Introduction
Atopic dermatitis (AD) has a multifactorial
pathology (immunological, genetical, environmental factors and skin
barrier damage) with specific complex mechanisms (1,2). There
are certain foods that can trigger atopy, such as peanut allergy
due to the MALT1 gene (3), chicken
egg allergy (4), cow's milk or
breast milk [due to changes in the sCD14 gene caused by
environmental factors in today's society, that include the immune
modulators from breast milk (5)] or
fish (6). Also, the consumption of
processed foods and/or energy drinks (7), antenatal exposure to some heavy metals
(Plumb and Chrome) could determine the AD development after 24
months (8). Other substances that
can cause AD are: Sodium monoglutamate (9), olive pollen (due to β-1, 3-glucanase
rOle e9), allergen produces by Aspergillus (due to MnSOD rAsp f6
IgE) (10) or dust mites (11). So far, there are few
dermato-endocrinological studies regarding the involvement of
adipokines in the AD pathogenesis. Banihani et al (12), from Jordan, found that 38.7% of the
patients with AD had been associated with some leptin genes that
are polymorphic. The study showed that one of them (rs2167270) has
the most important role.
Nutrition is one of the most important elements in
improving the patients' quality of life in AD. There are
preclinical studies that have shown that the systemic
immunoregulatory effect of the probiotic bacterium Lactobacillus
pentosus KF340 (LP340) (present in different fermented foods)
was induced by interleukin (IL)-10, produced by Tr1 cells (13). Also, Bifidobacterium adolescentis
and lactis, Lactobacillus sakei, acidophilus and casie and
Longum are beneficial probiotic bacteria (14,15).
In order to suppress the allergic effect of pasteurized cow's milk,
Abbring et al (16) treated
it with alkaline phosphatase and obtained positive preclinical
results.
The manufacture of safe food products for human
health is performed in compliance with the rules of good hygiene
and manufacturing norms (the HACCP principles). In translation,
HACCP means: Risk analysis and determination of crucial control
points, just like the production recipes specific to each economic
agent or of the established/traditional recipes. The Regulation
(EU) 218/2016 is available and presents the specific requirements
in order to respect the composition and the information applicable
to foods intended for particular medical purposes. According to the
4th paragraph, ‘The composition of foods intended for special
medical purposes may vary greatly depending on, among other things,
the specific pathology, disorder or disease for which the diet is
designed, the age of the patients and the place where they receive
medical care or for the intended use of these products. In
particular, foods intended for special medical purposes may be
classified by composition into different categories, standard
nutritional formula or an adapted nutritional formula, specific to
a pathology, disorder or disease, or whether or not they constitute
the only food source for the persons whom they are intended’
(17).
The efficacy of the active substances (for which
there is preclinical/clinical research presented in this paper) and
the fact that they have been administered orally are the basis for
developing foods to special nutritional states, in our case for
AD.
The food industry operators who want to research and
develop foods for special nutritional states, in our case for AD,
must form multidisciplinary teams that consist of food industry
specialists and health professionals. Paragraph 3 of the
aforementioned European Regulation, states that ‘Foods for special
medical purposes are developed in close cooperation with health
professionals for the nutrition of patients with pathological
disorders, suffering from a disorder or a specific disease or
malnutrition due to these diseases which makes it impossible or
very difficult to meet the nutritional needs of these patients
through the consumption of other foods’ (17). According to this paragraph ‘For this
reason, foods intended for special medical purposes should be used
under medical supervision which can be provided with the assistance
of other competent persons working in the health field’ (17).
Materials and methods
The PubMed database was analyzed for the period
2018-2020. The search criteria were ‘chronic dermatitis’, ‘atopic
dermatitis’, ‘psoriasis’ ‘alternative treatments’, ‘natural
treatments’, ‘complementary treatments’, ‘treatments for dermatitis
chronic’. We also looked for undesirable or side effects related to
foods, potential food ingredients and the action mechanisms of the
analyzed foods. In the period 2018-2020 we identified 461 articles,
of which 95 were preclinical researches and 265 clinical trials.
Out of all the analyzed articles, only 31 were important for us, in
order to achieve our objectives.
In this investigation we have tried to identify and
to present foods and potential food ingredients for which there is
scientific evidence and we formed a proposal the principles of
research and food development for special nutritional states, for
atopic dermatitis.
Results
The foods with systemic immunoregulatory effect used
preclinically in the treatment of AD were determined. These are
summarized in Table I. The foods
and substances which efficacy has been clinically determined in
vitro are presented in Tables
II and III.
 | Table ISystemic immunoregulatory effect of
food in the treatment of AD determined preclinically. |
Table I
Systemic immunoregulatory effect of
food in the treatment of AD determined preclinically.
| | Preclinical
determination of the efficacy of the products/preparations |
|---|
| Product/preparation,
dose, mode of administration | Pathology
followed | Species/line, sex,
age (weeks) | Number animals/number
groups | Period, number of
days | Induction mechanism
pathology | Main action monitored
and demonstrated | Action mechanism of a
product/preparation |
|---|
| LP340a, 800 pg/ml, food (13) | AD (13) | Mice/BALB/c (13) | NMa (13) | 77(13) | 20 µl of 1.5%
DNCBa-topical
(13) | - ILa-10 and IL-12 ↑; -
PD-L1aSocs-3a, Idoa ↑; - TGF-β1a ↓; -CDa40, CD80 și CD86 ↑;
-IgEa ↓; - thickness
of ear lobe ↓; - the injuries caused by the DNCB ↓ (13) | The IL-10-producing
Tr1 induced by LP340 are functional: IL-10 ↑; IL-27↓ (13) |
| B.
adolescentisa,
suspension/0.2 ml (1x109 UFC), oral (15) | AD (15) | Mice/C57bl/6,
females/6 weeks (15) | 20/4(15) | 35(15) | 0.5% DNCB solution:
acetone/olive oil + 0.2% DNCB-topical (15) | - Ear thickness ↓;
- Mast ↓; - Spleen regulatory T cells ↑; - Th2 ↓; -IgE seric ↓; -
IL-4 ↓; -IL-13 ↓; - chemokines derived from macrophages (CDM/CCL22)
↓; - Firmicutes ↑; - Chao1 index ↑; - Colonial SCFA ↑
(15). | Metabolism of
fructose and mannose, SCFA ↑; IFN-γ ↑; - Regulatory T cells ↑; -
Th2 ↓: IL-4 and IL-13 ↓ (15) |
| Alnus
sibirica, fermented extract/100 mg/kg, 100 µM/kg, oral
(18) | AD (18) | Mice/BALB/c Male/7
weeks (18) | 30/6(18) | 28(18) | 20 µl 1% DNCB and
20 µl 0.5% DNCB (18) | - Antioxidant
activity ↑; - Antioxidant activity ↑: - IgE ↓; Th2 ↓; - Th1 ↑;
-IL-4, -5, -10, - 13 ↓; -TNF-α and IFN-γ ↓ (18) | Hirsute-nona,
Muricar-pon B (18) |
| Cow's milk
pasteurized and improved with ALPa, 0.5 ml, oral (16) | Allergy (16) | Mice
C3H/HeOuJ/females/3 weeks (16) | 48/6(16) | 28(16) | 20 mg chicken egg
protein dissolved in 0.5 ml PBSa containing 10 µg CTa for 5 days; 0.5 ml raw milk 8
consecutive days (16) | IgE ↓; Th2 ↓; IL-13
↓; CD103 ↑; CD11b ↑; DC ↑; TGF-β ↑ (16) | Suppression of
allergic effect: IgE ↓; Th2 ↓; IL-13 ↓; CD103 ↑; CD11b ↑; DC ↑;
TGF-β ↑ (16) |
| Apigenin, 150 mg/kg
body, oral (19) | AD (19) | Mice ICR (19) | 20/4(19) | 7(19) | Compound 48/80, 50
µg injection for induction of scratching behavior (19) | - IL-31 ↓; -IL-31
release in HMC-1 cells; Suppression of scratches behavior (19) | mARN IL-31 ↓;
Inhibition of MAPKa
and phosphorylation NF-κBa (19) |
|
Cheongguk-janga, oral (20) | AD (20) | Hairless
mice/males/4 weeks (20) | 20/4(20) | 20(20) | Compound 48/80, for
inducing scratching and pruritus behavior, 0.1 ml 0.15% DNCB
(prepared with acetone/olive oil in a ratio of 3:1) (20) | - SCORAD ↓; - The
thickness of the epidermis ↓; - Deposition of collagen fibers on
the skin of mice with AD ↓; - Prevention of mast cell infiltration
in the dermis of mice (20) | - IgE ↓; -Th2 ↓; -
IL -4 ↓; - IL-31 ↓; - mARN IL-31 ↓; - Inhibition of
MAPKa and
phosphorylation NF-κBa
(20) |
| Korean red
ginsenga (21), capsules, 500 mg RGa/capsule (2.5 g/kg body), oral
(dissolving RG powder in water) (21) | AD (21) | Rats
SDa, male, 6 weeks
(21) | 20/4(21) | 27(21) | Making two round
wounds with a diameter of 2 cm (21) | - Moisture in the
skin ↑; - Leather lipids ↑; - Angiogenesis ↑; - Epitheliazation ↑;
- Very active fibroblasts; - Active neovascularization; - Collagen
accumulation ↑; - The regeneration time ↓ (21) | -
TGF-β1a ↑; -
VEGFa ↑; - MMP-1 ↑; -
MMP-9 ↑ (21) |
| Cinamamide | AD (22) (NCT and NCPA)a 50 mg/kg/day, oral (22) | Mice/BALB/c, male,
8 weeks (22) | 18/6(22) | 28(22) | 20 ml of
DEEa, 20 ml 1% DNCB
(22) | - Thickness of the
epidermis and dermis of the ear ↓; - Mast cell infiltration; - IgE
↓; - IgG2 ↓; -IL-4 ↓; - Weight and size of cervical lymph nodes
(22) | - mRNA of Jurkat
cells ↓; - mRNA of Th1 and Th2 cytokines ↓ (22) |
| Duolac
ATPa, 2x106
CFU/200 µl/day, oral (23) | AD (23) | Mice/BALB/c,
females, 7-10 weeks mice NC/Nga 4 weeks (23) | 18/3(23) | 59(23) | - DEEa, - DNFBa (23) | - S.F.a - No;- BMDCsa- regulated/immune response: PD-L1
↑, IL-10 ↑, TGF-β↑, IL-12p40 ↓, - Improvement of AD symptoms: •
Erythema, scaling/drying, excoriation/erosion, edema and itching ↓;
• apoptotic cells ↓; • IgE seric total ↓; -Achieving cell balance
T: Th1 ↑, Th2 ↓ and Th17 ↓; - IL-2 ↑, IFN-γ ↑; -Maintaining the
balance of T cells: yes (23) | - mARNm of IL-10 ↑;
- TGF-β-unchanged; - Intestinal Treg cell population ↑ (23) |
| β-GdAPa SM-2001, oral: 1 mg/kg
bodya - group 1; 10
mg/kg bodya - group 2;
20 mg/kg bodya - group
3, oral (24) | AD (24) | Rats SD, male, 6
weeks, mice ddY, male, 6 weeks (24) | 60/6(24) | 14(24) | - COM in ddY mice,
and after 7 days 10 µl topically (24) | • Vasodilation ↓; •
serum histamine ↓; -effects of bGdAP on allergic itching and
contact dermatitis: • inflammation ↓; • scratches behavior ↓; •
thickness of the epidermis ↓; • pruritus ↓; - FOXP3* and galectin-9
stimulation: • inflammation ↓; • scratches behavior ↓ (24). | - Th2 ↓ (24) |
| CAPSa, 0.2-1% powder, oral (25) | AD/Hydration of the
skin/UV (25) | Mice Hairless Hos:
HR-1, females, 5 weeks (25) | 40/4(25) | 25(25) | - UV radiation
(25) | - IL-6 ↓; -MMP-13
↓; - Moisture in the skin ↑; - IL-10 ↑ (25) | - Non-itemized
(25) |
| L. sakei
WIKIM30, CFU, oral (26) | AD (26) | Mice/BALB/c, male,
6 weeks (26) | 20/4(26) | 63(26) | DNCB in
acetone/olive oil (3:1); 0,2% DNCB (26) | - Th2 ↓; -
CD4+ ↓, cell T ↓; CD8+ ↓, CD19+ ↓,
Cell B ↓; - IL-4, -5 and -13 ↓; - MLN: CD25+↑,
Foxp3+ ↑; - IL-10 ↑ (26) | - Non-itemized
(26) |
| Bacterial mixture
of lactic acida
(conc.a 2%: t
2x109 CFU/g in 0.2 ml PBS), with sodium butyrate (conc.
100 mM/0.2 ml in 0.2 ml PBS, oral (27) | AD (27) | Mice/BALB/c, male,
3 weeks rats SD, male, 4-6 Weeks (27) | 20/5(27) | 41(27) | Whey protein; TC
(27) | - The thickness of
the ears ↓; -GATA3 and the expression Rorγt mRNA ↓; - Galectin9
mRNA expression in MLN ↑; -Th1 ↓; -The quality of the gut
microbiota ↑: Firmicutes ↑ (27) | - IL-10 ↑; -Th2 ↓;
- Galectin9 modulates mast cell degranulation and cell
differentiation (27) |
 | Table IISystemic immunoregulatory effect of
food clinically determined in treating AD. |
Table II
Systemic immunoregulatory effect of
food clinically determined in treating AD.
| Active
substance/dose | Main action
monitored and demonstrated | Action mechanism of
the drug/subst. Assets | Side effects |
|---|
| L-92a/20 mg (14) | -The median value
of SCORAD ↓; -Median value of drug scores ↓; -IDQOLa ↑ (14). | -Total IgE
a ↓; -Th2 ↓;
-TARCa ↓; -Lecithinase
(-) Clostridium ↓; -Enterobacteriaceae ↓ (14). | No (14) |
| Korean Red Ginseng
Extract (28) | -Severity of the
disease ↓; -EASI score a↓; -Transepidermal water loss ↓;
-VAS a for sleep
disorder and itching ↓; -The amount of topical agents used ↓;
-IGAa ↑ (28). | -IgE ↓; -TNF-α ↓;
-IFN-γ ↓; -IL-31 ↓; -mARN TNF-α ↓ (28). | Yes (28) |
 | Table IIIAlternative foods/treatments
determined in vitro with systemic immunoregulatory effect in
treating AD. |
Table III
Alternative foods/treatments
determined in vitro with systemic immunoregulatory effect in
treating AD.
| Active
substance | Main action
monitored and demonstrated |
|---|
| Alnus
sibirica, fermented (18,29),
fermentation | - In vitro
cytokine regulation; - Missing cytotoxicity (on RBL-2H3 cells);
-IL-12 ↑; -IFN-γ and IL-4 ↓ (on RAW 264.7 cell lines) (18,29). |
| Duolac
ATPa, x106
CFU/ 200 µl/day (23) Strawberry
seed extract (tiliroside), 1.0-3.0 µg/ml (30) | - Treg
differentiation: proliferation of CD4+ T ↑, Foxp3
+/Tregs ↑, IL -10 ↑; -IFN-γ ↑, IL-4↓ (23). - Ceramide synthesis in the stratum
corneum ↑, with the exception of ceramide [EOS], [AP]; - Skin
barrier function and moisture retention ↑; -GCS and GBA ↑; SPT2 and
CerS3, not influenced (30). |
| L. sakei
WIKIM30, 2x109 CFU, oral (26) | - Modulation of DC
and T cells: • TNF-α ↑, IL-6 ↑, IL-12p70 ↑, IL-10 ↑; • CD40 ↑, CD69
↑, CD80 ↑, CD86 ↑ și MHCII ; • PD-L1 ↑and CD103 ↑; • D4
+ ↑, CD25+ ↑, Foxp3+ ↑, Tregs ↑;
-Modulation of T cell immune responses: • Th2 ↓, IL-4 ↓, IL-10 ↑; •
Improvement of specific lesions AD; • IgE ↓ (26). |
Considering the multidisciplinary character of the
teams (medical, pharmaceutical and food industry) necessary to
develop the food for special nutritional states (in our case for
AD), we propose the general principles underlying this process as
indicated in Fig. 1.
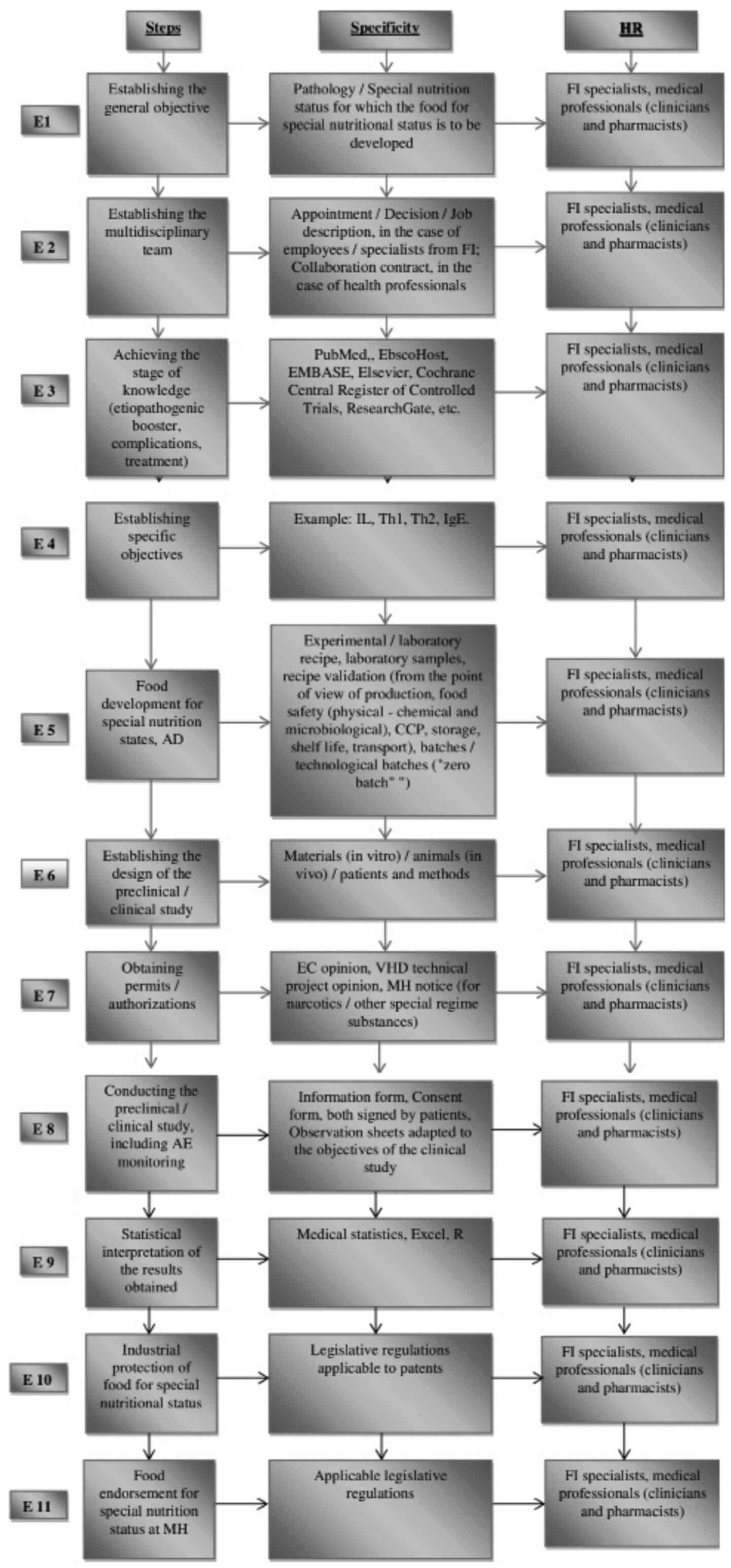 | Figure 1Food development for special nutrition
states. General principles. E, stage in the research process -
development of foods for special nutrition states, in
vitro/preclinical/clinical evaluation and their approval to the
MH; HR, human resources; FI, food industry; IL, interleukins; Th, T
helper cells; IgE, immunoglobulin E; CCP, critical control point
(it is essential for food safety. It is the stage in which a
control measure can be used to prevent or eliminate a food safety
hazard or to reduce it to an accepTable level); AD, atopic
dermatitis; EC, Ethics Committee; VHD, Veterinary Health
Department; MH, Ministry of Health; R, program of medical
statistics; AE, adverse events. |
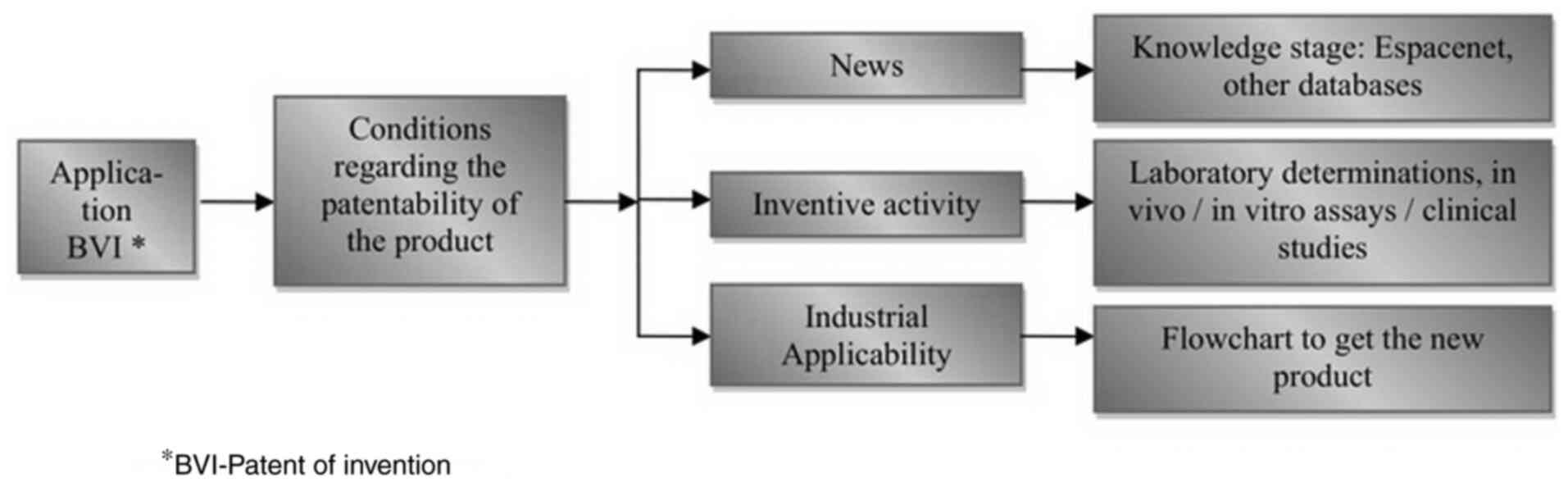
The industrial protection of foodstuffs for special
nutrition states is shown in Fig.
2.
Discussion
Preclinical research was performed over a large
range of days from 7 to 77 days (Table
I). The animal behavior did not change during the research. In
these studies, the topical use of some substances (DNCB, DNFB and
DEE) (13,15,18,20-24,26),
some nutrients (cow milk, red Korean ginseng) (16,21),
UV radiations (25) and injections
with Apigenin (19) could explain
the appearance of skin lesions in AD.
Both in vivo and in vitro research has
demonstrated the efficacy of prebiotics and probiotics
[LP340(13), L-92(14), B. adolescentis (15), Cheongguk-jang (20), Duolac ATP (23), L. sakei WIKIM30(26), Bacterial mixture of lactic acid
(27)], plant extracts [Alnus
sibirica (18), Korean red
ginseng (21), Cinamamide (22)] and certain plant resources [β-GdAP
(24), CAPS (25), Korean Red Ginseng Extract (28), Strawberry seed extract (tiliroside)
(30)] in alleviating the symptoms
of atopic dermatitis (erythema, scaling/drying, erosion, edema and
itching) (Tables I and II). This is due to the increase of IL-10,
IL-12, CD40, CD80 and CD86, T cells spleen regulators, Firmicutes,
Chao1 index, colonic SCFAs, Th1, TGF-β, IL-2, IFN-γ, Galectin9 mRNA
expression in MLN, GCs and GBA, IL-6 and to the decrease of TGF-β1,
IgE, macrophage-derived chemokines (MDC/CCL22), IL-12 p40,
apoptotic cells, B cells, T cells, CD4+,
CD8+, CD19+, IL-4, IL-5, IL-13, GATA3 and
RoRγt mRNA expression.
There are meta-analyses that sustain the beneficial
anti-inflammatory effects of topical Janus kinase inhibitors in
some inflammatory diseases (psoriasis, atopic dermatitis) (31). An other attractive therapy for AD is
the topical phosphodiesterase inhibitors that act by reducing the
release of proinflammatory cytokines (32).
The efficacy of Korean red ginseng in the treatment
of AD has been demonstrated preclinically and clinically. This
product determines the growth of: Hydration degree, lipid layers,
angiogenesis and neovascularization, epithelization, fibroblast
activity, collagen accumulation (21) and IGA (28) (Tables
I and II). It also determines
the decrease of skin regeneration time, severity of the disease,
EASI score, transepidermal water loss, visual analog scale, SCORAD
(that quantified also the itching and lack of sleep) and the number
of topical agents used (28).
From a legislative point of view, we consider that
the European authorities (Commissions, Medicine Agency and Food
Safety Authority) and national authorities (the Ministry of
Health), should issue a common guide for all the countries in the
EU. This guide must provide the principles of the development of
food for special nutritional states, such as: The etiopathogenic
booster, the diagnostic problems, the diagnosis of complications,
the treatment (hygienic-dietary regimens, drug treatment). It
should complete the legislative regulations in order to obtain the
Notice of the Ethics Commission for conducting preclinical/clinical
studies and also for foods for special nutritional states. This
notice is not only for drugs and/or dietary supplements but also
for the nutrivigilance system that identifies and monitors the side
effects caused by the new foods for special nutritional states,
similar to the existing pharmacovigilance system.
The development of foods for special medical
purposes requires multidisciplinary knowledge and multiple
resources. Out of all the resources, time is the most expensive one
because the determination of the efficacy of these products takes a
lot of time.
To develop the foods for special nutritional states
we need to know the etiopathogeny of the disease, the
symptomatology and the pharmacological principles (Fig. 1).
The top management of food factories that want to
manufacture food for special nutritional conditions, must have
multidisciplinary teams, specialized in research and development of
these products, or collaborate with health professionals. The
principles proposed in Fig. 1 are
also valid for the development of food supplements.
Industrial protection (obtaining the invention
patent) is a long-term process. It is based on the verification of
the stage of knowledge (including patents filed worldwide),
physico-chemical and preclinical/clinical analyzes. Also, it is
necessary to know the advantages and disadvantages of the new
developed food for special nutritional status in comparison with
others existing patents (Fig.
2).
In conclusion, the development of foods for special
nutrition states represents a solution for improving the quality of
life of atopic dermatitis patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MR contributed in the conception and design of the
study, analysis and interpretation of the data, manuscript drafting
and critical revision of the manuscript for important intellectual
content. GMI was responsible for the analysis and interpretation of
the data, manuscript drafting and design, and critical revision of
the manuscript for important intellectual content. IMM contributed
in the conception and design of the study, data acquisition,
analysis and interpretation of the data, manuscript drafting and
design, and critical revision of the manuscript for important
intellectual content. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests and they have no financial relationships to disclose.
References
|
1
|
Rotaru M, Ionescu A, Rotaru BI and Iancu
GM: Etiopathogenic and therapeutic considerations in atopic
dermatitis. Acta Medica Transilvanica. 24:34–38. 2019.
|
|
2
|
Solomon I, Ilie MA, Draghici C, Voiculescu
VM, Caruntu C, Boda D and Zurac S: The impact of lifestyle factors
on evolution of atopic dermatitis: An alternative approach. Exp
Ther Med. 17:1078–1084. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rudman Spergel AK and Togias A:
Observational human studies in allergic diseases: Design concepts
and highlights of recent National Institute of Allergy and
Infectious Diseases-funded research. Curr Opin Allergy Clin
Immunol. 20:208–214. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Grimshaw KEC, Roberts G, Selby A, Reich A,
Butiene I, Clausen M, Dubakiene R, Fiandor A, Fiocchi A,
Grabenhenrich LB, et al: Risk factors for Hen's Egg allergy in
Europe: EuroPrevall birth cohort. J Allergy Clin Immunol Pract.
8:1341–1348.e5. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fikri B, Tani Y, Nagai K, Sahara M,
Mitsuishi C, Togawa Y, Nakano T, Yamaide F, Ohno H and Shimojo N:
Soluble CD14 in breast milk and its relation to atopic
manifestations in early infancy. Nutrients. 11(2118)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yuan M, Tan M, Moore D, Shen S, Qiu X,
Thomas GN and Cheng K: Timing of Cow's Milk or Cow's milk formula
introduction to the infant diet and atopic risk in children: A
systematic review and meta-analysis. Clin Rev Allergy Immunol.
59:46–60. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cho SI, Lee H, Lee DH and Kim KH:
Association of frequent intake of fast foods, energy drinks, or
convenience food with atopic dermatitis in adolescents. Eur J Nutr:
Dec 10, 2019 (Epub ahead of print). doi:
10.1007/s00394-019-02157-4.
|
|
8
|
Kim J, Kim S, Woo SY, Chung JY, Hong YS,
Oh SY, Choi SJ, Oh SY, Kim KW, Shin YH, et al: Prenatal exposure to
lead and chromium is associated with IL-13 levels in umbilical cord
blood and severity of atopic dermatitis: COCOA atudy. Immune Netw.
19(e42)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zanfirescu A, Ungurianu A, Tsatsakis AM,
Nițulescu GM, Kouretas D, Veskoukis A, Tsoukalas D, Engin AB,
Aschner M and Margină D: A review of the alleged health hazards of
monosodium glutamate. Compr Rev Food Sci Food Saf. 18:1111–1134.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Scala E, Abeni D, Guerra EC, Pirrotta L,
Locanto M, Meneguzzi G, Giani M, Russo G and Asero R:
β-1,3-glucanase rOle e 9 and MnSOD rAsp f 6 IgE reactivity are the
signature of atopic dermatitis in the Mediterranean area. Clin Exp
Allergy. 50:487–498. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Emran H, Chieng CSE, Taib S and Cunningham
AC: House dust mite sensitisation and association with atopic
dermatitis in Brunei: Allergen sensitization and allergic disease
in Brunei. Clin Transl Allergy. 9(65)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Banihani SA, Elmadhoun RA, Khabour OF and
Alzoubi KH: The rs2167270 polymorphism of leptin gene is associated
with atopic dermatitis. Dermatoendocrinol.
10(e1454191)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kim JE, Sharma A, Sharma G, Lee SY, Shin
HS, Rudra D and Im SH: Lactobacillus pentosus modulates
immune response by inducing IL-10 producing Tr1 cells. Immune Netw.
19(e39)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nakata J, Hirota T, Umemura H, Nakagawa T,
Kando N, Futamura M, Nakamura Y and Ito K: Additive effect of
Lactobacillus acidophilus L-92 on children with atopic
dermatitis concomitant with food allergy. Asia Pac Allergy.
9(e18)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fang Z, Li L, Zhao J, Zhang H, Lee YK, Lu
W and Chen W: Bifidobacteria adolescentis regulated immune
responses and gut microbial composition to alleviate DNFB-induced
atopic dermatitis in mice. Eur J Nutr: Nov 30, 2019 (Epub ahead of
print). doi: 10.1007/s00394-019-02145-8.
|
|
16
|
Abbring S, Ryan JT, Diks MA, Hols G,
Garssen J and van Esch BC: Suppression of food allergic symptoms by
raw cow's milk in mice is retained after skimming but abolished
after heating the Milk-A promising contribution of alkaline
phosphatase. Nutrients. 11(1499)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Official Journal of the European Union:
Commission Delegated Regulation (EU) 2016/128 of 25 September 2015
supplementing Regulation (EU) No 609/2013 of the European
Parliament and of the Council as regards the specific compositional
and information requirements for food for special medical purposes.
Document 32016RO128, 2016. urihttp://data.europa.eu/eli/reg_del/2016/128/ojsimplehttp://data.europa.eu/eli/reg_del/2016/128/oj.
|
|
18
|
Yin J, Yoon SH, Ahn HS and Lee MW:
Inhibitory activity of allergic contact dermatitis and atopic
dermatitis-like skin in BALB/c mouse through oral administration of
fermented barks of Alnus sibirica. Molecules.
23(450)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Che DN, Cho BO, Shin JY, Kang HJ, Kim JS,
Oh H, Kim YS and Jang SI: Apigenin inhibits IL-31 cytokine in human
mast cell and mouse skin tissues. Molecules.
24(1290)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cho BO, Shin JY, Kim JS, Che DN, Kang HJ,
Jeong DY and Jang SI: Soybean fermented with Bacillus
amyloliquefaciens (Cheonggukjang) ameliorates atopic
dermatitis-like skin lesion in mice by suppressing infiltration of
mast cells and production of IL-31 cytokine. J Microbiol
Biotechnol. 29:827–837. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Park KS and Park DH: The effect of Korean
Red Ginseng on full-thickness skin wound healing in rats. J Ginseng
Res. 43:226–235. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Choi EJ, Ryu YB, Tang Y, Kim BR, Lee WS,
Debnath T, Fan M, Kim EK and Lee HS: Effect of cinnamamides on
atopic dermatitis through regulation of IL-4 in CD4+
cells. J Enzyme Inhib Med Chem. 34:613–619. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kim HW, Hong R, Choi EY, Yu K, Kim N,
Hyeon JY, Cho KK, Choi IS and Yun CH: A probiotic mixture regulates
T cell balance and reduces atopic dermatitis symptoms in mice.
Front Microbiol. 9(2414)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kim IS, Lee SH, Kim JA, Yu DY, Hong YH,
Kim JY, Lim JM, Lee SS, Yun CH, Choi IS and Cho KK: Effect of oral
administration of β-glucans derived from Aureobasidium
pullulans SM-2001 in model mice and rat with atopic
dermatitis-like phenotypes. Food Sci Biotechnol. 27:1185–1192.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ashigai H, Komano Y, Wang G, Kawachi Y,
Sunaga K, Yamamoto R, Takata R and Yanai T: Orally administered
polysaccharide derived from blackcurrants (Ribes nigrum L.)
improves skin hydration in ultraviolet-irradiated hairless mice. J
Nutr Sci Vitaminol (Tokyo). 64:301–304. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kwon MS, Lim SK, Jang JY, Lee J, Park HK,
Kim N, Yun M, Shin MY, Jo HE, Oh YJ, et al: Lactobacillus
sakei WIKIM30 ameliorates atopic dermatitis-like skin lesions
by inducing regulatory T cells and altering gut microbiota
structure in mice. Front Immunol. 9(1905)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kim JA, Kim SH, Kim IS, Yu DY, Kim SC, Lee
SH, Lee SS, Yun CH, Choi IS and Cho KK: Anti-inflammatory effects
of a mixture of lactic acid bacteria and sodium butyrate in atopic
dermatitis murine model. J Med Food. 21:716–725. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kim H, Park CW and Cho SH: The beneficial
effect of Korean red ginseng extract on atopic dermatitis patients:
An 8 weeks open, noncomparative clinical study. Ann Dermatol.
30:304–308. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang HS, Hwang YJ, Yin J and Lee MW:
Inhibitory effects on no production and DPPH radicals and NBT
superoxide activities of Diarylheptanoid isolated from
enzymatically hydrolyzed ehthanolic extract of Alnus
sibirica. Molecules. 24(1938)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Takeda S, Shimoda H, Takarada T and
Imokawa G: Strawberry seed extract and its major component,
tiliroside, promote ceramide synthesis in the stratum corneum of
human epidermal equivalents. PLoS One. 13(e0205061)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hosking AM, Juhasz M and Mesinkovska NA:
Topical Janus kinase inhibitors: A review of applications in
dermatology. Am Acad Dermatol. 79:535–544. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yang H, Wang J, Zhang X, Zhang Y, Qin ZL,
Wang H and Luo XY: Application of topical phosphodiesterase 4
inhibitors in mild to moderate atopic dermatitis. JAMA Dermatol.
155:585–593. 2019.PubMed/NCBI View Article : Google Scholar
|