Introduction
Thromboangiitis obliterans (TAO) is a
non-atherosclerotic inflammatory disease of unknown etiology,
usually affecting the small and medium arteries of the upper and
lower limbs. Long-term smoking is closely related to the incidence
of TAO (1). Epidemiology shows that
TAO is distributed all over the world. The prevalence of peripheral
arterial diseases can reach 16-66% in Korean and Japanese patients.
For Indian patients the value can be as high as 45-63%. In recent
years, it has been shown that the prevalence of TAO in women has
also increased, reaching 11-23% (2). Early TAO manifests as ischemic
symptoms, and in the advanced stage, symptoms such as limb ulcers
and gangrene appear (3). Although
there is a detailed understanding of the occurrence and development
of TAO, TAO has the characteristics of being difficult to cure
clinically. This condition is concurrent to vascular reocclusion
and eventually leads to amputation (4).
At present, the main treatment used for TAO includes
drug therapy and revascularization. In these methods the medical
team tries to enrich the collateral vessels, increase the blood
supply of the limb ischemic area and reduce the blood viscosity
(5,6). Usually, antiplatelet and vasodilator
drugs are used for the treatment of TAO, these includes 5-HT2
receptor antagonists, prostaglandins, and vascular endothelin-1
receptor antagonists. Prostaglandin sodium and cilostazol are the
widely used medications due to their good efficiency on TAO
(7,8). There are also surgical treatment
available to patients, such as endometrial ablation, vascular
bypass, lumbar sympathectomy, balloon dilatation or catheter
thrombolysis and amputation. Although a good clinical efficacy has
been achieved, the specific effects of treating TAO are still
controversial (9,10).
The pathological manifestations of TAO are mainly
the presence of white blood cells and inflammatory thrombus in the
diseased vessel wall, and the end stage is often accompanied by
fibrotic mature thrombus (11). The
immune system and inflammatory response play an important role in
the pathogenesis of TAO. The interaction between inflammation and
hemostasis increases the tendency of thrombosis, and chronic
inflammation can induce endothelial damage (12). Vascular endothelial growth factor
(VEGF) is an endogenous angiogenic growth factor that acts on
vascular endothelial cells and promotes vascular growth and
endothelial proliferation (13).
VEGF has an effect on limb ischemia caused by TAO and may play an
important role in the occurrence, development and treatment of TAO
(14). Interleukin-1 (IL-1) can
regulate the expression of various growth factors and inflammatory
factors, and its expression in vascular injury is elevated, which
plays an important role in the development of vascular injury
(15). Tumor necrosis factor-α
(TNF-α) is an inflammatory cytokine promoting thrombus formation by
inducing macrophage/monocyte expression (16). Previous studies have shown that
TNF-α may be involved in the pathological process of TAO (17).
Clinical treatment of TAO is still controversial
(18). In this study, patients with
TAO were treated with revascularization combined with drugs to
observe the clinical efficacy of the treatment and to evaluate the
therapeutic and prognostic role of VEGF, IL-1 and TNF-α in TAO.
Patients and methods
General materials
A total of 117 patients with TAO admitted to the
First Hospital of Lanzhou University (Lanzhou, China) from April
2012 to August 2015 were selected for this study. Patients treated
with revascularization combined with prostaglandin sodium and
cilostazol were included in group A (67 patients), and patients
treated with sodium and cilostazol were enrolled in group B (50
patients). There were 63 males and 4 females in group A, the age
range was 46-69 years, mean age 55.3±8.6 years, and mean disease
duration was 3.8±1.2 years. We had 36 cases of unilateral lesions
of lower extremities and 31 cases of bilateral lesions of lower
limbs. In group B, there were 48 males and 2 females, the age range
was 45-67 years, and mean age was 54.7±9.4 years. There were 29
cases of unilateral lesions of lower extremities and 21 cases of
bilateral lesions of lower limbs. All enrolled patients understood
the study and signed an informed consent form. This study program
was submitted to the Ethics Committee of the First Hospital of
Lanzhou University for review and was implemented after the
approval was obtained.
Inclusion and exclusion criteria
Inclusion criteria: Confirmed as TAO by limb
angiography; age between 44 to 70 years; Buerger test (19) was positive; clinical data were
complete. Patients in both groups were treated with prostate sodium
and cilostazol on the same day.
Exclusion criteria: Anti-inflammatory and
immunosuppressive drugs had been used in the past month; allergic
to this drug; surgical related contraindications; combined with
severe liver and kidney dysfunction, arteriosclerosis obliterans,
acute arterial embolism, hematopoietic dysfunction, malignant
tumors, endocrine systemic diseases, diabetic gangrene, mental
diseases; severe tissue necrosis and limb infections.
Treatment
Group B was given IV infusion of alprostadil 10 µg
(Beijing Tide Pharmaceutical Co., Ltd., batch no. H10980024) in
0.9% sodium chloride injection (100 ml), once a day for 2 weeks.
Cilostazol tablets (Shandong Lukang Pharmaceutical Group Saite Co.,
Ltd., batch no. H20054770) were given orally, twice a day for
long-term, each oral dose was 100 mg. Patients in group A underwent
revascularization, 45 patients underwent thrombolysis and balloon
angioplasty. Twenty-two patients underwent vascular bypass surgery,
and after the surgery low-molecular-weight heparin 0.4 ml was
injected subcutaneously. The follow-up treatment was consistent
with group B.
Arterial interventional angioplasty: Continuous
epidural anesthesia was performed, and the ipsilateral femoral
artery was punctured antegradely to perform a ‘turning over the
mountain’ approach. Limb angiography was used to confirm the
location of the lesion, the inflow and outflow tract, and degree.
5F catheter was used to dredge the occlusion artery under the
guidance of the circuit diagram, then followed the catheter to
dredge the lesion segment to the distal artery of the lesion. We
confirmed that the distal end of the catheter was located in the
true lumen of the vessel, and introduced a balloon with a diameter
of 2.5 mm to expand occlusive vessel from the distal end to the
proximal end. The superficial femoral artery or the aplicular
artery were often expanded with a 4.0 mm balloon.
Vascular bypass surgery: a continuous epidural
anesthesia was performed by dissociating the great saphenous vein
trunk, followed by a ligature of the great saphenous vein branch.
The proximal and distal end of the great saphenous vein was then
cut and ligatured. The proximal end was inserted with a thin tube,
which was used for injection of physiological saline, and the upper
part of the valve was pressurized, to assure that the physiological
saline would flow out from the distal end of the great saphenous
vein along the proximal end. The posterior tibial artery was
exfoliated from the anterior tibial muscle layer, and the
artificial blood vessel patch or the autologous great saphenous
vein surgery was performed. Then, the upper and lower great
saphenous veins were anastomosed to the common femoral artery and
posterior tibial artery.
Determination of efficacy
After 3 months of treatment, the curative effect was
evaluated. Healed: Normal walking 2.0 km without discomfort, skin
temperature and skin color improved obviously or returned to
normal, limp, rest pain and gangrene improved markedly or
disappeared, wound healing was good, distal arterial pulsation is
fully restored. Markedly effective: Normal walking 0.5 km without
discomfort, skin temperature, skin color and ischemic symptoms
improved, wound surface narrowed, distal arterial pulsation
partially recovered. Effective: Feeling uncomfortable after walking
300 m, body often feels numb, and skin temperature, skin color and
ischemic symptoms are alleviated. Ineffective: Failure to walk
normally, skin temperature, skin color and ischemic symptoms did
not improve or worsen. (Healed + markedly effective +
effective)/total number of cases x100% = treatment effective
rate.
Observation indicators
The intermittent claudication distance was observed
before the surgery and 6 months after surgery. The patient walked
at a constant speed and recorded the distance traveled until the
affected limb was forced to stop due to pain. The ES-100V3 Doppler
blood flow detector (Changsha Tengjian Medical Devices Co., Ltd.)
was used to detect the ankle brachial index (ABI), and the blood
flow waveform and contraction of the bilateral brachial artery and
foot dorsal artery were measured. The peak of bilateral brachial
artery systolic pressure was brachial artery pressure, and the
middle systolic pressure of foot dorsal artery was ankle artery
pressure. The ankle artery pressure was divided by the brachial
artery pressure in order to calculate the ABI value. Patients were
recorded for 12 months for presence of nausea and vomiting, skin
pruritus, abdominal pain, and coagulation abnormalities. Patients
were followed up once a year for 3 years in order to record the
occurrence of amputation. Patients with amputation in both groups
were enrolled in the study group, and those without amputation were
included in the control group.
Detection method
Venous blood samples (5 ml) were collected before
and 1 month after the beginning of the treatment. Serum VEGF, IL-1
and TNF-α levels were evaluated using enzyme-linked immunosorbent
assay (ELISA), referring to human VEGF, IL-1 and TNF-α ELISA
(Shanghai Hengfei Biotechnology Co., Ltd., CSB-E11718h-1,
CSB-E04620h-1, 130-094-023) instructions. The sample and the kit
were equilibrated for 30 min. Fifty microliters of the standard was
added to the standard well, and 50 µl of the sample was added to
the sample well. Fifty microliters of streptavidin-HRP was the
added and wells were covered with sealing film. Plates were
incubated at 37˚C for 60 min, and after the incubation period all
liquids were discarded, and wells were rinsed (5 times). Reagents A
and B were added to each well (50 µl), and plates were incubated at
37˚C for 10 min in the dark. Fifty microliters of the stop solution
was then added to each well, and the OD value was read at a
wavelength of 450 nm using a Biotek automatic microplate reader
(Shanghai Biyou Biotechnology Co., Ltd.), and the concentrations of
VEGF, IL-1 and TNF-α were calculated.
Statistical methods
Statistical analysis was performed using SPSS 20.0
(IBM Corp). The count data were expressed as the number/count
percentage (n/%). Chi-square test was used to compare the count
data between groups. When the theoretical frequency in the
Chi-square test was less than 5, the continuity correction
Chi-square test was used. Measurement data were expressed as mean ±
standard deviation (mean ± SD). The t-test was used to compare the
measurement data between groups, and paired t-test was used to
compare that before and after treatment. Risk factors were analyzed
by Logistic multivariate regression analysis. P<0.05, indicates
the difference is statistically significant.
Results
General materials of the two
groups
There were no significant differences in general
clinical data between the groups. General clinical data included
sex, age, body mass index (BMI), duration of disease, smoking
history, drinking history, hypertension, erythrocyte sedimentation
rate (ESR), lesion site, and ischemic staging (P>0.05) (Table I).
 | Table IGeneral materials of group A and group
B [n(%)] (mean ± SD). |
Table I
General materials of group A and group
B [n(%)] (mean ± SD).
| Category | Group A (n=67) | Group B (n=50) | t/χ2
value | P-value |
|---|
| Sex | | | 0.228 | 0.633 |
|
Male | 63 (94.03) | 48 (96.00) | | |
|
Female | 4 (5.97) | 2 (4.00) | | |
| Age (years) | 55.3±8.6 | 54.7±9.4 | 0.359 | 0.720 |
| BMI
(kg/m2) | 23.2±2.4 | 23.6±2.5 | 0.876 | 0.383 |
| Duration (year) | 3.8±1.2 | 3.6±1.4 | 0.830 | 0.408 |
| Smoking history | | | 0.632 | 0.427 |
|
Yes | 64 (95.52) | 46 (92.00) | | |
|
No | 3 (4.48) | 4 (8.00) | | |
| Drinking
history | | | 0.400 | 0.527 |
|
Yes | 59 (88.06) | 42 (84.00) | | |
|
No | 8 (11.94) | 8 (16.00) | | |
| Hypertension | | | 0.037 | 0.848 |
|
Yes | 6 (8.96) | 5 (10.00) | | |
|
No | 61 (91.04) | 45 (90.00) | | |
| ESR (mm/h) | | | 0.025 | 0.873 |
|
≥30 | 10 (14.93) | 8 (16.00) | | |
|
<30 | 57 (85.07) | 42 (84.00) | | |
| Lesion site | | | 0.211 | 0.646 |
|
Unilateral
lower limb | 36 (53.73) | 29 (58.00) | | |
|
Bilateral
lower limbs | 31 (46.27) | 21 (42.00) | | |
| Ischemic
staging | | | 2.613 | 0.271 |
|
Ischemic
period (stage I) | 28 (41.79) | 27 (54.00) | | |
|
Nutritional
disorder period (stage II) | 26 (38.81) | 18 (36.00) | | |
|
Tissue
necrosis period (stage III) | 13 (19.40) | 5 (10.00) | | |
Intermittent claudication distance and
ABI before and after treatment in the two groups
There was no significant difference in the
intermittent claudication distance and ABI between groups before
treatment (P>0.05). After treatment, the intermittent
claudication distance and ABI in both groups were significantly
higher than those measured before treatment (P<0.05). After
treatment, the intermittent claudication distance and ABI in group
A were significantly higher than those in group B (P<0.05)
(Table II).
 | Table IIComparison of intermittent
claudication distance and ABI before and after treatment in group A
and group B (mean ± SD). |
Table II
Comparison of intermittent
claudication distance and ABI before and after treatment in group A
and group B (mean ± SD).
| | Intermittent
claudication distance (km) | | | ABI | | |
|---|
| Group | n | Before
treatment | After
treatment | t value | P-value | Before
treatment | After
treatment | t value | P-value |
|---|
| Group A | 67 | 0.21±0.09 | 2.42±0.51 | 34.930 | <0.001 | 0.41±0.10 | 0.84±0.16 | 18.650 | <0.001 |
| Group B | 50 | 0.23±0.08 | 2.13±0.43 | 30.720 | <0.001 | 0.39±0.11 | 0.76±0.13 | 15.360 | <0.001 |
| t value | - | 1.246 | 3.249 | - | - | 1.025 | 2.893 | - | - |
| P-value | - | 0.215 | 0.002 | - | - | 0.307 | 0.005 | - | - |
Effective treatment rate of the two
groups
Effective rate in group A was 95.52%. Nineteen cases
(28.36%) were fully healed, treatment in 33 cases (49.25%) were
markedly effective, 12 cases (17.91%) were effective, 3 cases
(4.48%) were ineffective, and the effective treatment rate was
95.52%. Effective treatment rate in group B was 84.00%. Nine cases
were fully healed (18.00%), 15 cases (30.00%) were markedly
effective, 18 cases (36.00%) were effective, and 8 cases (16.00%)
were ineffective. The effective treatment rate of group A was
significantly higher than that of group B (P<0.05) (Table III).
 | Table IIIComparison of treatment effective
rate between group A and group B [n(%)]. |
Table III
Comparison of treatment effective
rate between group A and group B [n(%)].
| Group Group A | n 67 | Healed 19
(28.36) | Markedly effective
33 (49.25) | Effective 12
(17.91) | Ineffective 3
(4.48) | Effective treatment
rate (%) 95.52 |
|---|
| Group B | 50 | 9 (18.00) | 15 (30.00) | 18 (36.00) | 8 (16.00) | 84.00 |
| χ2
value | - | - | - | - | - | 4.463 |
| P-value | - | - | - | - | - | 0.035 |
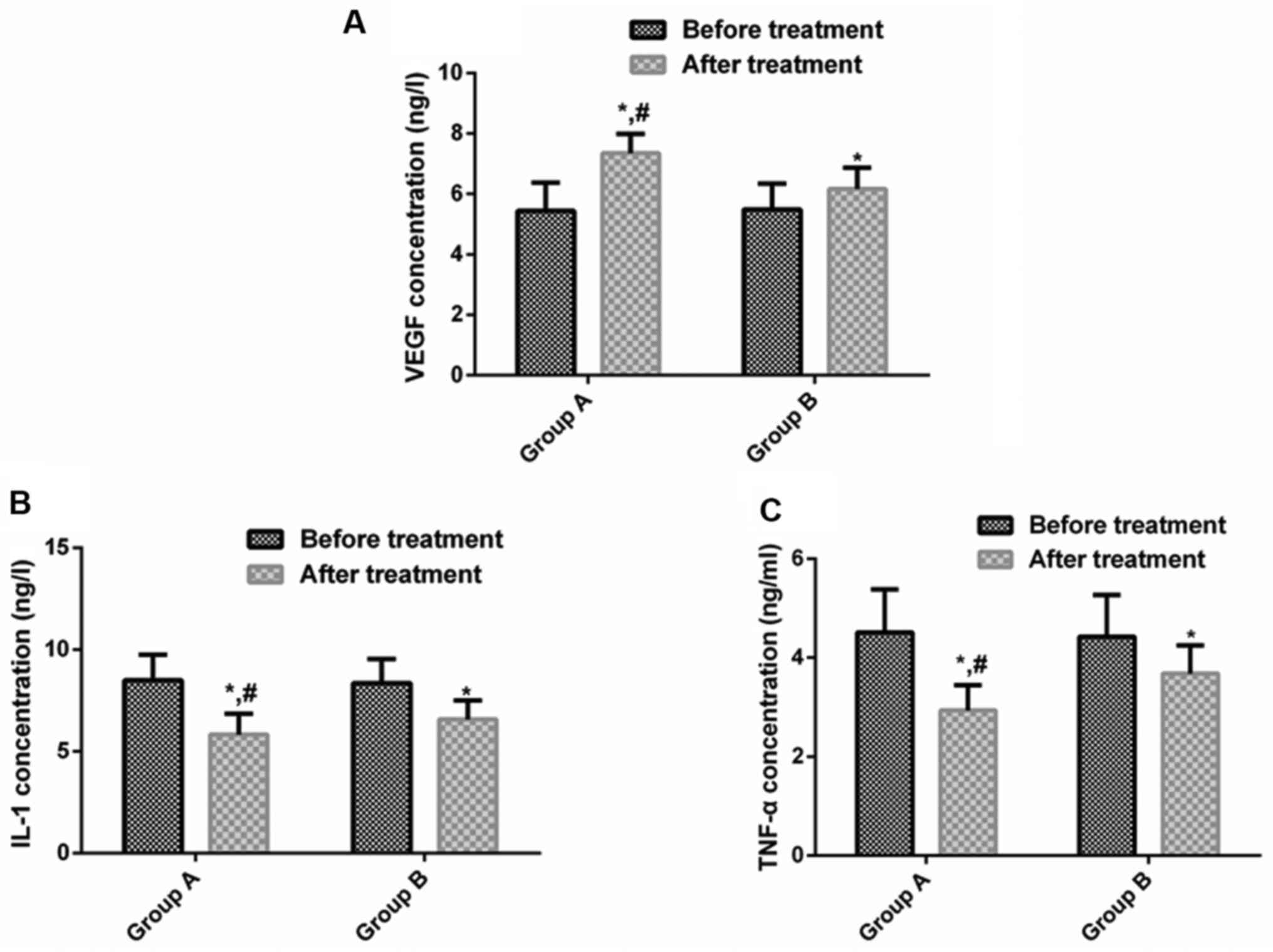
Changes of serum VEGF, IL-1 and TNF-α
concentrations before and after treatment in the two groups
There was no significant difference in serum VEGF,
IL-1 and TNF-α levels between groups before treatment (P>0.05).
After treatment, serum VEGF levels in both groups were both
obviously higher than those before treatment (P<0.05). IL-1 and
TNF-α levels were significantly declined (P<0.05). After
treatment, serum VEGF level in group A was distinctly higher than
that in group B (P<0.05), and IL-1 and TNF-α levels were
noticeably lower than those in group B (P<0.05) (Table IV and Fig. 1).
 | Table IVComparison of serum VEGF, IL-1 and
TNF-α concentrations before and after treatment in group A and
group B (mean ± SD). |
Table IV
Comparison of serum VEGF, IL-1 and
TNF-α concentrations before and after treatment in group A and
group B (mean ± SD).
| Category | Group A (n=67) | Group B (n=50) | t value | P-value |
|---|
| VEGF (ng/l) | | | | |
|
Before
treatment | 5.44±0.93 | 5.49±0.85 | 0.298 | 0.766 |
|
After
treatment | 7.35±1.02 | 6.16±0.97 | 6.462 | <0.001 |
|
t value | 15.150 | 8.492 | - | - |
|
P-value | <0.001 | <0.001 | - | - |
| IL-1 (ng/l) | | | | |
|
Before
treatment | 8.49±1.25 | 8.36±1.19 | 0.568 | 0.571 |
|
After
treatment | 5.83±1.02 | 6.58±0.93 | 4.084 | <0.001 |
|
t value | 13.500 | 8.334 | - | - |
|
P-value | <0.001 | <0.001 | - | - |
| TNF-α (ng/l) | | | | |
|
Before
treatment | 4.51±0.87 | 4.42±0.95 | 0.559 | 0.577 |
|
After
treatment | 2.93±0.51 | 3.68±0.57 | 7.482 | <0.001 |
|
t value | 12.820 | 5.113 | - | - |
|
P-value | <0.001 | <0.001 | - | - |
Adverse reactions and amputation rate
of the two groups
In group A, there were 3 cases (4.48%) with nausea
and vomiting, 1 case (1.49%) with skin pruritus, 2 cases (2.99%)
with abdominal pain, 2 cases (2.99%) with coagulation abnormality,
and 9 cases (13.43%) with amputation. In group B, there were 2
cases (4.00%) with nausea and vomiting, 1 case (2.00%) with skin
pruritus, 1 case (2.00%) with coagulation abnormality, and 15 cases
(30.00%) with amputation. There was no significant difference in
the incidence of nausea and vomiting, skin pruritus, abdominal
pain, coagulation abnormality and amputation between groups
(P>0.05). The amputation rate in group A was significantly lower
than that in group B (P<0.05) (Table
V).
 | Table VComparison of adverse reactions and
complications between group A and group B [n(%)]. |
Table V
Comparison of adverse reactions and
complications between group A and group B [n(%)].
| Category | Group A (n=67) | Group B (n=50) | χ2
value | P-value |
|---|
| Nausea and
vomiting | 3 (4.48) | 2 (4.00) | 0.016 | 0.899 |
| Skin pruritus | 1 (1.49) | 1 (2.00) | 0.044 | 0.834 |
| Abdominal pain | 2 (2.99) | 0 (0.00) | 1.518 | 0.218 |
| Coagulation
abnormality | 2 (2.99) | 1 (2.00) | 0.111 | 0.739 |
| Amputation | 9 (13.43) | 15 (30.00) | 4.820 | 0.028 |
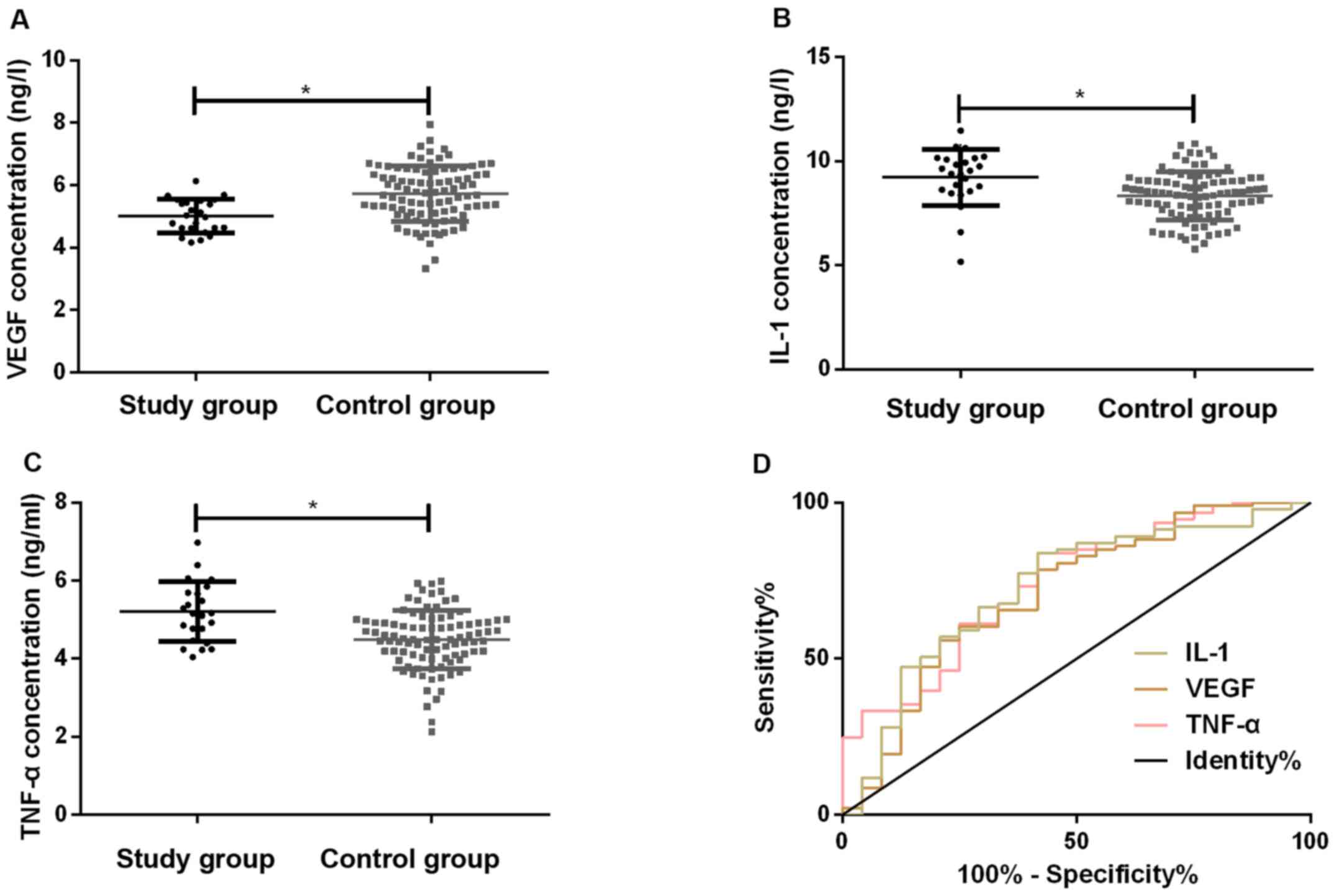
Diagnostic value of serum VEGF, IL-1
and TNF-α levels before treatment for amputation
Patients with amputation in both groups (24 cases)
were enrolled in the study group, and those without amputation were
included in the control group (93 cases). Before treatment, the
concentrations of serum VEGF, IL-1 and TNF-α were 5.04±0.53,
9.15±1.37, 5.23±0.78 ng/l in the study group, and 5.72±0.79,
8.21±1.36, 4.35±0.64 ng/l in the control group, respectively. The
serum VEGF level in the study group before treatment was
significantly lower than that in the control group (P<0.05),
while the levels of IL-1 and TNF-α were markedly higher than those
of the control group (P<0.05). The ROC curve prognosis of
amputation was evaluated in patients with TAO by pretreatment serum
VEGF, IL-1 and TNF-α levels. The most appropriate cutoff was
determined, taking sensitivity and specificity into account. The
AUC value of pretreatment serum VEGF level to determine the need
for amputation in TAO patients was 0.709, the sensitivity was
79.17%, the specificity was 58.06%, and the cutoff was 5.78 ng/l.
AUC value of pretreatment serum IL-1 level to determine the need
for amputation in TAO patients was 0.727, the sensitivity was
83.33%, the specificity was 59.14%, and the cutoff was 9.17 ng/l.
The AUC value of pretreatment serum TNF-α concentration to
determine the need for amputation in TAO patients was 0.741, the
sensitivity was 83.33%, the specificity was 60.22%, and the cutoff
was 5.08 ng/l (Table VI and
Fig. 2).
 | Table VIDiagnostic value of serum VEGF, IL-1
and TNF-α concentrations before treatment for amputation in
patients with TAO. |
Table VI
Diagnostic value of serum VEGF, IL-1
and TNF-α concentrations before treatment for amputation in
patients with TAO.
| Diagnostic
indicator | AUC | 95% CI | Std. Error | Cut-off (ng/l) | Sensitivity
(%) | Specificity
(%) |
|---|
| VEGF | 0.709 | 0.583-0.835 | 0.064 | 5.78 | 79.17 | 58.06 |
| IL-1 | 0.727 | 0.607-0.847 | 0.061 | 9.17 | 83.33 | 59.14 |
| TNF-α | 0.741 | 0.629-0.523 | 0.057 | 5.08 | 83.33 | 60.22 |
Analysis of risk factors affecting
amputation in patients with TAO
Logistic multivariate analysis of risk factors for
amputation in patients with TAO showed that sex, age, BMI, duration
of disease, drinking history, hypertension, ESR, and lesion site
were not associated with amputation in TAO patients (P>0.05).
Smoking history, ischemic staging, revascularization, VEGF, IL-1
and TNF-α were independent risk factors for amputation in TAO
patients (P<0.05). Low concentration of VEGF and high
concentration of IL-1 and TNF-α were risk factors for amputation
(Table VII).
 | Table VIILogistic multivariate analysis of
risk factors for amputation in patients with TAO. |
Table VII
Logistic multivariate analysis of
risk factors for amputation in patients with TAO.
| Variable | Regression
coefficients | Standard error | Wald value | P-value | OR value | 95% CI |
|---|
| Sex | 0.070 | 0.167 | 0.173 | 0.675 | 1.072 | 0.774-1.483 |
| Age (years) | 0.433 | 0.407 | 1.131 | 0.287 | 1.541 | 0.694-3.423 |
| BMI | 0.058 | 0.034 | 3.030 | 0.083 | 1.061 | 0.994-1.132 |
| Duration | 1.273 | 0.726 | 4.348 | 0.184 | 0.843 | 1.131-4.476 |
| Smoking
history | 1.521 | 0.391 | 15.183 | <0.001 | 4.573 | 2.129-9.823 |
| Drinking
history | 0.059 | 0.033 | 3.030 | 0.083 | 1.060 | 0.994-1.132 |
| Hypertension | 1.335 | 0.703 | 3.548 | 0.060 | 3.798 | 0.948-15.216 |
| ESR | 1.089 | 0.598 | 3.328 | 0.068 | 2.972 | 0.923-9.576 |
| Lesion site | 2.723 | 0.562 | 6.462 | 0.097 | 0.514 | 1.461-3.473 |
| Ischemic
staging | 1.118 | 0.373 | 9.057 | <0.001 | 3.062 | 1.478-6.345 |
|
Revascularization | 0.439 | 0.227 | 6.579 | <0.001 | 3.569 | 1.346-2.357 |
| VEGF | 0.345 | 0.166 | 4.273 | 0.038 | 1.412 | 1.018-1.957 |
| IL-1 | 0.781 | 0.347 | 5.083 | 0.026 | 2.183 | 1.108-4.305 |
| TNF-α | 0.882 | 0.391 | 4.871 | 0.026 | 2.416 | 1.105-5.283 |
Discussion
TAO has a variety of clinical treatment methods,
such as antiplatelet drugs, anticoagulants, and vasodilators,
although there is no evidence that they have a palliative effect
(20). Prostaglandin analogues are
also beneficial in the treatment of TAO, but there are still some
inadequacies in recent effects. Revascularization, sympathectomy,
Ilicavor technique and autologous omental fixation are still
controversial in the treatment of TAO (21).
Revascularization includes vascular bypass grafting
and percutaneous transluminal angioplasty (22). Vascular bypass grafting is widely
used in atherosclerotic diseases. Artificial blood vessels or
autologous blood vessels are used for bridging at the site of
diseased blood vessels, thereby improving the blood circulation of
tissues (23). Balloon dilatation
and stent implantation are important methods for percutaneous
transluminal angioplasty, with the characteristics of minimal
trauma, safety, and exact curative effect. However, these methods
are limited by the distal outflow tract and may cause reocclusion
of the diseased vessel (20). In
the study of Dilege et al (24), vascular bypass grafting was
performed on TAO, and the patency rates of 1, 2, and 3 years after
surgery were 59.2, 48.0 and 33.3%. The patency rate decreased with
time. Therefore, the long-term efficacy of revascularization is
questionable. In the study of Bozkurt et al (25), the 4-week effective rate of
prostacyclin in treating TAO patients was 61.9%, and the effective
rate was up to 85.3% with time. TAO was treated with
revascularization and supplemented with alprostadil and cilostazol
tablets. The results showed that the intermittent claudication
distance and ABI in both groups increased significantly after
treatment, and the intermittent claudication distance, ABI, and
effective treatment rate in group A were obviously higher than
those in group B. The amputation rate in group A was significantly
lower than that in group B. This suggested that revascularization
combined with drug therapy for TAO had a good clinical effect,
which can reduce the amputation rate of patients.
TAO is a thrombotic occlusive and inflammatory
peripheral arterial disease. Most scholars believe that the
occurrence of TAO is caused by the interaction of internal and
external factors (26). VEGF is a
secreted endothelial cell-specific mitogen which is the most
critical cytokine responsible for neovascularization (27). IL-1 is a central mediator that
regulates the inflammatory and immune responses in the body and is
an important pro-inflammatory factor (28). TNF-α has a direct cytotoxic effect,
which can destroy the structure of vascular endothelial cells,
leading to vascular endothelial dysfunction, which in turn leads to
the secretion of pro-inflammatory factor IL-1(29). Wan et al (30) reported that postoperative lower
extremity symptoms were significantly improved in TAO patients, and
VEGF levels were markedly elevated. The combination of VEGF with
other growth factors (such as angiopoietin-1, hepatocyte growth
factor) may become an alternative strategy to promote neovascular
growth. Yong et al (31)
reported that cilostazol combined with aspirin can improve the
clinical symptoms of patients, and can effectively reduce the level
of serum inflammatory factors in patients. Thus, promotion of
angiogenesis and inhibition of inflammatory factor levels may be
the key to treat TAO. The results of our study showed that serum
VEGF levels in both groups increased significantly after treatment,
while IL-1 and TNF-α levels declined significantly. After
treatment, serum VEGF level in group A was markedly higher than
that in the group B, while IL-1 and TNF-α levels were both
evidently lower than that in the group B. This suggests that
promotion of angiogenesis and inhibition of inflammatory factor
levels may be one of the therapeutic mechanisms of TAO. Wu et
al (32) showed that
revascularization can visibly improve the clinical symptoms and
dysfunction of patients with TAO. This was probably achieved by
inhibiting IL-6, IL-8 and TNF-α, and reducing inflammation. Whether
to perform revascularization and ischemic staging is one of the
factors affecting vascular reocclusion or amputation, which is
similar to this study. Other studies have shown that smoking
history, ischemic staging and whether vascular reconstruction is
performed are the risk factors for amputation in TAO patients.
However, Wu et al (32)
concluded that smoking history was not related to amputation in TAO
patients. This may be due to the fact that the patients in their
study were older and had longer smoke history than those used in
our study. Amputation of TAO patients often affects their life
quality (33). Previously, there
have been many studies on the risk factors of amputation in
patients with TAO (34-36),
but there is no report on whether VEGF, IL-1 and TNF-α levels are
risk factors for amputation in patients with TAO before treatment.
Results showed that VEGF, IL-1, and TNF-α had a certain diagnostic
value for amputation in patients with TAO before treatment, and low
concentrations of VEGF and higher concentrations of IL-1 and TNF-α
were risk factors for amputation in TAO patients. Therefore,
observation of VEGF, IL-1 and TNF-α levels may have predictive
value for amputation prognosis of patients with TAO.
This study confirmed the feasibility of
revascularization combined with alprostadil and cilostazol in the
treatment of TAO, and initially confirmed the predictive value of
VEGF, IL-1 and TNF-α in amputation in patients with TAO.
In conclusion, revascularization combined with
alprostadil and cilostazol in the treatment of TAO patients had a
good clinical efficacy with a possible therapeutic mechanism by
upregulating VEGF and inhibiting IL-1, TNF-α. Pretreatment serum
VEGF, IL-1 and TNF-α had a positive diagnostic value for poor
prognosis of patients with amputation, and high concentrations of
VEGF and high concentrations of IL-1 and TNF-α are risk factors for
amputation in patients with TAO.
Acknowledgements
Not applicable
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZFL wrote the manuscript. ZFL and WHW collected and
analyzed general data of patients. YWC, SYL and LD were responsible
for evaluation of treatment efficacy. YQS, YWB and XJS performed
ELISA. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the First Hospital of Lanzhou University (Lanzhou, China) and
informed consents were signed by the patients and/or the
guardians.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sun XL, Law BY, de Seabra Rodrigues Dias
IR, Mok SW, He YZ and Wong VK: Pathogenesis of thromboangiitis
obliterans: Gene polymorphism and immunoregulation of human
vascular endothelial cells. Atherosclerosis. 265:258–265.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rivera-Chavarría IJ and Brenes-Gutiérrez
JD: Thromboangiitis obliterans (Buerger's disease). Ann Med Surg
(Lond). 7:79–82. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Neufang A, Vargas-Gomez C, Ewald P,
Vitolianos N, Coskun T, Abu-Salim N, Schmiedel R, von Flotow P and
Savvidis S: Very distal vein bypass in patients with
thromboangiitis obliterans. Vasa. 46:304–309. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Klein-Weigel PF, Köning C, Härtwig A,
Krüger K, Gutsche-Petrak B, Dreusicke S, Thieme U, Enke-Melzer K,
Urbach B and Kron J: Immunoadsorption in Buerger's disease
(thromboangiitis obliterans): A promising therapeutic option:
Results of a consecutive patient cohort treated in clinical routine
care. Zentralbl Chir. 137:460–465. 2012.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
5
|
Jiménez-Gallo D, Albarrán-Planelles C,
Arjona-Aguilera C, Blanco-Sánchez G, Rodríguez-Mateos ME and
Linares-Barrios M: Treatment of thromboangiitis obliterans
(Buerger's disease) with high-potency vasodilators. Dermatol Ther
(Heidelb). 28:135–139. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Narváez J, García-Gómez C, Álvarez L,
Santo P, Aparicio M, Pascual M, López de Recalde M, Borrell H and
Nolla JM: Efficacy of bosentan in patients with refractory
thromboangiitis obliterans (Buerger disease): A case series and
review of the literature. Medicine (Baltimore).
95(e5511)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sugimoto M and Komori K: Buerger's disease
(thromboangiitis obliterans). In: Systemic Vasculitides: Current
Status and Perspectives. Dammacco F (ed). Springer, Switzerland,
pp361-376, 2016.
|
|
8
|
Galyfos G, Kerasidis S, Kastrisios G,
Giannakakis S, Sachmpazidis I, Anastasiadou C, Geropapas G,
Papapetrou A, Papacharalampous G and Maltezos C: Conservative
treatment of patients with thromboangiitis obliterans or
cannabis-associated arteritis presenting with critical lower limb
ischaemia. Vasa. 46:471–475. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fazeli B, Dadgar Moghadam M and Niroumand
S: How to treat a patient with thromboangiitis obliterans: A
systematic review. Ann Vasc Surg. 49:219–228. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Guo J, Guo L, Cui S, Tong Z, Dardik A and
Gu Y: Autologous bone marrow-derived mononuclear cell therapy in
Chinese patients with critical limb ischemia due to thromboangiitis
obliterans: 10-year results. Stem Cell Res Ther.
9(43)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Fazeli B and Rezaee SA: A review on
thromboangiitis obliterans pathophysiology: Thrombosis and
angiitis, which is to blame? Vascular. 19:141–153. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Huang ZH, Kuo SY, Chiu YH, Chen HC and Lu
CC: Treatment of multiple refractory ankle ulcerations in
thromboangiitis obliterans: A case report. Medicine (Baltimore).
97(e10798)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ferrara N and Adamis AP: Ten years of
anti-vascular endothelial growth factor therapy. Nat Rev Drug
Discov. 15:385–403. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Akar AR, İnan MB and Baran Ç:
Thromboangiitis obliterans. Curr Treatm Opt Rheumatol. 2:178–195.
2016.
|
|
15
|
Fazeli B, Rafatpanah H, Ravari H, Farid
Hosseini R, Tavakol Afshari J, Hamidi Alamdari D, Valizadeh N,
Moheghi N and Rezaee SA: Sera of patients with thromboangiitis
obliterans activated cultured human umbilical vein endothelial
cells (HUVECs) and changed their adhesive properties. Int J Rheum
Dis. 17:106–112. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nosaka M, Ishida Y, Kimura A, Kuninaka Y,
Taruya A, Furuta M, Mukaida N and Kondo T: Contribution of the
TNF-α (tumor necrosis factor-α)-TNF-Rp55 (tumor necrosis factor
receptor p55) axis in the resolution of venous thrombus.
Arterioscler Thromb Vasc Biol. 38:2638–2650. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shapouri-Moghaddam A, Saeed Modaghegh MH,
Rahimi HR, Ehteshamfar SM and Tavakol Afshari J: Molecular
mechanisms regulating immune responses in thromboangiitis
obliterans: A comprehensive review. Iran J Basic Med Sci.
22:215–224. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Olin JW: Thromboangiitis obliterans: 110
years old and little progress made. J Am Heart Assoc.
7(e011214)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Taniguchi T, Higuchi T, Tazaki J, Saito N
and Kimura T: Successful percutaneous transcatheter angioplasty of
radial artery in thromboangiitis obliterans (Buerger's disease).
JACC Cardiovasc Interv. 10:e205–e206. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kim DH, Ko YG, Ahn CM, Shin DH, Kim JS,
Kim BK, Choi D, Hong MK and Jang Y: Immediate and late outcomes of
endovascular therapy for lower extremity arteries in Buerger
disease. J Vasc Surg. 67:1769–1777. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Narváez J, García-Gómez C, Álvarez L,
Santo P, Aparicio M, Pascual M, López de Recalde M, Borrell H and
Nolla JM: Efficacy of bosentan in patients with refractory
thromboangiitis obliterans (Buerger disease): A case series and
review of the literature. Medicine (Baltimore).
95(e5511)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Del Conde I and Peña C: Buerger disease
(thromboangiitis obliterans). Tech Vasc Interv Radiol. 17:234–240.
2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jun HJ: Endovascular revascularization for
the obstruction after patch angioplasty in Buerger's disease.
Korean J Thorac Cardiovasc Surg. 47:174–177. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Dilege S, Aksoy M, Kayabali M, Genc FA,
Senturk M and Baktiroglu S: Vascular reconstruction in Buerger's
disease: Is it feasible? Surg Today. 32:1042–1047. 2002.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bozkurt AK, Köksal C, Demirbas MY, Erdoğan
A, Rahman A, Demirkiliç U, Ustünsoy H, Metin G, Yillik L, Onol H,
et al: Turkish Buerger's Disease Research Group: A randomized trial
of intravenous iloprost (a stable prostacyclin analogue) versus
lumbar sympathectomy in the management of Buerger's disease. Int
Angiol. 25:162–168. 2006.PubMed/NCBI
|
|
26
|
Fazeli B and Ravari H: Mechanisms of
thrombosis, available treatments and management challenges
presented by thromboangiitis obliterans. Curr Med Chem.
22:1992–2001. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Campochiaro PA, Khanani A, Singer M, Patel
S, Boyer D, Dugel P, Kherani S, Withers B, Gambino L, Peters K, et
al: TIME-2 Study Group: Enhanced benefit in diabetic macular edema
from AKB-9778 Tie2 activation combined with vascular endothelial
growth factor suppression. Ophthalmology. 123:1722–1730.
2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Palomo J, Dietrich D, Martin P, Palmer G
and Gabay C: The interleukin (IL)-1 cytokine family - Balance
between agonists and antagonists in inflammatory diseases.
Cytokine. 76:25–37. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Jain A, Barrile R, van der Meer AD,
Mammoto A, Mammoto T, De Ceunynck K, Aisiku O, Otieno MA, Louden
CS, Hamilton GA, et al: Primary human lung alveolus-on-a-chip model
of intravascular thrombosis for assessment of therapeutics. Clin
Pharmacol Ther. 103:332–340. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Wan J, Yang Y, Ma ZH, Sun Y, Liu YQ, Li GJ
and Zhang GM: Autologous peripheral blood stem cell transplantation
to treat thromboangiitis obliterans: Preliminary results. Eur Rev
Med Pharmacol Sci. 20:509–513. 2016.PubMed/NCBI
|
|
31
|
Yong J, Zhang S, Gao Y, Guo W, Shi P and
Zhou Q: Effects of aspirin combined with cilostazol on
thromboangiitis obliterans in diabetic patients. Exp Ther Med.
16:5041–5046. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wu S, Sun X, Wu W, Shi D and Jiang T:
Effect of revascularization on IL-6 and TNF-α in patients with
thromboangiitis obliterans. Exp Ther Med. 15:3947–3951.
2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Idei N, Soga J, Hata T, Fujii Y, Fujimura
N, Mikami S, Maruhashi T, Nishioka K, Hidaka T, Kihara Y, et al:
Autologous bone-marrow mononuclear cell implantation reduces
long-term major amputation risk in patients with critical limb
ischemia: A comparison of atherosclerotic peripheral arterial
disease and Buerger disease. Circ Cardiovasc Interv. 4:15–25.
2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sambandam MT, Boologapandian V and
Amalorpavanathan J: Limb salvage in critical limb ischaemia in
thromboangiitis obliterans patients - revascularisation - a study.
J Evol Med Dent Sci. 7:3679–3684. 2018.
|
|
35
|
Le Joncour A, Soudet S, Dupont A, Espitia
O, Koskas F, Cluzel P, Hatron PY, Emmerich J, Cacoub P,
Resche-Rigon M, et al: French Buerger's Network: Long-term outcome
and prognostic factors of complications in thromboangiitis
obliterans (Buerger's disease): A multicenter study of 224
patients. J Am Heart Assoc. 7(e010677)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ye K, Shi H, Qin J, Yin M, Liu X1, Li W1,
Jiang M and Lu X: Outcomes of endovascular recanalization versus
autogenous venous bypass for thromboangiitis obliterans patients
with critical limb ischemia due to tibioperoneal arterial
occlusion. J Vasc Surg. 66:1133–1142.e1. 2017.PubMed/NCBI View Article : Google Scholar
|