Introduction
Abdominal aortic aneurysm (AAA) is a lethal, age-
and sex-related chronic inflammatory degenerative disease, with a
prevalence ranging from 5-10% amongst men >65 years old, and
increasing with age (1-3).
Recently, endovascular aneurysm repair (EVAR) is a new technology
that is used to treat patients with AAA when the anatomy is
suitable (4,5). Greenhalgh et al (4) reported that the 30-day mortality of
AAA in the EVAR group was 1.7 vs. 4.7% in the open repair group
within British hospitals. Although treatment methods for AAA have
improved significantly in the last few decades, the mortality rate
of patients with ruptured AAA remains very high worldwide (5,6). At
present, surgical repair is an effective treatment for advanced
AAAs (diameter >55 mm). Public screening programmes frequently
detect AAA at an early stage (7).
However, surgical repair does not provide a significant benefit for
AAA at an early stage (8), and the
current practice is to watch and wait until the diameter exceeds 55
mm, resulting in a risk of rupture or subsequent complications. AAA
has a natural process of progressive growth, which indicates that
early stage AAA can grow and subsequently require surgical
treatment (9). To date, no
pharmacological inhibition strategies have been effective in
suppressing AAA development or eventual rupture (7). Discovering novel drugs that could
inhibit the development of AAA is therefore crucial.
AAA is characterized by increased mural
inflammation, neoangiogenesis, degeneration of smooth muscle cells
(SMCs), degradation of extracellular matrix, activation of matrix
metalloproteinases (MMPs) and accumulation of intraluminal thrombi
(10). Therapeutic strategies for
stopping or decelerating AAA progression must target the underlying
events that promote its development (11). Mural inflammation has been
implicated in AAA formation, development and rupture. Lindberg
et al (12) reported that
numerous inflammatory biomarkers are significantly elevated and
positively correlated with aortic diameter in abdominal aortic
aneurysms. Furthermore, anti-inflammation-based therapy has been
demonstrated to attenuate aneurysmal development in AAA models
(11,13-15).
Xiong et al (13)
demonstrated that blocking TNF-α expression can attenuate aneurysm
formation in a murine model. Li et al (11) reported that cold-inducible
RNA-binding protein (CIRP) is a novel proinflammatory cytokine, and
that anti-CIRP-based therapy could attenuate aneurysm formation by
inhibiting mural inflammation in an experimental murine model.
Numerous studies have therefore focused on exploring
anti-inflammatory agents that could alleviate AAA development.
Previous studies have demonstrated that some natural products can
be used to treat coronary heart disease, atherosclerosis, diabetes
mellitus due to their powerful anti-inflammatory activities
(16-18).
Furthermore, previous studies reported that natural products have
antioxidant and anti-inflammatory effects. Fan et al
(19) found that curcuma, which is
the source of the spice turmeric widely used in Asian countries,
can attenuate rat thoracic aortic aneurysm formation. In addition,
Hao et al (16) reported
that curcumin attenuates AAA by inhibiting the inflammatory
response in a murine model. Importantly, Kaluza et al
(20) demonstrated that an
anti-inflammatory diet is associated with a reduced risk of AAA,
which is an association that was even more significant for AAA
rupture. Natural products are therefore considered as ideal sources
of potential agents capable of managing AAA because of their low
toxicity and the absence of clear side effects.
Daphnetin (7,8-dihydroxycoumarin; DAP) is a natural
anti-inflammatory, anti-oxidative and anti tumour product (21-25)
that is extracted from Daphne odora var. Liu et al
(26) reported that DAP has some
protective effects on severe acute pancreatitis in a rat model by
inhibiting the expression of inflammatory cytokines. Subsequently,
DAP was found to prevent and treat numerous diseases, including
cerebral ischaemia/reperfusion injury, rheumatoid arthritis,
colitis and endotoxin-induced lung injury, due to its powerful
anti-inflammatory activities (24,27-32).
At present, DAP is an anti-inflammatory agent used in some
inflammation-related diseases (33-35).
Li et al (33) reported that
DAP inhibited inflammation in the systemic lupus erythematosus
murine model via inhibition of NF-κB activity. Wang et al
(34) demonstrated that DAP
alleviated experimental autoimmune encephalomyelitis via regulating
dendritic cell activity. Chronic inflammation of the aortic mural
is a major characteristic of AAA and is related to AAA formation,
development and rupture (1).
However, the anti-inflammatory effect of DAP on AAA remains to be
determined.
The present study hypothesized that DAP could
prevent aneurysm development by inhibiting the inflammatory
response. To investigate this hypothesis, this study established an
AAA mice model induced by intra-aortic infusion of porcine
pancreatic elastase (PPE) and the effect of DAP on AAA mice were
evaluated.
Materials and methods
Experimental animals
The present study used 14 male C57BL/6 mice weighing
24-27 g (Beijing Vital River Laboratory Animal Technology Co.,
Ltd.) aged 8-10 weeks. All experimental protocols were approved by
the Medical Ethics Committee of Shandong Shanxian Central Hospital.
All animals were cared for in accordance with the recommendations
of the Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health (36).
Animal model establishment
The AAA model was induced by intra-aortic infusion
of type I porcine pancreatic elastase (PPE) as described previously
(3,37-39).
In the present study, mice were anesthetized using 4% isoflurane
prior to surgery followed by 2.5% isoflurane to maintain the
anaesthesia. Once mice were anesthetized, a vertical midline
abdominal incision was performed. After exposing the posterior
abdominal wall, the abdominal aorta from the left renal vein to the
bifurcation was isolated, and the lumbar arteries were ligated
under sterile conditions. The proximal and distal abdominal aortas
were temporarily blocked with 6-0 silk sutures. Subsequently, a
polyethylene catheter (PE-10, Instech Laboratories, Inc.) was
inserted into the controlled aorta above the bifurcation, and the
aorta was infused with type 1 PPE (1.5 U/ml in saline;
Sigma-Aldrich; Merck KGaA) for 5 min under constant pressure (100
mm Hg).
During the 14 days following the operation, mice
were intraperitoneally injected with DAP or an equal volume of
vehicle every day. On the 14th day after the operation, mice were
euthanized under CO2 exposure (flowrate of
CO2, 2 l/min; air displacement rate, 20%/min). Death was
confirmed by the absence of breathing, pulse, corneal reflex,
response to firm toe pinch, heart beat and respiratory sounds, the
graying of the mucous membranes and rigor mortis. Exception of
rigor mortis, none of these signs can independently confirm death.
Blood was collected after mice euthanasia cardiac puncture. Blood
was collected (0.6 ml) and stored in 1.5 ml tube for 2 h at 4˚C.
Then, 0.2 ml serum was collected after centrifugation at 3,000 x g
at 4˚C for 10 min. Aortic infused segments (about 10 mm long) were
also collected, embedded in optimal cutting compound media (cat.
no. 4583; Sakura Finetek USA, Inc.) and then stored in -80˚C.
Experimental groups and DAP
treatment
Mice were randomly assigned into two groups as
follows: The AAA+ vehicle group (n=7, surgery was not performed on
one mouse as a abdominal aorta aberrance which was identified via
ultrasound, which measured the maximum diameter of the abdominal
aorta before surgery) and the AAA+DAP group (n=7). Briefly, DAP
(purity >98%) was obtained from Sigma-Aldrich; Merck KGaA. DAP
was dissolved at the concentration of 1 mg/ml in a solvent composed
of 5% DMSO and 95% saline. Mice were intraperitoneally injected
with DAP (20 mg/kg/day) or an equal volume of vehicle immediately
after PPE infusion and lasted for 14 days. The dose of DAP was
determined according to a previous study demonstrating that
improved survival in a rodent cerebral ischaemia/reperfusion model
(24). Aortic segments were
subsequently collected on day 14 following surgery operation.
Aortic size measurements via
ultrasound
The maximum diameter of the abdominal aorta was
measured in anesthetized mice by using a 30 MHz ultrasound system
(VisualSonics, Inc.) before surgery and on days 3, 7 and 14
following surgery. Two experienced operators who were blinded to
study group assignments independently completed the full-scale
measurement and quantitative analysis of the ultrasonic data. AAA
was defined as a 50% or greater increase in infrarenal aortic
diameter compared with the baseline assessment (Vevo 770 ultrasound
system).
Elasticavan Gieson staining
Aortas were collected on the 14th day after the
operation and embedded in a straight strip shape in optimal cutting
compound media (cat. no. 4583; Sakura Finetek USA Inc.). To observe
changes in the aortic tissues at the morphological level, 8-µm
sections of aortic tissue were sectioned and fixed with cold
acetone for 8 min at 4˚C. To evaluate the integrity of elastin,
aortic tissue sections were stained with Elasticavan Gieson (EVG;
cat. no. G1042, Wuhan Servicebio Technology Co. Ltd.) according to
the manufacturers' protocol. As previously described, the analysis
of medial elastin destruction was graded from mild (I) to severe
(IV) (3).
Immunohistochemical staining
Immunohistochemical staining was performed as
described previously (3). Briefly,
8-µm sections of aortic tissue were prepared as afore mentioned for
EVG staining. The sections were incubated with antibodies against
α-smooth muscle actin (α-SMA; 1:400; cat. no. ab32575; Abcam), CD68
(1:400; cat. no. ab125212, Abcam), CD8 (1:100; cat. no. ab22378;
Abcam), B220 (1:100; cat. no. ab64110; Abcam) and CD31 (1:200; cat.
no. ab28364; Abcam) overnight at 4˚C. Then, sections were incubated
with a biotinylated secondary antibody kit (cat. nos. PV-9001 and
PV-9004; Beijing zhongshan Jinqiao Biotechnology Co., Ltd.), and
immune complexes were visualized using a 3-amino-9-ethylcarbazole
(AEC) peroxidase substrate kit (cat. no. A2010; Beijing Solarbio
Science & Technology Co., Ltd) according to the standard
procedure. Subsequently, the sections were counterstained with
haematoxylin. After dehydration overnight at 25˚C incubator,
clearing and mounting using aqueous non-fluorescing mounting media
(cat. no. HS-106; National Diagnostics), sections were visualized
under x10 magnification by microscopy (CX31; Olympus Corporation).
The degeneration of SMCs was graded from mild (I) to severe (IV) as
described previously (3). The
infiltration of macrophages was graded as follows: i) I,
infiltration limited to the adventitia or <1/4 of the aortic
circumference; ii) II, infiltration involving the medial layer and
≤1/2 of the aortic circumference; iii) III, infiltration involving
the medial layer and >1/2 and ≤3/4 of the aortic circumference;
and iv) IV, infiltration involving all aortic layers and expanded
>3/4 of the aortic circumference. Mural T cell infiltration, B
cell infiltration and angiogenesis were quantified as
CD8+, B220+ and CD31+ blood
vessels, respectively, per aortic cross section analysis.
Statistical analysis
Data are presented as the means ± standard
deviation. Statistical analysis was performed using GraphPad Prism
6.0 (GraphPad Software, Inc.). Student'σ t-test was used to
evaluate the significance of differences between two groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
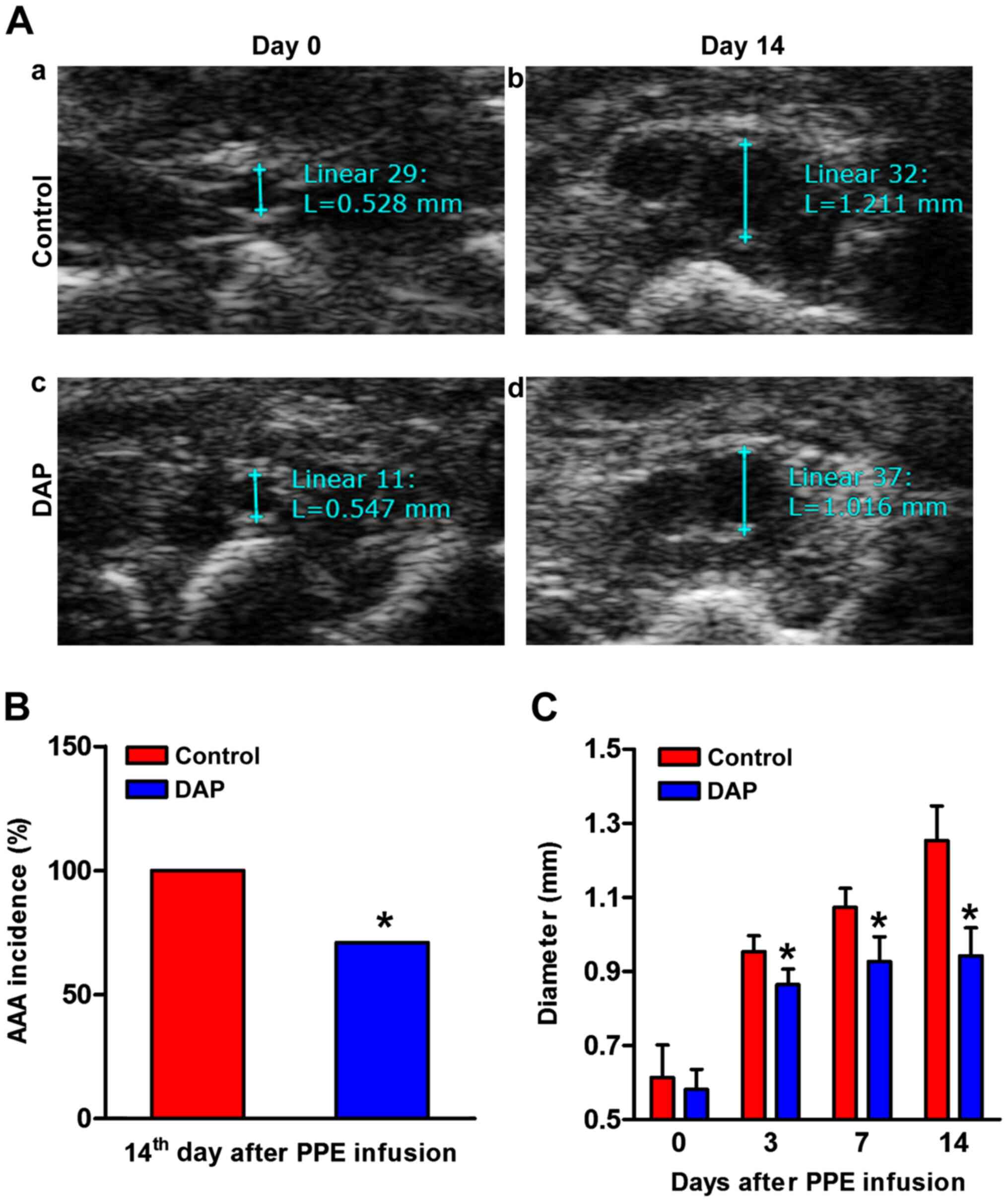
DAP treatment attenuates experimental
AAA formation and development
The maximum aortic diameter was measured with by
ultrasound before the operation and on days 3, 7 and 14 after
operation (Fig. 1A). In the control
group, the infused abdominal aorta sustained enlargement in a
time-dependent manner. In the DAP treatment group, aortic
enlargement was significantly decreased compared with the control
group. The maximum aortic diameter decreased significantly compared
with that of the control group at each checkpoint after the
operation (Fig. 1C). In the DAP
treatment group, aneurysm incidence (>50% baseline diameter
increase) was significantly decreased compared with the control on
the 14th day after operation (71 vs. 100%, DAP group vs. vehicle
group; P<0.05; Fig. 1B).
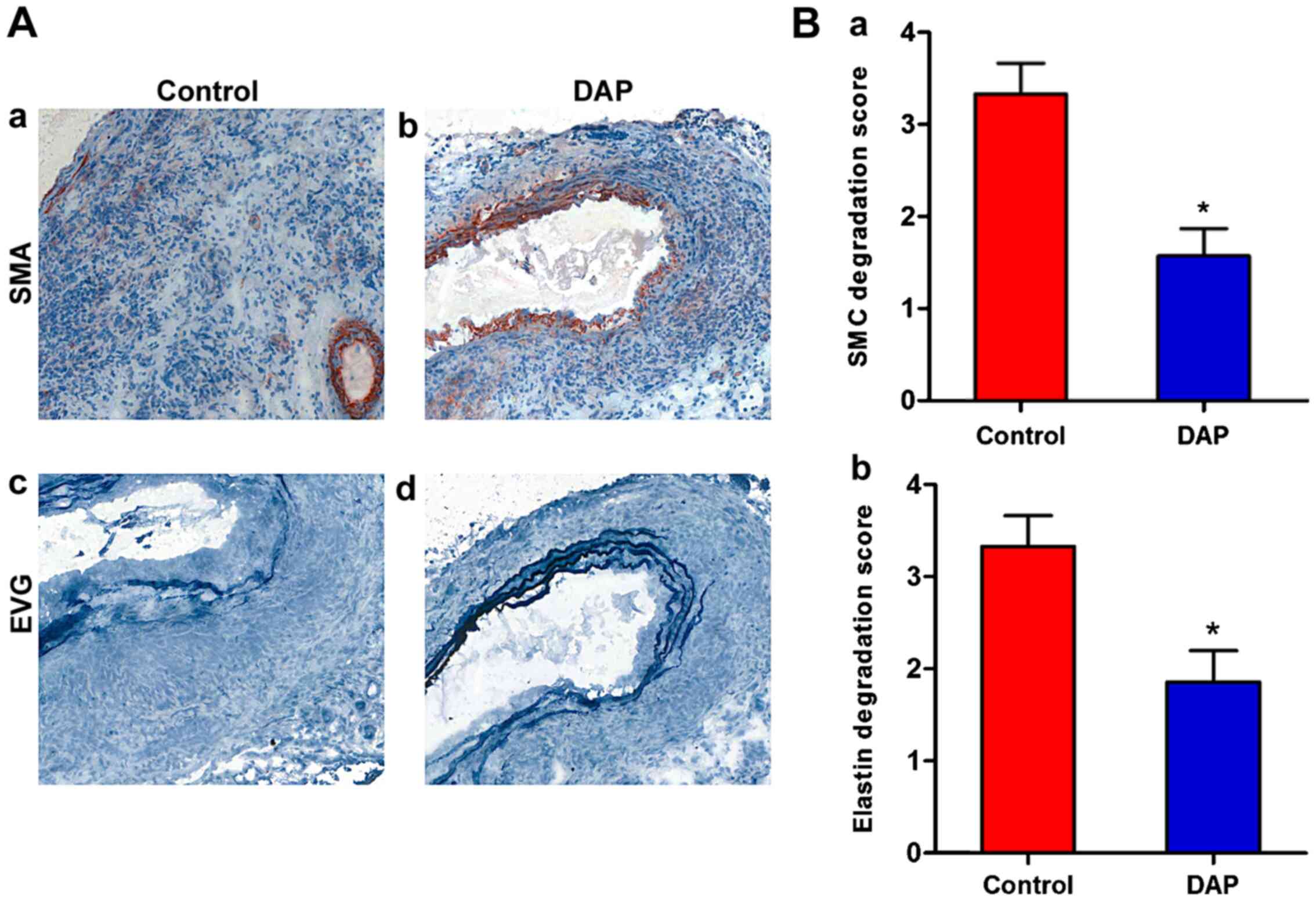
DAP treatment attenuates medial
elastin and SMC destruction
To further investigate DAP-mediated protection
against experimental AAA, changes in the aortic tissues at the
morphological level were observed. Medial elastin and SMC
destruction are the main histological characteristics of clinical
and experimental AAAs (10,40). In the DAP treatment group, SMCs
stained with α-SMA were degenerated and had almost fully
disappeared in the aortic mural (Fig.
2A). The degeneration of SMCs was significantly decreased in
the presence of DAP (20 mg/kg/day). Furthermore, in the control
group, elastin stained with EVG was degraded and had almost fully
disappeared in the aortic mural (Fig.
2B). However, in the DAP treatment group, the EVG-stained
elastic lamellae exhibited partial aortic elastin preservation,
which was determined by semi-quantitative analysis (P<0.05).
Therefore, the preservation of medial elastin and
SMC cellularity of aortic wall are the mural structural factors for
explaining reduced aortic diameter enlargement in DAP-treated mice.
These findings suggested that DAP may protect against AAA by
attenuating aortic elastin and SMC destruction.
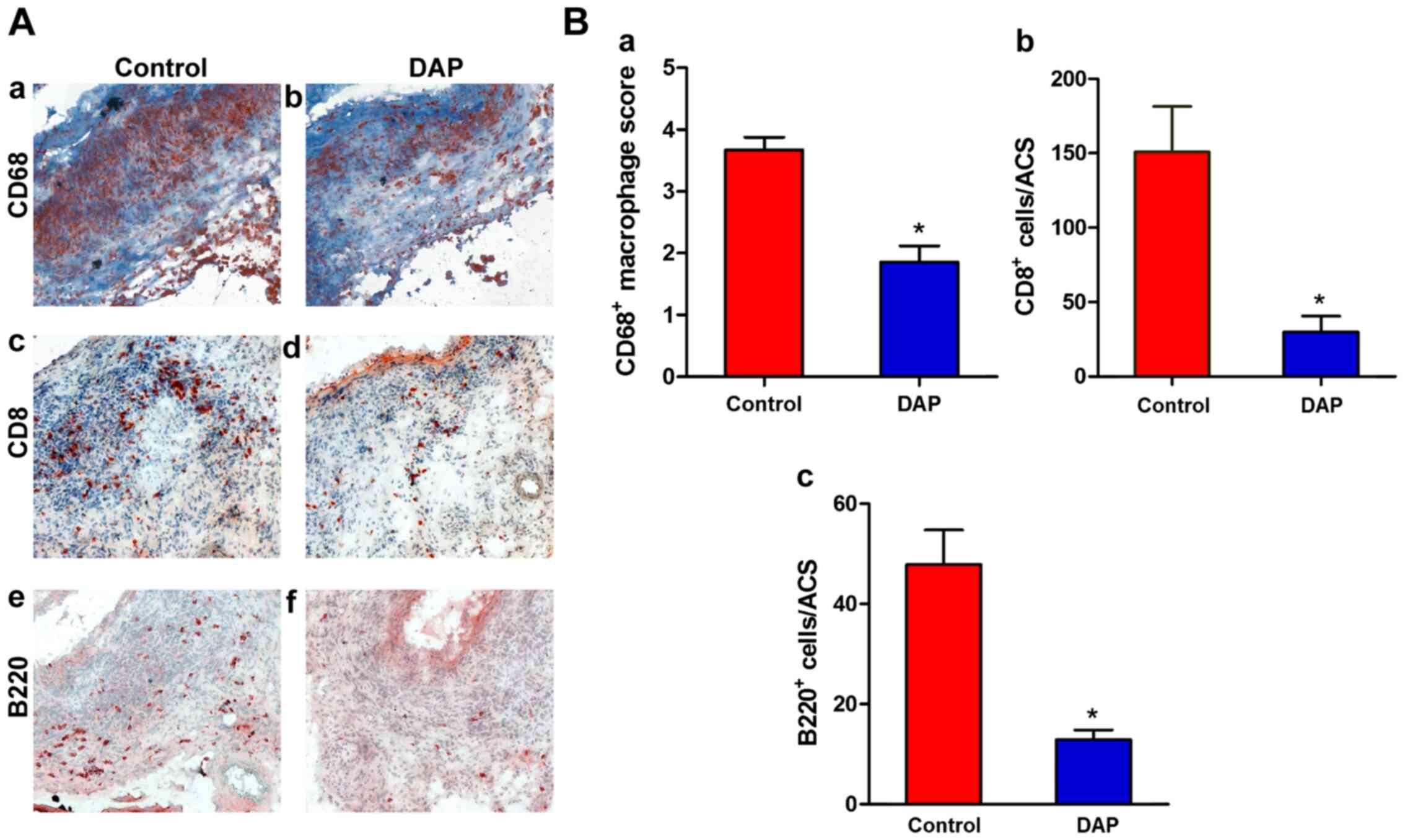
DAP treatment attenuates mural
leukocyte infiltration
Mural leukocyte accumulation is another pathologic
hallmark of AAA disease (10,40).
DAP is an anti-inflammatory agent used in inflammation-related
diseases. To evaluate the influence of DAP treatment on mural
inflammation, leukocyte infiltration was detected. CD68, CD8 and
B220 staining was performed on aortic samples. As presented in
Fig. 3, significant accumulation of
macrophages and T and B lymphocytes was present in the media and
adventitia in the control group. However, in the DAP treatment
group, the increased numbers of mural macrophages, T cells and B
cells were significantly decreased, which indicated that mural
inflammation was attenuated. These results indicated that DAP
treatment may limit experimental AAA progression in part by
diminishing aortic accumulation of proinflammatory leukocytes.
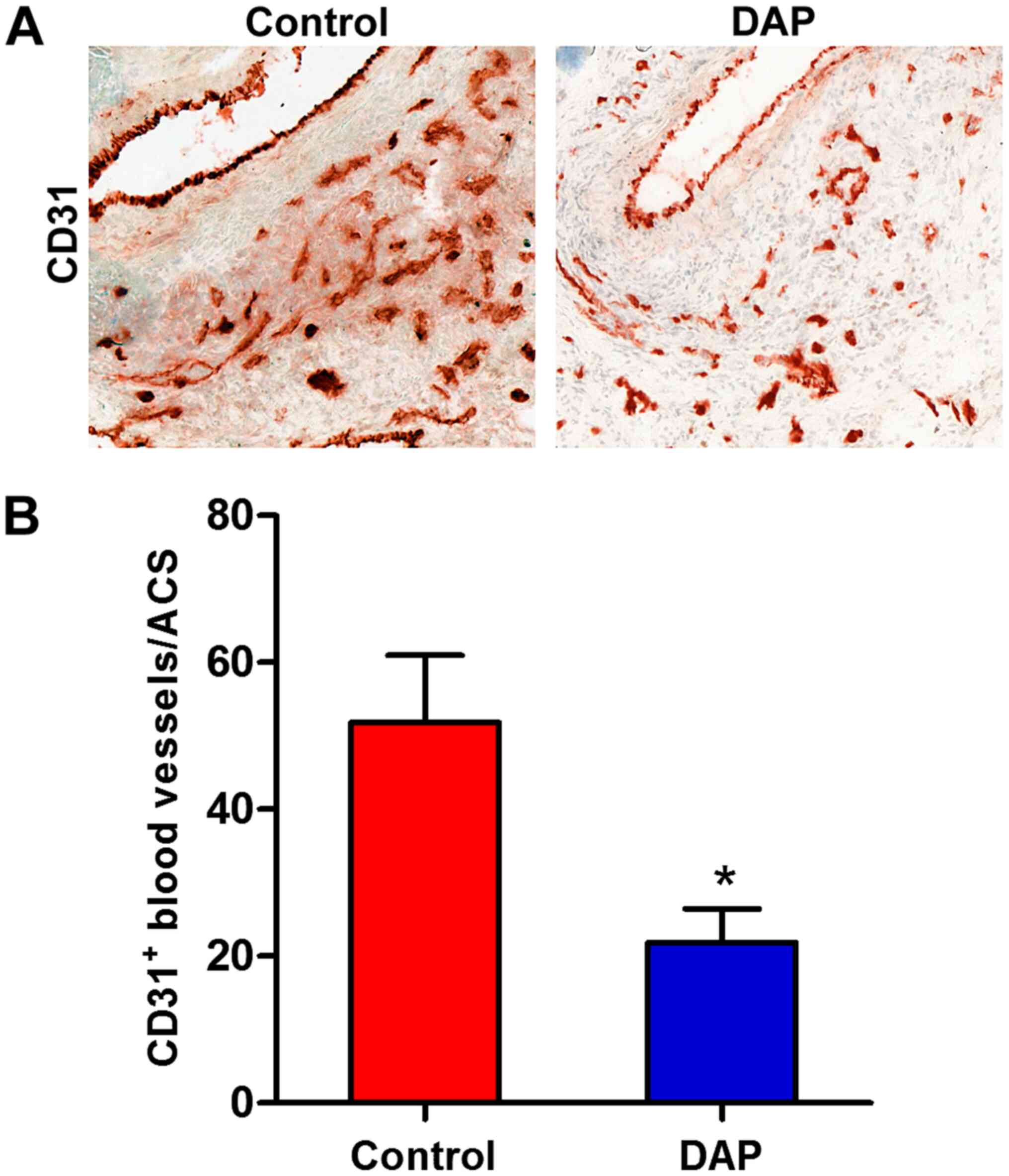
DAP treatment attenuates mural
angiogenesis
Neoangiogenesis is an important histopathological
feature of AAA (41-43).
The influence of DAP treatment on mural angiogenesis was therefore
evaluated, and CD31 staining was performed on aortic samples. As
presented in Fig. 4, the quantity
of mural CD31+neovessels decreased significantly in the
DAP treatment group compared with the control group. These results
suggested that DAP treatment may suppress AAA by impairing mural
angiogenesis during aneurysm formation and progression.
Discussion
DAP is a natural product extracted from Daphne
odora var with anti-inflammatory, antioxidant and antitumour
effects (21-25).
Liu et al (24) reported
that DAP protects against cerebral ischaemia/reperfusion injury in
mice via inhibition of inflammatory responses. Yao et al
(44) demonstrated that DAP has
therapeutic effects on an autoimmune arthritis model by modulating
inflammation. Yu et al (31)
found that DAP possesses some anti-inflammatory activities in
endotoxin-induced lung injury. Furthermore, Liu et al
(26) reported that DAP has
protective effects on severe acute pancreatitis in a rat model by
inhibiting the expression of inflammatory cytokines. Ji et
al (32) demonstrated that DAP
ameliorates experimental colitis by modulating microbiota
composition and the Treg/Th17 balance. Currently, an increasing
number of studies have demonstrated that DAP serves a crucial role
in the prevention and treatment of inflammation-related diseases
due to its powerful anti-inflammatory activities (33-35).
The pathogenesis of AAA is characterized by
inflammation with leukocyte infiltration, degradation of
extracellular matrix and depletion of vascular smooth muscle cells
(10,40). Local chronic inflammation of the
aortic wall has been implicated in the formation, development and
rupture of AAA (1). Leukocytes are
the principal effector cells of aneurysmal disease, contributing to
aortic degradation via the production of extracellular MMPs,
reactive oxygen species and proinflammatory cytokines. Leukocyte
accumulation in the aortic wall is an early feature of PPE-induced
AAAs and is present throughout the process of AAA. The present
study demonstrated that high numbers of macrophages and T and B
lymphocytes assembled in the media and adventitia in experimental
AAAs. Mural macrophages and lymphocytes secrete various types of
inflammatory cytokines and induce mural injury and subsequent
aneurysm formation (10,12,40).
Previous studies have demonstrated that increased levels of
inflammatory cytokines, including interleukin (IL)-1β, IL-6 and
tumour necrosis factor (TNF)-α, are positively correlated with AAA
formation and expansion (12,16,45,46). Lindberg et al
(12) investigated the relationship
between AAA and the inflammatory biomarkers IL-6, TNF-α,
endothelin-1 and soluble urokinase-type plasminogen activator
receptor and reported that inflammatory cytokines play important
roles in AAA progression. Xiong et al (13) reported that blocking TNF-α
attenuates aneurysm formation in a murine model. De et al
(14) found that systemic blockade
of monocyte chemoattractant protein 1 inhibits aortic aneurysm
formation in Ang II-induced AAA in apolipoprotein E-deficient mice.
Mural inflammation serves therefore a crucial role in AAA formation
and progression.
In the present study, following DAP treatment, the
increased numbers of mural macrophages, T and B cells were
significantly attenuated in the aortic wall. Previous studies also
reported that DAP inhibits the infiltration of inflammatory cells
to alleviate inflammatory injury in mice (25,31,44).
The results of DAP inhibiting the infiltration of inflammatory
cells from the current study were consistent with previous studies.
These results indicated that DAP may prevent the formation and
progression of AAA via anti-inflammatory effects. Furthermore,
treatment with DAP resulted in a significant reduction in mural
neovessels. Neoangiogenesis is an important histopathological
feature of AAA (41-43).
Previous studies demonstrated that aortic mural neovascularization
serves a key role in AAA progression and rupture (47-49).
Kaneko et al (50) found
that inhibition of vascular endothelial growth factor A(VEGF-A) by
soluble VEGF-A receptor-1 attenuates experimental AAA development.
Vijaynagar et al (48)
reviewed studies on the role of angiogenesis in AAA and
demonstrated that angiogenesis plays important roles in AAA
progression. Recently, anti-angiogenesis therapy has been used as a
potential intervention to treat AAA (48,51,52).
DAP treatment may therefore suppress AAA via impaired mural
leukocyte infiltration and angiogenesis during aneurysm formation
and progression.
The present study aimed to examine the influence of
DAP on the formation and progression of experimental AAAs; however,
this study had some limitations. A previous study reported that DAP
inhibits the TLR4/NF-κB signalling pathway (24,53).
Liu et al (24) found that
DAP protects against cerebral ischaemia/reperfusion injury in mice
via inhibition of the TLR4/NF-κB signalling pathway. Song et
al (53) demonstrated that DAP
downregulates the activation of concanavalin A-induced NF-κB signal
transduction pathways in mouse T lymphocytes. A previous study has
also found that TLR4/NF-κB signalling pathway is activated in AAA
and mediates AAA formation and progression (54). DAP may therefore suppress
experimental AAA by inhibiting the TLR4/NF-κB-mediated inflammatory
signalling pathway, and further investigation is thus required. In
addition, the dose-range experiments and the clinical dose for
humans was not determined in the current study. Therefore,
substantial work will be required before clinical trials.
In conclusion, the findings from the present study
suggested that DAP attenuated AAA formation and progression. This
inhibitory effect may be mediated by inhibition of mural leukocyte
infiltration and angiogenesis. These results suggested that DAP may
be considered as a clinical candidate in early AAA disease
suppression.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CH and LW conceived and designed the experiments.
SX, LM, HG, SG and LW performed the experiments. SX and CH analysed
the data. CH wrote the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Medical Ethics Committee of Shandong Shanxian Central Hospital
(Shanxian, China; approval no. IACUC A1027). All animals were cared
for in accordance with the recommendations of the Guide for the
Care and Use of Laboratory Animals of the National Institutes of
Health (28).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Golledge J, Muller J, Daugherty A and
Norman P: Abdominal aortic aneurysm: Pathogenesis and implications
for management. Arterioscler Thromb Vasc Biol. 26:2605–2613.
2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Miyake T and Morishita R: Pharmacological
treatment of abdominal aortic aneurysm. Cardiovasc Res. 83:436–443.
2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rouer M, Xu BH, Xuan HJ, Tanaka H,
Fujimura N, Glover KJ, Furusho Y, Gerritsen M and Dalman RL:
Rapamycin limits the growth of established experimental abdominal
aortic aneurysms. Eur J Vasc Endovasc Surg. 47:493–500.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Greenhalgh RM, Brown LC, Kwong GP, Powell
JT and Thompson SG: EVAR trial participants: Comparison of
endovascular aneurysm repair with open repair in patients with
abdominal aortic aneurysm (EVAR trial 1), 30-day operative
mortality results: Randomised controlled trial. Lancet.
364:843–848. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Badger S, Forster R, Blair PH, Ellis P,
Kee F and Harkin DW: Endovascular treatment for ruptured abdominal
aortic aneurysm. Cochrane Database Syst Rev.
5(CD005261)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Anderson RN: Deaths: Leading causes for
2000. Natl Vital Stat Rep. 50:1–85. 2002.PubMed/NCBI
|
|
7
|
Xuan H, Xu B, Wang W, Tanaka H, Fujimura
N, Miyata M, Michie SA and Dalman RL: Inhibition or deletion of
angiotensin II type 1 receptor suppresses elastase-induced
experimental abdominal aortic aneurysms. J Vasc Surg.
67:573–584.e2. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lederle FA, Wilson SE, Johnson GR, Reinke
DB, Littooy FN, Acher CW, Ballard DJ, Messina LM, Gordon IL, Chute
EP, et al: Immediate repair compared with surveillance of small
abdominal aortic aneurysms. N Engl J Med. 346:1437–1444.
2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rehm JP, Grange JJ and Baxter BT: The
formation of aneurysms. Semin Vasc Surg. 11:193–202.
1998.PubMed/NCBI
|
|
10
|
Michel JB, Martin-Ventura JL, Egido J,
Sakalihasan N, Treska V, Lindholt J, Allaire E, Thorsteinsdottir U,
Cockerill G and Swedenborg J: FAD EU consortium: Novel aspects of
the pathogenesis of aneurysms of the abdominal aorta in humans.
Cardiovasc Res. 90:18–27. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li G, Yang L, Yuan H, Liu Y, He Y, Wu X
and Jin X: Cold-inducible RNA-binding protein plays a central role
in the pathogenesis of abdominal aortic aneurysm in a murine
experimental model. Surgery. 159:1654–1667. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lindberg S, Zarrouk M, Holst J and
Gottsater A: Inflammatory markers associated with abdominal aortic
aneurysm. Eur Cytokine Netw. 27:75–80. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xiong W, MacTaggart J, Knispel R, Worth J,
Persidsky Y and Baxter BT: Blocking TNF-alpha attenuates aneurysm
formation in a murine model. J Immunol. 183:2741–2746.
2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
de Waard V, Bot I, de Jager SC, Talib S,
Egashira K, de Vries MR, Quax PH, Biessen EA and van Berkel TJ:
Systemic MCP1/CCR2 blockade and leukocyte specific MCP1/CCR2
inhibition affect aortic aneurysm formation differently.
Atherosclerosis. 211:84–89. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhu H, Qu X, Zhang C and Yu Y:
Interleukin-10 promotes proliferation of vascular smooth muscle
cells by inhibiting inflammation in rabbit abdominal aortic
aneurysm. Int J Clin Exp Pathol. 12:1260–1271. 2019.PubMed/NCBI
|
|
16
|
Hao Q, Chen X, Wang X, Dong B and Yang C:
Curcumin attenuates angiotensin ii-induced abdominal aortic
aneurysm by inhibition of inflammatory response and ERK signaling
pathways. Evid Based Complement Alternat Med.
2014(270930)2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sikora E, Scapagnini G and Barbagallo M:
Curcumin, inflammation, ageing and age-related diseases. Immun
Ageing. 7(1)2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sunagawa Y, Wada H, Suzuki H, Sasaki H,
Imaizumi A, Fukuda H, Hashimoto T, Katanasaka Y, Shimatsu A, Kimura
T, et al: A novel drug delivery system of oral curcumin markedly
improves efficacy of treatment for heart failure after myocardial
infarction in rats. Biol Pharm Bull. 35:139–144. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fan J, Li X, Yan YW, Tian XH, Hou WJ, Tong
H and Bai SL: Curcumin attenuates rat thoracic aortic aneurysm
formation by inhibition of the c-Jun N-terminal kinase pathway and
apoptosis. Nutrition. 28:1068–1074. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kaluza J, Stackelberg O, Harris HR, Bjorck
M and Wolk A: Anti-inflammatory diet and risk of abdominal aortic
aneurysm in two Swedish cohorts. Heart. 105:1876–1883.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Qi Z, Qi S, Gui L, Shen L and Feng Z:
Daphnetin protects oxidative stress-induced neuronal apoptosis via
regulation of MAPK signaling and HSP70 expression. Oncol Lett.
12:1959–1964. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Fylaktakidou KC, Hadjipavlou-Litina DJ,
Litinas KE and Nicolaides DN: Natural and synthetic coumarin
derivatives with anti-inflammatory/antioxidant activities. Curr
Pharm Des. 10:3813–3833. 2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Venugopala KN, Rashmi V and Odhav B:
Review on natural coumarin lead compounds for their pharmacological
activity. Biomed Res Int. 2013(963248)2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Liu J, Chen Q, Jian Z, Xiong X, Shao L,
Jin T, Zhu X and Wang L: Daphnetin protects against cerebral
ischemia/reperfusion injury in mice via inhibition of TLR4/NF-κB
signaling pathway. Biomed Res Int. 2016(2816056)2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tu L, Li S, Fu Y, Yao R, Zhang Z, Yang S,
Zeng X and Kuang N: The therapeutic effects of daphnetin in
collagen-induced arthritis involve its regulation of Th17 cells.
Int Immunopharmacol. 13:417–423. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liu ZY, Liu J, Zhao KL, Wang LK, Shi Q,
Zuo T, Liu TY, Zhao L and Wang WX: Protective effects of daphnetin
on sodium taurocholateinduced severe acute pancreatitis in rats.
Mol Med Rep. 9:1709–1714. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Du G, Tu H, Li X, Pei A, Chen J, Miao Z,
Li J, Wang C, Xie H, Xu X and Zhao H: Daphnetin, a natural coumarin
derivative, provides the neuroprotection against glutamate-induced
toxicity in HT22 cells and ischemic brain injury. Neurochem Res.
39:269–275. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhi J, Duan B, Pei J, Wu S and Wei J:
Daphnetin protects hippocampal neurons from oxygen-glucose
deprivation-induced injury. J Cell Biochem. 120:4132–4139.
2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chen X, Kuang N, Zeng X, Zhang Z, Li Y,
Liu W and Fu Y: Effects of daphnetin combined with Bcl2siRNA on
antiapoptotic genes in synovial fibroblasts of rats with
collageninduced arthritis. Mol Med Rep. 17:884–890. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Shu K, Kuang N, Zhang Z, Hu Z, Zhang Y, Fu
Y and Min W: Therapeutic effect of daphnetin on the autoimmune
arthritis through demethylation of proapoptotic genes in synovial
cells. J Transl Med. 12(287)2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yu WW, Lu Z, Zhang H, Kang YH, Mao Y, Wang
HH, Ge WH and Shi LY: Anti-inflammatory and protective properties
of daphnetin in endotoxin-induced lung injury. J Agric Food Chem.
62:12315–12325. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ji J, Ge X, Chen Y, Zhu B, Wu Q, Zhang J,
Shan J, Cheng H and Shi L: Daphnetin ameliorates experimental
colitis by modulating microbiota composition and
Treg/Th17 balance. FASEB J. 33:9308–9322.
2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li M, Shi X, Chen F and Hao F: Daphnetin
inhibits inflammation in the NZB/W F1 systemic lupus erythematosus
murine model via inhibition of NF-κB activity. Exp Ther Med.
13:455–460. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wang D, Lu Z, Zhang H, Jin SF, Yang H, Li
YM and Shi LY: Daphnetin alleviates experimental autoimmune
encephalomyelitis via regulating dendritic cell activity. CNS
Neurosci Ther. 22:558–567. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Shen L, Zhou T, Wang J, Sang X, Lan L, Luo
L and Yin Z: Daphnetin reduces endotoxin lethality in mice and
decreases LPS-induced inflammation in Raw264.7 cells via
suppressing JAK/STATs activation and ROS production. Inflamm Res.
66:579–589. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Guide for the Care and Use of Laboratory
Animals. In: National Research Council of the National Academes.
8th edition. Washington, DC, 2011.
|
|
37
|
Fujimura N, Xiong J, Kettler EB, Xuan H,
Glover KJ, Mell MW, Xu B and Dalman RL: Metformin treatment status
and abdominal aortic aneurysm disease progression. J Vasc Surg.
64:46–54.e8. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Iida Y, Xu B, Xuan H, Glover KJ, Tanaka H,
Hu X, Fujimura N, Wang W, Schultz JR, Turner CR and Dalman RL:
Peptide inhibitor of CXCL4-CCL5 heterodimer formation, MKEY,
inhibits experimental aortic aneurysm initiation and progression.
Arterioscler Thromb Vasc Biol. 33:718–726. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Iida Y, Xu B, Schultz GM, Chow V, White
JJ, Sulaimon S, Hezi-Yamit A, Peterson SR and Dalman RL: Efficacy
and mechanism of angiotensin II receptor blocker treatment in
experimental abdominal aortic aneurysms. PLoS One.
7(e49642)2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Halloran BG and Baxter BT: Pathogenesis of
aneurysms. Semin Vasc Surg. 8:85–92. 1995.PubMed/NCBI
|
|
41
|
Wolanska M, Bankowska-Guszczyn E,
Sobolewski K and Kowalewski R: Expression of VEGFs and its
receptors in abdominal aortic aneurysm. Int Angiol. 34:520–528.
2015.PubMed/NCBI
|
|
42
|
Trollope AF and Golledge J: Angiopoietins,
abdominal aortic aneurysm and atherosclerosis. Atherosclerosis.
214:237–243. 2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Li ZZ and Dai QY: Pathogenesis of
abdominal aortic aneurysms: Role of nicotine and nicotinic
acetylcholine receptors. Mediators Inflamm.
2012(103120)2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Yao R, Fu Y, Li S, Tu L, Zeng X and Kuang
N: Regulatory effect of daphnetin, a coumarin extracted from Daphne
odora, on the balance of Treg and Th17 in collagen-induced
arthritis. Eur J Pharmacol. 670:286–294. 2011.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Juvonen J, Surcel HM, Satta J, Teppo AM,
Bloigu A, Syrjälä H, Airaksinen J, Leinonen M, Saikku P and Juvonen
T: Elevated circulating levels of inflammatory cytokines in
patients with abdominal aortic aneurysm. Arterioscler Thromb Vasc
Biol. 17:2843–2847. 1997.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Flondell-Site D, Lindblad B and Gottsater
A: High levels of endothelin (ET)-1 and aneurysm diameter
independently predict growth of stable abdominal aortic aneurysms.
Angiology. 61:324–328. 2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Chapple KS, Parry DJ, McKenzie S,
MacLennan KA, Jones P and Scott DJ: Cyclooxygenase-2 expression and
its association with increased angiogenesis in human abdominal
aortic aneurysms. Ann Vasc Surg. 21:61–66. 2007.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Vijaynagar B, Bown MJ, Sayers RD and Choke
E: Potential role for anti-angiogenic therapy in abdominal aortic
aneurysms. Eur J Clin Invest. 43:758–765. 2013.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Choke E, Cockerill GW, Dawson J, Wilson
RW, Jones A, Loftus IM and Thompson MM: Increased angiogenesis at
the site of abdominal aortic aneurysm rupture. Ann NY Acad Sci.
1085:315–319. 2006.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kaneko H, Anzai T, Takahashi T, Kohno T,
Shimoda M, Sasaki A, Shimizu H, Nagai T, Maekawa Y, Yoshimura K, et
al: Role of vascular endothelial growth factor-A in development of
abdominal aortic aneurysm. Cardiovas Res. 91:358–367.
2011.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Xu B, Iida Y, Glover KJ, Ge Y, Wang Y,
Xuan H, Hu X, Tanaka H, Wang W, Fujimura N, et al: Inhibition of
VEGF (vascular endothelial growth factor)-a or its receptor
activity suppresses experimental aneurysm progression in the aortic
elastase infusion model. Arterioscler Thromb Vasc Biol.
39:1652–1666. 2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Ma X, Yao H, Yang Y, Jin L, Wang Y, Wu L,
Yang S and Cheng K: miR-195 suppresses abdominal aortic aneurysm
through the TNF-α/NF-κB and VEGF/PI3K/Akt pathway. Int J Mol Med.
41:2350–2358. 2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Song B, Wang Z, Liu Y, Xu S, Huang G,
Xiong Y, Zhang S, Xu L, Deng X and Guan S: Immunosuppressive
activity of daphnetin, one of coumarin derivatives, is mediated
through suppression of NF-κB and NFAT signaling pathways in mouse T
cells. PLoS One. 9(e96502)2014.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Lai CH, Wang KC, Lee FT, Tsai HW, Ma CY,
Cheng TL, Chang BI, Yang YJ, Shi GY and Wu HL: Toll-like receptor 4
is essential in the development of abdominal aortic aneurysm. PLoS
One. 11(e0146565)2016.PubMed/NCBI View Article : Google Scholar
|