Introduction
Hepatitis C virus (HCV) infection is highly
prevalent worldwide and has caused an extensive medical burden
(1). Hepatic fibrosis is a crucial
pathological process associated with chronic viral hepatitis, which
facilitates the progressions towards severe hepatic outcomes,
including liver cirrhosis, liver failure and hepatocellular
carcinoma. With the development of therapeutic interventions, most
patients are able to achieve sustained virological clearance and
improved fibrosis, while the above-mentioned progressive features
may not be completely reversed. Hence, it is critical to evaluate
and monitor the stage of liver fibrosis prior to and after
antiviral treatment (2).
Cells of the innate immune system regulate the
fibrotic process in chronic liver diseases (3). Macrophages and their progenitor cells,
monocytes, are key factors in the immune system. Liver fibrosis is
characterized by extracellular matrix accumulation and hepatic
stellate cell activation. Macrophages, which release
pro-inflammatory and pro-fibrogenic cytokines, including tumor
necrosis factor α (TNF-α) and transforming growth factor β1
(TGF-β1) may promote liver fibrosis by degrading matrix collagen
and regulating hepatic stellate cells (4-7).
Macrophages may differentiate into ‘classically activated’ M1 and
‘alternatively activated’ M2 macrophages (7). The function of M2 macrophages is to
inhibit the inflammatory reaction and participate in tissue repair,
anti-inflammatory cytokine production and extracellular matrix
synthesis and stabilization (8,9). CD163
is predominantly expressed on M2 macrophages, particularly on
Kupffer cells, the resident macrophages of the liver, which
represent the largest population of macrophages in the mammalian
body. Soluble CD163 (sCD163) is a scavenger receptor, which is
released from M2 macrophages upon activation. The activation of
macrophages, mainly Kupffer cells, may be reflected by the levels
of sCD163 in the blood circulation (10). Cytokines produced by
CD14+ cells, including interleukin-6 (IL-6), IL-8, TNF-α
and IL-10, contribute to the pathogenesis of HCV-induced liver
disease.
However, to date, the immunopathogenic role of
peripheral blood monocytes and intrahepatic macrophages in
HCV-associated fibrosis have remained to be fully elucidated. The
present study aimed to investigate the effect of
monocytes/macrophages and its associated cytokines in the fibrosis
of chronic hepatitis C (CHC) by detecting peripheral
CD14+ monocyte frequencies and intrahepatic
CD163+ macrophage levels in HCV-associated liver
fibrosis.
Various studies have demonstrated the important role
of liver macrophages (Kupffer cells) during liver fibrosis
(11,12), therefore, macrophage-specific
markers may be useful tools to monitor liver fibrotic processes.
Previous data have indicated that in patients with liver diseases,
sCD163 may be used to monitor Kupffer cell activation (13). Thus, in the present study, the
diagnostic relevance of sCD163 was assessed by comparing it to
other well-known biomarkers of liver fibrosis.
Materials and methods
Subjects
A total of 87 patients with CHC were recruited at
The Third Hospital of Hebei Medical University (Shijiazhuang,
China) between January 2013 and October 2013. HCV infection was
diagnosed based on positivity for IgG antibodies to HCV in the
serum, the presence of plasma HCV RNA and a liver biopsy with
histology consistent with chronic HCV. Participants with the
following conditions were excluded: i) Decompensated cirrhosis; ii)
co-infection with human immunodeficiency virus (HIV); iii)
co-infection with hepatitis A (HAV), B (HBV)or D virus; and iv)
other chronic liver diseases. Furthermore, 20 age- and sex-matched
healthy subjects with no presence of HAV, HBV, HCV, HIV or other
causes of chronic liver disease were used as controls.
Liver biopsies were performed on all 87 HCV
patients. In addition, 20 normal liver tissues as controls were
collected from donor livers for transplantation. H&E and Masson
trichrome staining were used for observation of hepatic
inflammation and fibrosis in the liver sections. The grade of
hepatic fibrosis was determined using the Metavir scoring system
(12). A Metavir stage of F2, F3 or
F4 was defined as indicating significant fibrosis. Patients were
classified into two groups according to the F-score (F≥2 and
F<2, respectively). The demographic and clinical data of the
cohort are provided in Table I.
 | Table IClinical characteristics of the
patients and controls enrolled in the present study. |
Table I
Clinical characteristics of the
patients and controls enrolled in the present study.
| Item | Healthy controls
(n=20) | Patients with CHC
(n=87) | P-value |
|---|
| Sex
(male/female) | 9/11 | 38/49 | 1.000 |
| Age (years) | 44.9±11.7 | 46.6±13.8 | 0.627 |
| Body mass index
(kg/m2) | 23.4±3.3 | 22.3±2.5 | 0.588 |
| ALT (0-40
IU/l) | 28.66±1.9 | 83.2±9.4 | 0.001 |
| AST (0-40
IU/l) | 23.1±6.3 | 67.1±7.9 | 0.006 |
| HCV RNA
(IU/ml) | | 9.1x105
(128-2.5x107) | |
| Possible route of
contamination | | | |
|
Transfusion | | 62 (71.3) | |
|
Previous
surgery | | 6 (6.9) | |
|
Stomatologic
treatments | | 3 (3.4) | |
|
Others or
unknown | | 16 (18.4) | |
|
HCV
genotype, 1b/2a | | 81/6 | |
| Fibrosis score | | | |
|
F<2 | | 43 | |
|
F≥2 | | 44 | |
In the present study, the values of three biomarkers
for liver fibrosis, namely the aspartate aminotransferase (AST) to
platelet ratio index (APRI), AST to alanine aminotransferase (ALT)
ratio (AAR) and fibrosis 4 score (FIB-4), were also calculated
based on the following formulas: APRI=100x[AST(U/l)/upper limit of
normal range]/platelets(109/l), FIB-4=age(years) x
AST(U/l)/[platelets(109/l) x ALT(U/l)1/2],
where the age of the patient is the age at the time of liver
biopsy, and AAR=AST/ALT (14-16).
Blood sample collection
Blood was obtained from each patient at the
time-point of enrollment in this study. Samples were aliquoted and
stored at -80˚C for further use.
Biochemical assays
The liver and kidney functions were analyzed by a
Mindray BS-800M automatic chemical analyzer at the Central
Laboratory of the 3rd Hospital of Hebei Medical University
(Shijiazhuang, China).
HCV antibody tests and quantitative
detection of HCV RNA
The serum antibodies to HCV were detected by ELISA
with a commercial detection kit (Livzon Diagnostics Inc.). The
plasma HCV RNA load was measured by using qualitative reverse
transcription PCR (RT-PCR) assay (Cobas Taqman HCV Test; Roche
Diagnostics) and the lower limit of quantification was 15
IU/ml.
Immunohistochemistry detection of
CD163 in liver tissues
Paraffin-embedded liver sections (5 µm) were
incubated with anti-CD163 (specific for M2 macrophages) (1:100
dilution; cat. no. MCA1853; AbD Serotec) and EnVision System
HRP-conjugated secondary antibody (cat. no. K4001; Dako; Agilent
Technologies, Inc.). Freshly prepared 3,3'-diaminobenzidine
solution was used as the substrate, followed by counterstaining
with hematoxylin according to previously described protocols
(17).
Measurement of the sCD163
concentration
Serum sCD163 levels were detected with an ELISA kit
(cat. no. DC1630; R&D Systems) according to the manufacturer's
protocol.
Flow cytometric analysis of
CD14+ monocytes and inflammatory cytokines expressing
CD14+ monocytes
All the antibodies were purchased from BD
Biosciences. For marker staining with FITC-conjugated anti-human
CD14 (cat. no. 347493) and phycoerythrin (PE)-conjugated anti-human
IL-2 (cat. no. 340450), interferon-gamma (IFN-γ; cat. no. 554701),
IL-6 (cat. no. 340527), TNF-α (cat. no. 340517), IL-8 (cat. no.
554720), IL-4 (cat. no. 559333) and IL-10 (cat. no. 559330; all
1:100 dilution), the methods were according to previously described
protocols (18,19).
Statistics analysis
Values are expressed as the mean ± standard
deviation. One-way analysis of variance (ANOVA) was used for
multiple comparisons and Student's t-test to study differences of
normally distributed variables between the groups. Following ANOVA,
the Student-Newman-Keuls post-hoc test was applied. The association
between sCD163 and CD163 in liver tissues was analyzed by simple
linear regression. Spearman's rank correlation test was used to
study associations between sCD163, CD163, CD14 and histological
scores. P<0.05 was considered to indicate statistical
significance.
The diagnostic values of four markers (sCD163, APRI,
FIB-4 and AAR) were assessed by calculating the area under the
receiver operating characteristic (ROC) curves (AUROC) as the best
cut-off values. The diagnostic performance was evaluated by
determining the sensitivity, specificity, positive predictive value
(PPV), and negative predictive value (NPV). All data were analyzed
using SPSS version 17.0 for Windows software (SPSS, Inc.).
Results
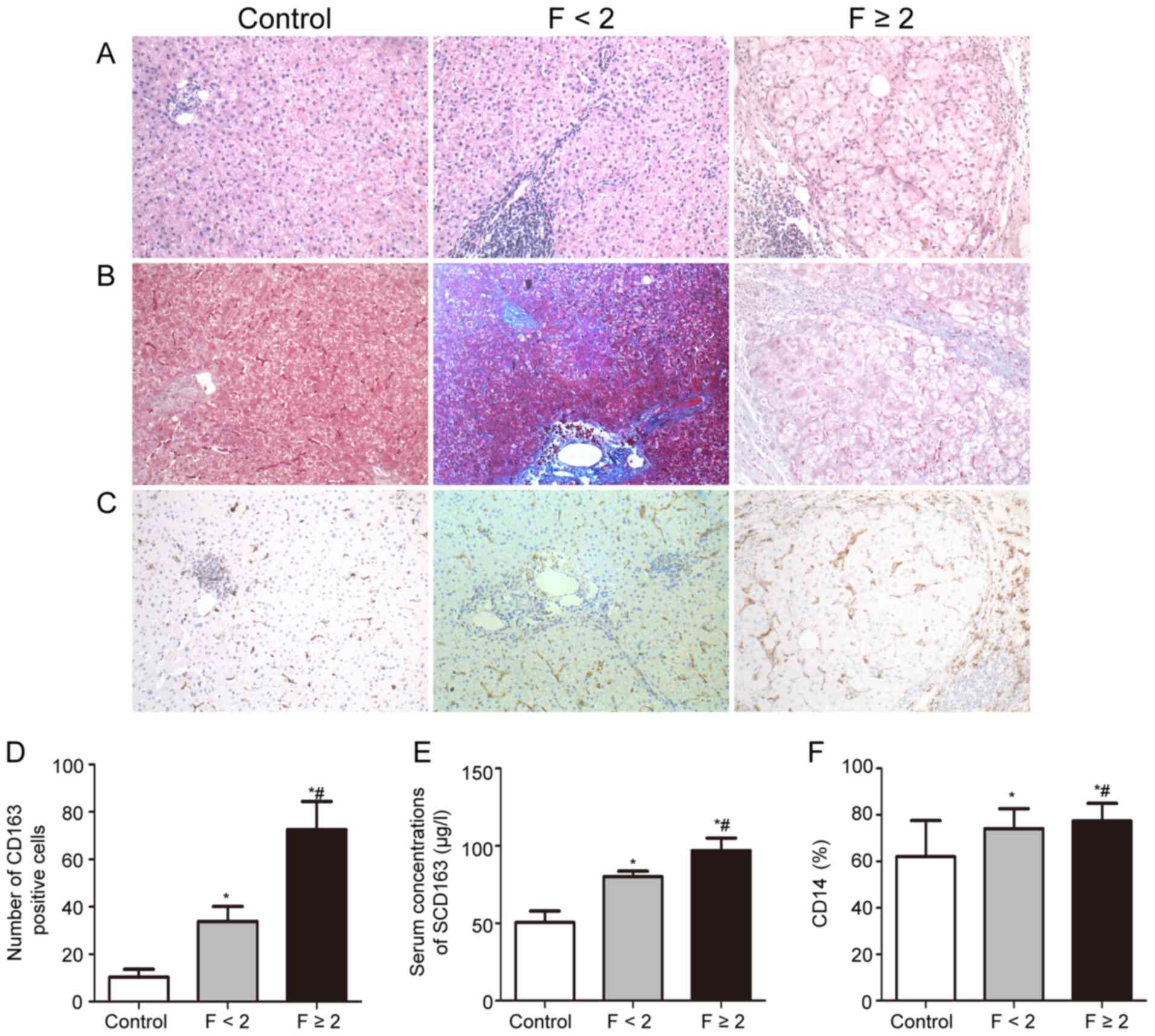
Hepatic macrophages are markedly
increased in HCV infection patients with fibrosis
The hepatic distribution of CD163+ cells
of patients with CHC and healthy controls was examined. As
presented in Fig. 1, patients with
F≥2 had a higher CD163+ cell density in the liver than
patients with F<2. As CD163 was widely expressed on Kupffer
cells in the lobular area, CD163+ cells in the portal
area were taken as the macrophages for quantitative analysis. It
was revealed that the number of CD163+ cells in the
portal area was markedly higher in CHC patients with high fibrosis
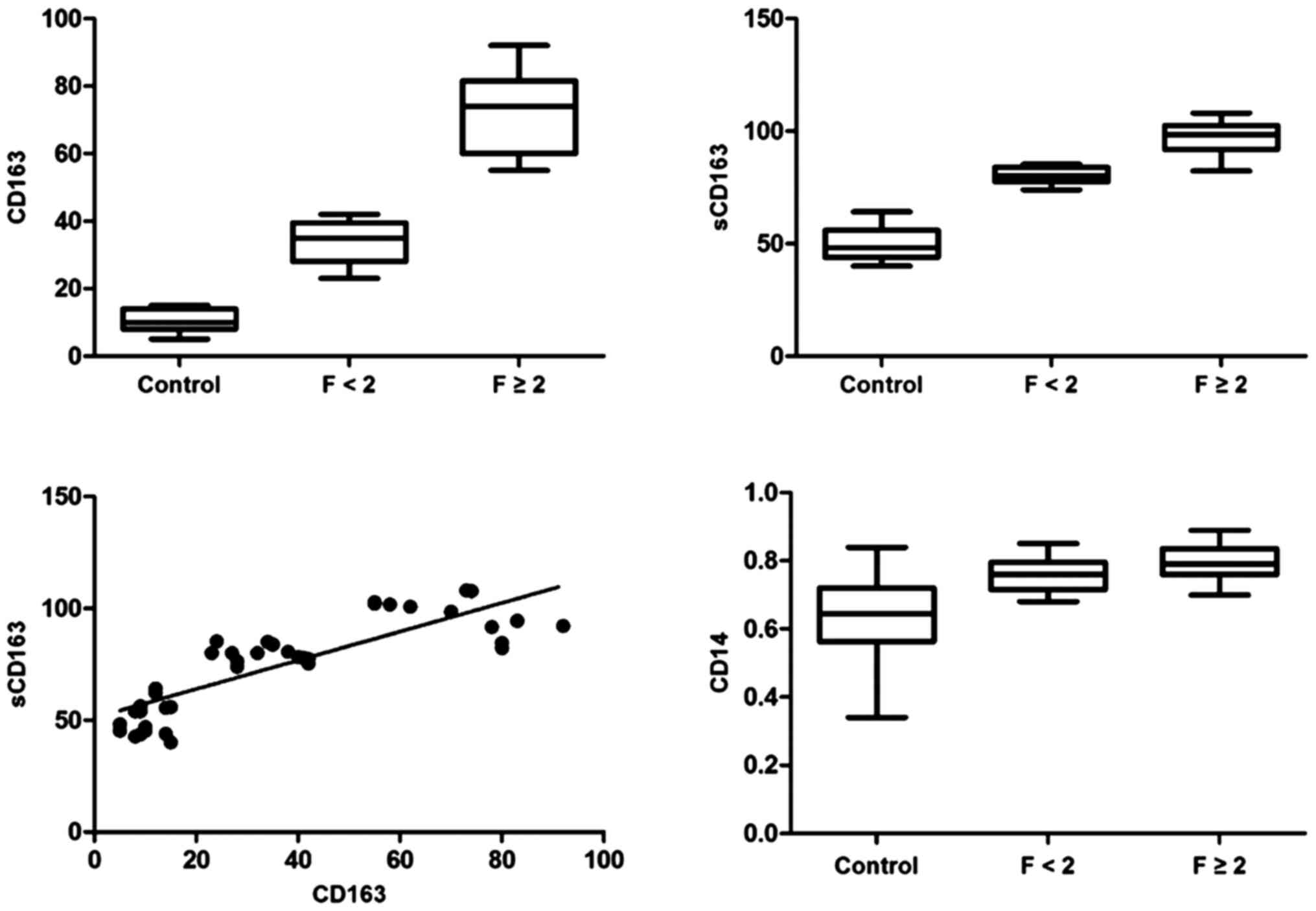
scores (Fig. 1). Box plots of the
CD163 count in relation to the fibrosis stage are presented in
Fig. 2 (r2=0.942,
P<0.001). There were no differences among the groups regarding
the ratios of CD163 to CD68 (CD163/CD68 data not shown).
Serum sCD163 levels are significantly
higher and gradually increased with the progression of hepatic
fibrosis in CHC patients
The serum sCD163 levels of CHC patients and in
healthy subjects are presented in Fig.
1D. The mean serum sCD163 levels in patients with CHC were
markedly higher than those in the control subjects (88.3±11.2 µg/l
vs. 49.5±7.6 µg/l, P<0.001). Serum sCD163 levels were markedly
elevated in patients with F≥2 as compared with those in patients
with F<2 (102.3±9.98vs. 76.0±12.2 µg/l, P<0.001). There was a
positive correlation between sCD163 and fibrosis
(r2=0.899, P<0.001; Fig.
2). Furthermore, serum sCD163 and the number of
CD163+ cells in the portal area increased in parallel in
association with the histological fibrosis stage in HCV patients.
There was a correlation between sCD163 and hepatic
CD163+ cells in CHC patients (r2=0.701,
P<0.001; Fig. 2).
Frequencies of CD14+
monocytes andCD14+ monocytes expressing IL-2, IFN-γ,
IL-6, TNF-α, IL-8, IL-4 and IL-10
CD14+ monocyte frequencies were higher in
CHC patients than those in healthy controls. Of note,
CD14+ monocyte frequencies were increased in patients
with F≥2 as compared with those in patients with F<2 (72±7% vs.
78±5%, P=0.04; Fig. 1). The
correlation between the frequencies of CD14+ monocytes
and fibrosis was then analyzed in these patients (Fig. 2). There was a significant positive
correlation between CD14+ monocyte frequencies and the
fibrosis stage (r2=0.604, P<0.001), but no
correlation was observed with serum HCV RNA (data not shown).
Although CD14 is a marker of anti-inflammatory
monocytes, it also transduces signals upon binding its ligands that
leads to the release of anti-inflammatory mediator IL-10 and
pro-inflammatory cytokines, including IL-6, TNF-α and IL-8. To
further investigate the changes of CD14+ monocytes
expressing associated cytokines during the progression of
HCV-associated liver fibrosis, the levels of IL-2-, IFN-γ-, IL-6-,
TNF-α-, IL-8-, IL-4- and IL-10-expressing CD14+
monocytes were determined. Compared with the controls, the IL-2-,
IL-4-, IFN-γ-, IL-6-, IL-8- and TNF-α-expressing CD14+
monocytes were increased in patients with CHC, but IL-10-expressing
CD14+ monocytes were decreased in the F<2 group
(P<0.05; Fig. 3). With the
progression of fibrosis, IL-8-expressing CD14+ monocytes
were significantly upregulated as compared with those in the F<2
group (IL-8, P<0.05; TNF-α, P>0.05; Fig. 3), while the IL-2 and
IFN-γ-expressing CD14+ monocytes were significantly
downregulated (IL-2 and IFN-γ, P<0.05; IL-4, IL-6 and IL-10;
P>0.05; Fig. 3).
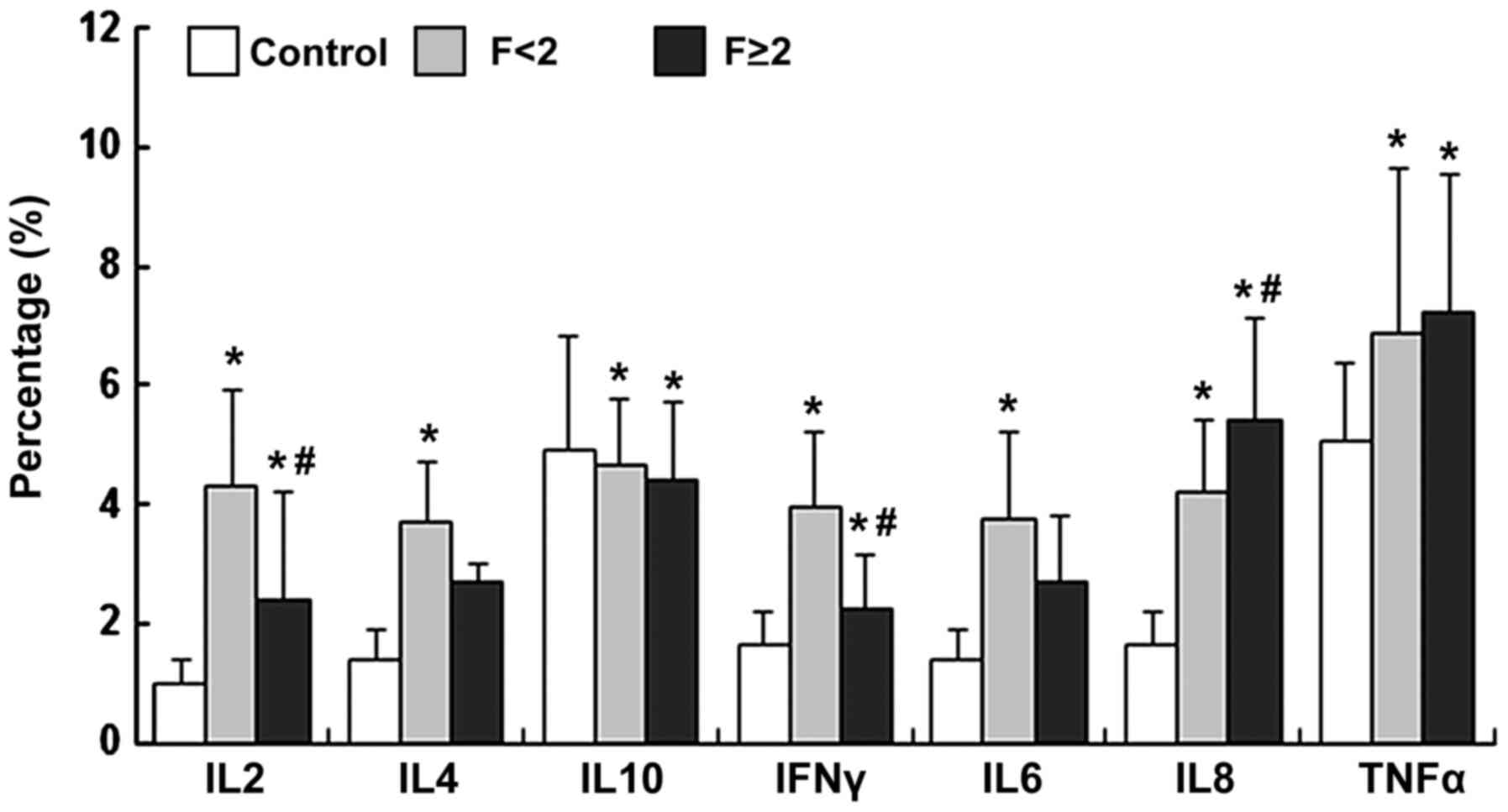 | Figure 3Proportions of IL-2-, IL-4-, IL-10-,
IFN-γ-, IL-6-, IL-8- and TNF-α-expressing CD14+
monocytes were measured by flow cytometry and data were presented
as the mean ± standard deviation. *P<0.05, compared
with control; #P<0.05, compared with F<2. IL,
interleukin; IFN, interferon; TNF, tumor necrosis factor. |
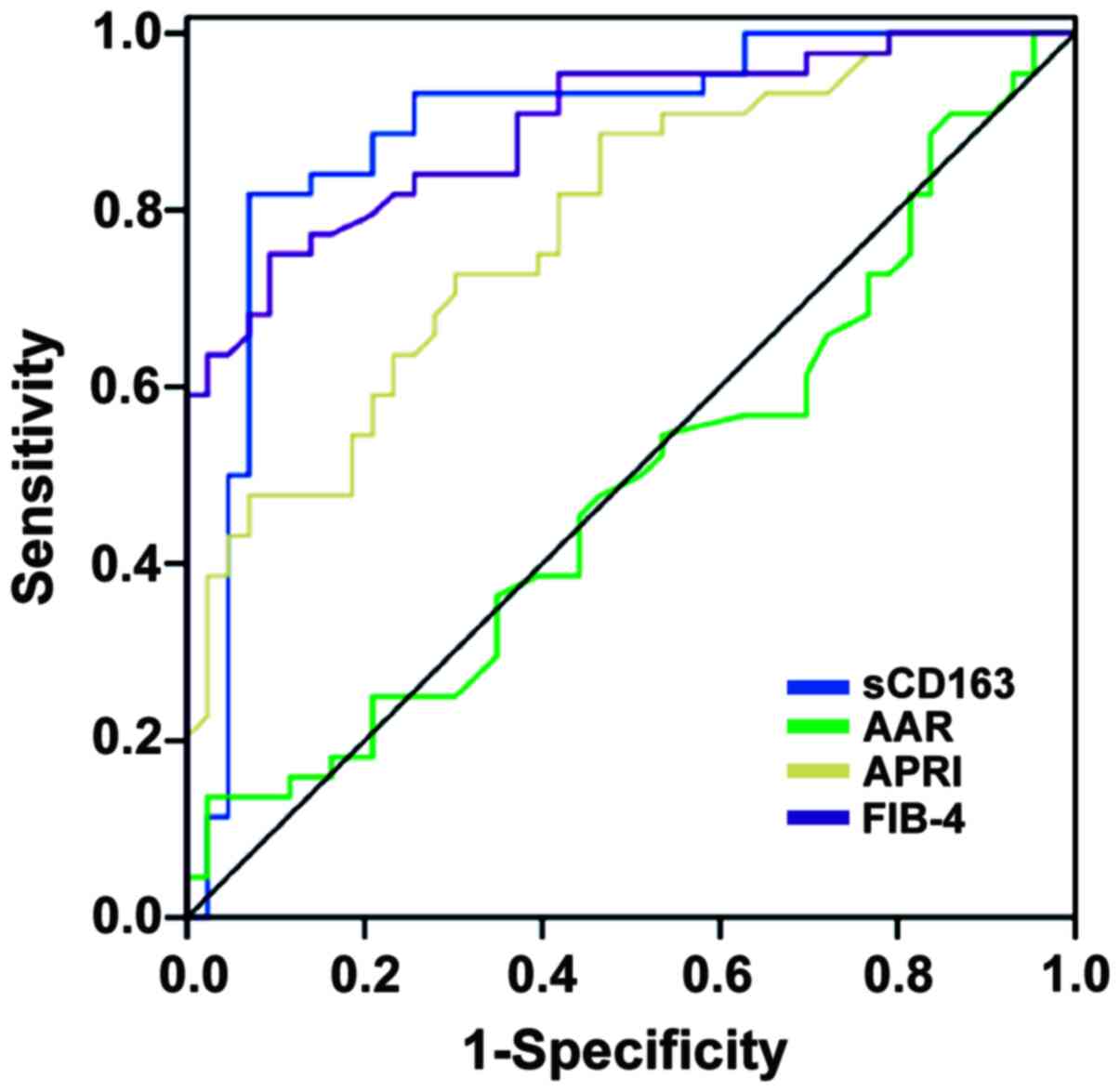
Predictive value of sCD163 as a
non-invasive biomarker of fibrosis in patients with CHC
As sCD163 was higher in patients with considerable
hepatic fibrosis, a ROC curve analysis for sCD163 with the cut-off
Metavir score F≥2 was performed to distinguish patients with F≥2
from those with F<2 (Fig. 4).
The AUROC for sCD163 to differentiate patients with F≥2 from those
with F<2 was 0.876 (95% confidence interval: 0.795-0.958,
P<0.001) with an optimal cut-off value of 73.985 µg/l. Serum
sCD163 levels of ≥116.54 µg/l had a >90% specificity to identify
subjects with F≥2, with an optimal cut-off value of 73.985 µg/l.
Regarding the discrimination of subjects with significant fibrosis,
the AUROCs for APRI, FIB-4 and AAR were 0.785, 0.825 and 0.488,
respectively (Fig. 4). The optimal
cut-off values of APRI, FIB-4 and AAR were 1.549, 0.74 and 0.583,
respectively. In the comparison of the AUROCs, sCD163 exhibited a
significantly higher AUROC as compared with APRI and AAR (P=0.028,
P<0.001, respectively), while no differences were observed for
sCD163 vs. FIB-4 (P=0.48). When compared to liver biopsy, AAR
values >1.2 had a PPV of 77% for the diagnosis of significant
fibrosis, while AAR<0.5 was able to exclude significant fibrosis
with an NPV of 77%. Similar results were obtained by applying the
APRI, FIB-4 and sCD163 original cutoffs. FIB-4>3.25 had a PPV of
92%, while FIB-4<1.45 was able to exclude significant fibrosis
with an NPV of 81%.
Discussion
Kupffer cells are involved in liver cirrhosis
development. Studies have indicated that macrophage subsets have
bidirectional roles in the progression and reversal of liver
fibrosis (8,20). Macrophages not only initiate and
accentuate inflammatory responses after tissue injury, but also
participate in the resolution of inflammation and injury. In
certain relevant studies, liver tissues from only a small number of
cases or no liver tissues were included, or studies were limited to
females only (21-23).
The exact role of hepatic macrophages in CHC remains elusive.
CD163, a member of the scavenger receptor cysteine-rich family, is
involved in anti-inflammatory functions and is predominantly
expressed on M2 macrophages (24,25).
The present results suggested that CD163 expression was
significantly increased in liver tissues of CHC patients, which
correlated with the degree of hepatic fibrosis. Therefore, M2
macrophages are considered pro-fibrotic under certain
conditions.
Activation of Kupffer cells, the resident
macrophages in the liver, is an important component of
inflammation, cell death and fibrosis development (20). sCD163 is a surrogate parameter for
macrophage activation, which may be a useful tool to assess the
prognosis and complications of liver cirrhosis. In the present
cohort of CHC patients, the serum sCD163 levels were higher in
patients with significant fibrosis as compared to subjects with no
or mild fibrosis. Furthermore, a strong correlation between sCD163
levels and the severity of liver fibrosis has been observed in the
present study, which is in line with a recent publication
confirming sCD163 as a fibrosis predictor (26). In addition, the present study
indicated a positive correlation between the serum levels of sCD163
and hepatic CD163 expression. Therefore, to a certain extent,
sCD163 levels reflect the changes of CD163 in liver tissue. These
results all support the notion that hepatic macrophage activation
is linked to fibrosis in CHC patients. Hiraoka et al
(27) reported elevated levels of
plasma sCD163 in patients with acute and chronic viral hepatitis.
They also demonstrated that the cells expressing CD163 in the liver
were Kupffer cells. Another study indicated increased hepatic
expression of CD163 mRNA in patients with CHC (25). In patients with cirrhosis, sCD163
levels are associated with portal hypertension (12,28)
and a recent study, sCD163 was demonstrated to be an independent
predictor of variceal bleed and mortality in cirrhotic patients
(10). These studies support the
present results of elevated CD163 and sCD163 in patients with CHC.
The present study suggested that the elevation of sCD163 and CD163
are associated with the progression of liver fibrosis, the most
severe outcome of chronic viral hepatitis. Thus, the present study
provided strong evidence for macrophage activation in patients with
CHC.
Macrophages have a critical role in innate and
adaptive immune responses (22).
Monocytes are precursors of tissue macrophages and may exhibit
special functions in the progression of HCV infection. The presence
of elevated levels of CD14+ monocytes has been
demonstrated in various pathological conditions, including
infection, inflammatory syndrome, sepsis and cancer. Most monocytes
express cell surface CD14. In the present study, the levels of
CD14+monocytes increased in CHC patients, which was
associated with the severity of fibrosis. There were significant
positive correlations between CD163, CD14 and fibrosis, which
suggested the involvement of CD14+ monocytes and
CD163+ macrophages in CHC-associated liver fibrosis.
CD14+ monocytes represent 90% of
circulating monocytes, which produce cytokines including IL-6,
IL-8, TNF-α and IFN-γ. In the present study, changes in the levels
of CD14+ monocytes expressing IL-6, IL-8 and TNF-α
obtained from CHC patients were observed. The results confirmed
that the frequencies of IL-6- and TNF-α-expressing CD14+
monocytes were significantly increased in CHC patients compared
with those in the normal control group, and higher levels of IL-8-
and TNF-α-expressing CD14+ monocytes in patients with
fibrosis were determined. IL-6-, IL-8- and TNF-α-expressing
CD14+ monocytes are composed mainly of mononuclear
macrophages induced by external stimuli (such as viral infection or
endotoxin), whose levels may reflect the activation of monocytes
and macrophages and have an important role in the pathogenesis of
HCV infection. This suggests that IL-8- and TNF-α-expressing
CD14+ monocyte levels have a certain utility in the
evaluation of HCV fibrosis.
IL-10 is a multifunctional negative regulatory
cytokine, mainly produced by monocytes and macrophages. IL-10
activates B cells and type 2 T-helper (Th2) cells. CD14+
monocytesexpressingIL-10 regulate immune and other cells and have a
pivotal role in various diseases, including autoimmune diseases,
severe infections and cancer. In the present study,
IL-10-expressing CD14+ monocyte levels were decreased in
CHC patients. IL-10 has strong immune suppressive effects and an
immune regulatory function. Therefore, it was speculated whether
decreased levels of IL-10 expressing CD14+ monocytes are
insufficient to inhibit inflammation, thus resulting in fibrosis.
Thus, modulation of IL-10 expressing CD14+ monocytes in
the early stage of HCV may slow the progression of fibrosis.
Aroucha et al (29)
indicated a protective role of IL-10 in patients with moderate
fibrosis, confirming the present hypothesis that IL-10 has a
protective role in HCV infection regarding the progression of
hepatic fibrosis. Another study emphasized the protective role of
IL-10 used in the treatment of CHC, which decreased the severity of
fibrosis in the patients enrolled (30). In another study on animal models, it
was demonstrated that the absence of IL-10 was associated with
liver fibrosis (31).
The present study also indicated that IL-2- and
IFN-γ-expressing CD14+ monocytes were significantly
increased in CHC patients as compared to controls, while they
declined gradually with the progression of fibrosis. IL-2 and IFN-γ
expressing CD14+ monocytes were predominant in CHC
patients with no or mild fibrosis. IL-2 and IFN-γ are Th1
cytokines. It was therefore presumed that patients with HCV
infection and fibrosis exhibited a distinct immunoregulatory
cytokine pattern that was shifted towards the Th2 response.
Liver biopsy has traditionally been considered the
gold standard for the evaluation of liver fibrosis. However, the
liver biopsy technique is an invasive procedure with a risk of
complications (32). Noninvasive
biomarkers of liver fibrosis have been proposed and their clinical
utilities have been evaluated (33,34).
Hence, several noninvasive indexes, including the APRI, FIB-4 and
AAR, have been developed, compared and validated as markers of
liver fibrosis in patients with chronic liver diseases. In the
present study, the serum concentration of sCD163 was consistently
higher in patients with significant fibrosis as compared with that
in patients with no/mild fibrosis and the AUROC (0.88) was on a par
with what was obtained by combined marker algorithms, including
liver biopsy, APRI, FIB-4 and AAR. The present results suggested
that the sCD163 had the best-performing ROC curve in the diagnosis
of moderate and severe fibrosis. FIB-4 was the second-best
noninvasive biomarker of liver fibrosis after sCD163. Since blood
samples are readily obtainable, it is appealing to search for serum
markers that are able to replace liver biopsies or liver stiffness
measurements. Soluble CD163 is readily available as a promising
parameter for the noninvasive determination of HCV-associated
fibrosis.
Taken together, the present results demonstrated
that CD14+ monocytes participate in the modulation of
fibrosis in patients with CHC. Targeting inflammatory monocytes in
CHC patients may not only lead to a decrease in pro-inflammatory
cytokine production but also reduce liver fibrosis.
The macrophage-associated marker sCD163 is
significantly higher in CHC patients with advanced fibrosis than in
those with no/mild liver fibrosis. Furthermore, serum sCD163
correlated with CD163 in liver tissue and its AUROC was higher than
that for APRI, AAR, representing a promising novel fibrosis marker
for the non-invasive diagnosis of fibrosis in patients with
CHC.
In conclusion, serum sCD163 levels are increased in
patients with CHC, reflecting hepatic macrophage activation.
Increased sCD163 is positively correlated with fibrosis. It may be
used to monitor the progression of liver fibrosis in the management
of CHC. The levels of CD14+ monocytes and
CD163+ macrophages may serve as markers for the disease
progression in patients with CHC and pathogenic macrophage targets
for specific drug development.
Acknowledgements
Data included in the present study were previously
presented as a poster on 11th April 2014 at The
International Liver Congress TM 2014, London, United Kingdom
(abstract no. P772) and at the 26th Annual Conference of
The Asian Pacific Association for the Study of the Liver, February
15-19, 2017, Shanghai, China and published as an abstract no.
PP1849 in Hepatol Int 11, 1-1093: 2017.
Funding
This work was funded by the Key Science and
Technology Research Project of Health and Family Planning
Commission of Hebei Province (grant no. 201601543), the Key Program
for Science and Technology Development (grant no. 10276102D) of
Hebei Province and the Project of International Cooperation Program
(grant no. 2015043571). The funders had no role in the study
design, data collection and analysis, decision to publish or
preparation of the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YN designed the study; SZ, LK, YZ NF, QZ, JD, BW, RW
and WR performed the experiments; SZ, WL, LK, FH and PC analyzed
data; YN, SZ and RW wrote the manuscript. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The study protocol conforms to the ethical
guidelines of the 1975 Declaration of Helsinki (6th revision,
2008). The present study was approved by the Ethics Committee of
the 3rd Hospital of Hebei Medical University (Shijiazhuang, China;
Oct 13th, 2010). All participants provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
European Association for Study of Liver.
EASL clinical practice guidelines: Management of hepatitis C virus
infection. J Hepatol. 60:392–420. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang Y, Rao H, Chi X, Li B, Liu H, Wu L,
Zhang H, Liu S, Zhou G, Li N, et al: Detection of residual HCV-RNA
in patients who have achieved sustained virological response is
associated with persistent histological abnormality. EBioMedicine.
46:227–235. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Liaskou E, Wilson DV and Oo YH: Innate
immune cells in liver inflammation. Mediators Inflamm.
2012(949157)2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pellicoro A, Ramachandran P, Iredale JP
and Fallowfield JA: Liver fibrosis and repair: Immune regulation of
wound healing in a solid organ. Nat Rev Immunol. 14:181–194.
2014.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Pinzani M: Pathophysiology of liver
fibrosis. Dig Dis. 33:492–497. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wallace K, Burt AD and Wright MC: Liver
fibrosis. Biochem J. 411:1–18. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wynn TA and Barron L: Macrophages: Master
regulators of inflammation and fibrosis. Semin Liver Dis.
30:245–257. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Saha B, Kodys K and Szabo G: Hepatitis C
virus-induced monocyte differentiation into polarized M2
macrophages promotes stellate cell activation via TGF-β. Cell Mol
Gastroenterol Hepatol. 2:302–316.e8. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Murthy S, Larson-Casey JL, Ryan AJ, He C,
Kobzik L and Carter AB: Alternative activation of macrophages and
pulmonary fibrosis are modulated by scavenger receptor, macrophage
receptor with collagenous structure. FASEB J. 29:3527–3536.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Waidmann O, Brunner F, Herrmann E, Zeuzem
S, Piiper A and Kronenberger B: Macrophage activation is a
prognostic parameter for variceal bleeding and overall survival in
patients with liver cirrhosis. J Hepatol. 58:956–961.
2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Liu C, Tao Q, Sun M, Wu JZ, Yang W, Jian
P, Peng J, Hu Y, Liu C and Liu P: Kupffer cells are associated with
apoptosis, inflammation and fibrotic effects in hepatic fibrosis in
rats. Lab Invest. 90:1805–1816. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Grønbaek H, Sandahl TD, Mortensen C,
Vilstrup H, Møller HJ and Møller S: Soluble CD163, a marker of
Kupffer cell activation, is related to portal hypertension in
patients with liver cirrhosis. Aliment Pharmacol Ther. 36:173–180.
2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bedossa P and Poynard T: An algorithm for
the grading of activity in chronic hepatitis C: The METAVIR
Cooperative Study Group. Hepatology. 24:289–293. 1996.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wai CT, Greenson JK, Fontana RJ,
Kalbfleisch JD, Marrero JA, Conjeevaram HS and Lok AS: A simple
noninvasive index can predict both significant fibrosis and
cirrhosis in patients with chronic hepatitis C. Hepatology.
38:518–526. 2003.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sterling RK, Lissen E, Clumeck N, Sola R,
Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT,
Thomas DL, et al: Development of a simple noninvasive index to
predict significant fibrosis in patients with HIV/HCV coinfection.
Hepatology. 43:1317–1325. 2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Iwata Y, Enomoto H, Sakai Y, Aizawa N,
Tanaka H, Ikeda N, Takashima T, Ishii A, Hasegawa K, Yuri Y, et al:
Elevation of the AST to ALT ratio in association with the severity
of esophageal varices in patients with HCV-related compensated
liver cirrhosis. Hepatogastroenterology. 60:149–152.
2013.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Wan J, Benkdane M, Teixeira-Clerc F,
Bonnafous S, Louvet A, Lafdil F, Pecker F, Tran A, Gual P, Mallat
A, et al: M2 Kupffer cells promote M1 Kupffer cell apoptosis: A
protective mechanism against alcoholic and nonalcoholic fatty liver
disease. Hepatology. 59:130–142. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Xu D, Fu J, Jin L, Zhang H, Zhou C, Zou Z,
Zhao JM, Zhang B, Shi M, Ding X, et al: Circulating and liver
resident CD4+CD25+ regulatory T cells actively influence the
antiviral immune response and disease progression in patients with
hepatitis B. J Immunol. 177:739–747. 2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ziegler-Heitbrock L: The CD14+ CD16+ blood
monocytes: Their role in infection and inflammation. J Leukoc Biol.
81:584–592. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Beljaars L, Schippers M, Reker-Smit C,
Martinez FO, Helming L, Poelstra K and Melgert BN: Hepatic
localization of macrophage phenotypes during fibrogenesis and
resolution of fibrosis in mice and humans. Front Immunol.
5(430)2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kuniholm MH, Hanna DB, Landay AL, Kaplan
RC and Ley K: Soluble CD163 is associated with noninvasive measures
of liver fibrosis in hepatitis C virus- and hepatitis C virus/human
immunodeficiency virus-infected women. Hepatology. 61:734–735.
2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lidofsky A, Holmes JA, Feeney ER, Kruger
AJ, Salloum S, Zheng H, Seguin IS, Altinbas A, Masia R, Corey KE,
et al: Macrophage activation marker soluble CD163 is a dynamic
marker of liver fibrogenesis in human immunodeficiency
virus/hepatitis C virus coinfection. J Infect Dis. 218:1394–1403.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lidofsky A, Holmes JA, Feeney ER, Kruger
AJ, Salloum S, Zheng H, Seguin IS, Altinbas A, Masia R, Corey KE,
et al: Macrophage Activation Marker Soluble CD163 Is a Dynamic
Marker of Liver Fibrogenesis in Human Immunodeficiency
Virus/Hepatitis C Virus Coinfection. J Infect Dis. 218:1394–1403.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lee J, French B, Morgan T and French SW:
The liver is populated by a broad spectrum of markers for
macrophages. In alcoholic hepatitis the macrophages are M1 and M2.
Exp Mol Pathol. 96:118–125. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Melino M, Gadd VL, Walker GV, Skoien R,
Barrie HD, Jothimani D, Horsfall L, Jones A, Sweet MJ, Thomas GP,
et al: Macrophage secretory products induce an inflammatory
phenotype in hepatocytes. World J Gastroenterol. 18:1732–1744.
2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kazankov K, Barrera F, Møller HJ, Bibby
BM, Vilstrup H, George J and Grønbaek H: Soluble CD163, a
macrophage activation marker, is independently associated with
fibrosis in patients with chronic viral hepatitis B and C.
Hepatology. 60:521–530. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hiraoka A, Horiike N, Akbar SM, Michitaka
K, Matsuyama T and Onji M: Soluble CD163 in patients with liver
diseases: Very high levels of soluble CD163 in patients with
fulminant hepatic failure. J Gastroenterol. 40:52–56.
2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Holland-Fischer P, Grønbæk H, Sandahl TD,
Moestrup SK, Riggio O, Ridola L, Aagaard NK, Møller HJ and Vilstrup
H: Kupffer cells are activated in cirrhotic portal hypertension and
not normalised by TIPS. Gut. 60:1389–1393. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Aroucha DC, do Carmo RF, Moura P, Silva
JL, Vasconcelos LR, Cavalcanti MS, Muniz MT, Aroucha ML, Siqueira
ER, Cahú GG, et al: High tumor necrosis factor-α/interleukin-10
ratio is associated with hepatocellular carcinoma in patients with
chronic hepatitis C. Cytokine. 62:421–425. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Nelson DR, Lauwers GY, Lau JY and Davis
GL: Interleukin 10 treatment reduces fibrosis in patients with
chronic hepatitis C: A pilot trial of interferon nonresponders.
Gastroenterology. 118:655–660. 2000.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Thompson K, Maltby J, Fallowfield J,
McAulay M, Millward-Sadler H and Sheron N: Interleukin-10
expression and function in experimental murine liver inflammation
and fibrosis. Hepatology. 28:1597–1606. 1998.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Van Thiel DH, Gavaler JS, Wright H and
Tzakis A: Liver biopsy. Its safety and complications as seen at a
liver transplant center. Transplantation. 55:1087–1090.
1993.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Castera L: Noninvasive methods to assess
liver disease in patients with hepatitis B or C. Gastroenterology.
142:1293–1302.e4. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Adams LA: Biomarkers of liver fibrosis. J
Gastroenterol Hepatol. 26:802–809. 2011.PubMed/NCBI View Article : Google Scholar
|