Introduction
The lytic benign bone tumors are usually surgically
treated using curettage and filling the defect using bone graft
(autograft or allograft) or bone substitutes, such as
hydroxyapatite crystals and tricalcium phosphate (1-4).
The location and the size of the bone defect are critical elements
that determine the bone regeneration (5). The autologous bone graft is a very
effective method, usually harvested from the iliac crest. The major
flaw of this resource is the very limited bone that can be
harvested and requires an additional surgical intervention that
usually creates a local postoperative chronic pain (6,7). The
allografts need to be preserved in special conditions, that may not
be available in some clinical centers and, besides the methods of
sterilization, some studies show an increased rate of infections,
but also a decreased integration (8,9). For
this reason, various alternatives had been studied, such as
xenografts or bone substitutes. Unfortunately, the xenograft has
issues because of a slower integration and a greater risk of
allergic reactions and infections (7). The bone substitutes are biomaterials
that are either of human, animal, vegetal or synthetic origin,
especially prepared for filling bone defects, enhancing bone
structure or bone replacement (10-12).
The tricalcium phosphate combined with hydroxyapatite, used as
scaffold, is a good composition with a similar structure to the
bone, with a good integration. They are generally used in dental
and maxillofacial surgeries. The major advantage of this composite
is that the tricalcium phosphate is resorbed, leaving a new formed
bone tissue (8). One of the
disadvantages is the fragility of the calcium phosphate, thus, it
is not indicated in large bone defects. The aim of the present
study is to compare the efficiency of bone substitute formed of 35%
tricalcium phosphate and 65% hydroxyapatite with the simple bone
grafts in the surgical treatment of bone lesions.
Materials and methods
Fifteen patients with benign lesions in different
localizations were included in our prospective randomized
comparative study at ‘Foisor’ Orthopedics Hospital, Bucharest,
Romania, between 2016 and 2019. They underwent a curettage and
filling of the bone defect using allograft or a biphasic mixture
made of 35% tricalcium phosphate, with 60-85% pore volume and 65%
hydroxyapatite.
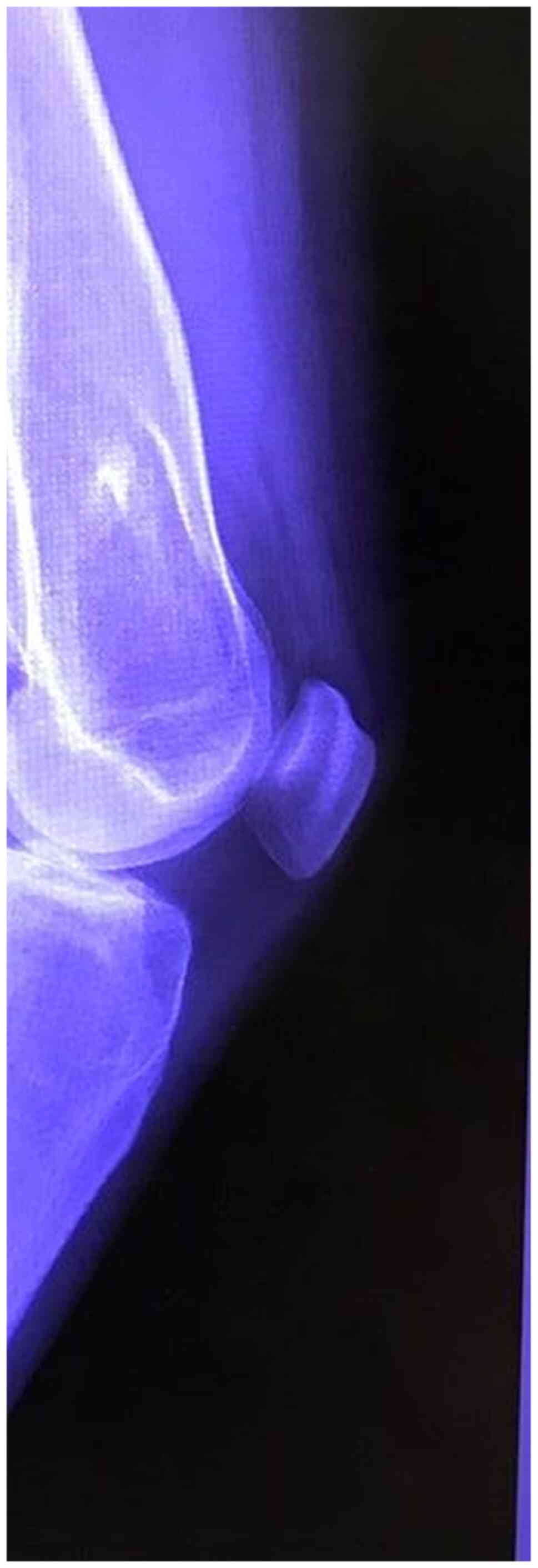
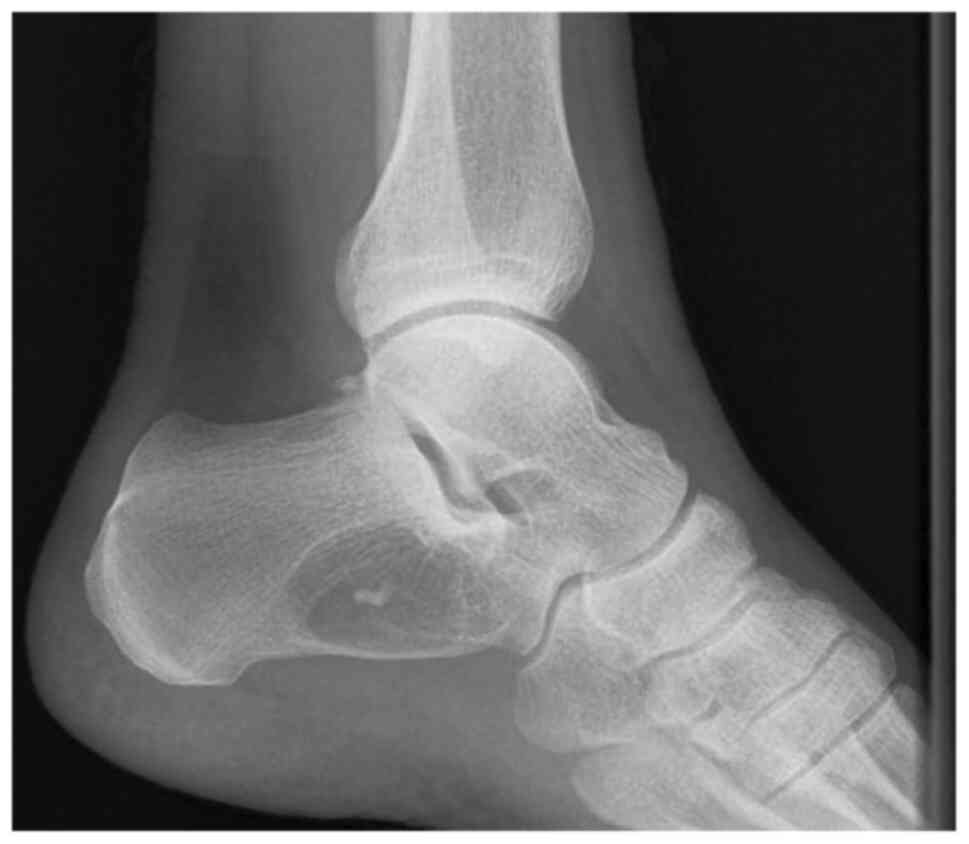
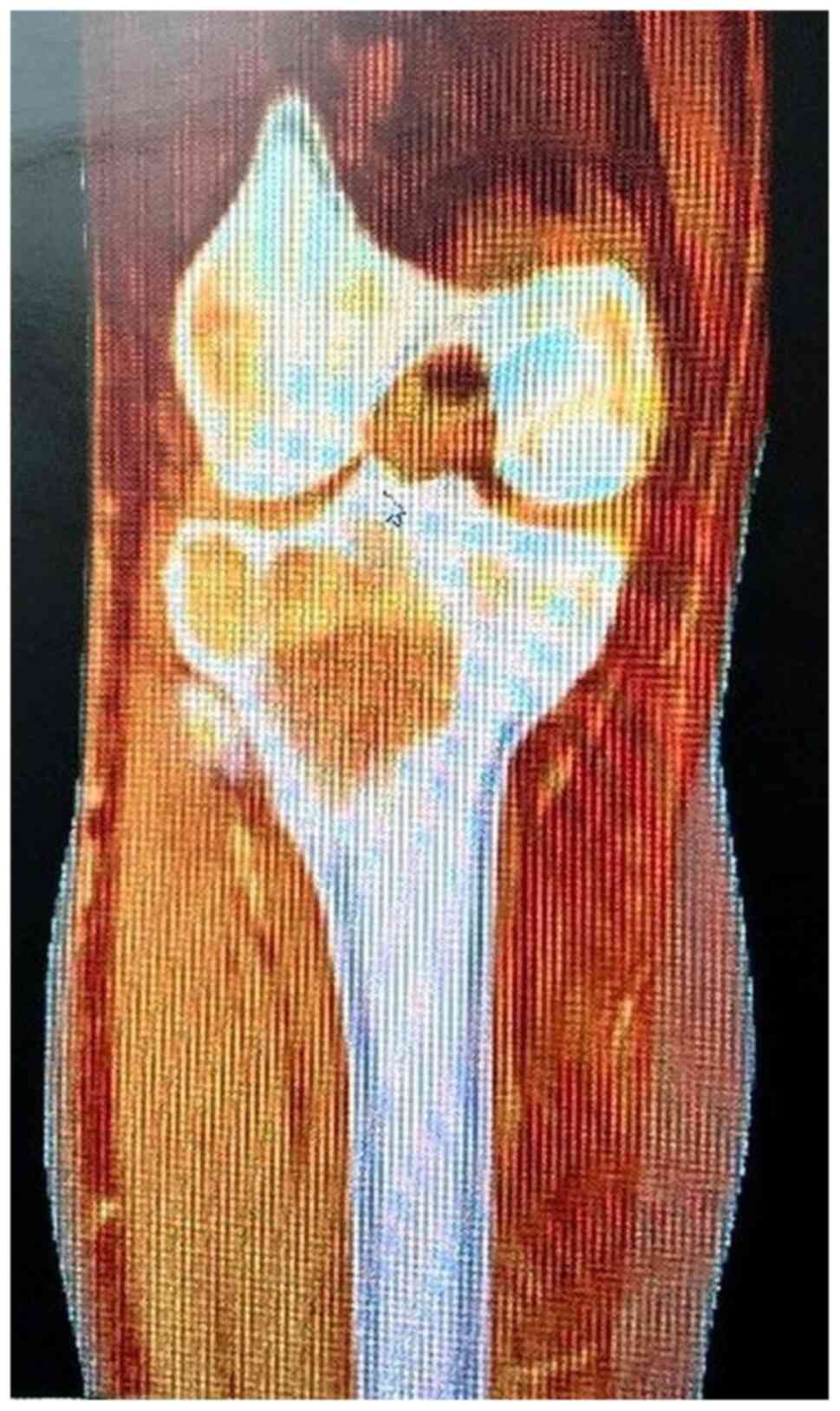
Any patient with benign small or medium sized bone
lytic lesions (Fig. 1), unicameral
bone cyst (Fig. 2), non-ossifying
fibroma or giant cell tumor (Fig.
3) localized in a non-structural area were included. The
exclusion criteria were patients suspect of malignant bone tumors,
local infections, pre-existing metabolic calcium disorders or
patients with large lesions that required supplementary internal
fixation.
All the patients were under general anesthesia and
the surgical procedures were guided under fluoroscopy. The surgical
approach was different depending on the localization of the bone
lesion. After the dissection and the periosteal incision, the wall
of the cyst was opened and the fluid content aspirated. The cavity
wall or the tissue within the lesion was curetted and cauterized
and, after that, the cavity was filled with morselized allograft in
7 cases or a composite ceramic granular material in 8 cases. In the
end, the periosteum was sutured over the filling. The specimens
from the bone were sent for pathological examination. After the
surgery, no weight bearing was recommended for patients with bone
lesions in the lower limb for 6 weeks. For the upper limb, a 6
weeks sling immobilization was recommended.
The patient age, sex, bone size and localization
were recorded preoperatively using the X-ray and CT scan imaging
and Cedara I-View 6.3.3 (Cedara Software Corp.). After the surgery,
all patients were followed up every 3 weeks until 6 months, and
then every 2 months until 1 year for the clinical and radiological
assessment. We used the modified Neer grading system to assess the
amount of bone healing after adding the bone graft (13,14)
(Table I). All the data was
assessed using IBM SPSS Statistics 25.0.0 for Microsoft Windows
(IBM Corporation) and the statistical significance was set at
P<0.05. The baseline characteristics were assessed using the
Independent samples t-test. The study was approved by the Ethics
Committee of ‘Foişor’ Orthopedics-Traumatology and Osteoarticular
TB Hospital (Bucharest, Romania). All patients provided a signed
informed consent.
 | Table IThe modified Neer grading system. |
Table I
The modified Neer grading system.
| Score | Classification | Description |
|---|
| 1 | Healed | The lesion is
completely filled with new formed bone. |
| 2 | Partially healed | Radiolucent areas
smaller than 50% of the diameter of the bone and no increase of
size or lucencies over time. |
| 3 | Persistent cyst | Radiolucent areas
bigger than 50% of the diameter of the bone that may increase over
time and may need to continue the restrictions or repeat the
surgical procedure. |
| 4 | No response | No bony healing
evidence. Repetition of the surgical treatment is required. |
Results
Fifteen patients were followed for 12 months in our
clinic. The average age was 35.4 years (from 18 to 54) for the
allograft group and 41 years (from 22 to 58) for the patients
treated with bone substitute. Eight patients were male and seven
female with relatively equal distribution between both groups
(Table II). The localizations of
the lesions are presented in Table
III. The average bone defect was relatively equal 14 cc (4-25
cc) for the allograft group and 15.1 cc (4-33 cc) for the ceramic
group (P>0.1).
 | Table IIGeneral data regarding the age, sex
and the average size of the bone lesions. |
Table II
General data regarding the age, sex
and the average size of the bone lesions.
| Variable | Allograft | Ceramic | P-value |
|---|
| Age | 35.4 | 41 | n/a |
| Sex (M/F) | 4/3 | 4/4 | n/a |
| Bone defect size | 14 cc | 15.1 cc | >0.1 |
 | Table IIILocalizations of bone lesions. |
Table III
Localizations of bone lesions.
| Localization | Allograft | Ceramic |
|---|
| Clavicle | 1 | - |
| Proximal humerus | 1 | 2 |
| Humeral
diaphysis | 3 | 1 |
| Distal femur | - | 1 |
| Proximal tibia | 1 | 2 |
| Calcaneus | 1 | 2 |
During the follow-up, all the lesions gradually
disappeared after 12 months, with a time of healing of 18.8 weeks
(15-24 weeks) for the allograft group and 20.37 weeks (15-28 weeks)
for the bone substitute group. The amount of healing was almost
similar in both groups. In the allograft group, 5 patients
presented a substantial healing (Neer 1) and 2 patients were
partially healed (Neer 2). In the second group, only 3 patients
presented a partial healing (Neer 2) (Table IV). None of the patients showed any
persistent cysts or lack of response to the surgical treatment.
After 12 months, the CT scan confirmed the integration of the
graft. We did not encounter any significant differences regarding
the functional or clinical status and no patient required extra
pain medication after 2 weeks. There were no local infections or
bone fractures recorded. We did not find any correlation between
the age, sex, location of the lesion and the outcome of the
treatment (P>0.1).
 | Table IVTreatment outcome. |
Table IV
Treatment outcome.
| Neer | Allograft group | Ceramic graft |
|---|
| Type 1 | 5 | 5 |
| Type 2 | 2 | 3 |
| Type 3 | - | - |
| Type 4 | - | - |
Discussion
The bone defects derived from bone tumors remain a
challenging surgical problem from economic and social standpoint
(15). The autograft is the golden
standard; however, factors such as the longer surgery time,
morbidity associated to the donor site and the limited bone that is
available make the allograft, if available, a good alternative. We
did not record any kind of postoperative complications regarding
the allograft technique, but in literature, the following are
mentioned: immune-rejections, bacterial infections and viral
transmission as the limitations of this procedure (16,17).
Tricalcium phosphate could be a good bone substitute because they
have a low cost; it could be an alternative for patients who do not
accept graft transplant for ethical reasons and it offers a
biological response similar to natural bone. The chemical
composition, porosity, and mechanical properties are the most
important elements when a bone substitute is developed (18). In the present study, we had the same
results regarding the grade of integration of the graft compared
with the group treated with allograft. It seems that this kind of
bone substitute enables, by microporosity itself, the bone fluids
and tissues to impregnate the implant that will promote the
ingrowth of an early bone (19).
Nonetheless, the bone healing was slower for the group treated with
tricalcium phosphate. It is mentioned in literature that this type
of graft needs over 9 to 12 months to achieve full integration and
transformation into bone (19,20).
In addition, the proportion of tricalcium phosphate and
hydroxyapatite influences the time for the bone remodeling. If the
hydroxyapatite has a bigger proportion than the phosphate, the bone
requires a longer time to remodel (21). Literature also mentions that grafts
made mainly from calcium phosphate may cause adverse soft tissue
reactions and they have the tendency to dissolve very quickly
(22,23).
One of the major disadvantages of the tricalcium
phosphate/hydroxyapatite is the fragile structure and the low
mechanical properties, which is an important detail to be taken
into account in cases where the bone is exposed to high mechanical
stress (24). That is why it is not
recommended in large bone defects without additional internal
fixation and graft augmentation. The age and the sex of the
patients did not influence the outcome of the treatment.
One of the main limitations of this study is the
small number of patients included and a short follow up. Although
both types of treatment demonstrated good results, a larger number
of cases and a longer time of follow up may be necessary to fully
assess the rate of integration and the complications. The size of
the lesion may influence the outcome, which may require a further
radiological and histological assessment.
In conclusion, the surgical treatment of small and
medium sized lytic benign tumors has good results with both type of
grafts that have been studied. Using tricalcium phosphate mixed
with hydroxyapatite as bone substitute is a good and low cost
alternative, but it is a relatively fragile material with a slower
time to integrate compared to the allograft.
Acknowledgements
Professional editing, linguistic and technical
assistance was performed by Irina Radu, Individual Service
Provider, certified translator in Medicine and Pharmacy
(certificate credentials: Series E no. 0048).
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SD planned the clinical study, performed the
surgical procedures, contributed to the conception and design of
the study, and the acquisition, analysis and interpretation of the
data. CDMD contributed to the conception and design of the study,
analysis of data, the drafting of the manuscript and its critical
revision for important intellectual content. DCC contributed to the
analysis and interpretation of the data and the critical revision
for important intellectual content. ACA contributed to the
acquisition, analysis and interpretation of the data and the
critical revision for important intellectual content. CID and HTS
contributed to the conception and design of the study and the
critical revision of the manuscript for important intellectual
content. CIS contributed to the conception and design of the study,
the interpretation of the data and the critical revision of the
manuscript for important intellectual content. All authors read and
approved the final version of the manuscript and agreed to be
accountable for all aspects of the study.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
‘Foişor’ Orthopaedics-Traumatology and Osteoarticular TB Hospital
(Bucharest, Romania). All patients provided a signed informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jäger M, Herten M, Fochtmann U, Fischer J,
Hernigou P, Zilkens C, Hendrich C and Krauspe R: Bridging the gap:
Bone marrow aspiration concentrate reduces autologous bone grafting
in osseous defects. J Orthop Res. 29:173–180. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Damron TA: Use of 3D beta-tricalcium
phosphate (Vitoss) scaffolds in repairing bone defects.
Nanomedicine (Lond). 2:763–775. 2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Delloye C, Cnochaert N and Corbu O: Bone
substitutes in 2003: An overview. Acta Orthop Belg. 69:1–8.
2003.PubMed/NCBI
|
|
4
|
Larsson S: Calcium phosphates: What is the
evidence? J Orthop Trauma. 24 (Suppl 1):S41–S45. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hirn M, de Silva U, Sidharthan S, Grimer
RJ, Abudu A, Tillman RM and Carter SR: Bone defects following
curettage do not necessarily need augmentation: A retrospective
study of 146 patients. Acta Orthop. 80:4–8. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Banwart JC, Asher MA and Hassanein RS:
Iliac crest bone graft harvest donor site morbidity. A statistical
evaluation. Spine (Phila Pa 1976). 20:1055–1060. 1995.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fernyhough JC, Schimandle JJ, Weigel MC,
Edwards CC and Levine AM: Chronic donor-site pain complicating
graft harvesting from the posterior iliac crest for spinal fusion.
Spine (Phila Pa 1976). 17:1474–1480. 1992.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Daculsi G, LeGeros RZ, Nery E, Lynch K and
Kerebel B: Transformation of biphasic calcium phosphate ceramics in
vivo: Ultrastructural and physicochemical characterisation. J
Biomed Mater Res. 23:883–894. 1989.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Khan Y, Yaszemski MJ, Mikos AG and
Laurencin CT: Tissue engineering of bone: Material and matrix
considerations. J Bone Joint Surg Am. 90 (Suppl 1):S36–S42.
2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Daculsi G, Weiss P, Bouler JM, Gauthier O,
Millot F and Aguado E: Biphasic calcium phosphate/hydrosoluble
polymer composites: A new concept for bone and dental substitution
biomaterials. Bone. 25 (2 Suppl):59S–61S. 1999.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Van Blitterswijk CA, Grote JJ, Kuijpers W,
Daems WT and De Grout K: Macrospore tissue ingrowths: A
quantitative and qualitative study on hydroxyapatite ceramics.
Biomaterials. 7:137–143. 1986.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Daculsi G and Passuti N: Effect of the
macro porosity for osseous substitution of calcium phosphate
ceramics. Biomaterials. 11:86–87. 1990.PubMed/NCBI
|
|
13
|
Neer CS II, Francis KC, Marcove RC, Terz J
and Carbonare P: Treatment of unicameral bone cysts: A follow up
study of one hundred seventy-five cases. J Bone Joint Surg Am.
48:731–745. 1966.PubMed/NCBI
|
|
14
|
Chapman MW, Bucholz R and Cornell C:
Treatment of acute fractures with collagen-calcium-phosphate graft
material. A randomized clinical trial. J Bone Joint Surg Am.
79:495–502. 1997.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Stanovici J, Le Nail LR, Brennanetal MA,
Vidal L, Trichet V, Rosset P and Layrolle P: Bone regeneration
strategies with bone marrow stromal cells in orthopaedic surgery.
Curr Res Transl Med. 64:83–90. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Calori GM, Mazza E, Colombo M and
Ripamonti C: The use of bone-graft substitutes in large bone
defects: Any specific needs? Injury. 42 (Suppl 2):S56–S63.
2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dumic-Cule I, Pecina M, Jelic M, Jankolija
M, Popek I, Grgurevic L and Vukicevic S: Biological aspects of
segmental bone defects management. Int Orthop. 39:1005–1011.
2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bohner M: Physical and chemical aspects of
calcium phosphates used in spinal surgery. Eur Spine J. 10 (Suppl
2):S114–S121. 2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nilsson M, Zheng MH and Tagil M: The
composite of hydroxyapatite and calcium sulfate: A review of
preclinical evaluation and clinical applications. Expert Rev Med
Devices. 10:675–684. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hatten HP Jr and Voor MJ: Bone healing
using a bi-phasic ceramic bone substitute demonstrated in human
vertebroplasty and with histology in a rabbit cancellous bone
defect model. Interv Neuroradiol. 18:105–113. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Schindler OS, Cannon SR, Briggs TW and
Blunn GW: Composite ceramic bone graft substitute in the treatment
of locally aggressive benign bone tumours. J Orthop Surg (Hong
Kong). 16:66–74. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Petruskevicius J, Nielsen S, Kaalund S,
Knudsen PR and Overgaard S: No effect of Osteoset, a bone graft
substitute, on bone healing in humans: A prospective randomized
double-blind study. Acta Orthop Scand. 73:575–578. 2002.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Welkerling H, Raith J, Kastner N,
Marschall C and Windhager R: Painful soft-tissue reaction to
injectable Norian SRS calcium phosphate cement after curettage of
enchondromas. J Bone Joint Surg Br. 85:238–239. 2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Golberg VM and Stevenson S: Natural
history of autografts and allografts. Clin Orthop Relat Res. 7–16.
1987.PubMed/NCBI
|