Introduction
Periodontal disease is a multifactorial,
polymicrobially triggered inflammatory disease whose pathogenesis
is dependent on numerous host-related factors that eventually
results in an individual's susceptibility to the disease (1). Although over 800 bacterial species can
colonize tooth surfaces and various artificial oral appliances, the
gingival margin and gingival sulcus, periodontal disease can
already be initiated by a relatively small number of pathogens
present in the microbial biofilm (2-5).
In order to ensure the efficacy and long-term success of the
periodontal treatment, periopathogens must be drastically decreased
if not completely eradicated.
In patients with a history of periodontitis
resulting in displaced teeth, possible orthodontic tooth movements
include changes in alignment, space redistribution, and intrusion
(6). The primary aim, before
orthodontic intervention might start, is to stabilize the
periodontal condition. Bone loss alters the position of the tooth's
center of rotation and the force required to achieve the movement;
however, the orthodontist can use reduced or increased force
moments to avoid excessive alveolar bone loss (6).
Orthodontic therapy has been shown to be a reliable
therapy for restoring compromised dentition, closing infrabony
defects, reducing gingival recessions, and improving interdental
papilla levels; thus, orthodontics can be considered for the
treatment of periodontal patients with tooth migration (7). Other studies have shown that
orthodontic treatment can safely be used in patients with previous
periodontal therapy, despite the fact that orthodontic appliances
worsen conditions for oral hygiene, complicate tooth care, and,
thereby, create an environment favorable to plaque accumulation
(8). However, orthodontic treatment
often employs the permanent or long-term use of a retainer which
can complicate dental hygiene self-cleaning procedures, and can
potentially harm the periodontal tissues (8). In time, periodontal parameters can
deteriorate, even if some of them may improve after the removal of
the orthodontic retainers (9,10). A
very recent systematic review showed a deterioration of periodontal
parameters after orthodontic treatment, indicating that it
influences the accumulation and composition of the subgingival
microbiota and subsequently induces more inflammation and higher
BOP (11).
Earlier studies hypothesized that the orthodontic
treatment can improve or prevent deterioration of periodontal
parameters in treated periodontal patients. Significant reductions
in pocket depth (PD) and clinical attachment levels (CAL), as well
as radiographical improvements of periodontal bone defects were
reported (12-14).
Since orthodontic therapy can be safely used in patients with
previous periodontal therapy, it is only coherent to use it as an
additional tool in periodontitis treatment, even if the inherent
risks of the therapy must be taken into consideration (15).
Therapeutical stepbacks as ‘black triagles’ and
reduced interdental papilla heights (16) and difficult prosthetic
rehabilitation can be prevented in orthodontic patients with
previous periodontal disease, together with the additional
attachment loss, through strict biofilm control and periodontal
maintenance. These procedures are essential during the active phase
of orthodontic treatment, in order to prevent inflammation in
gingival tissues (17,18).
Bone changes induced by orthodontic treatment may
impact the morphology of bone defects, decrease pocket depth, and
enhance connective tissue healing (16). The influence of tilting movements in
the presence of intrabony pockets is further evidence that
orthodontic movements may be performed in teeth with bone defects
without further damaging periodontal attachment (19). Thus, orthodontic forces must be
carefully applied in teeth with a reduced periodontium.
Despite the high number of published articles
debating the periodontal-orthodontic interrelationship, there is a
lack of good evidence on systematic treatments including both
orthodontic and periodontal therapy. The periodontic-orthodontic
interrelationship has been the subject of substantial
investigation, yet it remains a controversial issue (20). Thus far, the literature regarding
orthodontic treatment in subjects with treated periodontal disease
is represented mostly by case reports on subjects already treated
for chronic periodontitis (17). Of
more interest for clinical studies, however, might be the
relationship between aggressive periodontitis and orthodontic
treatment, probably because of the significant tooth displacement
related to this rapidly progressing form of periodontitis (17,21).
In the early 2000's, the Cardaropoli group (13,22-24)
reported that orthodontic treatment is no longer a contraindication
in the therapy of severe adult periodontitis. While orthodontics
has improved the ability to restore deteriorated dentition, over
the last decade there has been no clinical study regarding the
outcome of treated periodontium undergoing orthodontic movements.
There are also relatively few clinical studies comparing the
outcomes of combined periodontal-orthodontic treatment with the
outcomes of periodontal treatment alone in patients with severe
periodontitis (25).
The aim of the present study was to determine the
longitudinal changes in clinical and microbial parameters of the
periodontium at site-level during the initial remodeling processes
of the alveolar bone and periodontal ligament caused by light
continuous forces employed in the orthodontic treatment of adult
patients with a history of severe periodontal disease treated with
standard (non-surgical and conventional non-regenerative)
periodontal therapy.
Patients and methods
Ethical approval, selection of
patients and timeline of measurements
The study was conducted in the Department of
Periodontology of the Faculty of Dental Medicine of the ‘Victor
Babes’ University of Medicine and Pharmacy University, Timișoara,
Romania, from November 2013 to November 2014, with the approval no.
14/16.09.2013 of the University Committee on Research Ethics.
Before the start of the study, all patients received detailed
information regarding sampling procedures, time points, and
conditions to be met during the trial, as well as inclusion and
exclusion criteria. Patients received guidelines for specific
proper oral hygiene during the wearing of orthodontic appliances;
instructions were reinforced at every subsequent appointment. After
receiving all necessary information, patients signed an informed
consent agreement. The study protocol was conducted in conformity
with the Declaration of Helsinki.
Thirteen adult patients (8 women and 5 men, aged
23-53 years, mean age 36.5 years), with a history of severe
periodontitis as described by Armitage (26) treated with standard (initial and
conventional surgical) periodontal therapy received fixed
orthodontic appliances. The criteria for inclusion in the study
were: i) ≥21 yo; ii) good systemic health in terms of diabetes,
cardiovascular diseases and other conditions that may impact the
peridontal status; iii) absence of extended fixed and removable
prosthetic restorations; iv) no previous orthodontic treatments; v)
severe periodontitis treated by standard (non-surgical and
conventional surgical) procedures, the treatment being completed at
least one year before the onset of the orthodontic treatment; vi)
good compliance with a rigorous (with respect to the initial
3-months recall intervals, good personal oral hygiene), supportive
periodontal therapy; vii) stable periodontal status during the
previous six months (absence of inflammation and attachment loss);
viii) good oral hygiene (full mouth plaque and full mouth bleeding
scores under 25%); ix) teeth affected by periodontal attachment
loss, misaligned or displaced following the evolution of
periodontitis and x) indication for orthodontic treatment for
esthetic or functional reasons. Exclusion criteria were
administration of antibiotics during in the previous six months,
pregnancy, lactation, smoking, allergies to the materials included
in the orthodontic appliances, incapacity to read and understand
the aim and nature of the study.
Clinical measurements and subgingival plaque
sampling were performed for each individual patient on the same
tooth and site by the same intra-examiner calibrated investigator
(AJ). The selection criteria for experimental periodontal sites
were: i) residual PD ≥3 mm; ii) situated on single-rooted teeth;
iii) at the site where the periodontal ligament underwent
compression. Experimental teeth underwent orthodontic corporeal
movements predominantly in mesial direction (12 teeth) and distal
direction (one tooth). In each experimental tooth, at the
periodontal site that displayed the deepest residual pocket at the
beginning of the orthodontic treatment, the periodontal clinical
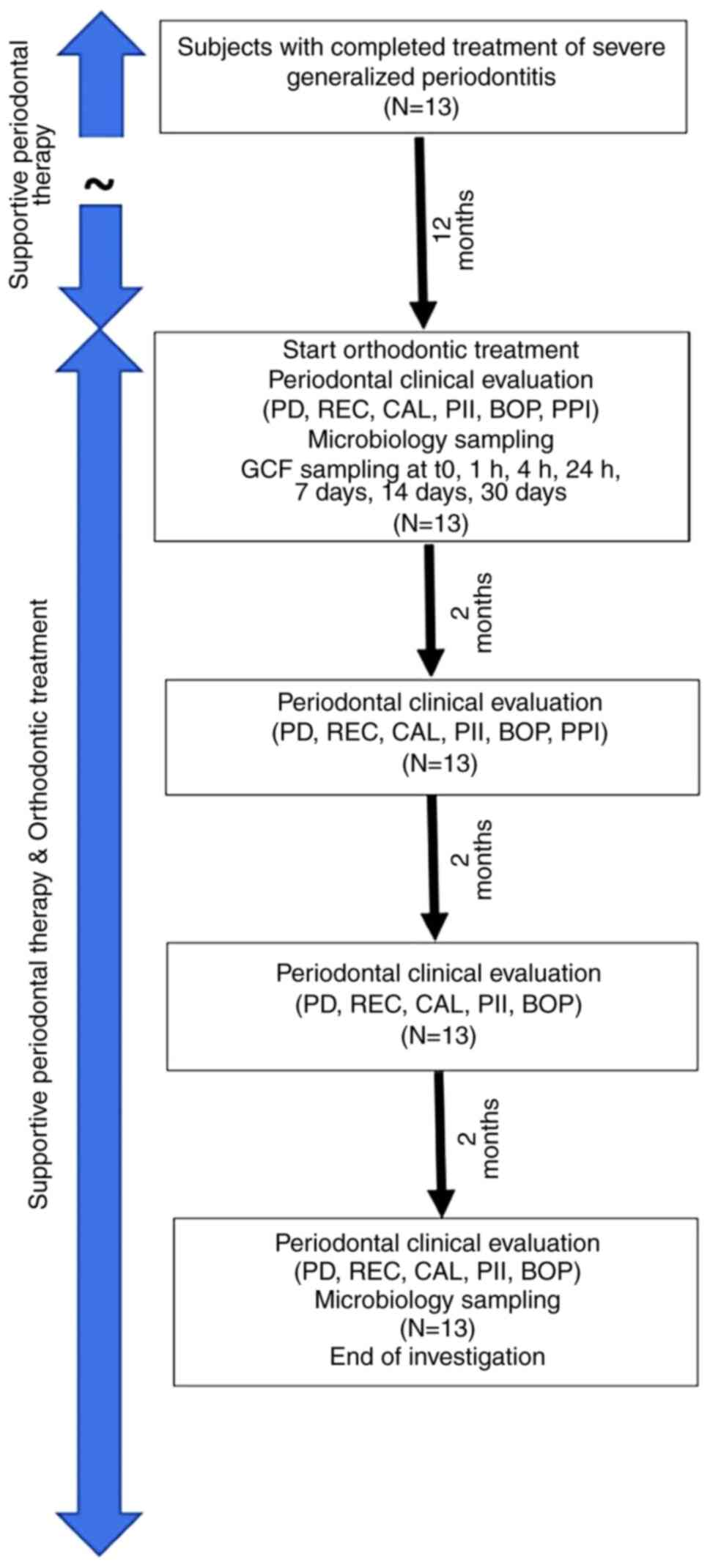
status was evaluated at baseline and at 2, 4, and 6 month
intervals; microbiological status was evaluated at baseline and
again after 6 months. Gingival crevicular fluid (GCF) sampling for
determining enzymatic and inflammatory changes during the
orthodontic treatment was performed on the same experimental sites
(data to be published elsewhere). Orthodontic treatment was
initiated 12 months after the completion of the planned active
periodontal therapy, even if a small number of pockets deeper than
3 mm persisted, within a well-controlled periodontal maintenance
program. The timeline of measurements is displayed as a flow chart
in Fig. 1.
Orthodontic treatment
Twelve months after the end of periodontal
treatment, orthodontic brackets (Omniarch®, Dentsply
GAC), slot 0.018 inch and Sentalloy Superelastic®
(Dentsply GAC), size 0.014 inch wires were applied to each patient
with a Roth Rx prescription.
Periodontal clinical parameter
measurements
In each selected site of the experimental teeth, the
following parameters were evaluated: PD (pocket depth), REC
(gingival recession), CAL (clinical attachment level), BOP
(bleeding on probing), and PPI (papilla presence index). The PlI
(plaque index) was evaluated in each patient in the experimental
tooth only. The measurements were made before applying the
orthodontic braces (T0) and again at 2, 4, and 6 months after
orthodontic treatment with the exception of PPI, which was assessed
at baseline and after 2 and 4 months of orthodontic treatment. PPI
is useful in evaluating the aesthetic success of
periodontal-orthodontic treatment (25) and was measured as scores (PPI-1 to
PPI-4) to quantify the loss of height of interdental papilla
following periodontitis. The last PPI assessment took place at 4
months, as it was considered that the orthodontic movement was
completed at that time. Before measurement, teeth were isolated
using dental cotton rolls and dried with warm air. In order to
measure the PlI, each experimental tooth was stained using a plaque
disclosing solution (micropellets soaked with a disclosing agent)
(Rondells Blue, Directa AB). Digital photos were taken at each
visit after staining for documentation. PD, REC, and CAL were
measured on the same tooth with a periodontal probe (PCP-UNC15);
measurements were rounded up by 0.5 mm if necessary. BOP was
recorded 20 sec after probing and scores were attributed (score 0,
absent, score 1, present).
Microbiological parameters sampling
and evaluation
The presence of five main periodontopathic bacteria
in the gingival sulcus was evaluated at baseline and after 6
months: Aggregatibacter acinomycetemcomitans (Aa), Porphyromonas
gingivals (Pg), Prevotella intermedia (Pi), Tannerella forsythia
(Tf), and Treponema denticola (Td). Sampling was
performed after complete removal of the supragingival plaque and
isolation of the tooth with cotton rolls. The tooth was dried with
gentle air flow in order to avoid contamination of the samples with
saliva. At each site, two #30 0.04 sterile paper points (Roeko
GmbH) were inserted and held in place for 30 sec until soaked.
After sampling, the cones were transferred to Eppendorf tubes
containing 700 µl PBS solution and kept refrigerated in a special
thermoisolated box during the transport to the laboratory.
Enzymatic and microbiological testing was performed in the
laboratories of the Department of Biochemistry of the ‘Victor
Babes’ University of Medicine and Pharmacy (Timișoara, Romania).
Each tube containing plaque sample received a code. It was vortexed
for 30 sec at room temperature. The points were removed and the
eluates clarified by centrifugation for 5 min at 3,000 x g at 4˚C.
Samples were stored for one day at -20˚C and then at -80˚C until
microbiological analysis (no longer than a month). The processing
of the samples included DNA extraction, amplification, and
hybridization. For DNA extraction the QIAamp DNA Micro kit (Qiagen
GmbH) was used, in accordance with the manufacturer's instructions.
Sample DNA hybridization was performed with a micro-IDent
plus® kit (Hain Lifescience GmbH). For amplification, a
HotStar Taq Polymerase kit (Qiagen GmbH) was employed; this is an
inactive polymerase that offers high specificity for PCR and
facilitates the amplification process by eliminating several
reaction steps. Amplification was performed using a thermocycler
(Thermo Fisher Scientific Inc.) for 32 cycles. After hybridization,
the reading strips were submerged in the sample tube and were
incubated at 45˚C for 30 min. Extracted DNA was quantified by
spectrophotometry (230 nm), using the NanoDrop ND-1000
spectrophotometer (Thermo Fisher Scientific Inc.). Accordingly,
semi-quantitative data were recorded.
Statistical analysis
The statistical unit was the patient, as one
orthodontically moved tooth per periodontal patient was included in
the study. Statistical analysis of the data was performed using the
software R 3.1.3. (R Core Group 2015). The data distribution was
checked for normality and the Friedman test was used for analyzing
continuous or ordinal clinical parameters, for which at least three
successive measurements were made (PD, CAL, REC, PlI, and PPI), in
order to describe the mean value variation during the observation
period. The Q Cochran test was employed for BOP. For P-values lower
than the significance level of 0.05, the null hypothesis stating no
difference between the samples was rejected, concluding that the
mean parameter values (or proportions in case of BOP) differ
significantly between at least two time points. For these cases,
post hoc tests (Conover and Holm adjustments for Friedman test;
Benjamini-Hochberg adjustment for Cochran test) were employed in
order to determine the exact point at which these differences
occurred. The microbiological results were recorded as the
following categories (scores) of detectability: 0=nondetectable,
1=104 (103 for Aa),
2=104-105 (103-104 for
Aa), 3=105-106
(104-105 for Aa), and
4=>107 (106 for Aa). The Wilcoxon
signed-rank test was used for analysis of microbial detectability
variations between baseline and the final timepoint. The
differences were considered significant at P<0.1.
Results
The changes in mean PD, REC, and CAL between the
measurements at different timepoints are given in Table I. There were no statistically
significant differences between the measurements at different
timepoints.
 | Table IDescriptive statistics for PD, REC,
CAL, and PlI measured at baseline and at 2, 4, and 6 months of
orthodontic treatment (mean ± SD, range specified in
parentheses).a |
Table I
Descriptive statistics for PD, REC,
CAL, and PlI measured at baseline and at 2, 4, and 6 months of
orthodontic treatment (mean ± SD, range specified in
parentheses).a
| Parameter | Baseline | 2 months | 4 months | 6 months | P-value |
|---|
| PD | 4.23±1.09
(3-7) | 3.77±1.24
(2-7) | 3.92±0.86
(2-5) | 4.00±0.82
(2-5) | 0.412 |
| REC | 0.77±1.01
(0-3) | 0.77±1.01
(0-3) | 0.85±0.99
(0-3) | 0.69±0.95
(0-3) | 0.494 |
| CAL | 4.92±1.50
(3-8) | 4.54±1.66
(2-8) | 4.77±1.42
(3-8) | 4.69±1.11
(3-7) | 0.559 |
| PlI | 1.04±0.43
(0-2) | 1.31±0.43
(1-2) | 1.23±0.44
(0.5-2) | 1.19±0.43
(0.5-2) | 0.019 |
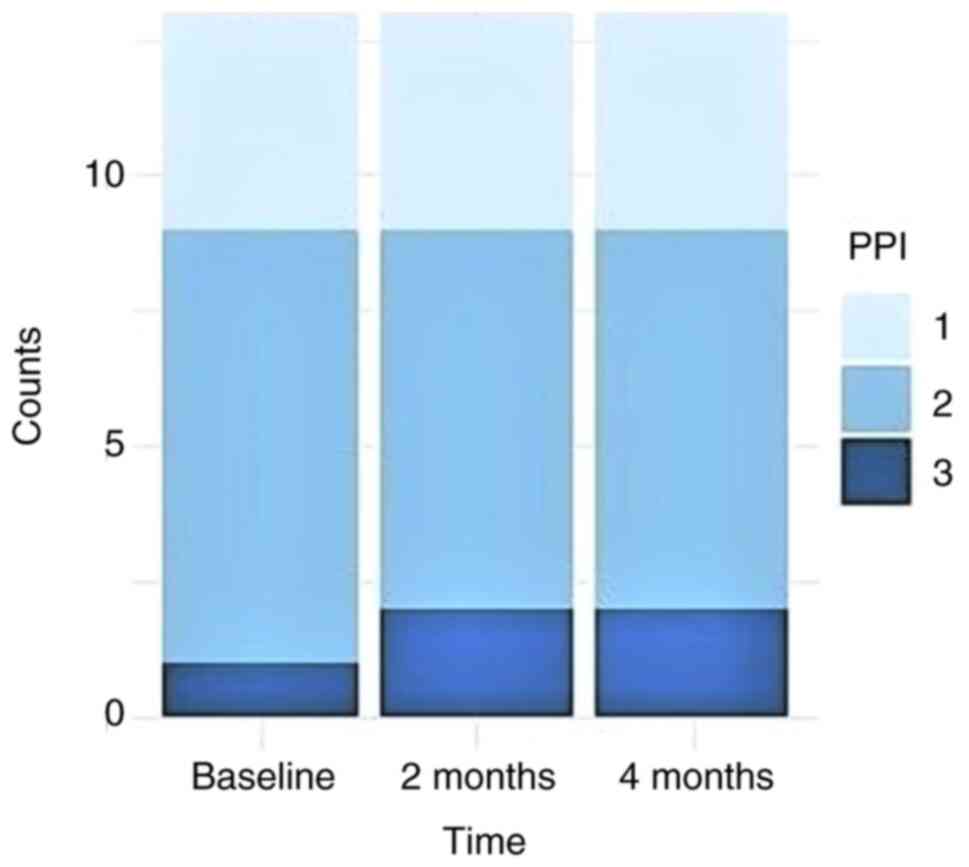
For PPI, there were no significant differences
between successive time points (Friedman test, P=0.36). At each
time point, PPI values ranged from 1 to 3 (median=2). For 12 out of
13 patients, no PPI changes occurred between the three observation
time points. For one patient, PPI increased from 2 to 3 in the
first interval, but remained unchanged afterwards (Fig. 2).
For the PlI, the Friedman test revealed significant
differences between the time points analyzed (P=0.019). Post-hoc
Conover tests showed that these differences are due to the fact
that the mean PlI at baseline was significantly lower than at later
time points. Furthermore, the PlI at 6 months also shows a
significant decrease as compared with 2 months (P<0.05 in each
case) (Table II).
 | Table IIResults (P-values) of post hoc
comparisons between PlI values at baseline and after 2, 4, and 6
months of orthodontic treatment (Holm adjustment). |
Table II
Results (P-values) of post hoc
comparisons between PlI values at baseline and after 2, 4, and 6
months of orthodontic treatment (Holm adjustment).
| Items | Baseline | 2 months | 4 months |
|---|
| 2 months |
<10-5 | - | - |
| 4 months | <0.001 | 0.165 | - |
| 6 months | 0.021 | 0.021 | 0.241 |
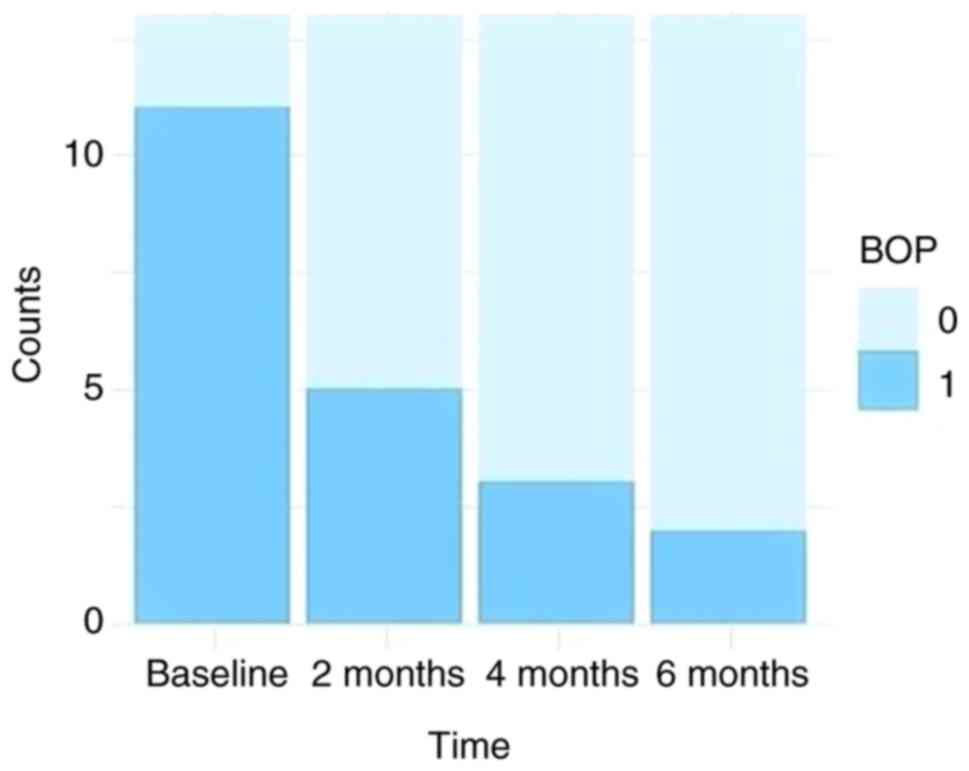
The prevalence of BOP at the four analyzed time
points is shown in Fig. 3.
The Cochran Q test showed that there are significant
differences between the proportions at various time points
(P=0.001). Post-hoc comparisons indicate that significant
differences occurred between baseline and after 4 and 6 months
(Fig. 3). BOP frequency of
occurrence decreased from 84.61 to 38.46%, 23.08, and 15.38% at 2,
4, and 6 months after orthodontic treatment, respectively (Table III).
 | Table IIIResults (P-values) of post hoc
comparisons between BOP prevalence ratios at baseline and after 2,
4, and 6 months of orthodontic treatment (Benjamini-Hochberg
adjustment). |
Table III
Results (P-values) of post hoc
comparisons between BOP prevalence ratios at baseline and after 2,
4, and 6 months of orthodontic treatment (Benjamini-Hochberg
adjustment).
| Items | Baseline | 2 months | 4 months |
|---|
| 2 months | 0.063 | - | - |
| 4 months | 0.023 | 0.824 | - |
| 6 months | 0.023 | 0.562 | 1 |
Assessing the presence of the periopathogens Aa,
Pg, Pi, and Tf, the Wilcoxon signed-rank test did not
reveal statistically significant differences between the values
recorded at baseline and after 6 months of treatment. For
Td, the only periopathogen that exhibited an increase
throughout the observation period, the differences were only
marginally significant (P<0.1) (Table IV).
 | Table IVComparative detection scores at
baseline and after 6 months of orthodontic treatment for
periopathogens Aa, Pg, Pi, Tf, and Td, and P-values
for the corresponding Wilcoxon signed-rank tests. |
Table IV
Comparative detection scores at
baseline and after 6 months of orthodontic treatment for
periopathogens Aa, Pg, Pi, Tf, and Td, and P-values
for the corresponding Wilcoxon signed-rank tests.
| | Aa | Pg | Pi | Tf | Td |
|---|
| Detection
score | Baseline | 6 months | Baseline | 6 months | Baseline | 6 months | Baseline | 6 months | Baseline | 6 months |
|---|
| Median | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Range
(min-max) | 0-4 | 0-4 | 0-1 | 0-4 | 0-2 | 0-2 | 0-3 | 0-4 | 0-1 | 0-2 |
| P-value | 0.1814 | 0.3447 | 0.8241 | 0.7252 | 0.0649 |
Discussion
To our knowledge, this is the first clinical study
to describe at site-level the evolution of clinical and
microbiological parameters in orthodontic patients previously
treated for severe periodontitis.
Naranjo et al noted a shift in the microflora
populating the subgingival plaque after orthodontic bracket
placement as well as a considerable increase of gingivitis in the
test group (27). Another study
found that levels of Pg, Pi, P. nigrescens, Tf, and
Fusobacterium spp. increased after bracket placement in
treated patients when compared with patients in the untreated
control group. Super-infectant microorganisms such as
Enterobacter cloacae, Klebsiella oxytoca, Klebsiella
pneumoniae, and Serratia marcescens were detected by the
authors in the treated group (28).
In an earlier study, clinical and bacteriological evaluations at
baseline and at 90 days after orthodontic bonding treatment; the
authors detected an increase in plaque and bleeding in periodontal
sites of bonded teeth in patients undergoing orthodontic treatment
as compared with the control group. Furthermore, there was no
increase in pocket depth (29).
Surprisingly, a microbiologic study from 2004 attributed the marked
improvement in the periodontopathogenic bacterial spectrum under
fixed appliance therapy with metal brackets, NiTi archwires, and
stainless steel wires to metal corrosion, which entailed the
release of nickel ions that are thought to be exclusively toxic to
periopathogenic bacteria (30).
In the present study, clinical and microbiological
evaluations were performed on each selected tooth at the site with
the deepest residual PD (≥3 mm) to allow for potential changes of
the respective surrogate parameters. All analyzed sites were chosen
on the aspect of the root that underwent compression so that GCF
sampling and analysis could also include inflammatory resorptive
activity on the alveolar bone induced by the orthodontic movements
(31,32).
The Friedman test for the mean values of PD, REC,
and CAL variations did not indicate changes between the timepoints.
The evolution of individual PDs revealed that, at 2 months, all 13
patients scored values equal to or lower than baseline.
The main periodontal objective of orthodontic
treatment in patients with treated periodontitis is to maintain or
improve attachment level. In our study, we concluded that PD did
not change significantly following orthodontic movement,
demonstrating that orthodontic treatment does not adversely affect
the periodontal condition in patients with a history of periodontal
disease. The results obtained are consistent with other clinical
studies in the literature. It was previously shown that orthodontic
treatment in patients with treated periodontal disease resulted in
a 0.7 mm decrease in PD after one year of treatment (33). Other authors, in a similar clinical
trial, concluded that orthodontic treatment has no negative impact
on PD variations. Although both studies used regenerative surgical
techniques, the effects of orthodontic treatment on PD between the
beginning of therapy and at the end of the assessment period are
relevant (34). Other studies have
reported significant reductions in PD values following orthodontic
treatment, concluding that orthodontic treatment can actually
improve periodontal conditions (21,22,35,36).
Differently to the aforementioned studies, in our study the reduced
magnitude of the PD changes could be potentially attributed to the
severity of the treated periodontitis.
REC and PD did not exhibit statistically significant
changes at the time points analyzed. The evolution of REC at
successive time points suggests that no change in the parameter
occurred during the first 2 month interval. After 4 months of
orthodontic treatment, there was a slight increase in mean values,
while at 6 months there was a decrease. One important observation
is that for 7 out of 13 patients, REC values did not undergo any
change during the study period. One patient experienced a 1 mm
increase over the 4 month interval that remained constant until the
end, and two patients experienced a 1 mm reduction in REC over the
6 month period. The maximum recorded value was 3 mm for the first
three intervals, and remained unchanged until the end of the
assessment period. Similar to PD, a slight reduction in mean REC
was observed in our study. These results provide some evidence for
possible beneficial effects on periodontal status following
orthodontic treatment. In the literature, the incidence of gingival
recession following orthodontic therapy is disputed and the results
are largely contradictory (37).
In our study, the mean CAL did not yield
statistically significant variations (P=0.559). However, a 0.2 mm
gingival attachment gain was noted at the end of the assessment
period, when compared with baseline. These results are consistent
with other clinical studies that did not identify statistically
significant differences (31) or
found no differences in CAL following orthodontic treatment
(38).
The only clinical parameter that recorded
statistically significant differences was BOP. Its incidence was
90% at baseline, 40% at 2 months, and 20% at 4 and 6 months of
orthodontic treatment. These results are somewhat surprising, given
the positive relationship between gingival inflammation and the
occurrence of BOP, as well as the pro-inflammatory effect of the
orthodontic appliances on the gums. However, BOP, as a clinical
symptom of inflammation, is largely influenced by plenty of other
host-related and external parameters, than the presence of
periopathogenic bacteria and subgingival calculus deposits
(39).
Although PlI showed a significant increase between
baseline and two months, reaching the maximum mean value of
1.31±0.43, it recorded a subsequent continuous decrease until the
six month timepoint (all differences were statistically
significant, P=0.019), reaching 1.19±0.43. Initial growth can be
explained by the deterioration of oral hygiene due to the insertion
of the orthodontic appliance, followed by a continuous decrease,
possibly due to continuous re-inforced instruction in oral hygiene
that was specific for patients undergoing supportive periodontal
therapy. Generally, these scores indicate good oral hygiene
throughout the study interval. However, there is no clear
correlation between the evolution of this parameter and the BOP.
While BOP scores exhibited statistically significant decreases
between baseline and 2 months, PlI increased statistically
significantly over the same interval and was the second largest
change in this parameter over the observation period. The reduction
in BOP could also be explained by the particular supportive therapy
program, including monthly visits with a particular emphasis on
preventing plaque accumulation in both supragingival and
subgingival areas.
The results from the present study related to the
evolution of BOP, PD, and PlI values are in line with those found
in a study from 2009(39), which
focused on the correlation of clinical parameters with the presence
of subgingival plaque deposits identified by endoscopy. The results
demonstrated a linear correlation between the presence of
subgingival plaque and the proportional increase of BOP and PD. As
in the present study, the authors concluded that differences in the
efficacy of oral hygiene among patients make this correlation
difficult, and failed to find a concrete link between PlI and the
evolution of BOP.
The variations in frequency of detection of the main
periopathogens Aa, Pg, Pi, and Tf did not reveal
statistically significant differences between the mean values
recorded at baseline and those at six months of orthodontic
treatment. The only periopathogen more frequently detected at the
end of the observation period was Td, but was only
marginally significant (P=0.0649). When analyzing the comparative
detection scores at baseline and after 6 months of treatment, a
very low but detectable presence of Aa, Pg, and
Pi was observed, with the median being 0 both at baseline
and after six months of treatment. Although the median was 1 at
baseline for Tf, the value remained unchanged after 6 months
of orthodontic treatment. Even in the case of Td, which
exhibited a slight increase at the 6 month time point as compared
with baseline, the highest score was 2, the lowest score to
ascertain detection.
These results demonstrate that the level of
subgingival pathogenic bacteria was very low, close to zero, during
the first months of orthodontic treatment. The low levels of
detection correlate with the results obtained for clinical
parameters, especially for BOP, demonstrating once again the
effectiveness of systematic periodontal therapy prior to
orthodontic therapy, and the role of properly performed oral
hygiene during the maintenance phase. Similar results were obtained
in a study on the incidence of Aa and Pg during
orthodontic treatment with lingual brackets (40) and in earlier study of Aa, Tf,
and Pi (41). The presence
of the main periopathogens in the gingival sulcus during classical
orthodontic treatment was investigated as well (42). In this study, Aa, Pg, Pi, and
Td did not show statistically significant differences
between baseline and the endpoint; Td exhibited only
marginally significant differences. Thus, the authors concluded
that patients with healthy periodontium prior to orthodontic
treatment have decreased risk of further periodontal deterioration
during orthodontic treatment. Although these results are not in
line with the data from our present study, they show that
orthodontic treatment does not have a negative effect on microbial
flora as long as oral hygiene is properly carried out and the level
of bacterial plaque accumulation is reduced to a minimum.
Nevertheless, other studies have reported significant increases in
pathogenic bacteria during orthodontic treatment (43,44).
Although in the mentioned studies the detectable
amount of periopathogens during orthodontic treatment increased
significantly, the changes did not appear to have negative clinical
effects; they returned to baseline with the removal of orthodontic
appliances. Of note, studies that reported increased levels of
periopathogens at the end of the treatment also showed
significantly higher frequencies of detection at baseline, in
contrast to studies that did not report significant differences and
where the frequency of detection was absent or slightly positive.
Thus, it can be argued that orthodontic treatment could have a
negative effect on patients with high levels of bacteria before
insertion of orthodontic devices, but does not cause them to occur
in a healthy periodontium. Orthodontic treatment had no detrimental
effect on the clinical parameters studied; however, we cannot draw
a conclusion that there were improvement in periodontal conditions
following orthodontic treatment, despite several studies that
reported such outcomes (13,14,23).
One limitation of the present study is the
relatively small number of investigated patients. This can be
explained by the high number of inclusion criteria (both for the
subjects and for the experimental sites), and by the numerous time
points at which evaluations occurred. On the contrary, similar
studies in the literature employed groups with a similar or a lower
number of patients (25,30,33).
As in the daily practice the periodontal status of
orthodontic patients previously treated for severe periodontitis is
being routinely monitored using articulated supportive therapy
sessions, there is clear need for further clinical studies to
identify the periodontal sites at risk of deterioration and the
measures necessary to mitigate the recurrence of the disease.
Within the limits of the present study, we concluded
that there were no significant changes in the clinical parameters
and microflora during the initial phase of orthodontic treatment in
patients with periodontal support reduced by severe periodontitis,
once the primary disease is systematically treated and the residual
inflammation controlled. By correlating the clinical parameters
with the microbiological ones, we inferred that residual levels of
periopathogens did not negatively influence the periodontal health
during orthodontic treatment in adult patients who underwent
therapy for severe periodontitis.
Acknowledgements
The authors would like to thank Mrs. Claudia Zaharia
for her kind assistance with the statistical analysis of the
data.
Funding
The study was partially funded by a doctoral grant
from the ‘Victor Babes’ University of Medicine and Pharmacy
(Timisoara, Romania) beginning in 2011, and partially by the
authors themselves.
Availability of data and materials
The datasets used/analyzed in the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
SIS, MT, AA, DR and AJ participated in the treatment
of the patients, the sample collection and data acquisition. HC,
LN, AO, AMR and DR participated in the study design. AMR drafted
the manuscript and critically revised it for important intellectual
content. SB, SM, MB, AR, PS and LS drafted the manuscript and
critically revised it for important intellectual content, and were
also involved in the conception of the study. AD and SS performed
the statistical analysis. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Research
Ethics Committee of the ‘Victor Babes’ University of Medicine and
Pharmacy (approval no. 14/16.09.2013). All subjects were informed
about the nature and purpose of the study, and each subject signed
an informed consent document giving permission for the dental
procedures and sampling of biological material.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Popova C, Dosseva-Panova V and Panov V:
Microbiology of periodontal diseases. A review. Biotechnol
Biotechnol Equip. 27:3754–3759. 2013.
|
|
2
|
Socransky SS and Haffajee AD: Periodontal
microbial ecology. Periodontol 2000. 38:135–187. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Paster BJ and Dewhirst FE: Molecular
microbial diagnosis. Periodontol 2000. 51:38–44. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
van Winkelhoff AJ and Winkel EG:
Microbiological diagnostics in periodontics: Biological
significance and clinical validity. Periodontol 2000. 39:40–52.
2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Calenic B, Greabu M, Caruntu C, Nicolescu
MI, Moraru L, Surdu-Bob CC, Badulescu M, Anghel A, Logofatu C and
Boda D: Oral keratinocyte stem cells behavior on diamond like
carbon films. Rom Biotechnol Lett. 21:11914–11922. 2016.
|
|
6
|
Willmot D: Orthodontic treatment and the
compromised periodontal patient. Eur J Dent. 2:1–2. 2008.PubMed/NCBI
|
|
7
|
Cardaropoli D: Orthodontics for the adult
periodontal patient: First or second choice treatment? Prog Orthod.
10:88–96. 2009.PubMed/NCBI
|
|
8
|
Zharmagambetova A, Tuleutayeva S,
Akhmetova S and Zharmagambetov A: Microbiological aspects of the
orthodontic treatment. Georgian Med News pp39-43, 2017.
|
|
9
|
Rego RO, Oliveira CA, dos Santos-Pinto A,
Jordan SF, Zambon JJ, Cirelli JA and Haraszthy VI: Clinical and
microbiological studies of children and adolescents receiving
orthodontic treatment. Am J Dent. 23:317–323. 2010.PubMed/NCBI
|
|
10
|
Van Gastel J, Quirynen M, Teughels W,
Coucke W and Carels C: Longitudinal changes in microbiology and
clinical periodontal parameters after removal of fixed orthodontic
appliances. Eur J Orthod. 33:15–21. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Verrusio C, Iorio-Siciliano V, Blasi A,
Leuci S, Adamo D and Nicolò M: The effect of orthodontic treatment
on periodontal tissue inflammation: A systematic review.
Quintessence Int. 49:69–77. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Corrente G, Abundo R, Re S, Cardaropoli D
and Cardaropoli G: Orthodontic movement into infrabony defects in
patients with advanced periodontal disease: A clinical and
radiological study. J Periodontol. 74:1104–1109. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Re S, Corrente G, Abundo R and Cardaropoli
D: Orthodontic treatment in periodontally compromised patients:
12-year report. Int J Periodontics Restorative Dent. 20:31–39.
2000.PubMed/NCBI
|
|
14
|
Amiri-Jezeh M, Marinello CP, Weiger R and
Wichelhaus A: Effect of orthodontic tooth intrusion on the
periodontium. Clinical study of changes in attachment level and
probing depth at intruded incisors. Schweiz Monatsschr Zahnmed.
114:804–816. 2004.PubMed/NCBI(In French and German).
|
|
15
|
Bollen AM, Cunha-Cruz J, Bakko DW, Huang
GJ and Hujoel PP: The effects of orthodontic therapy on periodontal
health: A systematic review of controlled evidence. J Am Dent
Assoc. 139:413–422. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gorbunkova A, Pagni G, Brizhak A,
Farronato G and Rasperini G: Impact of orthodontic treatment on
periodontal tissues: A narrative review of multidisciplinary
literature. Int J Dent. 2016(4723589)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Carvalho CV, Saraiva L, Bauer FPF, Kimura
RY, Souto MLS, Bernardo CC, Pannuti CM, Romito GA and Pustiglioni
FE: Orthodontic treatment in patients with aggressive
periodontitis. Am J Orthod Dentofacial Orthop. 153:550–557.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Castellanos-Cosano L, Machuca-Portillo G,
Mendoza-Mendoza A, Iglesias-Linares A, Soto-Pineda L and
Solano-Reina E: Integrated periodontal, orthodontic, and
prosthodontic treatment in a case of severe generalized aggressive
periodontitis. Quintessence Int. 44:481–485. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cirelli CC, Cirelli JA, da Rosa Martins
JC, Lia RC, Rossa C Jr and Marcantonio E Jr: Orthodontic movement
of teeth with intraosseous defects: Histologic and histometric
study in dogs. Am J Orthod Dentofacial Orthop. 123:666–675.
2003.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Meeran NA and Parveen MJ: The scope and
limitations of adult orthodontics. Indian J Multidiscip Dent.
2:383–387. 2011.
|
|
21
|
Khorsand A, Paknejad M, Yaghobee S,
Ghahroudi AA, Bashizadefakhar H, Khatami M and Shirazi M:
Periodontal parameters following orthodontic treatment in patients
with aggressive periodontitis: A before-after clinical study. Dent
Res J (Isfahan). 10:744–751. 2013.PubMed/NCBI
|
|
22
|
Re S, Cardaropoli D, Abundo R and Corrente
G: Reduction of gingival recession following orthodontic intrusion
in periodontally compromised patients. Orthod Craniofac Res.
7:35–39. 2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Corrente G, Abundo R, Re S, Cardaropoli D
and Cardaropoli G: Orthodontic movement into infrabony defects in
patients with advanced periodontal disease: A clinical and
radiological study. J Periodontol. 74:1104–1109. 2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cardaropoli D, Re S, Corrente G and Abundo
R: Intrusion of migrated incisors with infrabony defects in adult
periodontal patients. Am J Orthod Dentofacial Orthop. 120:671–675,
677. 2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Boyer S, Fontanel F, Danan M, Olivier M,
Bouter D and Brion M: Severe periodontitis and orthodontics:
Evaluation of long-term results. Int Orthod. 9:259–273.
2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Armitage GC: Development of a
classification system for periodontal diseases and conditions. Ann
Periodontol. 4:1–6. 1999.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Naranjo AA, Triviño ML, Jaramillo A,
Betancourth M and Botero JE: Changes in the subgingival microbiota
and periodontal parameters before and 3 months after bracket
placement. Am J Orthod Dentofacial Orthop. 130:275.e17–e22.
2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lauritano D and Caccianiga G: Periodontal
aspects in orthodontics. OA Dentistry. 1(1)2013.
|
|
29
|
Huser MC, Baehni PC and Lang R: Effects of
orthodontic bands on microbiologic and clinical parameters. Am J
Orthod Dentofacial Orthop. 97:213–218. 1990.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Speer C, Pelz K, Hopfenmüller W and
Holtgrave EA: Investigations on the influencing of the subgingival
microflora in chronic periodontitis. A study in adult patients
during fixed appliance therapy. J Orofac Orthop. 65:34–47.
2004.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Bennett JC and McLaughlin RP: Fundamentals
of orthodontic treatment mechanics. Le Grande Publishing, London,
Dubai, 2014.
|
|
32
|
Proffitt WR, Fields HW and Sarver DM:
Contemporary orthodontics. 4th edition. Mosby Elsevier, 2007.
|
|
33
|
Ghezzi C, Masiero S, Silvestri M, Zanotti
G and Rasperini G: Orthodontic treatment of periodontally involved
teeth after tissue regeneration. Int J Periodontics Restorative
Dent. 28:559–567. 2008.PubMed/NCBI
|
|
34
|
Cardaropoli D, Re S, Manuzzi W, Gaveglio L
and Cardaropoli G: Bio-Oss collagen and orthodontic movement for
the treatment of infrabony defects in the esthetic zone. Int J
Periodontics Restorative Dent. 26:553–559. 2006.PubMed/NCBI
|
|
35
|
Passanzaei D: Interdisciplinary treatment
of a localized juvenile periodontitis, a new prospective to an old
one. AJO-DO. 13:271–280. 2007.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhu B, Guo Y, Zhou H and Fu X: The
clinical results of combined periodontal-orthodontic treatment on
patients with periodontitis and labial displacement of incisors.
Shanghai Kou Qiang Yi Xue. 14:431–433. 2005.PubMed/NCBI(In Chinese).
|
|
37
|
Joss-Vassalli I, Grebenstein C, Topouzelis
N, Sculean A and Katsaros C: Orthodontic therapy and gingival
recession: A systematic review. Orthod Craniofac Res. 13:127–141.
2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gomes SC, Varela CC, da Veiga SL, Rösing
CK and Oppermann RV: Periodontal conditions in subjects following
orthodontic therapy. A preliminary study. Eur J Orthod. 29:477–481.
2007.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Checchi L, Montevecchi M, Checchi V and
Zappulla F: The relationship between bleeding on probing and
subgingival deposits. An endoscopical evaluation. Open Dent J.
3:154–160. 2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Demling A, Demling C, Schwestka-Polly R,
Stiesch M and Heuer W: Influence of lingual orthodontic therapy on
microbial parameters and periodontal status in adults. Eur J
Orthod. 31:638–642. 2009.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Sargolzaie N, Forghani M, Javadian
Langaroodi A and Moradi S: Evaluation of periodontal indices
following use of two incision techniques in apical surgery. J Dent
Mater Tech. 2:77–81. 2013.
|
|
42
|
Kim J and Amar S: Periodontal disease and
systemic conditions: A bidirectional relationship. Odontology.
94:10–21. 2006.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ristic M, Vlahovic Svabic M, Sasic M and
Zelic O: Clinical and microbiological effects of fixed orthodontic
appliances on periodontal tissues in adolescents. Orthod Craniofac
Res. 10:187–195. 2007.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Khan R and Antony VV: Investigation of the
periodontal and microbiological status of patients undergoing fixed
orthodontic therapy. IOSR J Dental Med Sci. 7:80–85. 2013.
|