2x105 cells/well) or 6-well (1x106
cells/well) plates and cultured for 24 h at 37˚C, before being
added with different concentrations of ATO (0, 5, 10, 15 and 20 µM)
at the indicated time points (0, 6, 12 and 24 h) and incubated at
37˚C.
Proliferation inhibition assay
The cells were inoculated at a density of
1x104 cells/well in 96-well culture plates. Each group
had three compound wells and continued to be cultured after adding
different concentrations of ATO (0, 5, 10, 15 and 20 µM) for 0, 6,
12 and 24 h. After the culture solution was removed at different
time-points, 10 µl MTT (5 mg/ml) was added to the culture. After
culturing for 4 h, the culture was terminated. After the culture
solution was absorbed, 100 µl DMSO was added to each well at 37˚C
for 10 min. The optical density (OD) values of each group were
measured at 570 nm using an enzyme labeling instrument (Type no.
Sunrise; Tecan Group, Ltd.). The cell survival curves were plotted
with the culture time as abscissa and OD as the ordinate.
Detection of ROS content
Intracellular ROS, such as
H2O2 and •OH, were determined on the basis of
fluorescent light detection using an oxidation-sensitive
fluorescent probe dye, 2',7'-dichlorodihydrofluorescein diacetate
(H2DCFDA; Invitrogen; Thermo Fisher Scientific, Inc.)
(25). The experiment was performed
according to the manufacturer's protocols (26). In brief, 1x106 cells were
incubated with the indicated concentrations (0, 5, 10, 15 and 20
µM) of ATO for 0, 6, 12 and 24 h at 37˚C. The cells were then
washed in PBS and incubated with 20 µM H2DCFDA at 37˚C
for 30 min according to the manufacturer's instruction.
Fluorescence was detected at 488 nm excitation wavelength and 525
nm emission wavelength using an enzyme labeling instrument (Type
no. Sunrise; Tecan Group, Ltd.).
Colony formation assay
Cells in the logarithmic growth phase were prepared
into single-cell suspensions and counted. In total, 1,000 cells
were inoculated in each 60-mm culture dish and cultured in a 5%
CO2 incubator for 37˚C. The medium was changed every 3
days and then removed. Cells were washed three times with PBS,
fixed with 100% anhydrous methanol for 15 min at room temperature
and stained with 0.1% crystal violet solution for 15 min at room
temperature. Subsequently, the number of colonies containing >15
cells was counted under a light microscope (magnification, x400;
Carl Zeiss AG).
Cell invasion assay
The final mass concentration of Matrigel was
adjusted to 1 mg/ml using 4˚C precooled serum-free medium. The
upper Transwell chamber with 8-µm pore-size filters (Corning, Inc.)
was coated with Matrigel for 3-5 h at 37˚C and set aside after
solidification. The cells were collected in each group before
4x105 cells were suspended in 400 µl serum-free medium,
and the Transwell culture chamber was inserted into the 24-well
culture plate. The cell mixture was added to the upper layer of the
chamber, and RPMI-1640 supplemented with 20% FBS was added to the
lower layer of the chamber as the chemokine source. The chamber was
removed, and the culture liquid was aspirated after 16 h. The upper
layer cells were carefully removed, washed twice with PBS and fixed
with 100% anhydrous methanol at room temperature for 5 min. Cells
were then stained for 5 min with 0.1% crystal violet at room
temperature, rinsed gently with water several times, dried while
inverted and mounted with a coverslip and neutral gum. Under a
light microscope (magnification x200; Carl Zeiss AG), six visual
fields were randomly selected, and the number of cells stained with
crystal violet solution (i.e., the number of invasive cells) was
counted.
Western blot analysis
The RIPA lysis buffer (including 1 µM PMSF protease
inhibitor) was added to SiHa cells at logarithmic growth phase and
then placed on ice for 40 min. The supernatant was collected via
13,000 x g centrifugation for 20 min at 4˚C. The total cell protein
was obtained, and the protein concentration was determined using
the BCA method. After separating the protein (40 µg protein/lane)
on a nitrocellulose membrane with 10% SDS-PAGE, membranes were
blocked in 5% skimmed milk powder at room temperature for 3 h.
Diluted HIF-1α (1:200; cat. no. ab113642; Abcam) or GAPDH
monoclonal antibodies (1:200; cat. no. ab8245; Abcam) were then
added and the membranes were incubated overnight on a rocker at
4˚C, followed by washing three times with PBS-0.1%Tween-20 (PBST)
for 10 min each. The polyclonal goat anti-mouse horseradish
peroxidase-conjugated secondary antibody (1:5,000; cat. no. ab6789;
Abcam) was added and incubated at 37˚C for 2 h. Membranes were
washed three times in PBST for 10 min each time. Odyssey infrared
laser scanning imaging (LI-COR Biosciences) and ImageJ 6.0
(National Institutes of Health) were used to analyze the
results.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
The total RNA of the SiHa cells from each group was
extracted using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), and the RNA purity and concentration were
determined using an ultraviolet spectrophotometer (Tecan Group,
Ltd.). The RNA to cDNA EcoDry™ Premix (cat. no. 639549; Takara
Biotechnology Co., Ltd.) was used to reverse-transcribe RNA to the
first chain of cDNA. The temperature protocol is 42˚C 50 min for
reverse transcription reaction, 99˚C for 5 min to inactivate the
reverse transcriptase and 4˚C to save the reverse transcription
product. The cDNA was subsequently used as a template to detect the
expression of HIF-1α using the BeyoFast™ SYBR-Green quantitative
PCR premix. The thermocycling conditions were as follows: Initial
denaturation at 95˚C for 10 min, followed by 40 cycles of 95˚C for
15 sec and 60˚C for 1 min. At the end of the PCR amplification, the
melting curve was plotted and the relative expression of HIF-1α was
assessed using the 2-ΔΔCq method (27). The primer sequences are presented in
Table I.
 | Table IReverse transcription-quantitative
PCR primer information. |
Table I
Reverse transcription-quantitative
PCR primer information.
| Gene | Primer | Primer
sequence | Annealing
temperature, ˚C |
|---|
| HIF-1α | F |
5'-ACAAGTCACCACAGGACAG-3' | 56.91 |
| | R |
5'-AGGGAGAAAATCAAGTCG-3' | 51.46 |
| GAPDH | F |
5'-AGGTCGGTGTGAACGGATTTG-3' | 62.14 |
| | R |
5'-GGGGTCGTTGATGGCAACA-3' | 60.75 |
Construction of HIF-1α knockdown
lentivirus plasmid
The recombinant lentivirus plasmid pLKO.1-HIF-1α
short hairpin (sh)RNA sequence 1, pLKO.1-HIF-1α shRNA sequence 2
and negative control plasmid pLKO.01-scrambled-shRNA were designed
and synthesized by Cyagen Biosciences Inc. 293T cells were cultured
in RPMI-1640 medium containing 10% FBS at 5% CO2, 37˚C.
To produce virus, 293T cells (1.6x106 cells/well) were
seeded into 6-well plates prior to transfection. The ratio of pMDL
(0.75 µg), VSVG (0.45 µg), and pRSV-Rev plasmids (0.3 µg) were
5:3:2, which were all purchased from Shanghai GeneChem Co., Ltd.
The three plasmids aforementioned (total DNA 1.5 µg), recombinant
lentivirus plasmid (1.5 µg) and Lipofectamine® 2000 (6
µl; Invitrogen; Thermo Fisher Scientific, Inc.) were then mixed.
After being kept at room temperature for 20 min, the mixture was
added to the culture medium of 293T cells drop by drop for
transfection. The culture medium was replaced with fresh medium
after 6 h, which then continued to cultivate for 24 or 48 h. The
supernatant was collected and the lentivirus particles were
harvested by ultracentrifugation (2 h at 50,000 x g; 4˚C). SiHa
cells were infected [multiplicity of infection (MOI)=10]. After
12-16 h, the medium was replaced with fresh medium. The cells were
harvested for subsequent experimentation after 48 h. The DNA
single-strand template sequences for the two pairs of interference
fragments shRNA1 and shRNA2 are as follows: HIF-1α shRNA sequence 1
forward,
5'-CCGGCTGCCCTTACGCAATAAATTGTCCATATTGGTCTGGAACAAGAGATAGCGGTTTTTG-3'
and reverse,
3'-AATTCAAAAACCGCAATTCAAATGTGACACTCTCCGGGTTAATATGTCATATTGGTCCAGG-5';
and HIF-1α shRNA sequence 2 forward,
5'-TGCAACATTTTATGATTAGACCCACAATCAGTGAGGATCAGATAACGTTATTCGGTTTTTG-3'
and reverse,
3'-AATTCAAAAACCAAGTTCTCTACTTCAGTAAAATATGTTCGTGATCAGATTAAAATTCTGG-5'.
Hoechst staining and flow cytometry
assay
Cells in the logarithmic growth phase were
collected, and a single-cell suspension with a concentration of
2.5x104 cells/ml was generated. Then, a 200 µl
suspension was added to each well of a 96-well plate. After the
cells adhered, lentivirus (MOI=10) was used to infect SiHa cells,
whilst the control group, which was transfected with the negative
control lentiviral vector, was set up, with three wells for each
group. After incubating for 72 h at 37˚C and 5% CO2, the
culture medium was aspirated and the cells were fixed in 4%
precooled paraformaldehyde at 37˚C for 30 min and stained at 37˚C
with Hoechst 33258 staining solution for 5 min (28). The cells were then observed and
imaged using fluorescence microscopy (magnification, x200). For the
flow cytometry detection, SiHa cells infected with lentivirus were
harvested, washed with PBS and stained with a Annexin V-FITC and PI
double-staining kit for 15 min for 37˚C (29). Subsequently, these cells were
analyzed via flow cytometry (BD FACSCalibur™; BD Biosciences)
within 1 h. The results were evaluated by CellQuest Pro software
(version 5.0; BD Biosciences). The apoptotic rate was calculated by
the percentage of early and late apoptotic cells.
Statistical analysis
All experiments were repeated three times. Data are
presented as the mean ± SD. Unpaired t-test was used to compare two
groups, and one-way ANOVA followed by Bonferroni post hoc test was
used to compare multiple groups. P<0.05 was considered to
indicate a statistically significant difference. All data were
analyzed using the SPSS 20 (IBM Corp.) software.
Results
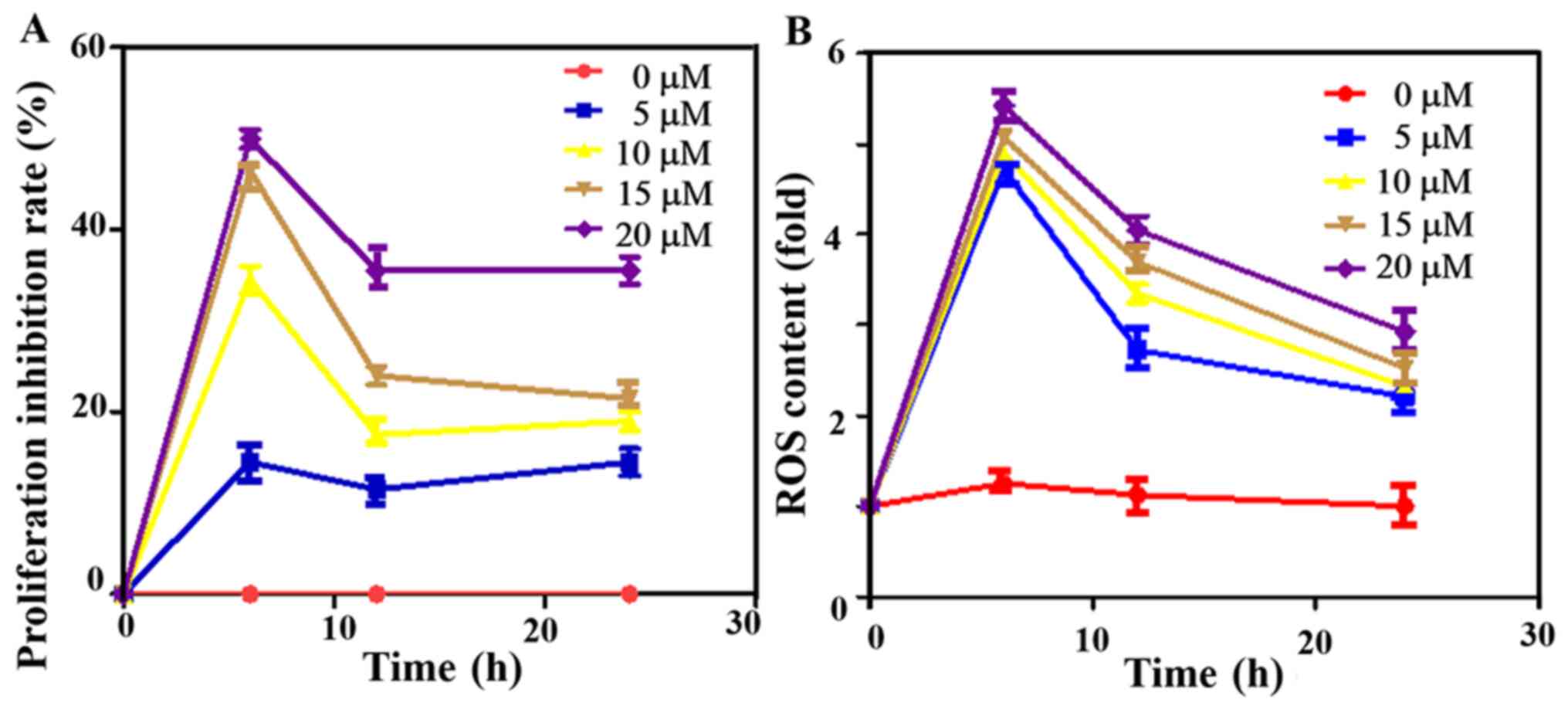
Cell viability inhibition rate and ROS
content in cells
The 50% inhibitory concentrations (IC50)
is the dose of ATO against proliferation of cervical cancer cells
within the prescribed time (30).
The cell viability inhibition rate was measured using the MTT
method after the cells were treated at different ATO concentrations
(0, 5, 10, 15 and 20 µM), which demonstrated the inhibition rate to
be dependent on the concentration, with the highest rat observed in
the 20 µM group. Besides, it was found that with the prolongation
of time, the inhibition rate of the ATO first increased, then
decreased (Fig. 1A). Subsequently,
the inhibition rate remained at a relatively stable level. At 6 h,
the inhibition rate was highest in all groups. Thus, ATO had a
time- and dose-dependent effect on cell viability.
After the cells were treated with different ATO
concentrations for 0, 6, 12 and 24 h, the intracellular ROS content
was detected. The ROS content first increased and then decreased in
a time- and dose-dependent manner, and it was the highest at 6 h,
which suggested that ATO inhibited the proliferation of cervical
cancer cells by mainly relying on ROS production (Fig. 1B).
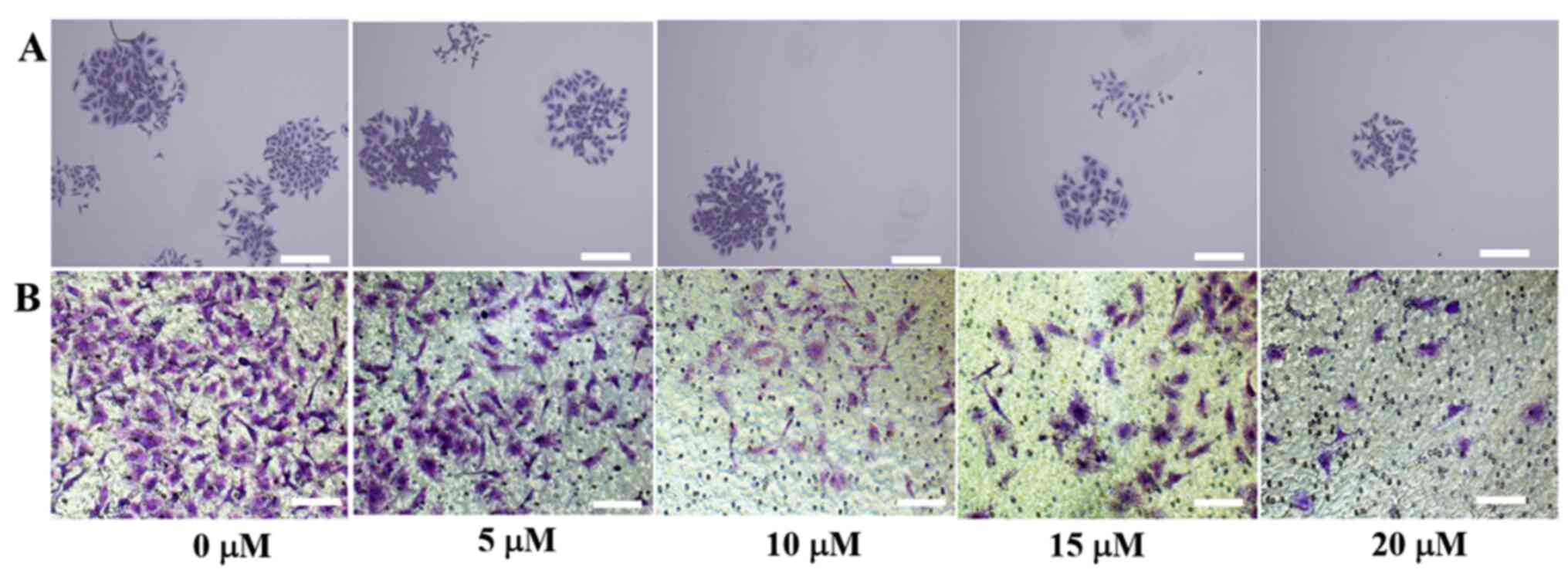
Colony formation assay and cell
invasion
SiHa cells were treated with different
concentrations ATO for 6 h, the colony formation and Transwell
assays were performed to detect cell proliferation and invasion
(Fig. 2A and B). When there was no drug treatment, the
colony-forming ability of the cells was increased, whilst higher
drug concentrations led to markedly fewer numbers of cell colonies
and lowered the invasive capabilities of cells. Therefore, the
results suggested that ATO inhibited SiHa cell proliferation and
invasion in a dose-dependent manner.
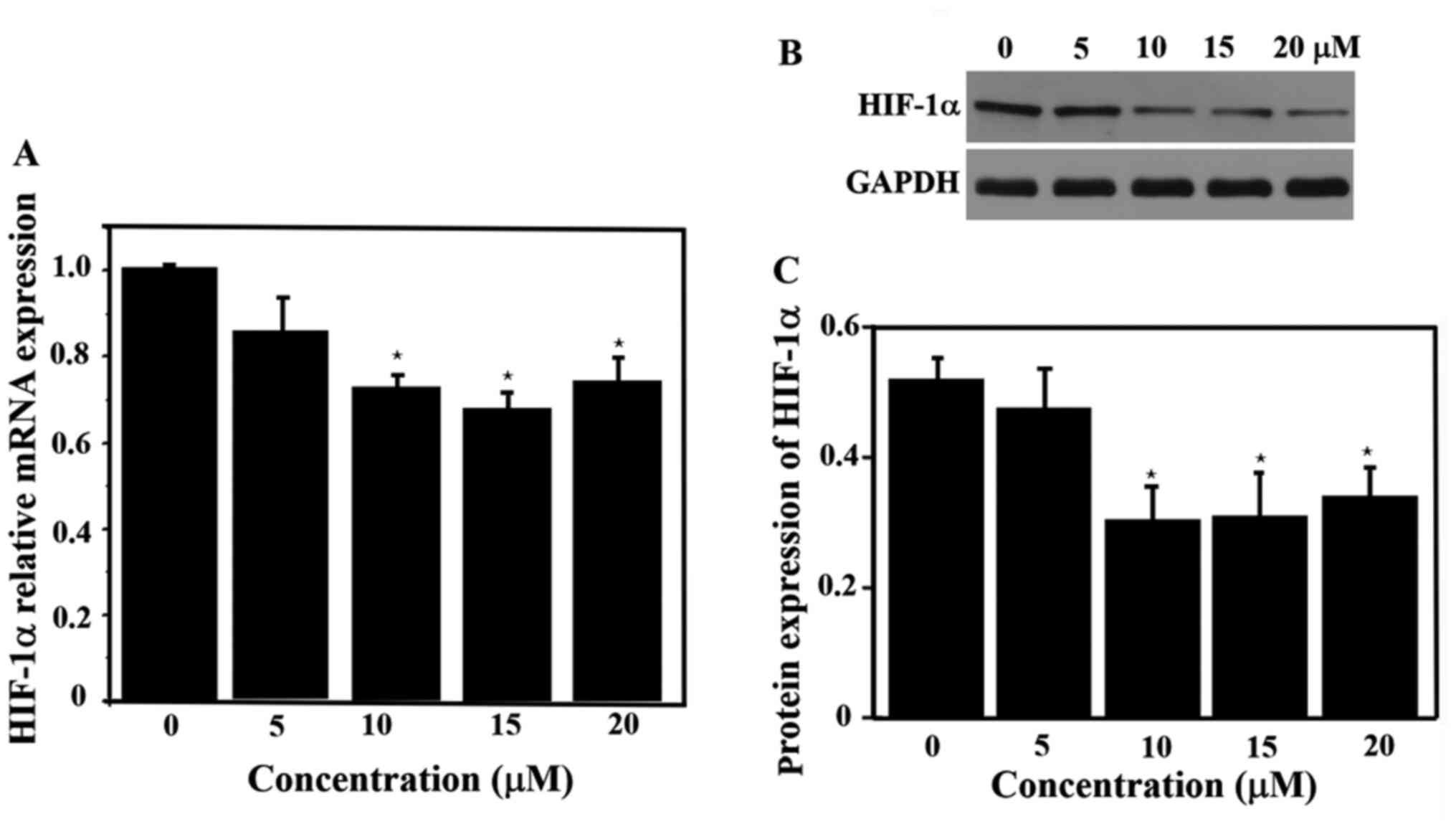
HIF-1α expression with ATO
treatment
The relative expression of HIF-1α mRNA was measured
with different concentrations ATO. The expression of HIF-1α mRNA
was significantly lower in the drug-treated groups (10, 15 and 20
µM) compared with the control group (P<0.05), but there was no
significant difference in the expression of HIF-1α mRNA among these
three experimental groups (Fig.
3A). Thus, ATO significantly inhibited the expression of HIF-1α
mRNA at 6 h. The expression of HIF-1α protein was also
significantly lower in the 10, 15 and 20 µM groups (Fig. 3B and C) compared with that in the control group,
which was in line with the RT-qPCR results for HIF-1α mRNA
expression.
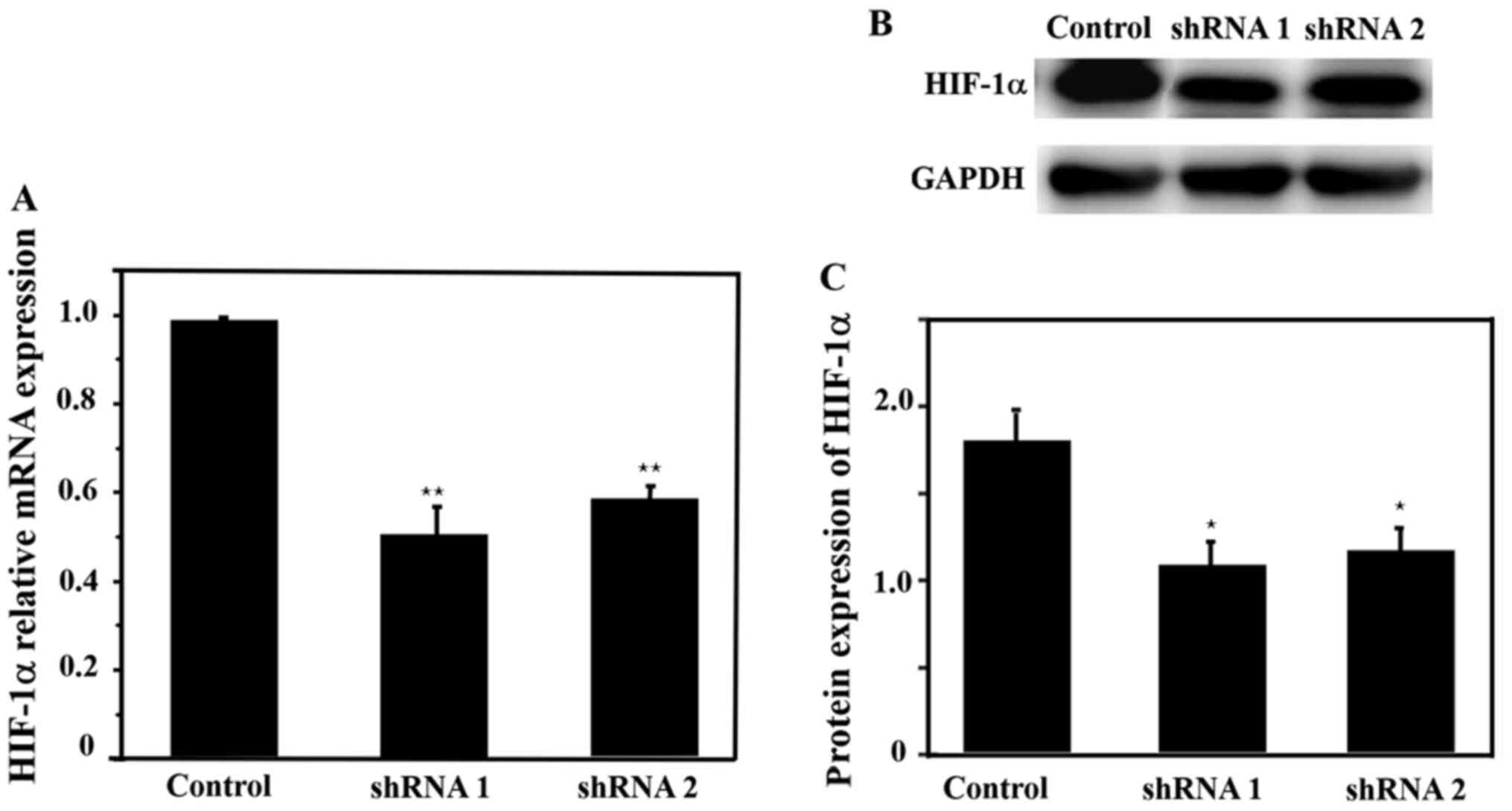
Colony formation and cell migration
after knockdown of HIF-1α
HIF-1α mRNA and protein expression levels were
detected after infection with lentivirus. The expression levels of
HIF-1α mRNA and protein were significantly lower in the HIF-1α
shRNA 1 and shRNA 2 groups compared with the control group
(Fig. 4). Moreover, HIF-1α shRNA 1
demonstrated a higher inhibitory effect compared with shRNA 2.
Therefore, HIF-1α shRNA 1 was selected for subsequent
experiments.
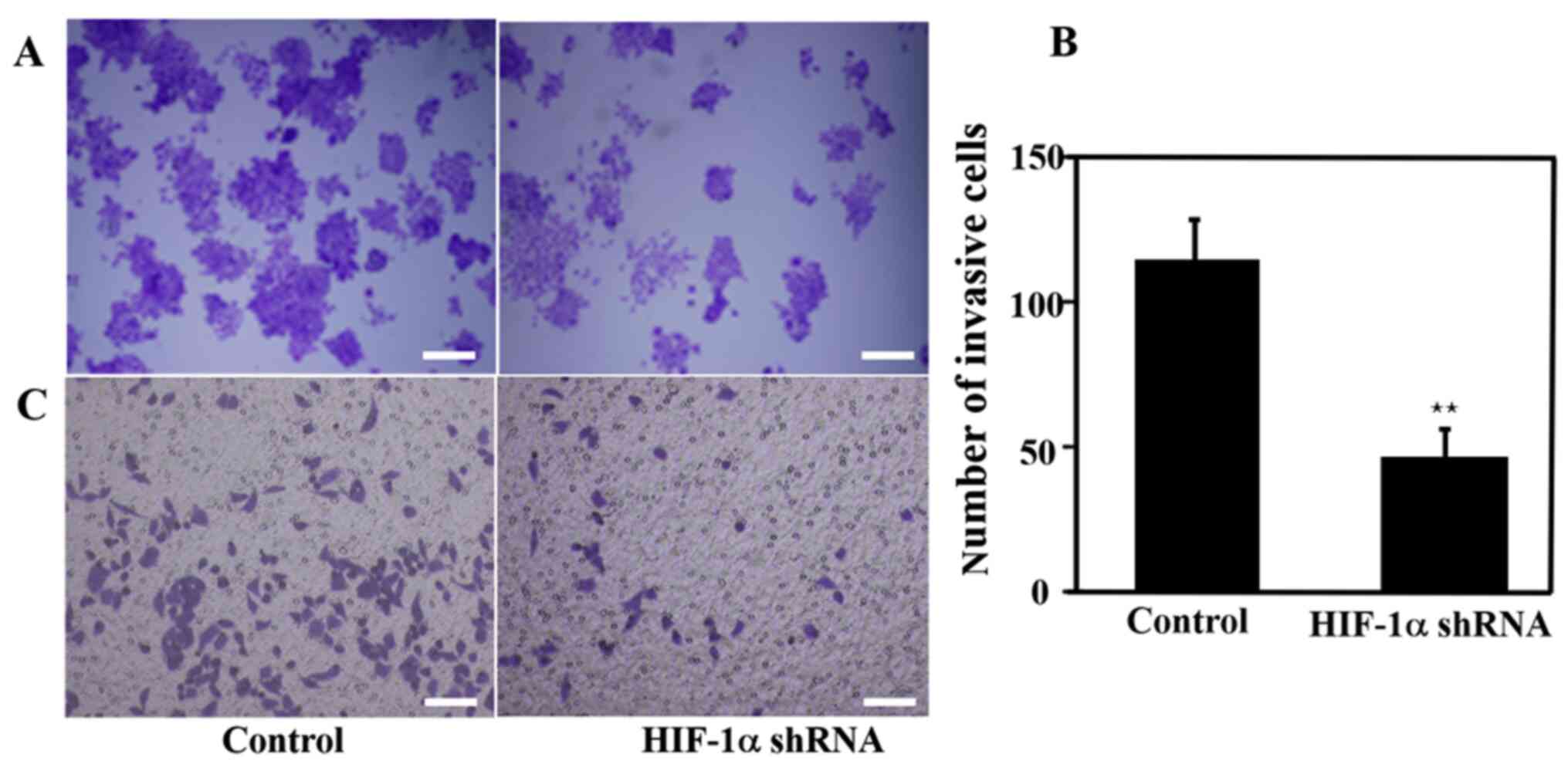
The results of the colony formation and cell
migration experiments are presented in Fig. 5. Compared with the control group,
the number of colonies formed in the HIF-1α knockdown group was
markedly lower, indicating that the knockdown of the HIF-1α gene
inhibited cell proliferation (Fig.
5A). Furthermore, the cell invasion rate was significantly
decreased, suggesting that knocking down HIF-1α reduced the cell
invasive ability (Fig. 5B and
C).
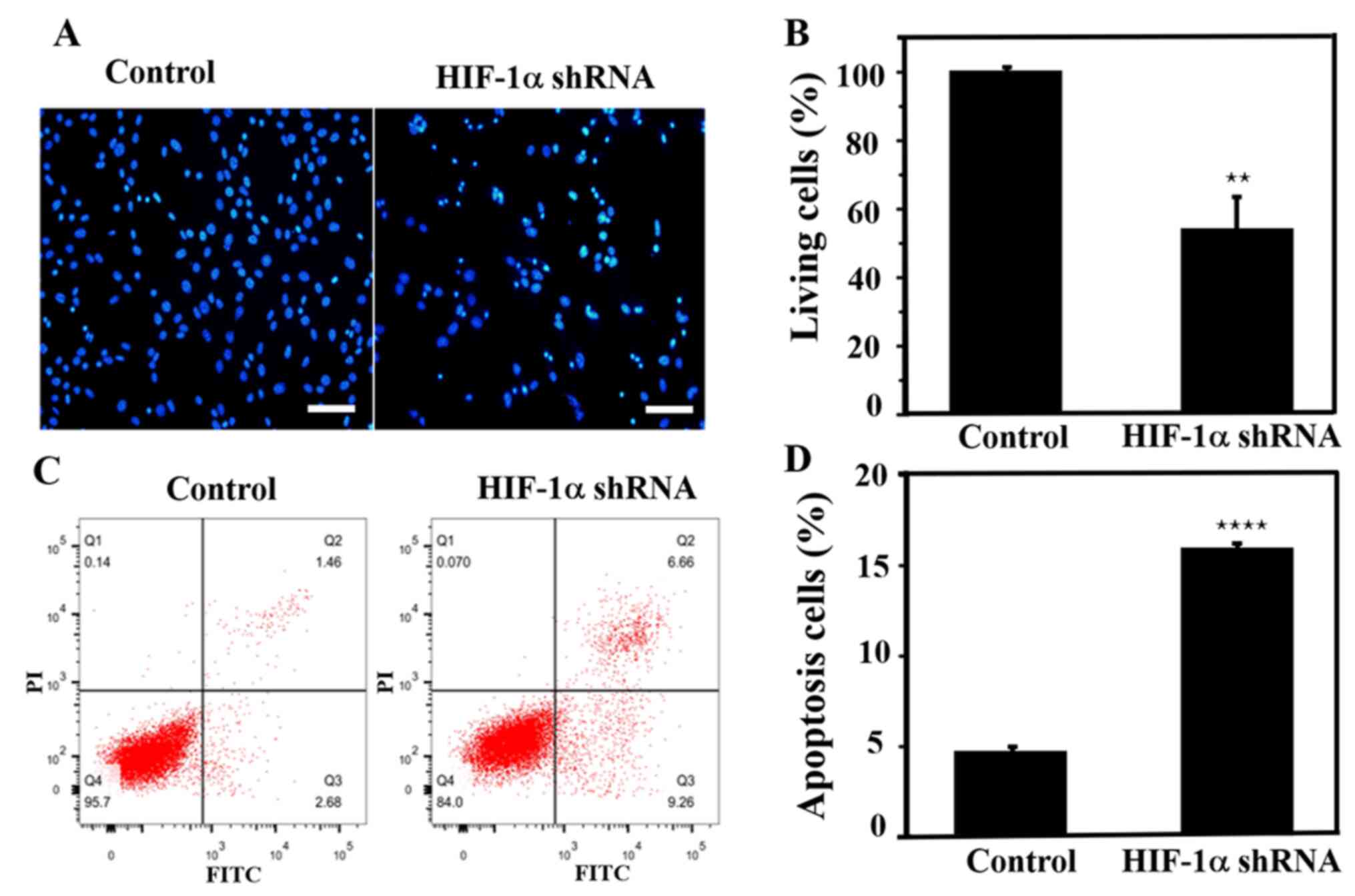
Apoptosis assay after HIF-1α
knockdown
Lentivirus-infected SiHa cells were stained with
Hoechst dye after 72 h. SiHa cells exhibited typical morphological
changes indicating apoptosis, such as nuclear condensation and
nucleosome and nuclear fragmentation, following knockdown of the
HIF-1α gene (Fig. 6A). In addition,
the apoptotic rate of cells after knockdown was ~50%, demonstrating
that HIF-1α knockdown promoted cell apoptosis (Fig. 6B). Flow cytometric analysis was
performed to further evaluate apoptosis, and it was identified that
after knocking down the HIF-1α gene, the early and late apoptotic
rate of SiHa cells was increased by ~3-fold compared with that in
the control group (Fig. 6C and
D), which was consistent with the
aforementioned Hoechst staining results.
Discussion
Cervical cancer is one of the most common
gynecological malignancies, which tends to be diagnosed in younger
women, and ~570,000 cases of cervical cancer and 311,000 deaths
from the disease occurred in 2018(31). The estimated age-standardized
incidence of cervical cancer was 13·1 per 100,000 women globally
(31). There is currently no
established standard for effective treatment, and there is yet no
commonly accepted highly effective drug. At present, the main
treatment principles for cervical cancer are surgery, followed by
radiotherapy and chemotherapy (32). Chemotherapy is of great significance
in the treatment of cervical cancer, but the main application of
chemotherapeutic drugs is not targeted therapy and there are a
number of side effects (33).
Chemotherapeutic resistance is a major challenge of recurrence and
metastasis of cervical cancer (33). Therefore, identifying novel,
high-efficiency and low-toxicity chemotherapy drugs will be the
primary direction of future research (28,33).
ATO is extracted from the traditional Chinese
medicine white arsenic, which is easy to obtain, cost-effective and
convenient (34). Recent studies
have reported that ATO not only serves an important role in the
treatment of hematological malignancies, but also has a beneficial
antitumor efficacy in solid tumors (11-13).
However, its specific mechanism of action requires further
investigation.
ATO-induced apoptosis is a complex process involving
multiple targets, and is one of its main antitumor effects
(35). Previous studies have
revealed that the antitumor effect of ATO is closely associated
with ROS (36,37). Moreover, the baseline levels of ROS
can stimulate the mitosis, DNA synthesis and the proliferation of
tumor cells (38). However, a high
level of ROS can induce tumor cell apoptosis and cause tumor cell
necrosis (39). It has been
reported that an important early cell activity in ATO treatment of
target cells is the change in the ROS level, and ATO can increase
the level of ROS in cells by acting on multiple pathways, including
the ATM-ATR-associated Chk pathway and caspase-3-dependent
apoptosis (37,40). However, the exact mechanism of
action of ATO in cervical cancer is yet to be fully elucidated
(41).
In the present study, different concentrations of
ATO were applied to SiHa cells to detect the inhibitory effect of
ATO on cellular processes. The results demonstrated that the
inhibition rate of 20 µM ATO was ~50% when the cells were treated
for 6 h, and the inhibitory effect of ATO on cells was time- and
dose-dependent. The level of ROS produced by ATO was also time- and
dose-dependent, which indicated that ATO inhibited cervical cancer
cellular functions via inducing ROS production in a time- and
dose-dependent manner.
HIF-1α is an important factor in determining the
prognosis of malignant tumors. For instance, excessive activation
of this factor can promote the proliferation, infiltration and
metastasis of tumor cells, as well as induce tumor cell resistance
to chemotherapy and radiotherapy (42). However, to the best of our
knowledge, the relationship of ATO and HIF-1α has been rarely
studied in cervical cancer. In the present study, it was identified
that ATO could inhibit the expression of HIF-1α, and that the
knockdown of HIF-1α can effectively suppressed SiHa cell
proliferation and invasion, and promote cell apoptosis.
In conclusion, with regards to the mechanism of
action of ATO against cervical cancer, the present results
suggested that ATO may inhibit cell proliferation and migration via
inducing ROS and inhibiting HIF-1α production. The present study
may provide a further theoretical basis for identifying effective
molecular targets for the prevention and treatment of cervical
cancer. However, the mechanism via which ATO induces ROS and
suppresses HIF-1α, and its downregulation of cervical cancer are
yet to be elucidated. In addition, additional animal experiments
need to further verify the safety of ATO.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Independent
Scientific Research Project of Wuhan University (grant no.
413000117) and the Chinese Medical Association Clinical Research
Fund-Reproductive Medicine Young Physicians Research and
Development Project (grant no. 17020310700).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ, YZ, YC and XY conceived and designed the
research. LZ, YZ, JK, YW, SX, LZ and MY conducted the experiments.
All authors analyzed and interpreted the data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen Y, Liu C, Xie B, Chen S, Zhuang Y and
Zhang S: miR96 exerts an oncogenic role in the progression of
cervical cancer by targeting CAV1. Mol Med Rep. 22:543–550.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Global Burden of Disease Cancer
Collaboration. Fitzmaurice C, Dicker D, Pain A, Hamavid H,
Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, et
alThe global burden of cancer 2013. JAMA Oncol. 4:505–527.
2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wei KR, Chen WQ, Zhang SW, Zheng RS, Wang
YN and Liang ZH: Epidemiology of uterine corpus cancer in some
cancer registering areas of China from 2003-2007. Zhonghua Fu Chan
Ke Za Zhi. 6:445–451. 2012.PubMed/NCBI(In Chinese).
|
|
4
|
Sousa DMDN, Chagas ACMA, Vasconcelos CTM,
Stein AT and Oriá MOB: Development of a clinical protocol for
detection of cervical cancer precursor lesions. Rev Lat Am
Enfermagem. 26(e2999)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
McClung NM, Gargano JW, Park IU, Whitney
E, Abdullah N, Ehlers S, Bennett NM, Scahill M, Niccolai LM,
Brackney M, et al: Estimated number of cases of high-grade cervical
lesions diagnosed among women-United States, 2008 and 2016. MMWR
Morb Mortal Wkly Rep. 68:337–343. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Su M, Gong XJ and Zhou X: Research
progress in mechanism of traditional Chinese medicine active
ingredients against cervical cancer. Zhongguo Zhong Yao Za Zhi.
44:675–684. 2019.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
7
|
Yang J, Li J, Sun M and Chen K: Studies of
traditional Chinese medicine monomer on HeLa cell of cervical
cancer. Pak J Pharm Sci. 27 (4 Suppl):S1063–S1068. 2014.PubMed/NCBI
|
|
8
|
Zhu J, Chen Z, Lallemand-Breitenbach V and
de Thé H: How acute promyelocytic leukaemia revived arsenic. Nat
Rev Cancer. 2:705–713. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM,
Qiu QY, Zhu J, Tang W, Sun GL, Yang KQ, et al: Use of arsenic
trioxide (As2O3) in the treatment of acute promyelocytic leukemia
(APL): II. Clinical efficacy and pharmacokinetics in relapsed
patients. Blood. 89:3354–3360. 1997.PubMed/NCBI
|
|
10
|
Wang ZY and Chen Z: Acute promyelocytic
leukemia: From highly fatal to highly curable. Blood.
111:2505–2515. 2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kozono S, Lin YM, Seo HS, Pinch B, Lian X,
Qiu C, Herbert MK, Chen CH, Tan L, Gao ZJ, et al: Arsenic targets
Pin1 and cooperates with retinoic acid to inhibit cancer-driving
pathways and tumor-initiating cells. Nat Commun.
9(3069)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sadaf N, Kumar N, Ali M, Ali V, Bimal S
and Haque R: Arsenic trioxide induces apoptosis and inhibits the
growth of human liver cancer cells. Life Sci. 205:9–17.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wu B, Tan M, Cai W, Wang B, He P and Zhang
X: Arsenic trioxide induces autophagic cell death in osteosarcoma
cells via the ROS-TFEB signaling pathway. Biochem Biophys Res
Commun. 1:167–175. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang H, Gao P and Zheng J: Arsenic
trioxide inhibits cell proliferation and human papillomavirus
oncogene expression in cervical cancer cells. Biochem Biophys Res
Commun. 441:556–561. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jambunathan N: Determination and detection
of reactive oxygen species (ROS), lipid peroxidation, and
electrolyte leakage in plants. Methods Mol Biol. 639:292–298.
2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang G, Li Y, Yang Z, Xu W, Yang Y and Tan
X: ROS mediated EGFR/MEK/ERK/HIF-1α loop regulates glucose
metabolism in pancreatic cancer. Biochem Biophys Res Commun.
500:873–878. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhao RZ, Jiang S, Zhang L and Yu ZB:
Mitochondrial electron transport chain, ROS generation and
uncoupling (Review). Int J Mol Med. 44:3–15. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lu L, Dong J, Wang L, Xia Q, Zhang D, Kim
H, Yin T, Fan S and Shen Q: Activation of STAT3 and Bcl-2 and
reduction of reactive oxygen species (ROS) promote radioresistance
in breast cancer and overcome of radioresistance with niclosamide.
Oncogene. 37:5292–5304. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Diwanji N and Bergmann A: An unexpected
friend-ROS in apoptosis-induced compensatory proliferation:
Implications for regeneration and cancer. Semin Cell Dev Biol.
80:74–82. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sun X, Jia H, Xu Q, Zhao C and Xu C:
Lycopene alleviates H2O2-induced oxidative stress, inflammation and
apoptosis in bovine mammary epithelial cells via the NFE2L2
signaling pathway. Food Funct. 10:6276–6285. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang Y, Yan J, Wang L, Dai H, Li N, Hu W
and Cai H: HIF-1α promotes breast cancer cell MCF-7 proliferation
and invasion through regulating miR-210. Cancer Biother Radiopharm.
32:297–301. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lai HH, Li JN, Wang MY, Huang HY, Croce
CM, Sun HL, Lyu YJ, Kang JW, Chiu CF, Hung MC, et al: HIF-1α
promotes autophagic proteolysis of Dicer and enhances tumor
metastasis. J Clin Invest. 128:625–643. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Sun RC, Board PG and Blackburn AC:
Targeting metabolism with arsenic trioxide and dichloroacetate in
breast cancer cells. Mol Cancer. 10(142)2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yang MH, Zang YS, Huang H, Chen K, Li B,
Sun GY and Zhao XW: Arsenic trioxide exerts anti-lung cancer
activity by inhibiting angiogenesis. Curr Cancer Drug Targets.
14:557–566. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Han YH, Kim SH, Kim SZ and Park WH:
Caspase inhibitor decreases apoptosis in pyrogallol-treated lung
cancer Calu-6 cells via the prevention of GSH depletion. Int J
Oncol. 5:1099–1105. 2008.PubMed/NCBI
|
|
26
|
Hsin IL, Ou CC, Wu TC, Jan MS, Wu MF, Chiu
LY, Lue KH and Ko JL: GMI, an immunomodulatory protein from
Ganoderma microsporum, induces autophagy in non-small cell lung
cancer cells. Autophagy. 7:873–882. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Dueñas-González A and Campbell S: Global
strategies for the treatment of early-stage and advanced cervical
cancer. Curr Opin Obstet Gynecol. 28:11–17. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liu H, Pei G, Song M, Dai S and Wang Y:
Influence of hsa-miR-6727-5p on the proliferation, apoptosis,
invasion and migration of Caski, Hela and SiHa cervical cancer
cells. J BUON. 22:973–978. 2017.PubMed/NCBI
|
|
30
|
Sakai C, Arai M, Tanaka S, Onda K,
Sugiyama K and Hirano T: Effects of arsenic compounds on growth,
cell-cycle distribution and apoptosis of tretinoin-resistant human
promyelocytic leukemia cells. Anticancer Res. 34:6489–6494.
2014.PubMed/NCBI
|
|
31
|
Arbyn M, Weiderpass E, Bruni L, de Sanjosé
S, Saraiya M, Ferlay J and Bray F: Estimates of incidence and
mortality of cervical cancer in 2018: A worldwide analysis. Lancet
Glob Health. 8:e191–e203. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Menderes G, Black J, Schwab CL and Santin
AD: Immunotherapy and targeted therapy for cervical cancer: An
update. Expert Rev Anticancer Ther. 16:83–98. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Geretto M, Pulliero A, Rosano C, Zhabayeva
D, Bersimbaev R and Izzotti A: Resistance to cancer
chemotherapeutic drugs is determined by pivotal microRNA
regulators. Am J Cancer Res. 7:1350–1371. 2017.PubMed/NCBI
|
|
34
|
Kuo YJ, Liu YJ, Way TD, Chiang SY, Lin JG
and Chung JG: Synergistic inhibition of leukemia WEHI-3 cell growth
by arsenic trioxide and Hedyotis diffusa Willd extract in
vitro and in vivo. Exp Ther Med. 13:3388–3396.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lunghi P, Giuliani N, Mazzera L, Lombardi
G, Ricca M, Corradi A, Cantoni AM, Salvatore L, Riccioni R,
Costanzo A, et al: Targeting MEK/MAPK signal transduction module
potentiates ATO-induced apoptosis in multiple myeloma cells through
multiple signaling pathways. Blood. 112:2450–2462. 2008.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sarkar S, Mukherjee S, Chattopadhyay A and
Bhattacharya S: Low dose of arsenic trioxide triggers oxidative
stress in zebrafish brain: Expression of antioxidant genes.
Ecotoxicol Environ Saf. 107:1–8. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Qu H, Tong D, Zhang Y, Kang K, Zhang Y,
Chen L and Ren L: The synergistic antitumor activity of arsenic
trioxide and vitamin K2 in HL-60 cells involves increased ROS
generation and regulation of the ROS-dependent MAPK signaling
pathway. Pharmazie. 68:839–845. 2013.PubMed/NCBI
|
|
38
|
Hassani S, Ghaffari SH, Zaker F, Mirzaee
R, Mardani H, Bashash D, Zekri A, Yousefi M, Zaghal A, Alimoghaddam
K and Ghavamzadeh A: Azidothymidine hinders arsenic
trioxide-induced apoptosis in acute promyelocytic leukemia cells by
induction of p21 and attenuation of G2/M arrest. Ann Hematol.
92:1207–1220. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Pollak M: Targeting oxidative
phosphorylation: Why, when, and how. Cancer Cell. 23:263–264.
2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kim JH, Kim JH, Yu YS, Kim DH, Kim CJ and
Kim KW: Antitumor activity of arsenic trioxide on retinoblastoma:
Cell differentiation and apoptosis depending on arsenic trioxide
concentration. Invest Ophthalmol Vis Sci. 50:1819–1823.
2009.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Chayapong J, Madhyastha H, Madhyastha R,
Nurrahmah QI, Nakajima Y, Choijookhuu N, Hishikawa Y and Maruyama
M: Arsenic trioxide induces ROS activity and DNA damage, leading to
G0/G1 extension in skin fibroblasts through the ATM-ATR-associated
Chk pathway. Environ Sci Pollut Res Int. 24:5316–5325.
2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Su W, Huang L, Ao Q, Zhang Q, Tian X, Fang
Y and Lu Y: Noscapine sensitizes chemoresistant ovarian cancer
cells to cisplatin through inhibition of HIF-1α. Cancer Lett.
350:94–99. 2011.PubMed/NCBI View Article : Google Scholar
|