Introduction
Ischemic stroke is widely recognized as a severe
cerebrovascular disease with high mortality and morbidity rates
(1), caused by arterial inflow
interruption and nutrient and oxygen deficits. Timely restoration
of cerebral blood flow perfusion in the ischemic area is pivotal to
minimize sustained brain injury. However, reperfusion following a
period of ischemia may lead to cerebral ischemia-reperfusion injury
(IRI). Furthermore, restored blood supply may trigger the
production of excessive amounts of reactive oxygen species (ROS),
oxidative stress-related factors (2), and inflammatory responses (3). Therefore, inflammation modulators and
ROS scavengers may serve as a promising treatment approach for
IRI.
In the inflammatory network, high mobility group box
chromosomal protein 1 (HMGB-1) is a potent inflammatory cytokine,
which activates the downstream inflammatory signaling pathway
(4). A previous study has
demonstrated that dynamic changes in the serum HMGB-1 levels are
associated with inflammatory responses following cardiopulmonary
bypass (5). Therefore, HMGB-1
levels are considered as a monitoring marker of inflammation during
cardiac surgery with cardiopulmonary bypass (5). Although a limited number of studies
have been conducted regarding the association between HMGB-1 and
cerebral IRI, HMGB-1 is hypothesized to serve an important role in
multiple-organ IRI through protecting against hepatic injury
following murine liver ischemia-reperfusion (6). As a ligand with high affinity to
HMGB-1, the receptor for advanced glycation end products (RAGE) is
able to specifically combine with HMGB-1, participating in the
inflammatory response in the late stages of sepsis (7). In addition, HMBG-1/RAGE axis may serve
a significant role in the development of chronic inflammatory
diseases such as neutrophilic asthma (8). Therefore, the present study
hypothesized that the inhibition of the HMBG-1/RAGE signaling
pathway may attenuate inflammatory responses, indicating a possible
neuroprotective effect in cerebral IRI.
Dioscin, a highly fat-soluble steroidal saponin, is
present in a number of plants such as ginseng (9) and Dioscorea nipponica Makino
(10). Modern pharmacological
studies have suggested that dioscin exerts anti-inflammatory
effects via alleviating the lipopolysaccharide-induced inflammatory
kidney injury (11). Previous
studies have revealed that dioscin not only has significant effects
on the anti-inflammatory responses, but also exhibits antiviral
(12), antioxidative (13), hepatoprotective (14) and antiapoptotic activities (15). Furthermore, numerous studies have
focused on the neuroprotective effects of dioscin following
peripheral and central nerve injury (16-19).
However, there is little information available regarding the
potential mechanisms of dioscin on inducing cerebral IRI. Whether
dioscin mediates its beneficial effects through a combination of
multiple mechanisms or a single pathway, or by an anti-inflammatory
mechanism, remains largely unknown. Therefore, the present study
aimed to assess whether the therapeutic effects of dioscin on
cerebral IRI were associated with its anti-inflammatory or other
medicinal properties, and to further investigate the role of the
HMGB-1/RAGE signaling pathway in these mechanisms.
Materials and methods
Cell culture
Primary hippocampal neuronal cultures were prepared
as previously described (20).
Briefly, following harvesting the Sprague-Dawley (SD) rat embryos
at embryonic day 18 (E18), hippocampi were chopped and digested
with 0.25% trypsin at 37˚C for 15 min. Animal experiments were
approved by the Animal Care and Use Committee of the Tangshan
Gongren Hospital (Tangshan, China; approval no. GRYY-LL-2019-15).
All animal experimental procedures were performed according to the
National Institutes of Health Guide for the Care and Use of
Laboratory Animals (NIH Publication no. 85-23, revised 1996)
(21). Following filtration, cells
were seeded at a density of 3x105 cells/well onto
12-well plates. Neurons were maintained at 37˚C in a neurobasal
medium (Invitrogen; Thermo Fisher Scientific, Inc.) supplemented
with 2% B-27 and 0.5 mM glutamine (both from Invitrogen; Thermo
Fisher Scientific, Inc.) and half of the cell-culture medium was
replaced every 3 days.
OGD/R model
To mimic cerebral IRI in vitro, an OGD/R
model was established as previously described (22). Briefly, primary hippocampal neurons
were cultured from E18 SD rats as aforementioned. Neurons were
maintained in glucose-free DMEM (cat. no. 11966-025; Gibco; Thermo
Fisher Scientific, Inc.) and cultured in an O2-free
chamber at 37˚C for 2 h. During the reoxygenation process, the
cell-culture medium was replaced with normal DMEM, and neurons were
then cultured in an incubator under normoxic conditions at 37˚C and
5% CO2 for an additional 12 h. Control cells were
treated in the same conditions without being exposed to OGD/R.
Drug administration
Dioscin was obtained from Sigma-Aldrich; Merck KGaA
(cat. no. SMB00576), and dissolved in dimethyl sulfoxide (DMSO), at
a final concentration of DMSO <0.1%. Neurons were then divided
into the following four groups: i) Control group, where neurons
were not treated; ii) Control + dioscin group, where primary
hippocampal neurons were treated with dioscin without OGD/R; iii)
OGD/R group, where primary hippocampal neurons subjected to OGD/R;
and iv) the OGD/R + dioscin group, where primary neurons subjected
to reoxygenation following oxygen-glucose deprivation. The optimum
concentration and action time of dioscin treatment was determined
using Cell Counting Kit-8 (CCK-8) assay.
CCK-8 assay
The viability of neurons was assessed by a CCK-8
assay (MedChemExpress). For CCK-8 analysis, cells were plated into
96-well plates at a density of 5x103 cells/well for 24 h
at 37˚C with 5% CO2. Subsequently, cells were subjected
to OGD for 2 h and reperfusion for 12 h, and then different
concentrations of dioscin (0, 100, 200, 400 and 800 ng/ml) were
added to the culture medium for a 24-h intervention period. A total
of 10 µl CCK-8 solution was carefully added to the culture medium,
and cells were incubated for an additional 2 h at 37˚C, according
to the manufacturer's instructions. Finally, cell viability was
determined by measuring the absorbance at a wavelength of 570 nm
using a microplate reader.
Western blot analysis
Neurons were collected, lysed in
radioimmunoprecipitation assay (RIPA) lysis buffer, centrifuged at
12,000 x g at 4˚C for 15 min and the concentration of the extracted
proteins was determined using the bicinchoninic acid Protein Assay
kit (Beyotime Institute of Biotechnology). A total of 25 µg protein
was then separated by SDS-PAGE and electro-transferred onto
nitrocellulose membranes using a Bio-Rad mini-protein-III wet
transfer unit (Bio-Rad Laboratories, Inc.) at 90 V/90 min.
Following blocking with non-fat milk for 2 h at room temperature,
membranes were incubated with primary antibodies at 4˚C overnight.
The primary antibodies used were against β-actin (cat. no.
sc-47778; 1:1,000, mouse; Santa Cruz Biotechnology, Inc.),
interleukin (IL)-1 (cat. no. sc-7884; 1:1,000, rabbit; Santa Cruz
Biotechnology, Inc.), IL-6 (cat. no. sc-7920; 1:1,000, rabbit;
Santa Cruz Biotechnology, Inc.), tumor necrosis factor α (TNF-α;
cat. no. sc-52746; 1:1,000, mouse; Santa Cruz Biotechnology, Inc.),
HMGB-1 (cat. no. 6893; 1:1,000, rabbit; Cell Signaling Technology,
Inc.), RAGE (cat. no. sc-5563; 1:1,000, rabbit; Santa Cruz
Biotechnology, Inc.), Bax (cat. no. 2772; 1:1,000, rabbit; Cell
Signaling Technology, Inc.), Bcl-2 (cat. no. sc-7382; 1:1,000,
mouse; Santa Cruz Biotechnology, Inc.), 3-NT (cat. no. ab61392;
1:1,000, mouse; Abcam) and cleaved caspase-3 (cat. No. 9661;
1:1,000; rabbit; Cell Signaling Technology, Inc.). Following
washing with PBS 3 times, membranes were incubated with horseradish
peroxidase (HRP) conjugated anti-rabbit IgG and anti-mouse IgG
(cat. nos. sc-2357 and sc-516102, 1:2,000; Santa Cruz
Biotechnology, Inc.) for 1.5 h at room temperature. Finally, the
protein density was measured using a Bio-Rad imaging system and
analyzed with the ImageJ software (Image Lab 4.1; National
Institutes of Health). Relative protein expression levels are
presented as the densitometric value (OD values) ratio of the
target protein band to β-actin band.
Reverse transcription-quantitative
(RT-q)PCR analysis
Total cellular RNA was isolated with the
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. Subsequently, RNA
concentration was measured using a UV spectrophotometer (NanoDrop
One Microvolume UV-Vis spectrophotometer; Thermo Fisher Scientific,
Inc.) and the RT assay was performed, using RNA as template, with
PrimeScript™ RT reagent kit (cat. no. RR037A; Takara Bio, Inc.) to
synthesize cDNA. The RT reactions were performed at 37˚C for 15
min, 85˚C for 5 sec and 4˚C for cooling. In total, 2 µg RNA from
each sample were reverse transcribed into cDNA. The RT-qPCR was
conducted with the TB Green® Premix Ex Taq™ II kit (cat.
no. RR820A; Takara Bio, Inc.) in a 20 µl reaction containing 10 µl
2X TB Green Premix Ex Taq II (Tli RNaseH Plus), 0.8 µl forward
primer (10 µM), 0.8 µl reverse primer (10 µM), 2 µl template DNA
and 6.4 µl ddH2O. The thermocycling conditions of the
qPCR were as follows: 37˚C for 30 min, 95˚C for 55 sec to
inactivate reverse transcriptase, followed by 40 cycles of two-step
PCR, 95˚C for 20 sec and 60˚C for 50 sec. The final extension was
performed at 75˚C for 10 min and samples were subsequently held at
4˚C. A total of 3 independent experiments were performed for each
group. The primer sequences used in this study were the following:
For β-actin, forward 5'-TGACGTGGACATCCGCAAAG-3' and reverse,
5'-CTGGAAGGTGGACAGCGAGG-3'; IL-1β, forward
5'-TGGGAGATGGAAACATCCAG-3' and reverse, 5'-GCATTTTACTGACTGCACGG-3';
IL-6, forward 5'-ACAGCCACTCACCTCTTCAG-3' and reverse,
5'-CCATCTTTTTCAGCCATCTTT-3'; TNF-a, forward
5'-AGAACCCCCTGGAGATAACC-3' and reverse, 5'-AAGTGCAGCAGGCAGAAGAG-3';
HMGB-1, forward 5'-GCAGATGACAAGCAGCCTTA-3' and reverse,
5'-TTTGCTGCATCAGGCTTTCC-3'; RAGE, forward
5'-GACTCTTAGCTGGCACTTGGAT-3' and reverse,
5'-GGACTTCACAGGTCAGGGTTAC-3'; cleaved caspase-3, forward
5'-AGCAATAAATGAATGGGCTGAG-3' and reverse,
5'-GTATGGAGAAATGGGCTGTAGG-3'; Bax, forward
5'-GTTGCCCTCTTCTACTTTGC-3' and reverse, 5'-ATGGTCACTGTCTGCCATG-3';
and Bcl-2 forward, 5'-GGTCCTCCAGTGGGTATTT-3' and reverse,
5'-TCCTCCTGAGACTGCCTTAT-3'. β-actin was used as an internal control
and the 2-ΔΔCq method was applied to determine the
relative gene expression (23).
Intracellular ROS detection
For the detection of intracellular ROS, the Reactive
Oxygen Species Assay kit was applied, according to the
manufacturer's instructions (cat. no. ab113851; Abcam). Following
treatment with and without OGD/R, dioscin or under control
conditions for 24 h, 2',7'-dichlorofluorescein diacetate (DCFH-DA;
concentration, 5 µmol/l) was added in the culture medium. The cell
mixture was then incubated at 37˚C in a 5% CO2 incubator
for 30 min for the conversion of DCFH-DA to
2',7'-dichlorofluorescein. Subsequently, cells were fixed with 1%
paraformaldehyde for 10 min at 4˚C and the levels of intracellular
ROS were determined using the cellular ROS detection assay kit with
flow cytometry (BD Accuri™ C6; BD Bioscience). Finally, the results
were analyzed using the FlowJo software version 7.6.5 (FlowJo,
LLC).
Determination of the glutathione
(GSH)/glutathione disulfide (GSSG) ratio
The effects of dioscin on non-enzymatic antioxidant
defense mechanisms were evaluated by detecting the GSH and GSSG
levels in neurons, according to the method proposed by Hissin and
Hilf (24), with slight
modifications. A proprietary non-fluorescent dye thiol green
indicator was used in the GSH/GSSG assay protocol, but without
O-phthalaldehyde as the fluorescent reagent. The concentration of
GSH and GSSG were measured using the GSH/GSSG Ratio Detection Assay
kit (cat. no., ab138881; Abcam). The levels of GSH (reduced form)
and total GSH (GSH + GSSG) were directly measured using their
standards provided by the kit. Then, the GSSG content was
indirectly determined by calculating the difference between total
GSH + GSSG and GSH. Finally, the GSH/GSSG ratio was calculated.
Measurement of glutathione peroxidase
(GPx), catalase (CAT) and superoxide dismutase (SOD) activity
The effects of dioscin on the enzymatic antioxidant
defense mechanisms were determined by measuring the GPx, CAT and
SOD activity. The activity of GPx was spectrophotometrically
detected in neurons as previously described (25). The GPx activity was expressed as the
amount of the oxidized NADPH protein in min/mg and the activity
rate was determined by the change in absorbance at 340 nm (A340).
When the substrate tert-butyl hydroperoxide was added, the reaction
was initiated and the reduction in GPx activity was recorded at
A340. Additionally, GPx activity was expressed in nmol/min/mg of
protein. Furthermore, CAT activity was assayed as previously
described (26), using
H2O2 as the substrate. The decomposition of
H2O2 was determined at 240 nm by measuring
the decrease in absorbance. The CAT activity was expressed as
µM/min/mg protein. Finally, SOD activity was spectrophotometrically
determined in kinetic mode at intervals of 1 min up to 3 min at 560
nm, as described by Hassan et al (27). SOD activity was expressed as U/mg of
protein.
Cell transfection
For cell transfection, cells were cultured at a
density of 1x105 cells/well. Neurons were grown in
six-well plates for 24 h and then transfected with the
pcDNA3.1-HMGB-1 overexpression plasmid (2 µg) or control vector for
24 h using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
The pcDNA3.1-HMGB-1 overexpression plasmid was synthesized by
Applied Biological Materials, Inc. Neurons transfected with control
vector, were considered as the negative control group. Furthermore,
the overexpression of the HMGB-1 was assessed using western blot
analysis 6 h post-transfection.
Analysis of neuronal apoptosis by
terminal deoxynucleotidyl transferase-mediated (dUTP) nick-end
labeling (TUNEL) staining
TUNEL staining was performed to detect cell
apoptosis according to the manufacturer's protocol. Briefly,
neurons at a density of 5x104 cells/cm2 were
fixed with 4% paraformaldehyde at 4˚C for 20 min. The ischemic
brain tissue was embedded in paraffin and sectioned at 5 µm
following fixing in 4% paraformaldehyde at room temperature for 48
h. Following dewaxing and rehydration, sections were incubated with
a proteinase K solution for 15 min at 37˚C. Subsequently, cells
were treated with a green fluorescein-labeled dUTP solution and
incubated with 4',6-diamidino-2-phenylindole (DAPI, 1 µg/ml) for 5
min at room temperature to detect cell nuclei. The TUNEL-positive
cells and morphology of neurons were observed under a fluorescence
microscope and cells were counted in 5 randomly selected
microscopic fields (magnification, x200). The cell apoptosis rate
was calculated as the number of TUNEL-positive cells/the number of
DAPI stained cells.
Animals and middle cerebral artery
occlusion (MCAO) model
A total of 50 12 weeks-old adult male SD rats were
obtained from the Experimental Animal Center, North China
University of Science and Technology. All rats were housed under a
controlled environment at 22-24˚C, with a 12/12 h light/dark cycle
and 50-55% humidity. All rats were provided with ad libitum
access to food and water throughout the whole trial. MCAO was used
to establish the cerebral IRI rat model. In brief, anesthesia was
administered to rats by intraperitoneal injection of 50 mg/kg
sodium pentobarbital. Following separation of the carotid arteries
including common carotid artery (CCA), internal carotid artery
(ICA) and external carotid artery (ECA), the right CCA was
carefully ligated with a microclip. Middle cerebral artery (MCA)
occlusion was induced by a monofilament inserted into the stump of
the ICA, following which the monofilament was advanced to the
origin of the MCA to occlude it. A Laser-Doppler flowmeter (Moor
Instruments, Ltd.) was used to monitor regional cerebral blood flow
(rCBF) of each rat. A 70-80% decrease in rCBF was regarded as
successful MCAO. Occlusion was maintained for a period of 60 sec.
The monofilament was then removed to reestablish carotid blood
flow. Sham-operated rats underwent only the carotid artery
separation without the monofilament insertion. A total of rats
succumbed during the establishment of the MCAO model.
Experimental groups and treatment
Rats were randomly allocated into the following five
groups: Sham; sham + dioscin; MCAO; MCAO + dioscin; and MCAO +
dioscin + HMGB-1. Dioscin was suspended in 0.5% sodium carboxyl
methyl cellulose solution prior to use. Rats were treated with
dioscin (60 mg/kg) by gavage at 1 h following establishment of the
MCAO rat model and once daily for the subsequent 6 days. In
addition, rats in the sham and MCAO groups were treated with equal
volumes of sodium carboxyl methyl cellulose solution. Following
treatment with dioscin or sham for 30 min, rats in the MCAO +
dioscin + HMGB-1 group were treated with HMGB-1 (50 ug/kg; R&D
Systems, Inc.) by intraperitoneal injection once daily for 6
days.
Detection of 8-oxo-deoxyguanosine
(8-OHdG)
8-OHdG is a biomarker of nucleic acid oxidation.
Therefore, 8-OHdG was assessed in frozen sections of brain tissue
by immunofluorescence. Following treatment with 0.4% Triton X-100
at room temperature for 10 min, sections were incubated with a
mixture containing a primary 8-OHdG antibody (cat. no. ab62623,
1:1,000, mouse, Abcam) and a nuclear protein (NeuN, ab104224,
1:1,000, mouse, Abcam) monoclonal antibody at 4˚C overnight. The
next day, sections were incubated with HRP-conjugated secondary
antibody (cat. no. ab205719, 1:500, Abcam) for 1 h at 37˚C.
Chromosomes were counterstained with DAPI (1 µg/ml) for 10 min at
room temperature and the fluorescence images were captured using an
Olympus F1000 laser scanning confocal fluorescence microscope
(magnification, x400; Olympus Corporation).
Statistical analysis
All statistical analyses were performed with the
SPSS 23.0 software (SPSS Inc.). All parameters were expressed as
the mean ± standard error of the mean (SEM) and were obtained from
≥3 replicates. Data were analyzed using one-way ANOVA followed by
Tukey's post hoc analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of dioscin on OGD/R-induced
cell viability
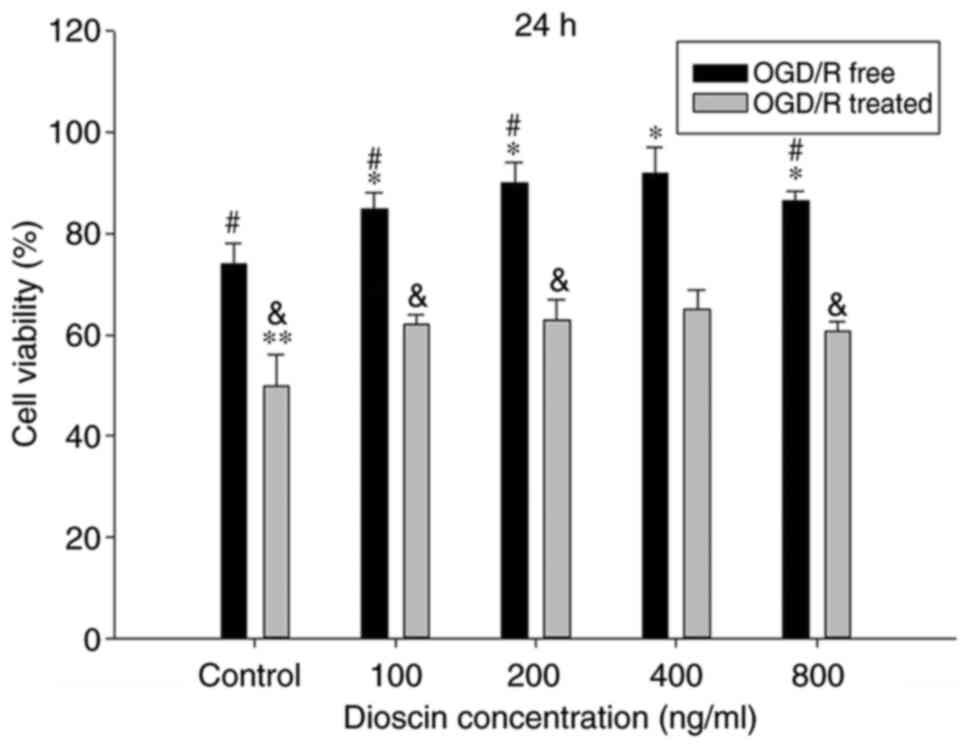
To evaluate the functional effects of dioscin on
neurons subjected to OGD/R or not, cells were treated with
different doses of dioscin (100, 200, 400 and 800 ng/ml) and cell
viability was assessed. OGD/R significantly inhibited cell
viability in cells treated with any concentration of dioscin.
Compared with the control group, dioscin at 100, 200 and 400 ng/ml
markedly promoted neuron viability in cells subjected to OGD/R or
not (P<0.05; Fig. 1). However,
dioscin at 800 ng/ml markedly inhibited cell viability compared
with cells treated with 400 ng/ml dioscin (P<0.05; Fig. 1). Therefore, dioscin at a
concentration of 400 ng/ml was used in subsequent experiments.
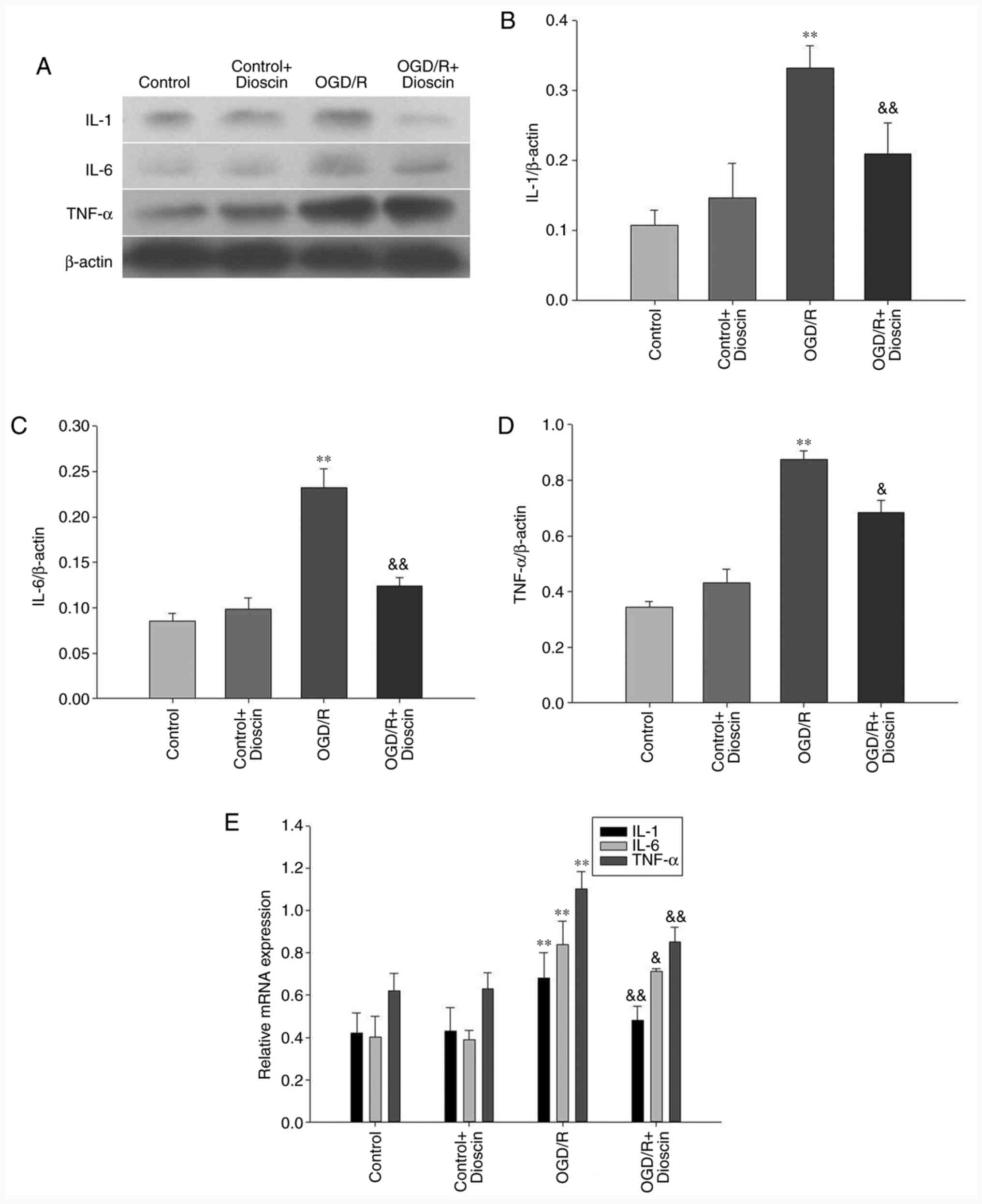
Dioscin inhibits the protein
expression levels of IL-1, IL-6 and TNF-α
IL-1, IL-6 and TNF-α were downregulated in the
control group. Following reperfusion, the expression levels of
IL-1, IL-6 and TNF-α were significantly increased in the OGD/R
group. However, treatment with dioscin restored the OGD/R-mediated
overexpression of IL-1, IL-6 and TNF-α (P<0.05; Fig. 2). The same results were also
observed in the mRNA level when RT-qPCR was applied (P<0.05;
Fig. 2E).
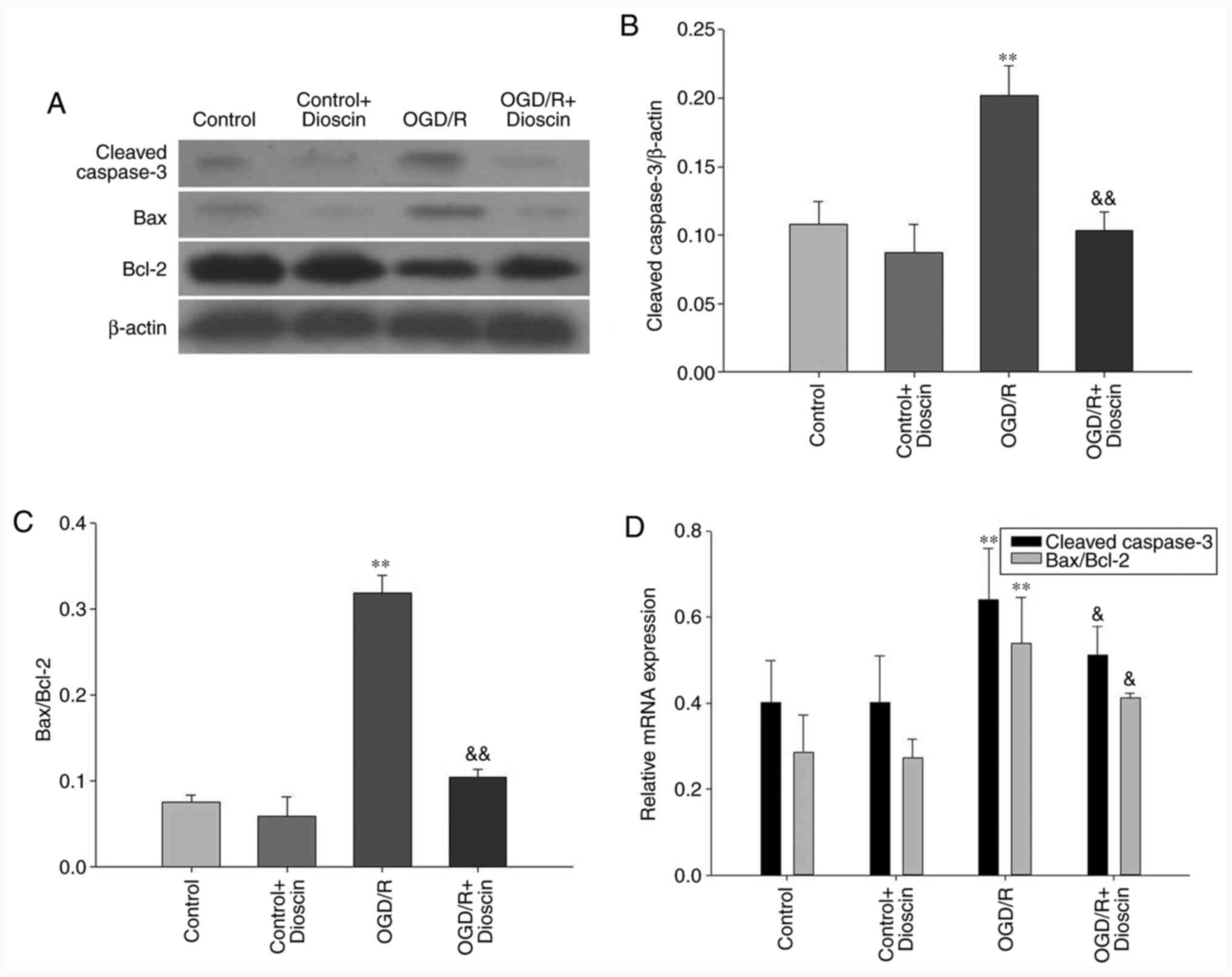
Dioscin inhibits the expression of
apoptosis-associated proteins
Cleaved caspase-3 and Bax protein expression levels
were decreased in the control groups. Furthermore, the protein and
mRNA expression levels of both cleaved caspase-3 and Bax were
markedly increased, and those of Bcl-2 were decreased in the OGD/R
group. However, cell treatment with dioscin significantly
attenuated the expression levels of cleaved caspase-3 and Bax. In
the OGD/R + dioscin group, the mRNA and protein expression levels
of Bcl-2 were notably increased compared with those in the OGD/R
group (P<0.05; Fig. 3).
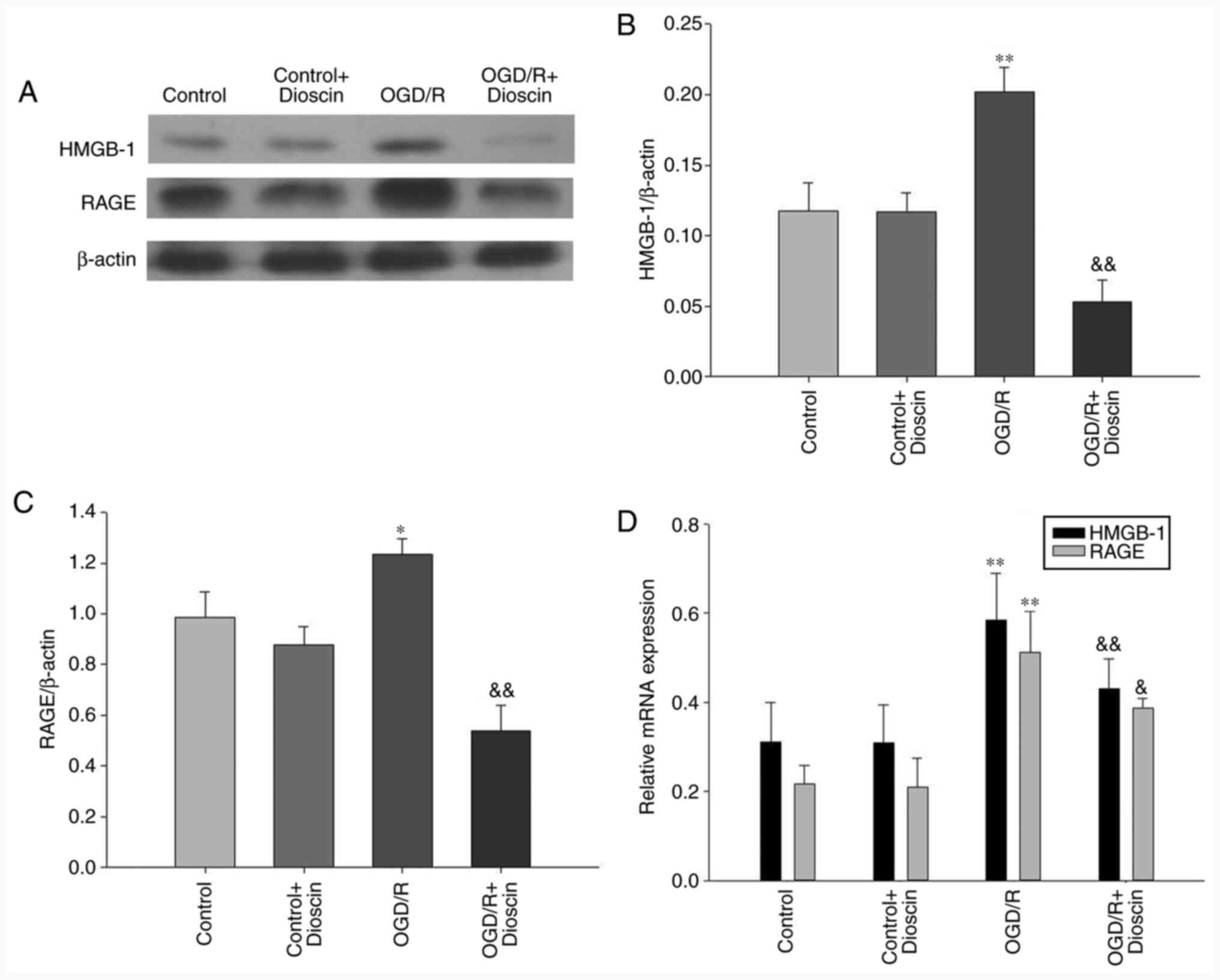
Dioscin inhibits the OGD/R-induced
activation of the HMGB-1/RAGE pathway
The results demonstrated that the expression levels
of HMGB-1 and RAGE were significantly elevated in the OGD/R group.
However, following treatment with dioscin, the expression of
proteins involved in the HMGB-1/RAGE pathway was downregulated
(OGD/R + dioscin group; P<0.05; Fig.
4A and B). The expression
levels of HMGB-1/RAGE at the mRNA level, detected by RT-qPCR, were
consistent with those observed at the protein level (Fig. 4C).
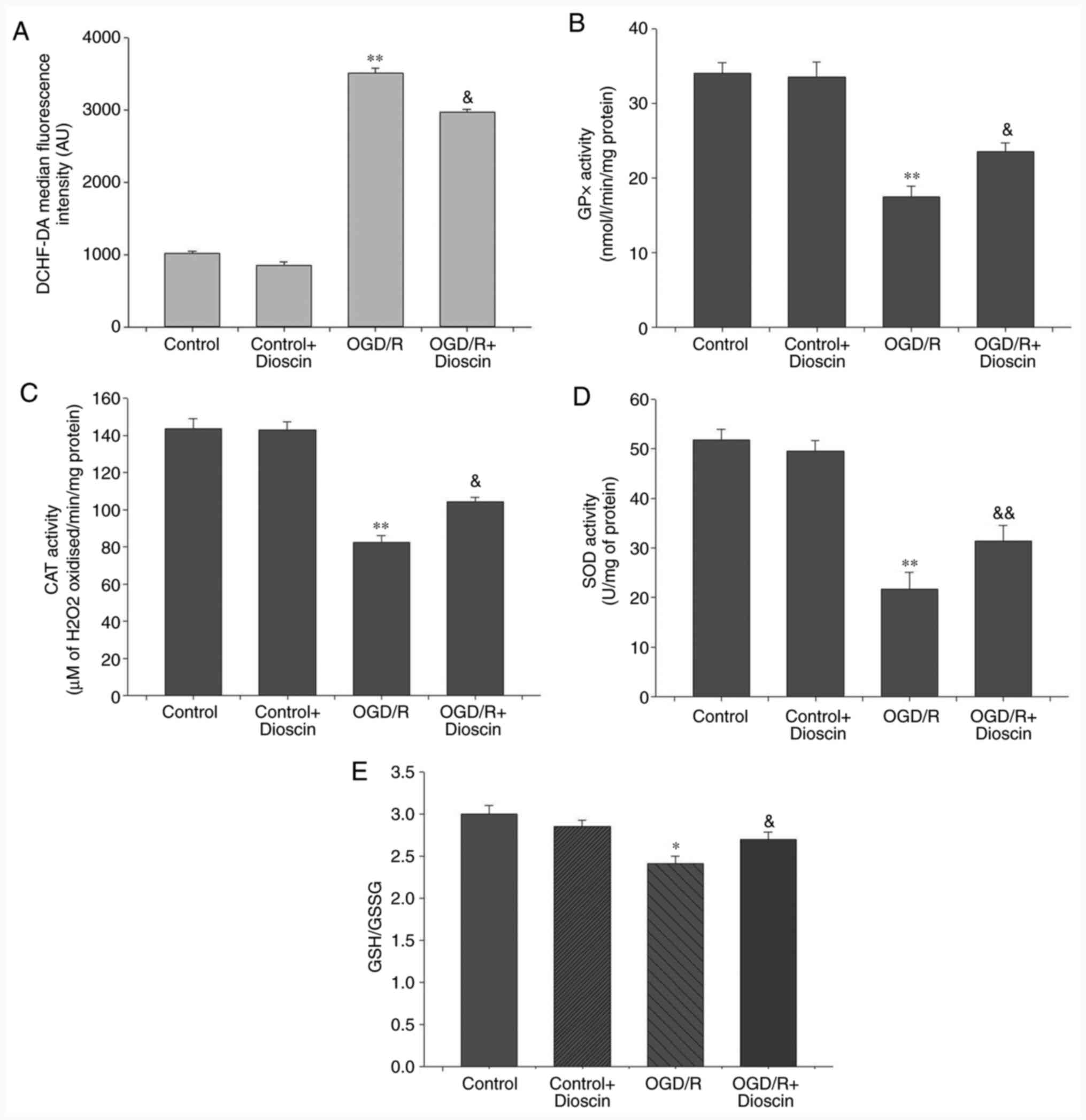
Dioscin markedly decreases ROS
production and promotes the antioxidant defense system
Compared with the control group, intracellular ROS
production in the neurons was significantly increased in the OGD/R
group (P<0.01). Additionally, dioscin treatment in the OGD/R +
dioscin group significantly inhibited ROS production compared with
the OGD/R group (P<0.05; Fig.
5A). Furthermore, to evaluate the antioxidant defense system,
the levels of the antioxidant enzymes, GPx, CAT and SOD, and the
ratio of the non-enzymatic antioxidants GSH/GSSG were measured. The
results indicated that the levels of GPx, CAT, SOD and the GSH/GSSG
ratio were decreased in neurons subjected to OGD/R compared with
those noted to the control group (P<0.05; Fig. 5). Nevertheless, co-treatment of
OGD/R-treated neurons with dioscin significantly elevated the
expression of the antioxidant compounds compared with the OGD/R
group (P<0.05).
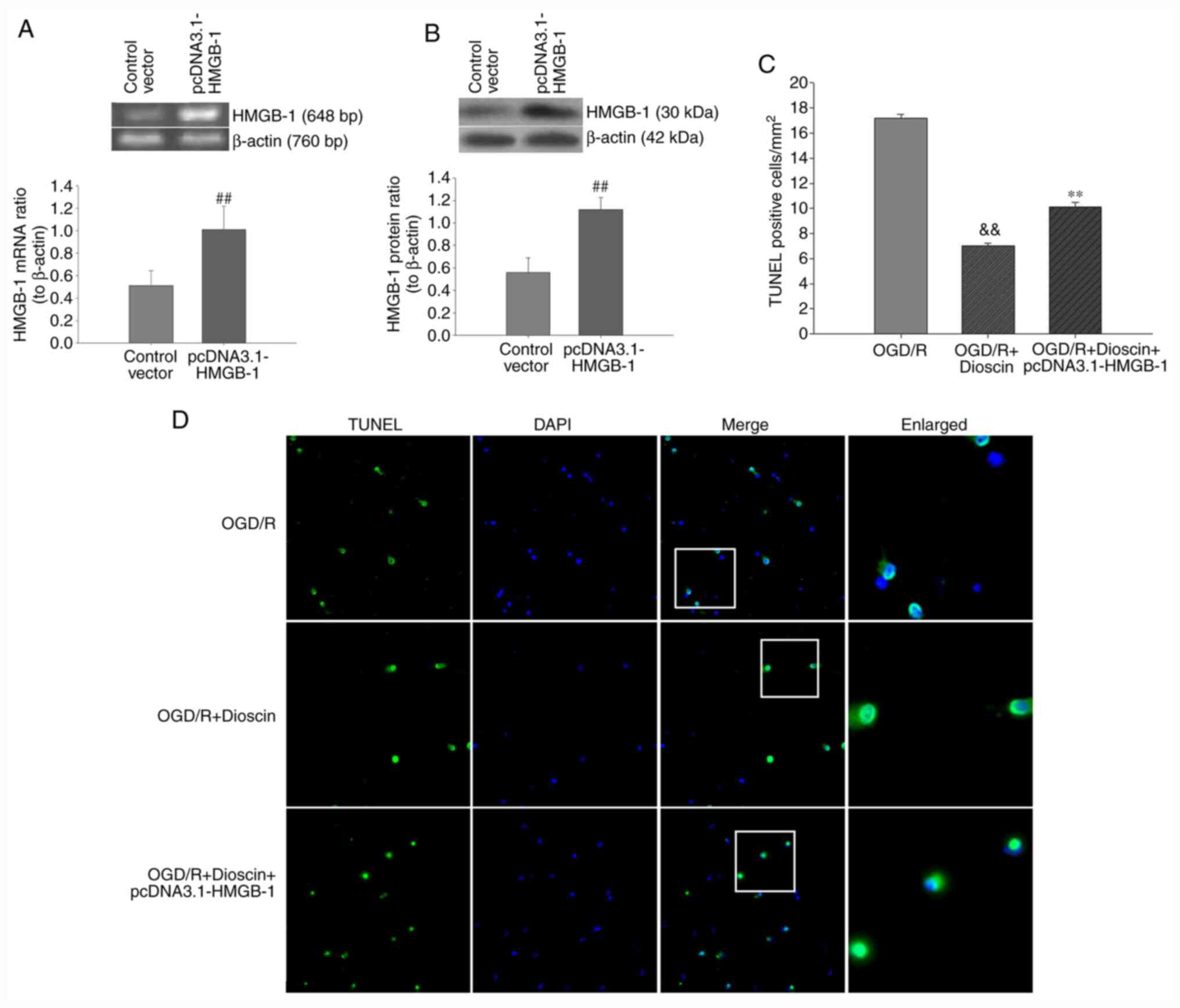
Upregulation of HMGB-1 with the
pcDNA3.1-HMGB-1 plasmid diminishes the protective effects of
dioscin on neuron apoptosis
Subsequently, neuronal apoptosis was evaluated using
TUNEL/DAPI staining. Therefore, hippocampal neurons were
transfected with the pcDNA3.1-HMGB-1 plasmid to overexpress HMGB1.
As demonstrated in Fig. 6, the
number of TUNEL-positive cells was markedly increased in the OGD/R
group. Furthermore, quantitative analysis indicated that dioscin
significantly decreased neuronal apoptosis in the OGD/R + dioscin
group. Following transfection with pcDNA3.1-HMGB1, the number of
TUNEL-positive cells was notably increased (P<0.01; Fig. 6). These results indicated that
upregulation of HMGB-1 may inhibit the protective effects of
dioscin against OGD/R-induced apoptosis on hippocampal neurons.
HMGB-1 overexpression may offset the
suppressive effects of dioscin on proinflammatory cytokine
expression
Therefore, compared with the OGD/R + dioscin group,
western blot analysis indicated that HMGB-1 overexpression
significantly increased the expression of inflammatory cytokines,
namely IL-1, IL-6 and TNF-α (P<0.05; Fig. 7A-C).
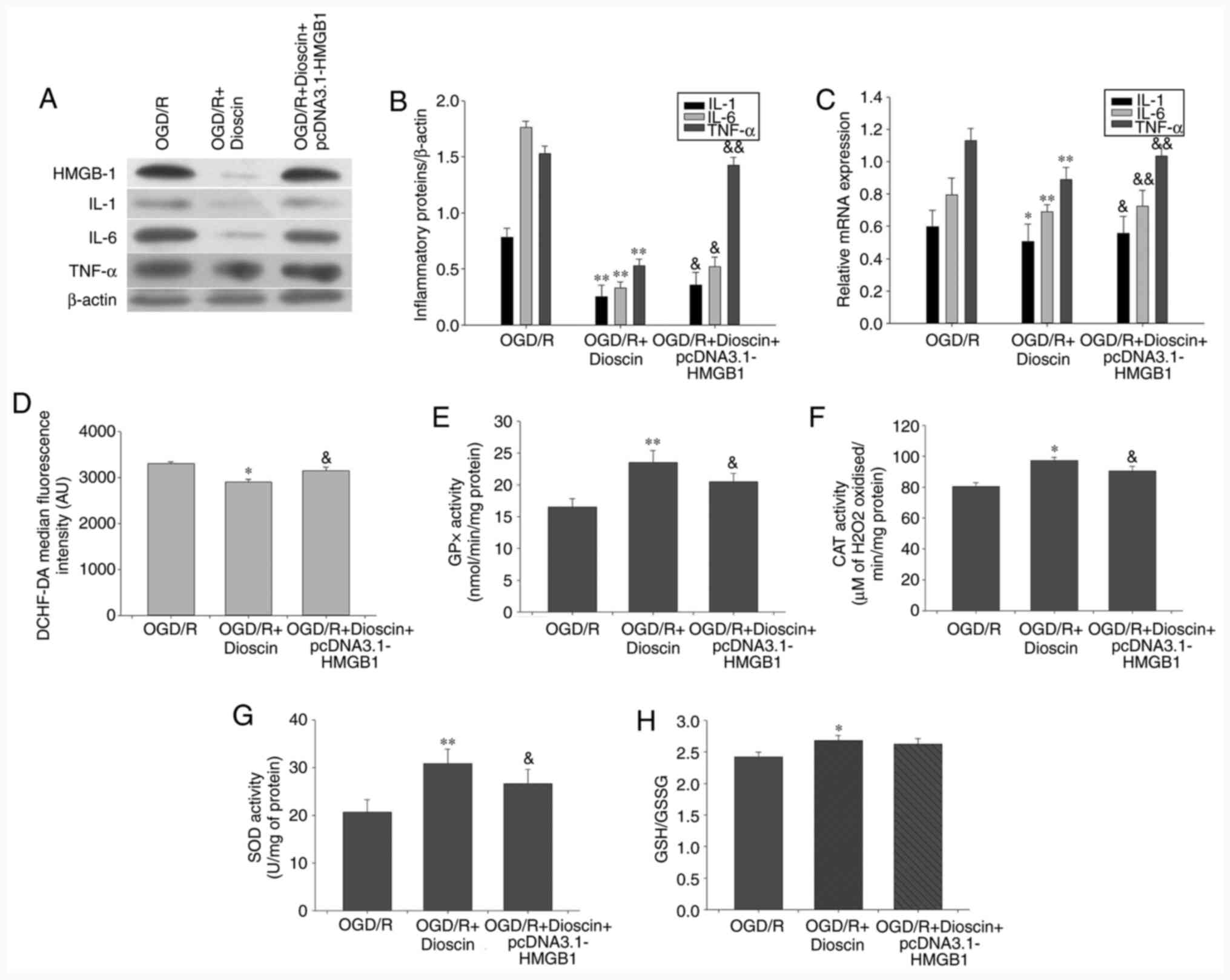 | Figure 7Inhibiting the activity of the
HMGB-1/RAGE pathway reversed the effect of dioscin on the
expression of proinflammatory cytokines, ROS generation and
antioxidant stress. (A) Western blot analysis results showing IL-1,
IL-6 and TNF-α protein expression. β-actin served as an internal
control. (B) Bar chart showing the IL-1/β-actin, IL-6/β-actin and
TNF-α/β-actin ratio from the western blot analysis results in
different groups. (C) Bar chart showing IL-1/β-actin, IL-6/β-actin
and TNF-α/β-actin ratio from RT-qPCR results in different groups.
(D) DCHF-DA fluorescence intensity in different groups using flow
cytometry. (E) GPx, (F) CAT and (G) SOD activity and (H) GSH/GSSG
ratio in different groups. Values are presented as the means ±
standard deviation (SD). *P<0.05 and
**P<0.01 vs. the OGD/R group.
&P<0.05 and &&P<0.01 vs.
the OGD/R + dioscin group. HMGB-1, high mobility group box
chromosomal protein 1; RAGE, receptor for advanced glycation end
products; ROS, reactive oxygen species; IL-1, interleukin 1; TNF-α,
tumor necrosis factor α; DCHF-DA, 2',7'-dichlorofluorescein
diacetate; GPx, glutathione peroxidase; CAT, catalase; SOD,
superoxide dismutase; GSH/GSSG, glutathione/glutathione disulphide;
OGD/R, oxygen-glucose deprivation/reperfusion. |
Activation of the HMGB-1/RAGE pathway
may reverse the effect of dioscin on antioxidant stress
The results demonstrated that HMGB-1 overexpression
reversed the protective effects of dioscin on ROS generation and
the activity of antioxidant enzymes (P<0.05; Fig. 7). Therefore, treatment of neurons
with the pcDNA3.1-HMGB-1 plasmid significantly elevated ROS
production compared with that observed in the OGD/R + dioscin group
(P<0.01; Fig. 7D). Furthermore,
compared with the OGD/R group, the activities of GPx, CAT and SOD
were increased in the OGD/R + dioscin group, while this effect was
restored following HMGB-1 overexpression (P<0.05; Fig. 7E-G). However, there was no marked
statistically significant difference in the degradation of GSH/GSSG
between the OGD/R + dioscin + pcDNA3.1-HMGB-1 and OGD/R + dioscin
groups (P<0.05; Fig. 7H).
Dioscin significantly alleviates
oxidative stress and apoptosis of hippocampal neurons in the MCAO
model via the HMGB-1/RAGE pathway
The results of the MCAO model demonstrated that
intracellular ROS production, and 3-NT and 8-OHdG expression in the
neurons were significantly elevated compared with the sham group
(P<0.01; Fig. 8A, B and G).
By contrast, MCAO significantly attenuated the activity of
antioxidant enzymes and non-enzymatic antioxidants compared with
the sham group (P<0.01; Fig.
8C-F). Furthermore, dioscin improved the antioxygenic ability
following MCAO. However, it did not elevate the activity of the
non-enzymatic antioxidants through the HMGB-1/RAGE pathway.
Additionally, HMGB-1 overexpression reversed the decreased ROS
production, and 3-NT and 8-OHdG expression, as well as the
increased activity of the antioxidant enzymes. In the MCAO model,
the apoptosis rate of the dioscin-treated hippocampal neurons was
attenuated compared with the MCAO group (P<0.05; Fig. 8H). In addition, HMGB-1
overexpression significantly increased neuronal apoptosis in rat
hippocampus in the MCAO + dioscin + HMGB-1 group. These results
suggested that dioscin may reverse the increased apoptosis rate via
the HMGB-1 pathway.
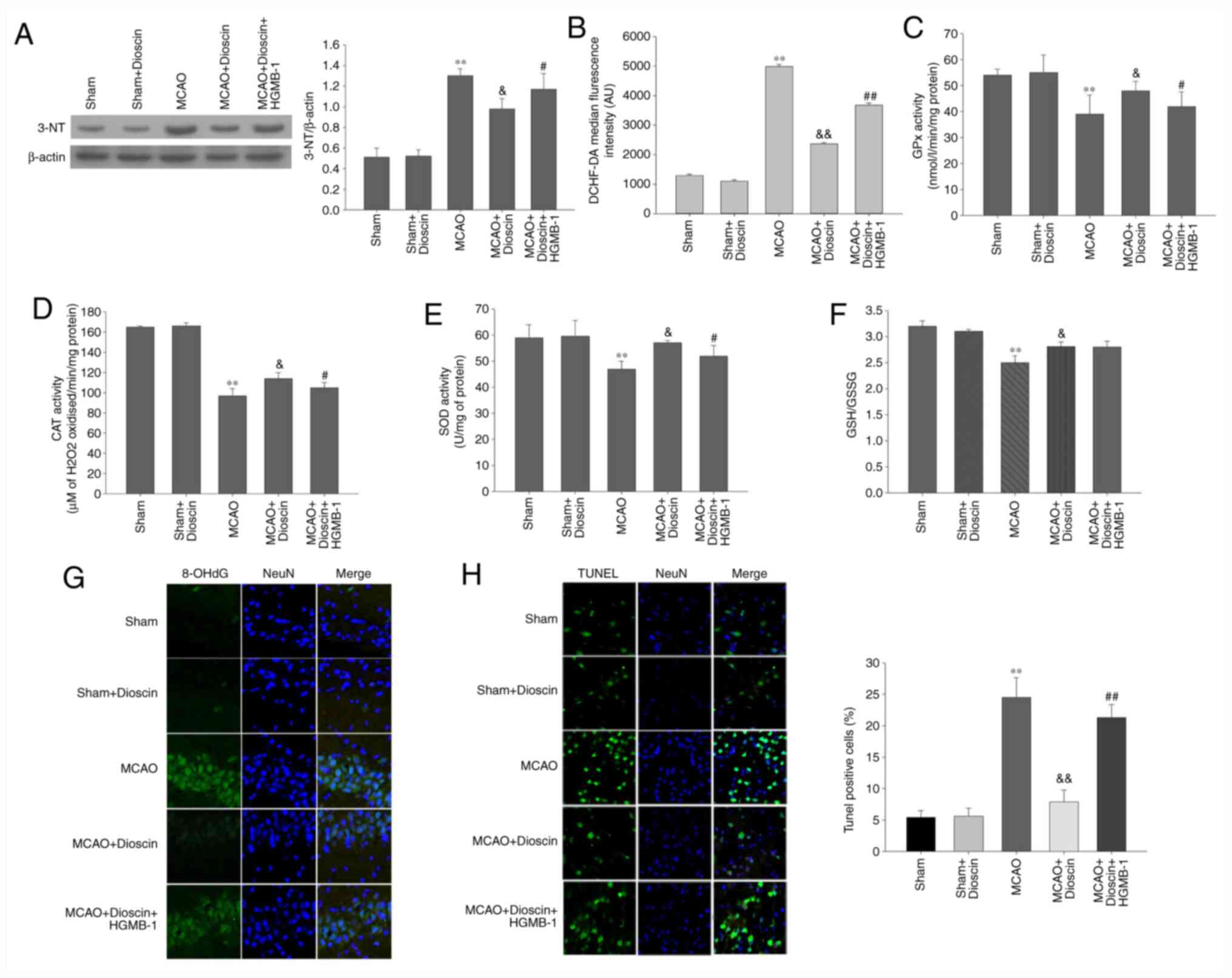 | Figure 8Dioscin remarkably alleviates
oxidative stress and apoptosis of hippocampal neurons in the MCAO
model via the HMGB-1/RAGE pathway. (A) The effect of dioscin and
HMGB-1 on the expression of the 3-NT protein in rat hippocampal
neurons. (B) DCHF-DA fluorescence intensity was quantified by flow
cytometry. Dioscin improved the enzymatic antioxidant defenses in
rat hippocampal neurons in the MCAO group via the HMGB-1 pathway.
(C) GPx, (D) CAT and (E) SOD activities were evaluated in rat
hippocampal neurons. Dioscin improved the non-enzymatic antioxidant
defenses in rat hippocampal neurons of the MCAO group. This effect
was not mediated via the HMGB-1 pathway. (F) The GSH/GSSG ratio was
evaluated in rat hippocampal neurons of the MCAO group. (G) The
effect of dioscin on the aggregation of the 8-OHdG protein in rat
hippocampal neurons. 8-OHdG (green) was immunolabeled in rat
hippocampal neurons. Magnification, x400. (H) Treatment with
dioscin decreased the number of TUNEL-positive apoptotic cells in
rat hippocampal tissue. Magnification, x200. Rat brain tissue
sections were stained with TUNEL (green) and DAPI (blue). The bar
chart shows the quantification of the TUNEL-positive cells (%).
Values are expressed as the mean ± standard error of the mean
(SEM). **P<0.01 vs. the sham group.
&P<0.05 and &&P<0.01 vs.
the MCAO group. #P<0.05 and ##P<0.01
vs. the MCAO + dioscin group. MCAO; middle cerebral artery
occlusion; HMGB-1, high mobility group box chromosomal protein 1;
RAGE, receptor for advanced glycation end products; DCHF-DA,
2',7'-dichlorofluorescein diacetate; GPx, glutathione peroxidase;
CAT, catalase; SOD, superoxide dismutase; GSH/GSSG,
glutathione/glutathione disulphide; 8-OHdG,
8-hydroxy-2'-deoxyguanosine; TUNEL, terminal deoxynucleotidyl
transferase-mediated (dUTP) nick-end labeling; DAPI,
4',6-diamidino-2-phenylindole. |
Discussion
With high fatality and morbidity rates, cerebral
ischemia not only seriously threatens patients' lives, but also
increases the financial burden placed on families and society
(28). An imbalance of energy
supply and demand is caused by cerebral artery occlusion, which in
turn leads to oxygen deficiency and glucose deprivation in the
brain, ultimately resulting in neuronal injury (29). Following recovery of blood supply to
the ischemic brain tissue, the excessive production of ROS may
cause neuronal damage, which may result in more severe cellular
dysfunction compared with hypoxia and ischemia only (30). The present study aimed to
investigate the protective effects of dioscin in OGD/R-subjected
neurons. The results indicated that dioscin exhibited
antioxidative, anti-inflammatory, and antiapoptotic effects.
Dioscin, a compound with several pharmacological and
biological activities, is a natural steroid saponin isolated from
multiple plants (31). It has been
reported that dioscin serves fundamental roles in several diseases,
including refractory apical periodontitis, acute renal injury, and
atherosclerosis (13,32,33).
The possible mechanisms underlying its effects include
anti-inflammatory, antioxidant stress and antiapoptotic activities.
A previous study has demonstrated that oral feeding of dioscin for
7 consecutive days downregulated the mRNA expression levels of
IL-1β, IL-6 and TNF-α in a hepatic IRI model (33). In addition, a recent study has
suggested that disocin exerts its antioxidant effects not only via
decreasing ROS production, but also by enhancing the activity of
antioxidant enzymes (13).
Furthermore, disocin mediates neuroprotection by suppressing
inflammation and inhibiting the HMGB-1/toll-like receptor 4 (TLR4)
pathway (34). These results were
generally consistent with the observations of the present study in
hippocampal neurons. Therefore, the results demonstrated that
OGD/R-subjected neurons increased the protein and mRNA expression
levels of IL-1β, IL-6 and TNF-α, concurrent with elevated ROS
generation and an attenuated activity of antioxidative enzymes.
Furthermore, treatment with dioscin (400 ng/ml) for 24 h remarkably
improved neuron viability and decreased the expression of
inflammatory cytokines. Subsequently, the balance of the oxidative
and antioxidative system was also evaluated. Therefore, the
activities of GPx, CAT and SOD, and the GSH/GSSG ratio were
significantly elevated in dioscin-treated neurons. These results
suggested that dioscin could exert neuroprotective effects,
possibly mediated by its anti-inflammatory and antioxidative
abilities.
Apart from its anti-inflammatory and antioxidant
effects, the results of the current study revealed that dioscin
attenuated neuron apoptosis via maintaining the homeostasis in the
expression of the apoptosis-associated genes Bax and Bcl-2, as well
as downregulating cleaved caspase-3 expression. These results were
consistent with those obtained in the study by Tao et al in
hepatic IRI (35). However, these
results were different from those in other studies. Therefore,
Zhang et al demonstrated that dioscin promoted cell
apoptosis (36). Additionally, in
hepatocellular carcinoma (HCC), dioscin significantly inhibited HCC
cell proliferation and upregulated the expression of cleaved
caspase-3 and Bax/Bcl-2. However, the possible mechanism underlying
the effects of dioscin on neuronal survival remains unclear.
Therefore, subsequent in-depth studies into its molecular
mechanisms of action are required.
Regarding the exact mechanisms underlying the
positive effects of dioscin, several studies have indicated that
dioscin exerts its anti-inflammatory, antioxidative and
antiapoptotic effects via the TLR4/myeloid differentiation factor
88 (MyD88) and peroxisome proliferator-activated receptor-γ
coactivator-1α (PGC-1α)/estrogen receptor α (ERα) signaling
pathways (13,37,38).
As an important injury-associated compound, dioscin has been
assumed to inhibit HMGB-1 in an IRI animal model (39). A previous study demonstrated that
dioscin downregulated the expression of HMGB-1 and other
inflammatory mediators, thus resulting in improving nerve injury
caused by ischemia/hypoxia (35).
HMGB-1 is considered as a crucial regulator in the early stages of
organ ischemia-reperfusion dysfunctions (40). It has been previously reported that
HMGB-1 exerts a variety of cytoprotective activities combined with
the downstream molecule RAGE (41).
In the present study, dioscin suppressed the oxidative and
inflammatory reactions, and attenuated OGD/R-associated apoptosis.
During this process, the HMGB-1/RAGE signaling pathway was
downregulated, thereby leading to a substantial decrease in the
cell apoptosis rate, in order to maintain an adequate number of
neurocytes. In addition, considerable changes in the intracellular
ROS production and the activity of antioxidant enzymes were
observed in the hippocampal neurons, probably mediated by the
downregulation of the HMGB-1/RAGE pathway. However, there were no
marked statistically significant differences in the GSH/GSSG ratios
between the OGD/R + dioscin + pcDNA3.1-HMGB-1 and OGD/R + dioscin
groups in vivo, as well as between the MCAO + dioscin and
MCAO + dioscin + HMGB-1 groups in vitro. Furthermore, to the
best of our knowledge, the association between the activation of
the HMGB-1/RAGE pathway and non-enzymatic antioxidant defenses has
not been previously investigated. This could be considered as
another mechanism of dioscin action associated with its antioxidant
effects, which requires further investigation. In addition to the
TLR4/MyD88 and PGC-1α/ERα signaling pathways described above
(13,37), numerous hypotheses have been
formulated to explain the internal mechanism of dioscin resistance
to OGD/R. As the neuroprotective effects of dioscin may involve
several mechanisms working together, a more comprehensive research
base is required to investigate the potential mechanisms that may
ensure the maintenance of neuronal function and survival.
In conclusion, treatment with dioscin decreased
nerve injury and enhanced cell viability. These neuroprotective
effects may primarily due to the maintenance of homeostasis in the
antioxidative/oxidative system, as well as antiapoptotic and
anti-inflammatory effects. Furthermore, the HMGB-1/RAGE signaling
pathway was also determined to be involved in these processes. The
results of the present study provided evidence in favor of
considering dioscin as a promising neuroactive compound for
ischemic stroke.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
available from the corresponding author on reasonable request.
Authors' contributions
AL, WZ and JH were involved in the study design, and
in the drafting and editing of the manuscript. AL, WZ, SW and YW
performed the experiments. JH, SW and YW were involved in data
interpretation. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Animal Care and Use
Committee, Tangshan Gongren Hospital, China (approval No.
GRYY-LL-2019-15).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Feigin V, Roth G, Naghavi M, Parmar P,
Krishnamurthi R, Chugh S, Mensah GA, Norrving B, Shiue I, Ng M, et
al: Global burden of stroke and risk factors in 188 countries,
during 1990-2013: A systematic analysis for the Global Burden of
Disease Study 2013. Lancet Neurol. 15:913–924. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Candelario-Jalil E: Injury and repair
mechanisms in ischemic stroke: Considerations for the development
of novel neurotherapeutics. Curr Opin Investig Drugs. 10:644–654.
2009.PubMed/NCBI
|
|
3
|
Stoll G, Kleinschnitz C and Nieswandt B:
Combating innate inflammation: A new paradigm for acute treatment
of stroke? Ann N Y Acad Sci. 1207:149–154. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Scaffidi P, Misteli T and Bianchi ME:
Release of chromatin protein HMGB1 by necrotic cells triggers
inflammation. Nature. 418:191–195. 2002.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang Z, Wu Y, Zhao Y, Xiao X, Liu J and
Zhou X: Dynamic changes in HMGB1 levels correlate with inflammatory
responses during cardiopulmonary bypass. Exp Ther Med. 5:1523–1527.
2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tsung A, Sahai R, Tanaka H, Nakao A, Fink
MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA and Billiar TR:
The nuclear factor HMGB1 mediates hepatic injury after murine liver
ischemia-reperfusion. J Exp Med. 201:1135–1143. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Xie J, Méndez J D, Méndez-Valenzuela V and
Aguilar-Hernández MM: Cellular signalling of the receptor for
advanced glycation end products (RAGE). Cell Signal. 25:2185–2197.
2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang F, Su X, Huang G, Xin XF, Cao EH,
Shi Y and Song Y: sRAGE alleviates neutrophilic asthma by blocking
HMGB1/RAGE signalling in airway dendritic cells. Sci Rep.
7(14268)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jang HJ, Han IH, Kim YJ, Yamabe N, Lee D,
Hwang GS, Oh M, Choi KC, Kim SN, Ham J, et al: Anticarcinogenic
effects of products of heat-processed ginsenoside Re, a major
constituent of ginseng berry, on human gastric cancer cells. J
Agric Food Chem. 62:2830–2836. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhao X, Tao X, Xu L, Yin L, Qi Y, Xu Y,
Han X and Peng J: Dioscin induces apoptosis in human cervical
carcinoma HeLa and SiHa cells through ROS-mediated DNA damage and
the mitochondrial signaling pathway. Molecules.
21(730)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang Y, Xu Y, Qi Y, Xu L, Song S, Yin L,
Tao X, Zhen Y, Han X, Ma X, et al: Protective effects of dioscin
against doxorubicin-induced nephrotoxicity via adjusting
FXR-mediated oxidative stress and inflammation. Toxicology.
378:53–64. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ikeda T, Ando J, Miyazono A, Zhu XH,
Tsumagari H, Nohara T, Yokomizo K and Uyeda M: Anti-herpes virus
activity of Solanum steroidal glycosides. Biol Pharm Bull.
23:363–364. 2000.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yang Q, Wang C, Jin Y, Ma X, Xie T, Wang
J, Liu K and Sun H: Disocin prevents postmenopausal atherosclerosis
in ovariectomized LDLR-/-mice through a PGC-1α/ERα pathway leading
to promotion of autophagy and inhibition of oxidative stress,
inflammation and apoptosis. Pharmacol Res.
148(104414)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lu B, Yin L, Xu L and Peng J: Application
of proteomic and bioinformatic techniques for studying the
hepatoprotective effect of dioscin against CCl4-induced liver
damage in mice. Planta Med. 77:407–415. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lu J, Zhang T, Sun H, Wang S and Liu M:
Protective effects of dioscin against cartilage destruction in a
monosodium iodoacetate (MIA)-indcued osteoarthritis rat model.
Biomed Pharmacother. 108:1029–1038. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dias KL, Correia Nde A, Pereira KK,
Barbosa-Filho JM, Cavalcante KV, Araújo IG, Silva DF, Guedes DN,
Neto Mdos A, Bendhack LM and Medeiros IA: Mechanisms involved in
the vasodilator effect induced by diosgenin in rat superior
mesenteric artery. Eur J Pharmacol. 574:172–178. 2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lee BK, Kim CJ, Shin MS and Cho YS:
Diosgenin improves functional recovery from sciatic crushed nerve
injury in rats. J Exerc Rehabil. 14:566–572. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gong G, Qin Y and Huang W: Anti-thrombosis
effect of diosgenin extract from Dioscorea zingiberensis CH
Wright in vitro and in vivo. Phytomedicine. 18:458–463.
2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zheng H, Wei Z, Xin G, Ji C, Wen L, Xia Q,
Niu H and Huang W: Preventive effect of a novel diosgenin
derivative on arterial and venous thrombosis in vivo. Bioorg Med
Chem Lett. 26:3364–3369. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liu J, Pasini S, Shelanski ML and Greene
LA: Activating transcription factor 4 (ATF4) modulates
post-synaptic development and dendritic spine morphology. Front
Cell Neurosci. 8(177)2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
National Research Council (US) Institute
for Laboratory Animal Research: Guide for the Care and Use of
Laboratory Animals. National Academies Press, Washington, DC, 1996.
urihttps://www.ncbi.nlm.nih.gov/books/NBK232589/simplehttps://www.ncbi.nlm.nih.gov/books/NBK232589/
doi: 10.17226/5140.
|
|
22
|
Xiong Y, Xia Y, Deng J, Yan X, Ke J, Zhan
J, Zhang Z and Wang Y: Direct peritoneal resuscitation with
pyruvate protects the spinal cord and induces autophagy via
regulating PHD2 in a rat model of spinal cord ischemia-reperfusion
injury. Oxid Med Cell Longev. 2020(4909103)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hissin PJ and Hilf R: A fluorometric
method for determination of oxidized and reduced glutathione in
tissues. Anal Biochem. 74:214–226. 1976.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Flohé L and Günzler WA: Assays of
glutathione peroxidase. Methods Enzymol. 105:114–121.
1984.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Aebi H: Catalase in vitro. Methods
Enzymol. 105:121–126. 1984.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hassan MQ, Akhtar MS, Akhtar M, Ansari SH,
Ali J, Haque SE and Najmi AK: Benidipine prevents oxidative stress,
inflammatory changes and apoptosis related myofibril damage in
isoproterenol-induced myocardial infarction in rats. Toxicol Mech
Methods. 25:26–33. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hankey GJ: Stroke. Lancet. 389:641–654.
2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Onwuekwe IO and Ezeala-Adikaibe B:
Ischemic stroke and neuroprotection. Ann Med Health Sci Res.
2:186–190. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cuzzocrea S, Riley DP, Caputi AP and
Salvemini D: Antioxidant therapy: A new pharmacological approach in
shock, inflammation, and ischemia/reperfusion injury. Pharmacol
Rev. 53:135–159. 2001.PubMed/NCBI
|
|
31
|
Tao X, Yin L, Xu L and Peng J: Dioscin: A
diverse acting natural compound with therapeutic potential in
metabolic diseases, cancer, inflammation and infections. Pharmacol
Res. 137:259–269. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yin W, Liu S, Dong M, Liu Q, Shi C, Bai H,
Wang Q, Yang X, Niu W and Wang L: A new NLRP3 inflammasome
inhibitor, Dioscin, promotes osteogenesis. Small.
16(e1905977)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Qi M, Zheng L, Qi Y, Han X, Xu Y, Xu L,
Yin L, Wang C, Zhao Y, Sun H, et al: Dioscin attenuates renal
ischemia/reperfusion injury by inhibiting the TLR4/MyD88 signaling
pathway via up-regulation of HSP70. Pharmacol Res. 100:341–352.
2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tao X, Sun X, Yin L, Han X, Xu L, Qi Y, Xu
Y, Li H, Lin Y, Liu K and Peng J: Dioscin ameliorates cerebral
ischemia/reperfusion injury through the downregulation of TLR4
signaling via HMGB-1 inhibition. Free Radic Biol Med. 84:103–115.
2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Tao X, Wan X, Xu Y, Xu L, Qi Y, Yin L, Han
X, Lin Y and Peng J: Dioscin attenuates hepatic
ischemia-reperfusion injury in rats through inhibition of
oxidative-nitrative stress, inflammation and apoptosis.
Transplantation. 98:604–611. 2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhang G, Zeng X, Zhang R, Liu J, Zhang W,
Zhao Y, Zhang X, Wu Z, Tan Y, Wu Y and Du B: Dioscin suppresses
hepatocellular carcinoma tumor growth by inducing apoptosis and
regulation of TP53, BAX, BCL2 and cleaved CASP3. Phytomedicine.
23:1329–1336. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yao H, Hu C, Yin L, Tao X, Xu L, Qi Y, Han
X, Xu Y, Zhao Y, Wang C and Peng J: Dioscin reduces
lipopolysaccharide-induced inflammatory liver injury via regulating
TLR4/MyD88 signal pathway. Int Immunopharmacol. 36:132–141.
2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhang W, Yin L, Tao X, Xu L, Zheng L, Han
X, Xu Y, Wang C and Peng J: Dioscin alleviates
dimethylnitrosamine-induced acute liver injury through regulating
apoptosis, oxidative stress and inflammation. Environ Toxicol
Pharmacol. 45:193–201. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhu S, Tang S and Su F: Dioscin inhibits
ischemic stroke-induced inflammation through inhibition of the
TLR4/MyD88/NF-κB signaling pathway in a rat model. Mol Med Rep.
17:660–666. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Liu A, Dirsch O, Fang H, Sun J, Jin H,
Dong W and Dahmen U: HMGB1 in ischemic and non-ischemic liver after
selective warm ischemia/reperfusion in rat. Histochem Cell Biol.
135:443–452. 2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Bortolotto V and Grilli M: Every cloud has
a silver lining: Proneurogenic effects of Aβ oligomers and HMGB-1
via activation of the RAGE-NF-κB axis. CNS Neurol Disord Drug
Targets. 16:1066–1079. 2017.PubMed/NCBI View Article : Google Scholar
|