Introduction
Hypoplastic left heart syndrome (HLHS), initially
described by Maurice Lev (1952), is a heterogeneous group of
congenital cardiac malformations (CCMs) that associates: The
hypoplastic/aplastic left ventricle (LV), the hypoplastic/atresia
mitral and aortic valve (M/AoV), and the aortic artery coarctation
(AoAC) in a severe form (1,2). The incidence of HLHS is 0.16-0.30% of
live births in the USA, and 0.22-0.37% of live births in the UK
(3,4). There are no studies for Romania that
record the exact incidence of HLHS. It represents 1.2-1.5% (up to
3%) of CCMs, and 7-9% of congenital heart diseases diagnosed in the
first year of life; before the surgical intervention, it caused 25%
of deaths because of neonatal heart disease (5-7).
Boys represent 55-70% of cases. The exact cause of the disease is
unknown, but it has been postulated that it has a multifactorial
transmission. There are family cases transmitted recessively and
autosomally; in 5-15% of cases, HLHS is included in genetic
syndromes, such as Turner, Noonan, Smith-Lemli-Opitz or Holt-Oram,
Edwards (trisomy 18), Patau (trisomy 13), Jacobson (chromosome
deletion 11q), Rubinstein-Taybi and partial trisomy 9 (1,8,9).
Case report
We present the case of a child with HLHS born at
‘Bega Maternity’ in Timișoara, operated using a mixed technique
cardiovascular repair surgery at the Vienna General Hospital, and
treated following the intervention at the ‘Louis Ţurcanu’ Emergency
Clinical Hospital for Children-Neonatology and Prematurity Unit
from Timișoara. The study was conducted in line with the CARE
criteria, following the CARE guidelines: Consensus-based clinical
case report guideline development. Ethics approval was obtained
from the Research Ethics Committee of the ‘Louis Ţurcanu’ Emergency
Clinical Hospital for Children in Timisoara (no. 3697/05.03.2020).
Consent to participate was also obtained.
Patient's data were retrieved from the child's
observation charts. The patient aged 1 month and 1 week, having a
35-year old mother, gravida 2 para 2 (G2 P2), dispensary pregnancy
(without an antenatal diagnosis of CCMs), was born by caesarean
section (for indication: Risk of uterine rupture on a scarred
uterus, previous caesarean delivery) at the gestational age of 39
weeks, having a birth weight of 3750 g, a length of 51 cm, an APGAR
score of 8/1 min (cyanosis), 9/5 min. The newborn had a good early
neonatal adaptation. After birth, the umbilical cord was cut, and
the infant was taken to the neonatal intensive care unit, in a
servo control-heated incubator, fed with free oxygen with a 2-4
l/min flow. In the first 24 h of life, the newborn was in good
clinical condition. After 24 h of life, the disease manifested
itself through a severe general condition, hypothermia (skin
temperature of the neonate below the normal values of
36.0-36.5˚C-96.8-97.7˚F, and below the normal rectal temperature of
36.5-37.5˚C-97.7-99.5˚F), loss of appetite, skin mildly jaundiced
on a pale-earthy background, declining oedema, respiratory
functional syndrome, mixed dyspnoea, precordial cardiac breath
grade IV/VI (auscultation), abdominal meteorism, bilious gastric
residue, oligo-/anuria (urine output of <1 ml/kg/h or the
absence of urinary output during the first 24 to 48 h of age), 3/3
cm anterior fontanelle slightly convex, lethargic, diminished
archaic reflexes; bilateral or global congestive heart failure
(characterised by tachypnoea, tachycardia, increased respiratory
effort, rales, hepatomegaly, oedema and delayed capillary refill),
as a result of a reduced right ventricle (RV) volume and pressure,
and shock.
Following the anamnesis, the clinical examination
and the paraclinical examinations, laboratory analysis,
electrocardiogram (EKG), echocardiography, abdominal ultrasound,
empty abdomen radiograph, cardiopulmonary radiograph, and thoracic
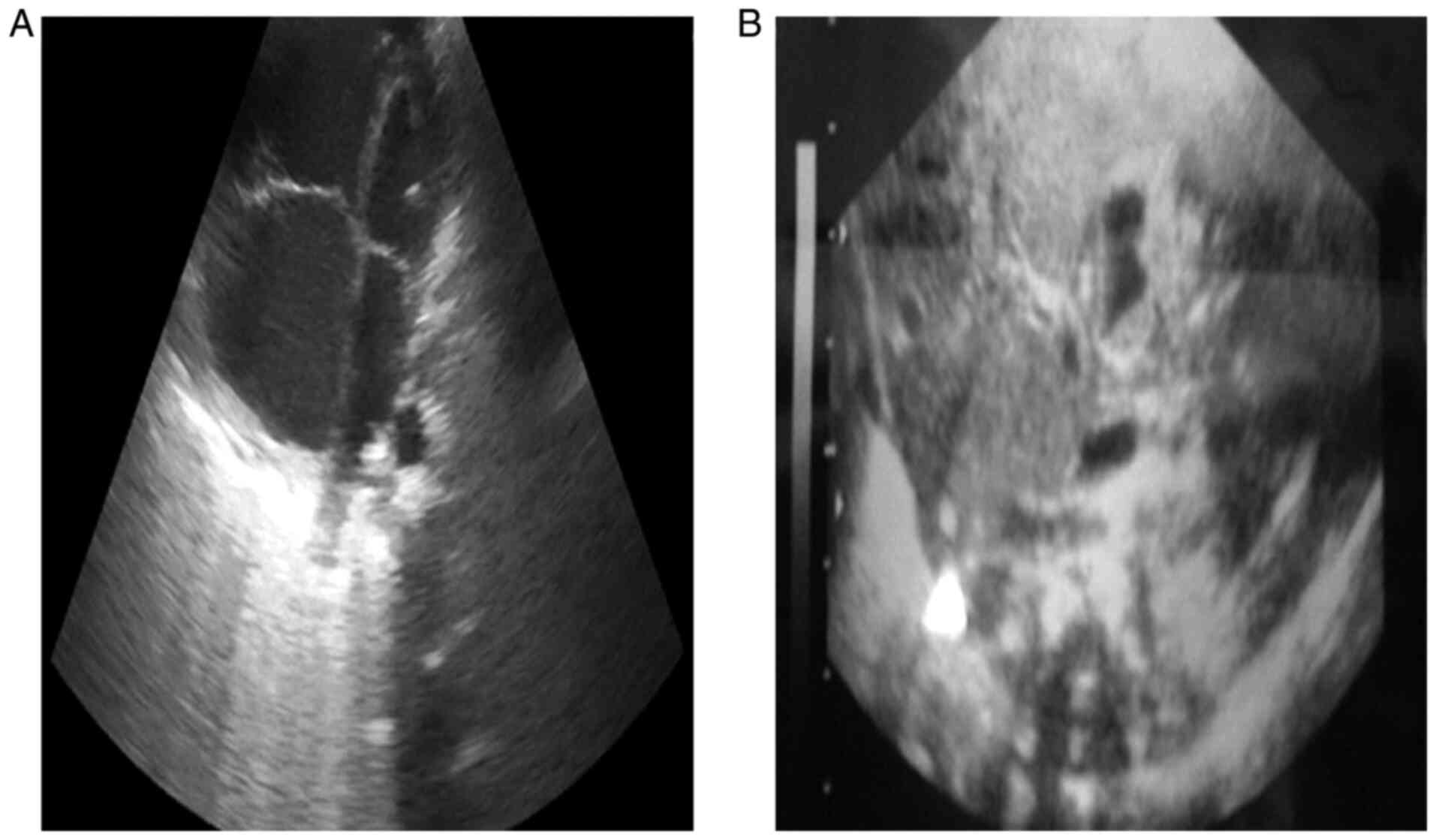
computed tomography angiography, a diagnosis was established: Left
heart hypoplasia (LHH), mitral valve stenosis, aortic valve
stenosis, persistent arterial duct (PAD), atrial septal defect
(ASD). The diagnosis of congenital heart malformation was
established in the first 24 h of life, not antenatally (Fig. 1). The arterial duct (AD)
permeability with prostaglandin E1 (PGE1) was maintained (infusible
Alprostadil, 200 ng/kg/min, then 50, and 15 ng/kg/min,
respectively).
The newborn was transferred to the Vienna General
Hospital. At 17 days old, bilateral pulmonary arteries (PAs)
banding was performed.
At 20 days old, cardiac catheterisation was
performed using the Rashkind manoeuvre (balloon atrial septostomy),
and two stents were implanted in the AD. Anticoagulant therapy
(heparin-dose of 28 IE/kg/h, infusion; acetylsalicylic acid-dose of
4 mg/kg/day, oral), intravenous broad-spectrum antibiotics
depending on the antibiogram (ceftriaxon 100 mg/kg/day and
gentamicin 5 mg/kg/day, IV, 3 days; meropenem 50 mg/kg/day and
amikacin 15 mg/kg/day, IV, 10 days; piperacillin/tazobactam
100/12.5 mg/kg/dose, sulfamethoxazole/trimethoprim 8
mg/kg/day-trimethoprim, IV infusion, caspofungin 2 mg/kg/day, IV
infusion, 14 days; ticarcillin/clavulanate 100 mg/kg/dose, 4
dose/day, IV infusion, and ciprofloxacin 10 mg/kg/day, IV infusion,
10 days, and gentamicin 5 mg/kg/day, IV, 2 days and micafungin 6
mg/kg/1st day-4 mg/kg/day, IV, 2 days), volume-expanders, blood
derivatives (erythrocyte concentrate, fresh plasma,
cryoprecipitate), immunoglobulin (for indications: Recurrent
neonatal infections and thrombocytopenia, dose of 0.4 g/kg/day, 3
days, infusion), inotropic drugs (adrenaline-for indication:
Bradycardia, 0.01-0.03 mg/kg/dose, IV push, and 0.2 mcg/kg/min, IV
infusion; atropine-for indication: Bradycardia, dose 0.01-0.03
mg/kg IV), amino acids (for indication: Hypoproteinaemia),
corticosteroids, antihypertensives (enalapril 0.02 mg/kg/day and
clonidine 0.02 mg/kg/day, oral), diuretics, proton pump inhibitors,
vitamin K and mannitol, were used.
At 7 days following the intervention, the child was
hemodynamically stable. The echocardiography showed LHH, a good
systolic function of systemic RV, grade I-II tricuspid
regurgitation, grade I pulmonary valve regurgitation, bilateral PAs
banding with saw-tooth systolic-diastolic doppler flow, patent
double ductal stent, right-left shunt 2.1 m/s, very good flow in
descending AoA, retrograde flow in AoA 1.8 m/s, antegrade flow via
very thin AoV, open, pericardial fluid 4-5 mm, patent foramen ovale
(PFO) type ASD (left-right shunt 0.8 m/s), and pericarditis with
pericardial drainage.
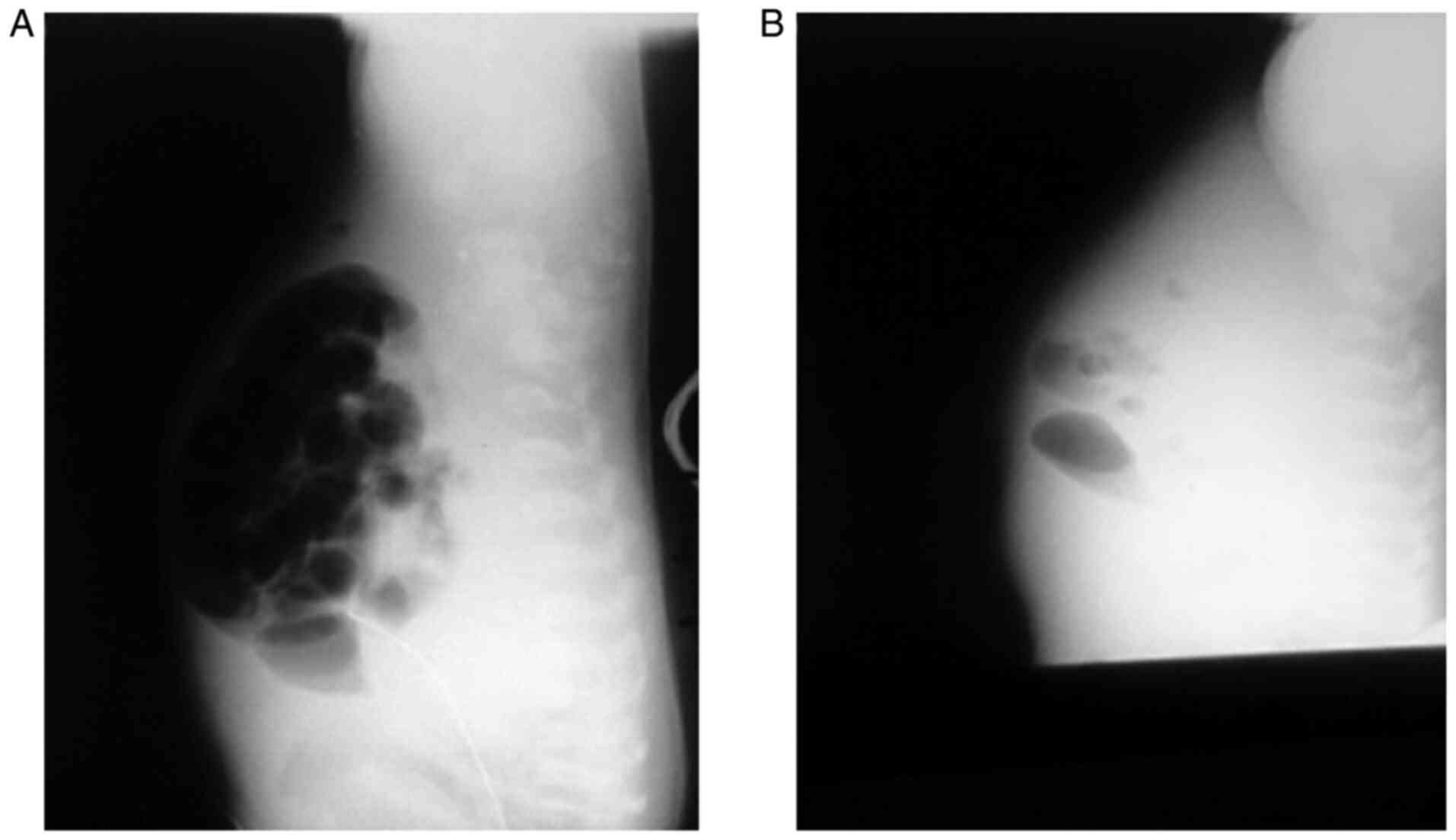
At 10 days following the intervention, the clinical
condition worsened, the child displaying increased cardiomegaly
with pulmonary stasis. The abdomen was relaxed with poorly visible
safety lysate and ascites (Fig. 2).
The following were performed: Pericardial drainage (12 days), left
pleural drainage (6 days), right pleural drainage (5 days). The
postprocedural echocardiography was favourable: Dilated RA, ASD
(following dilatation with a balloon), normal LA, RV with conserved
contractility and ejection fraction (EF) 42%. LV is rudimentary.
The AoA diameter at the ring was 4 mm and was retrogradely loaded.
The AD stent was permeable. After the surgery at the Vienna General
Hospital, 1-month old baby was transferred to the Neonatology
Clinic in Timisoara.
At 14 days following the intervention: Pericardial
drainage was suppressed, but the clinical condition was severe,
with earthy teguments and generalised oedema. Echocardiography
showed good RV function, tricuspid regurgitation (Vmax=5 m/s, band
on efficient PAs branches, stents on AD (Vmax=2 m/s), laminar flow
AoA hypoplasia. Clinically, functional respiratory syndrome,
melena, and severe metabolic acidosis appeared (Table I). The newborn required orotracheal
reintubation and mechanical ventilation.
 | Table IThe biochemical status of the patient
with hypoplastic left heart syndrome during the
hospitalization. |
Table I
The biochemical status of the patient
with hypoplastic left heart syndrome during the
hospitalization.
| Analyzes | Preoperation | Postoperation | Normal |
|---|
| Day | 1 | 2 | 3 | 7 | 9 | 1 | 3 | 7 | 10 | 12 | Values |
|---|
| CBC | | | | | | | | | | | |
| WBC |
23.9x103 | - |
19.9x103 | - | - |
7.19x103 | 16.38 |
18.78x103 |
17.58x103 |
20.49x103 |
6-17x103/µl |
| HGB | 12.6 | - | 13.7 | - | - | 9.6 | 9.9 | 7.3 | 13.2 | 8 | 9-18 g/dl |
| HCT | 36.5 | - | 42.8 | - | - | 28.1 | 29.2 | 21.8 | 36.9 | 24.7 | 35-50% |
| PLT |
126x103 | - |
61x103 | - | - |
151x103 |
141x103 | |
96x103 |
78x103 |
150-475x103/µl |
| Coagulation
indices | | | | | | | | | | | |
| Prothrombin time
(PT) | | | | | | 14.9 | 24.8 | 52 | | | 70-115% |
| INR | | | | | | 7.31 | 2.16 | 5.25 | 3.38 | 4.69 | |
| APTT | | | | | | 68 | 36 | >300 | >100 | 38.7 | 30-45 sec |
| Fibrinogen | | | | | | | | 147 | | | 200-400 mg/dl |
| Anti-Xa
(heparin) | | | | | | | | 0.41 | | | <0.1 UI/ml |
| Biochemistry | | | | | | | | | | | |
| ASAT | 151 | 542 | 4,962 | 409 | 129 | 11 | 32 | | 147 | 284 | |
| ALAT | 77 | 459 | 1,488 | 305 | 184 | 21 | 31 | | 1,213 | 2,487 | |
| Creatinine | | 1.4 | | 0.6 | 0.7 | 33 | 0.22 | | 49 | | 0.02-1.2 mg/dl |
| Urea | | | | 89 | 87 | 6.34 | | | 18.8 | | 15-39 mg/dl |
| Total bilirubin | | 8.2 | | 7 | 3.7 | | | | | | |
| Direct
bilirubin | | 1.3 | | 3.5 | 2.3 | | | | | | |
| Albumin | 2,5 | | | | | | | | | | |
| CK | | | | | | | | | | | |
| CKMB | - | 1,060 | - | 108 | 58 | - | - | - | - | - | 7-25 U/l |
| PCR | | | | | | 5.72 | 6.52 | 6.52 | 5.47 | 30 | |
| PCR-CMV | | | | | | | | | | | |
| Procalcitonin | | | | | | 0.28 | 5.36 | | 3.36 | 1.12 | |
| Pro-BNP | - | - | - | - | - | - | 5,533 | - | - | - | 0-125 pg/ml |
| Microbial
cultures | | | | | | | | | | | |
| Blood cultures | - | MRSA | - | - | Sterile | - | Sterile | - | Sterile | MRSA | Sterile |
| Nasal
discharge | No pathogenic
germs | - | - | - | - | - | - | - | - | - | No pathogenic
germs |
| Pharyngeal
exudate | No pathogenic
germs | - | - | - | - | - | - | - | - | - | No pathogenic
germs |
| Hypopharyngeal
aspirate | Sterile | - | - | - | - | - | - | Pseudomonas
aeruginosa | - | - | No pathogenic
germs |
| Aspirated
bronchially | - | - | - | - | Enterococcus
sp. | - | - | Stenotrophomonas
maltophilia | - | - | Sterile |
| Urinalysis | Sterile | - | - | - | Sterile | Klebsiella
sp. (contamination without infection) | - | - | - | - | Sterile |
| Coproculture | No pathogenic
germs | - | - | - | - | - | - | - | - | - | No pathogenic
germs |
At 21 days following the intervention: Massive
oedema with thoracic infiltration. During the chest auscultation,
bilateral sub-repeating rales were detected. Pulmonary radiography
showed acute pneumonia (Fig. 3).
Mild (beneficial) respiratory alkalosis was maintained.
Hypertension was more commonly caused by increased pulmonary blood
flow (at the expense of systemic flow) than intrinsic myocardial
dysfunction. After extubation, the patient was reintubated
orotracheally and mechanically ventilated. Following the surgery,
the complications were postcardiotomy syndrome, pneumonia with
Enterococcus faecalis and Stenotrophomonas
maltophilia, sepsis with methicillin-resistant
Staphylococcus aureus (at 21 days post-surgery in the
neonatal clinic in Timisoara), coagulopathy, mixed anaemia, and
metabolic acidosis (Table II). The
patient died one month after the intervention due to
cardiorespiratory arrest, bilateral CHF, HLHS with AD shunt and PAs
banding, multiorgan failure, and severe secondary haemorrhagic
disease.
 | Table IIComplications of hypoplastic left
heart syndrome. |
Table II
Complications of hypoplastic left
heart syndrome.
| Preoperative
complications | Postoperative
complications | Major surgical
complications |
|---|
| Acidosis (+) | Acidosis (+) | Norwood
Procedure: |
| Congestive heart
failure (+) | Acute kidney injury
(+) | Obstruction of the
AoA arch (at the anastomotic site) |
| Acute kidney injury
(+) | Chronic kidney
disease | Progressive
cyanosis (low blood flow through shunt) |
| Chronic kidney
disease | Liver failure
(+) | Bidirecțional
Glenn/hemi-Fontan Procedure: |
| Liver failure | Necrotizing
Enterocolitis | Upper vein
transient syndrome |
| Sepsis | Sepsis (+) | Heart
transplant: |
| Exitus | Pericardial
effusion (+) | Early acute
rejection of the graft |
| | Pleural
effusion | Opportunistic
infections |
| | Injuries of the
phrenic/recurrent laryngeal nerve | |
| | Stroke | |
| | Aortic artery
coarctation | |
| | Exitus (+) | |
Discussion
Clinical and haemodynamic forms include: 1) LV and
MV and AoV atresia/hypoplasia; Hypoplastic ascending and transverse
AoA, frequently associated with AoAC; the flow through the coronary
arteries is often retrograde from the AD into the small ascending
AoA; 2) another variant of HLHS, similar to the one presented, also
display narrow PAD (8,9). The case studied falls into the latter
form.
The newborn with HLHS may benefit from infusion with
prostaglandin E1 (PGE1) if he or she has a PFO; when foramen ovale
is low/absent, clinical status is critical, and will not improve
after PGE1. The patient in the study was infused with PGE1. Acute
vascular collapse secondary to AD closure induces shock (8-10).
The mandatory surgical treatment to be performed is reconstruction
through a series of three surgical interventions: Stage I, the
Norwood procedure (at 2 weeks of age), stage II, Hemi-Fontan or the
two-way Glenn procedure (at the age of 6-9 months), and stage III,
modified Fontan/Fontan procedure (at the age of 18-24 months);
orthotopic heart transplant, initially performed by L. Bailey, has
similar results (11-13).
The mixed technique performed at Vienna General Hospital was
performed safely.
The overall survival after stage I, mentioned in the
literature, is 75-93% when the RV's function is normal
preoperatively, and 47%, when the RV is dysfunctional (5,6,12).
Negative prognostic elements are high Aristotle comprehensive
complexity score (ACCS) >20 points (a useful tool for the
analysis of the outcome after congenital heart surgery, with values
between 1.5 and 25 points; it included two categories of complexity
factors: Procedure dependent factors and procedure independent
factors), low oxygen saturation in the first 48 h after stage I
(14). The severe metabolic
acidosis, progressive oedema and post-surgical infections caused an
unfavorable prognosis (in the case studied). Survival after
Glenn/Hemi-Fontan and Fontan bidirectional operations is 90-95%
(15). The survival rate at 5 years
after the reconstruction in stages is 70, 20% of infants on cardiac
transplant lists die preoperatively, and the survival rate at 5
years after the transplant is 80%; the Society of Congenital Heart
Disease Surgery shows that the survival rate of patients operated
by the RV-PAs shunt Norwood technique is the highest, and the
HYBRID technique has better results only in low birthweight infants
(16).
In conclusion: i) in the studied case, the HLHS is a
CCM that associates MV stenosis, AoV stenosis, PAD, ASD, and
represents a medical-surgical emergency; ii) the AD patent with
PGE1 is maintained in a continuous infusion, to allow pulmonary
venous blood to pass through the shunt from the left atrium to the
right atrium; iii) the initial treatment consists of a mixed
technique: Pas bilateral banding (at 17 days old), followed by
cardiac catheterisation using the Rashkind manoeuvre (balloon
atrial septostomy), and implanting two stents in the AD (at 20 days
old); iv) medical treatment includes anticoagulant therapy with
heparin, antibiotics (bacterial endocarditis prophylaxis to be
performed throughout life), volume-expansion, blood products,
inotropic agents, corticosteroid therapy, diuretics, and mechanical
ventilation; v) post-surgery complications (acute pneumonia with
Enterococcus faecalis and Stenotrophomonas
maltophilia, severe metabolic disorders, toxic-septic status,
and massive pulmonary and digestive bleeding) have aggravated the
patient's condition; vi) one month after the surgery, death occurs
by cardio-respiratory arrest, global congestive heart failure, HLHS
with shunt by AD and PAs bilateral banding, multiorgan
insufficiency, and severe secondary haemorrhagic disease; vii) the
prenatal diagnosis should lead to delivery in a specialized center,
and improving the prognosis.
Acknowledgements
The authors would like to thank Dorel-Viorel Cretu
and Sebastian Adam Puraci-technical for their support.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CD, AMM, VRE, AAML, AL and OIH were involved in the
conception and design of the study, acquired, analysed and
interpreted the data, and drafted the manuscript. MB was involved
in the conception of the study, analysed and interpreted the data,
and reviewed the manuscript. CI was involved in the conception of
the study, coordinated the study, reviewed the manuscript and gave
final approval of the version to be published. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was conducted in line with the CARE
criteria, following the CARE guidelines: Consensus-based clinical
case report guideline development. Ethics approval was obtained
from the Research Ethics Committee of the ‘Louis Ţurcanu’ Emergency
Clinical Hospital for Children in Timisoara, Romania (no.
3697/05.03.2020). Consent to participate was also obtained.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Si MS, Hirsch-Romano JC, Bove EL, Ohye RG,
Windle ML, Mancini MC, Berge S and Odim J: Surgical treatment of
pediatric hypoplastic left heart syndrome Medscape, 2014.
urihttp://emedicine.medscape.com/article/904137-overviewsimplehttp://emedicine.medscape.com/article/904137-overview.
Accessed April 11, 2014.
|
|
2
|
Si MS, Bove EL, Romano JC and Ohye RG: How
I teach the Norwood procedure. Ann Thorac Surg. 101:2045–2048.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cloherty J, Eichenwad E and Stark A:
Manual of neonatal care. 7th edition. Lippincott Williams &
Wilkins, Philadelphia, PA, 2017.
|
|
4
|
Gardner S, Carter B, Enzman-Hines M and
Hernandez J: Merenstein and Gardner's Handbook of neonatal
intensive care. 8th edition. Elsevier Inc., St. Louis, MO,
2016.
|
|
5
|
Sakurai T, Rogers V, Stickley J, Khan N,
Jones TJ, Barron DJ and Brawn WJ: Single-center experience of arch
reconstruction in the setting of Norwood operation. Ann Thorac
Surg. 94:1534–1539. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Anderson R, Pozzi M and Hutchinson S:
Hypoplastic left heart syndrome. Springer-Verlag, London, 2005.
|
|
7
|
Alabdulgader AA: Survival analysis:
Outcome of prenatal versus postnatal diagnosis of hypoplastic left
heart syndrome. J Invasive Cardiol. 1:8–12. 2018.
|
|
8
|
Roeleveld PP, Axelrod DM, Klugman D, Jones
MB, Chanani NK, Rossano JW and Costello JM: Hypoplastic left heart
syndrome: From fetus to Fontan. Cardiol Young. 28:1275–1288.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Beer M and Berkow R: The Merck manual of
diagnosis and therapy, 18th edition. Merck, 2006.
|
|
10
|
Connor JA and Thiagarajan R: Hypoplastic
left heart syndrome. Orphanet J Rare Dis. 2(23)2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Freedom R, Benson L and Smallhorn J:
Neonatal heart disease. 1st edition. Springer-Verlag, London,
1991.
|
|
12
|
Johnson S: Hypoplastic left heart
syndrome. Healthline Media, San Francisco, CA, 2016. urihttps://www.healthline.com/health/hypoplastic-left-heart-syndromesimplehttps://www.healthline.com/health/hypoplastic-left-heart-syndrome.
Updated March 30, 2017.
|
|
13
|
Riddick-Grisham S: Pediatric life care
planning and case management. Vol 3. 2nd edition. CRC Press LLC,
Boca Raton, FL, 2011.
|
|
14
|
Erek E, Yylmaz B, Kaya M, Onan YS, Þen O,
Oz K, Koçyigit ÖI, Güzeltaş A and Ödemiş E: Analysis of results
according to the Aristotle scoring system in congenital heart
surgery. Turkish J Thoracic Cardiovascular Surg. 22:509–516.
2014.
|
|
15
|
Kliegman R, Stanton B, Geme J and Schor N:
Nelson Textbook of pediatrics. Vol 2. 20th edition. Elsevier Inc.,
2016.
|
|
16
|
Wilder T, Hickey E, Ziemer G, Jacobs M,
Gruber P, Blackstone E, McCrindle B, Williams W, DeCampli W,
Caldarone AC and Pizarro C: Hybrid Alternatives to Norwood Stage-1
are not a lower risk alternative: Norwood-RVPA offers better
outcome in comparable neonates. Circulation. 130(19493)2018.
|