Introduction
An increasing number of cases of phalangeal defects
caused by surgical defects, palm defects or trauma, congenital
factors, malignant tumors and inflammation are encountered in the
clinic (1-3).
The unique anatomical structure of fingertips enables humans to
feel sensation and to perform fine processing and grasping
functions (4,5). Therefore, reconstruction and
restoration of metacarpal bone defects not only requires repair of
the wound surface but also to restore good fingertip sensation.
Finger length and aesthetic appearance also require to be
maintained (6,7). Repair of metacarpal defects has long
been a difficult challenge in plastic surgery (8).
Several methods may be used to treat bone defects,
including surgical treatment using autogenous, heterogeneous and
synthetic bone graft substitutes (9-11);
furthermore, gene therapy (12) and
growth factory therapy (13,14)
may be employed to accelerate bone growth and fusion. At present,
orthopaedic surgery using bone transplantation remains the most
commonly used method for repairing bone defects (15,16).
Autogenous bone transplantation is considered the gold standard of
bone transplantation (17),
although it has the disadvantages of limited bone, potential risk
of drug delivery sites and long-term hospitalization time (18,19).
Allogeneic bone transplantation is usually associated with immune
response and risk of disease transmission (20). Bone grafts and substitutes are the
most promising materials for bone defect repair and bone
implantation and they are also the focus of relevant research
(21,22).
It has been indicated that titanium alloys have the
advantages of light weight, good ductility, corrosion resistance
and high bone integration. Titanium alloys are now widely used in
orthopaedic and dental bone transplantation (23,24).
The present study was a retrospective cohort study of autogenous
bone transplantation for the repair of metacarpal defects. Its aim
was to explore the safety and clinical effect of titanium alloy
implantation in repairing metacarpal bone defect in order to
improve the clinical efficacy of bone transplantation.
Subjects and methods
Baseline information
A total of 64 cases of open metacarpal bone defect
treated with autologous bone graft or titanium alloy implantation
at The Third Hospital of Hebei Medical University (Shijiazhuang,
China) between June 2014 and December 2017 were included in the
present study. The inclusion criteria were as follows: Open
metacarpal bone defects within 3 weeks and one-stage debridement
and vacuum sealing drainage with a fresh wound surface. The
exclusion criteria were as follows: i) Age <18 years; ii) proper
digital artery defects; iii) proper digital nerve defects; iv)
combined with severe cardiovascular and cerebrovascular, kidney,
liver and hematopoietic diseases and endocrine system diseases, or
mental illnesses; v) severe brain injury and closed combined
thoracoabdominal injuries. Doctors and patients worked together to
formulate the surgical plan. Considerations included pain tolerance
of the patient, surgical tolerance of the patient, the economic
situation of the family of the patient and the patient's
willingness to undergo surgery. Patients with pain, low tolerance
to surgery and better economic conditions were recommended to opt
for new materials used in the surgery. During the study, 5 cases
dropped out and 8 cases were lost to follow-up. Hence, 53 patients
were finally recruited. There were 24 cases in the titanium alloy
implantation group (group A) and 29 cases in the autologous bone
grafting group (group B). Among these 53 cases, there were 41 males
and 12 females aged 21-56 years with an average of 36.54±9.56
years. A total of 31 cases had phalangeal bone defects and 22 cases
had metacarpal bone defects.
Surgical procedures
The implant in group A received titanium alloy
implants (Chinese patent no. 201620201366.X). The surgical
procedure was as follows: Brachial plexus blockage was performed.
An incision of appropriate length was made on the dorsal side of
the phalange for phalangeal defects or on the dorsum of hands for
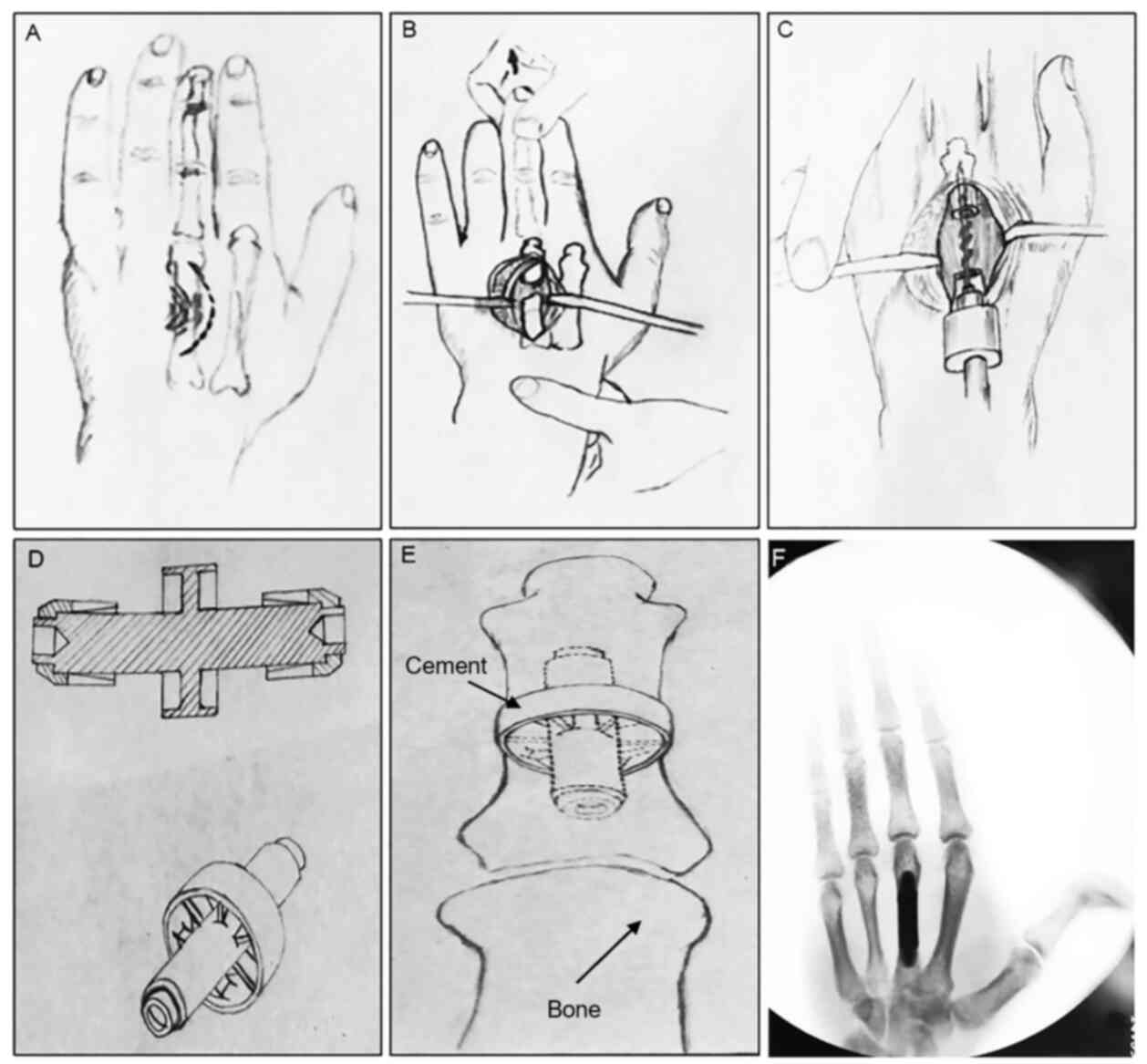
metacarpal bone defects (Fig. 1A).
The extensor tendon was tracted to expose the fractured bone and
bone defects (Fig. 1B).
Contaminated and non-vital tissues were removed within the surgical
field. The surrounding hardened edge was removed with bone nibbling
forceps. The edges of the fractured end were ground flat with a
bonesaw and flushed with normal saline-hydrogen peroxide. The wound
was soaked in benzalkonium chloride for 10 min. The medullary
cavity of phalange (or metacarpal bone) was dilated to accommodate
the proximal shaft of the prosthesis. Next, the reamer was inserted
to dilate the medullary cavity to accommodate the distal shaft of
the prosthesis (Fig. 1C). After
trial insertion of the prosthesis and determination of the proper
size, the prosthesis to be installed was taken out and bone cement
was applied to it (Fig. 1D). The
proximal prosthesis was first inserted, then the distal prosthesis.
The prosthesis was reduced, waiting for the hardening of the bone
cement 10 min later (Fig. 1E).
Finally, after X-ray film acceptance, the extensor tendon was
realigned and the incision was sutured layer by layer using
non-invasive thread (Fig. 1F).
Patients in group B received brachial plexus
blockage. For the harvesting of the iliac bone, epidural anesthesia
or local anesthesia was given. An appropriate length incision was
taken from the dorsal side of the phalanx, and an appropriate
length incision a was taken from the dorsal side of the metacarpus
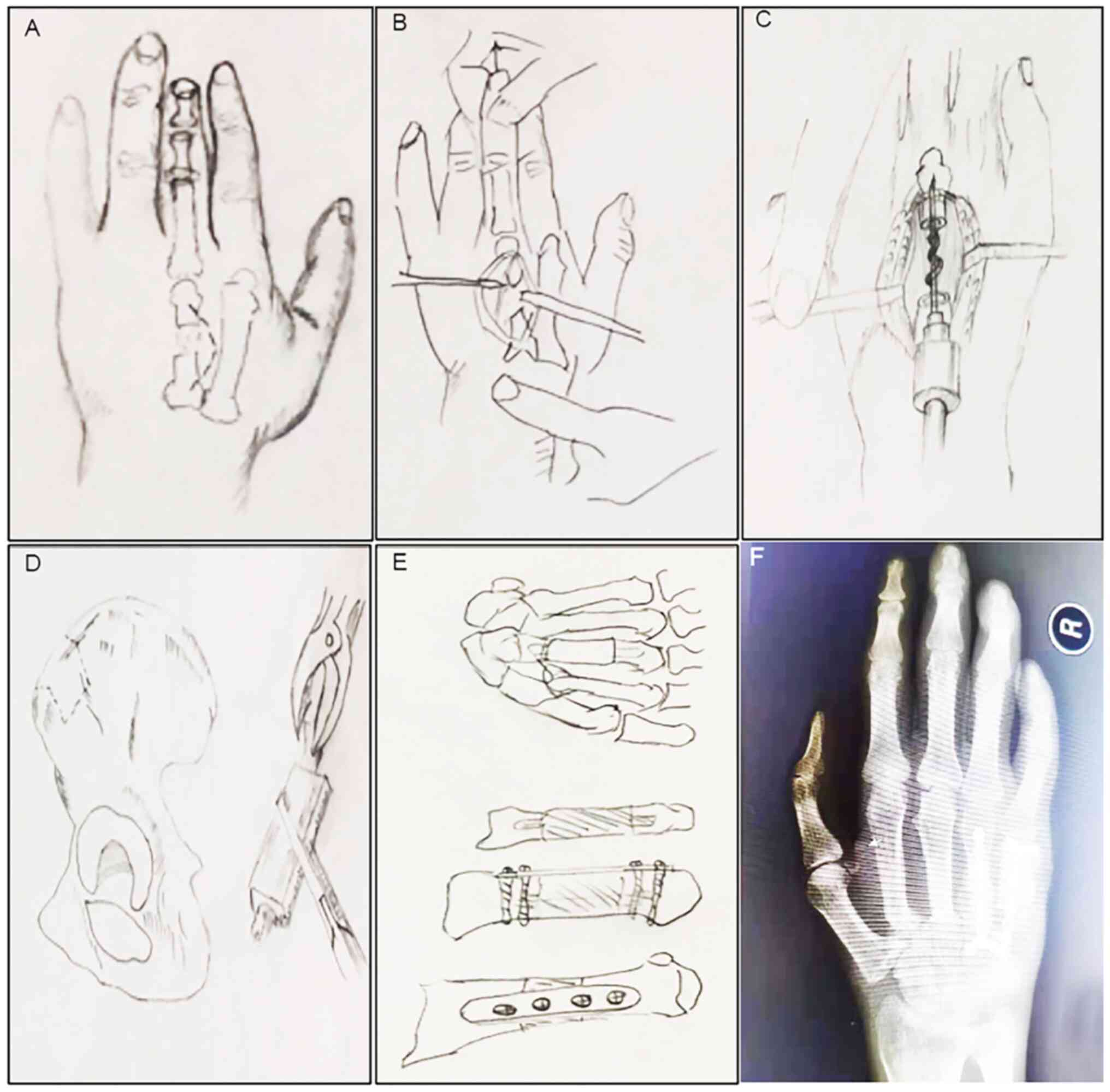
(Fig. 2A). The tendon was stretched
to expose the broken bone and bone defect site (Fig. 2B), and the contaminated and lifeless
tissue in the operation field was removed. The sclerotic edge
around the broken end was removed using bone biting forceps, and
the edge of the broken end was polished and leveled with a bone
saw, washed with normal saline hydrogen peroxide, and soaked with
benzalkonium chloride for 10 min. Then, the pulp cavity of phalanx
(or metacarpal bone) was expanded to accommodate the proximal stem
of prosthesis, and then the reamer was inserted to enlarge the
medullary cavity to accommodate the distal stem of prosthesis
(Fig. 2C). The bone needed for bone
grafting is typically autologous iliac bone (Fig. 2D). In cases with relatively few bone
defects, distal radius can also be selected. When the iliac bone is
removed, the bone block should be slightly longer than the defect
area by 0.5-1 cm, so that the two ends of the bone block are cut
into small wedges and embedded into the marrow cavity at the
fracture end. If there is only one broken end (such as end segment
defect), one end is cut into a wedge shape and inserted into the
medullary cavity near the fracture end, which not only increases
the contact area but also increases the stability (Fig. 2E). The periosteum of the donor site
was repaired after removal of the bone. According to the specific
situation, steel plate, screw or Kirschner wire were selected for
fixation, and then X-ray film was taken to accept (Fig. 2F). In order to reduce the tension of
the skin suture, cross or parallel Kirschner wires were used to fix
the defects of the middle and distal segments of the fingers.
Finally, the incision was sutured layer by layer with non-invasive
suture.
Post-operative treatment
Patients in the two groups received routine
antibiotics treatment for 5-7 days and detumescence with mannitol
for 3-5 days. The affected limb was elevated to facilitate
detumescence. The patients received frontal and oblique X-rays of
the affected hand at 1 day after surgery to assess bone length
after filling in the bone defect and the position of the implant or
bone graft. The patients wore a plaster caster support for 4-6
weeks. The patients began active and passive functional exercise at
1 month after surgery under guidance. Group A began partial
weight-bearing exercise at 1 month after surgery and group B began
partial weight-bearing exercise at 3 months after surgery according
to the extent of bone fusion and complete weight-bearing exercise
after the bone fracture completely healed.
Observation indicators
i) Post-operative pain intensity: Pain was assessed
by using the visual analog pain scale (VAS) (25) at day 3 and day 7 after surgery,
respectively, on which 10 points represented the maximal pain. ii)
Operation time: The operation time was the time span from the start
of surgery to the completion of incision suturing. iii) Time to
bone fusion: The patients were re-examined by X-rays at week 4, 8,
10, 12 and 16 after surgery to assess bone fusion. iv) Functional
recovery of the hand: Functional recovery of the hand was assessed
using the total active flexion scale (TAFS) developed by the
American Society for Surgery of the Hand (26). According to the scoring criteria of
TAFS, ‘Excellent’ recovery was defined as flexion of the
metacarpophalangeal joint and interphalangeal joint by >220̊,
‘good’ as 180-220̊ and ‘poor’ as <180̊. v) Surgical
complications: Infection of incision site, necrosis of the
fingertip, malunion and adhesion were recorded.
Statistical analysis
SPSS 20.0 (IBM Corp.) was used for statistical
analysis. Enumeration data were expressed as the n (%) and the
difference was compared by using the χ2 test. Continuous
variables with a normal distribution (assessed using the
Kolmogorov-Smirnov test) of data were expressed as the mean ±
standard deviation and differences were compared by using the
t-test, while the median and inter-quartile range (P25, P75) were
used to represent the continuous variables with a non-normal
distribution, and the Kruskal-Wallis test was used to compare the
differences. α=0.05, P<0.05 was considered to indicate a
statistically significant difference.
Results
Baseline information of the two
groups
Group A comprised 24 cases, including 19 males
(79.1%) and 5 females (20.8%), who were aged 21-55 years with an
average age of 37.58±8.47 years. The average size of the area
defect was 267.3±85.9 mm2. The causes of bone defects
were crush injury in 6 cases (25.0%), cut injury in 8 cases (33.3%)
and motor vehicle collisions in 10 cases (41.7%). There were 15
cases (62.5%) with phalangeal defects and 9 cases (37.5%) with
metacarpal bone defects. Group B comprised 29 cases, including 22
males (75.9%) and 7 females (24.1%), who were aged 21-56 years with
an average age of 35.69±10.43 years. The average size of the area
defect was 243.8±94.7 mm2. The causes of bone defects
were crush injury in 9 cases (31.0%), cut injury in 8 cases (24.1%)
and motor vehicle collisions in 13 cases (44.9%). There were 16
cases (55.2%) with phalangeal defects and 13 cases (44.8%) with
metacarpal bone defects. The two groups were comparable regarding
their baseline information without any significant differences
(P>0.05, Table I).
 | Table IComparison of baseline information
between the two groups. |
Table I
Comparison of baseline information
between the two groups.
| Item | Group A (n=24) | Group B (n=29) | χ2/t
statistic | P-value |
|---|
| Gender | | | 0.082 | 0.775 |
|
Male | 19 (79.1) | 22 (75.9) | | |
|
Female | 5 (20.8) | 7 (24.1) | | |
| Cause of injury | | | 0.592 | 0.744 |
|
Crush
injury | 6 (25.0) | 9 (31.0) | | |
|
Cut
injury | 8 (33.3) | 7 (24.1) | | |
|
Motor
vehicle collision | 10 (41.7) | 13 (44.9) | | |
| Site of injury | | | 0.290 | 0.590 |
|
Phalange | 15 (62.5) | 16 (55.2) | | |
|
Metacarpal
bone | 9 (37.5) | 13 (44.8) | | |
| Age (years) | 37.58±8.47 | 35.69±10.43 | 0.715 | 0.478 |
| Defect area
(mm2) | 267.3±85.9 | 243.8±94.7 | 0.946 | 0.344 |
Post-operative pain in the two
groups
The VAS scores in the two groups at day 3 and day 7
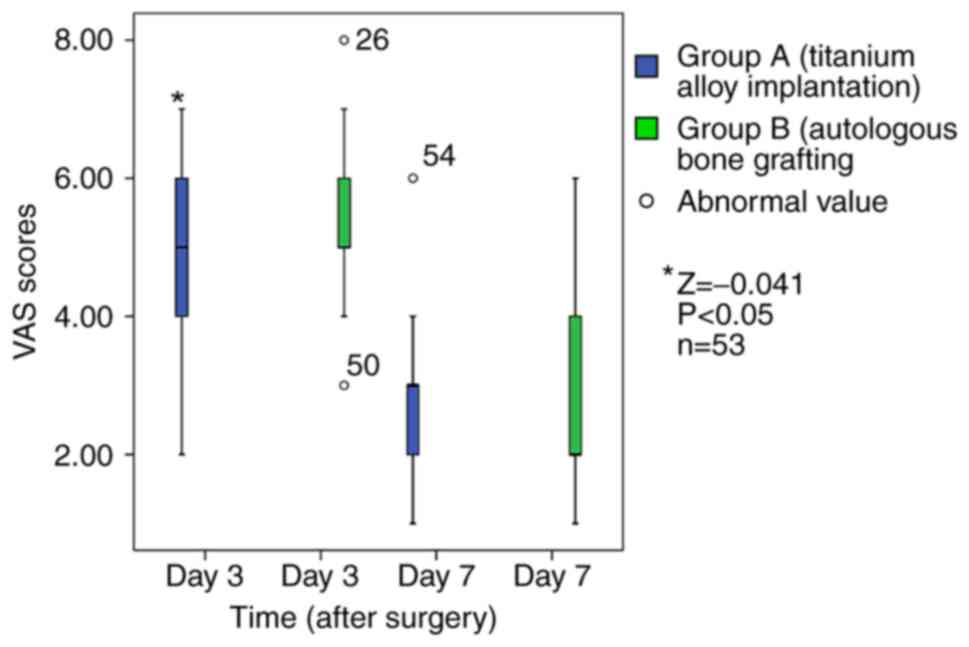
after surgery are shown in Fig. 3.
At day 3, the VAS score of group A [5(4, 6)] was significantly
lower than that of group B [5(5, 6)] (Z=-0.041, P<0.05). At day
7, there was no significant difference in the VAS scores between
the two groups (P>0.05; Table
II). Patients in both groups with a higher pain score and lower
pain tolerance were given pethidine as an analgesic aid within 24
h.
 | Table IIComparison of VAS scores between the
two groups at day 3 and day 7 after surgery. |
Table II
Comparison of VAS scores between the
two groups at day 3 and day 7 after surgery.
| Day after
surgery | Group A (n=24) | Group B (n=29) | t statistic | P-value |
|---|
| 3 | 4.75±1.32 | 5.51±1.58 | -2.132 | 0.025 |
| 7 | 2.75±1.07 | 2.89±1.47 | -0.406 | 0.686 |
Comparison of surgery and
hospitalization costs between the two groups
All 53 cases included were fully prepared prior to
surgery and all of the surgeries were successful. There were no
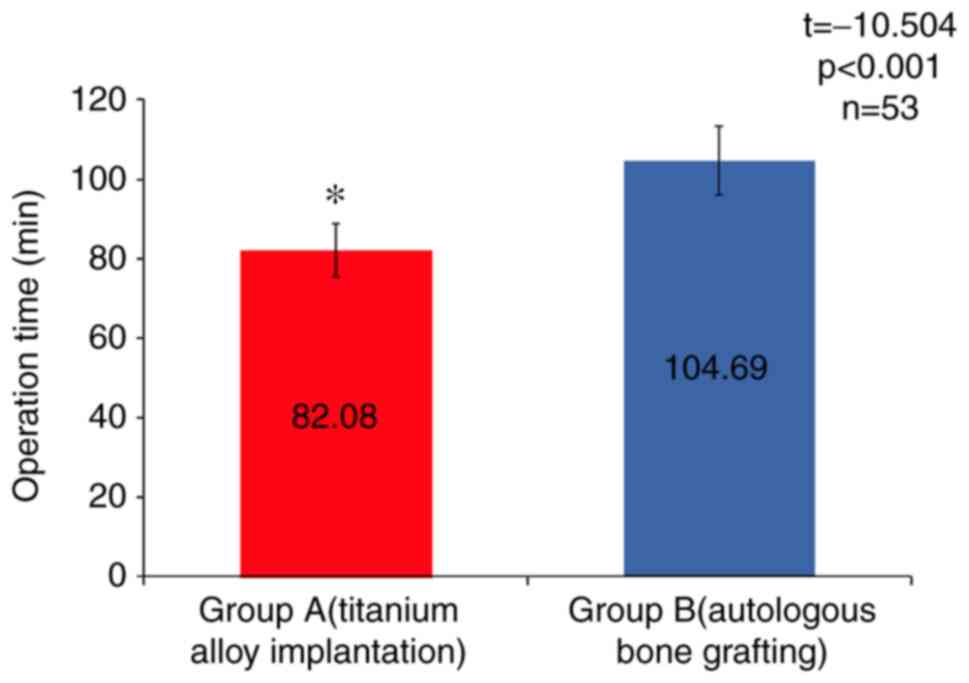
interoperative or anesthetic complications. The average operation
time was 82.08±6.64 min in group A, which was significantly shorter
than that in group B (104.69±8.63 min, t=-10.504, P<0.001;
Fig. 4). In all patients,
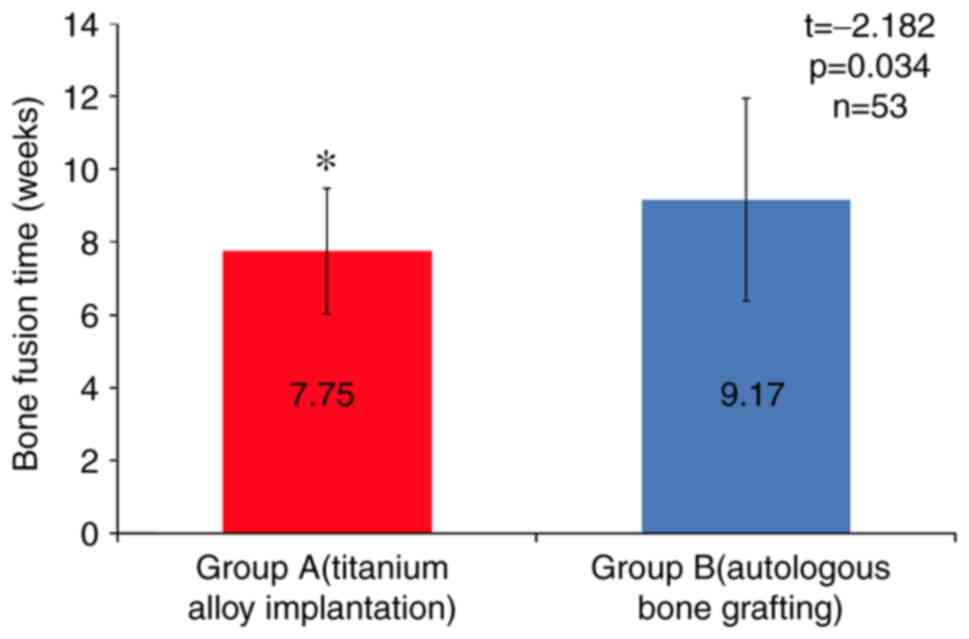
radiographs indicated satisfactory bone fusion. The average time to
bone fusion was 7.75±1.73 weeks in group A, which was significantly
shorter than that in group B (9.17±2.78 weeks; t=-2.182, P<0.05;
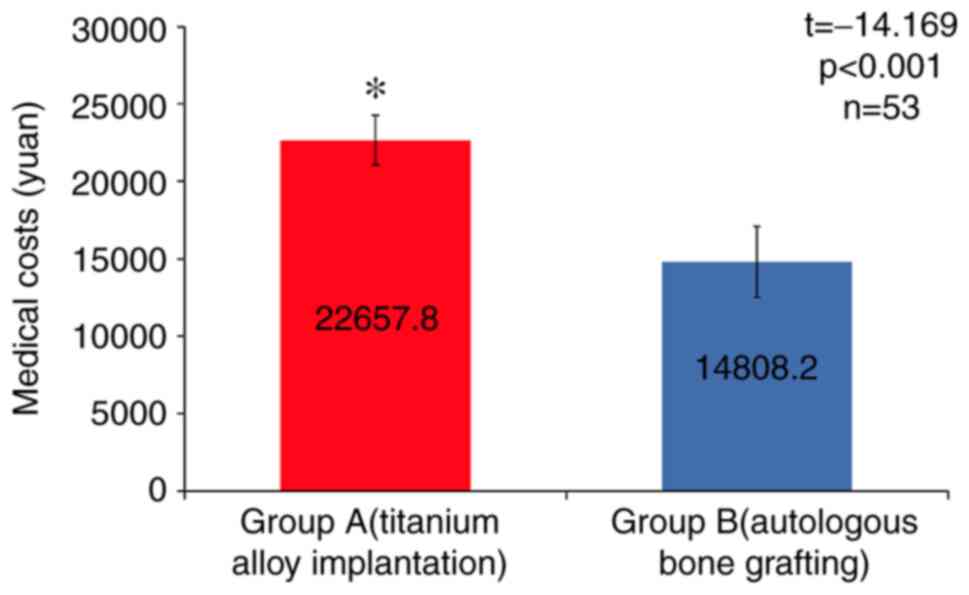
Fig. 5). The average medical cost
in group A was (22657.8±1595.4 Ұ), which was significantly higher
than that in group B (14808.2±2291.3 Ұ, t=144.169, P<0.05;
Fig. 6).
Comparison of TAFS scores between the
two groups
Functional recovery of the hand was assessed by TAFS
scoring 14 weeks postoperatively. As presented in Table III, group A comprised 15 cases
(62.5%) with excellent TAFS scores, 7 cases (29.2%) with good TAFS
scores and 2 cases (8.3%) with poor TAFS scores. The overall
excellent and good recovery rate was 91.7%. In group B, there were
165 cases (55.2%) with excellent TAFS scores, 10 cases (34.5%) with
good TAFS scores and 3 cases (10.3%) with poor TAFS scores. The
overall excellent and good recovery rate was 89.7%. The
χ2 test was used for comparison of excellent and good
recovery rates between the two groups, and χ2=0.062 and
P>0.05 was obtained, indicating no significant difference
(Table III).
 | Table IIIComparison of rating based on TAFS
scores between the two groups 14 weeks post-operatively. |
Table III
Comparison of rating based on TAFS
scores between the two groups 14 weeks post-operatively.
| | Rating | |
|---|
| Group | Excellent | Good | Poor | Excellent and
good | χ2
statistic | P-value |
|---|
| A (n=24) | 15 (62.5) | 7 (29.2) | 2 (8.3) | 22 (91.7) | 0.062 | 0.803 |
| B (n=29) | 16 (55.2) | 10 (34.5) | 3 (10.3) | 26 (89.7) | | |
Comparison of post-operative
complications between the two groups
The major post-operative complications for patients
with metacarpal bone defects included skin reddening and swelling,
infection of the incision site, malfusion and loosening of internal
fixation. For group A, the overall incidence of post-operative
complications was 33.3%, and that of group B was 41.3%. The
χ2 test provided χ2=0.362 and P>0.05,
indicating no significant difference (Table IV). All post-operative
complications were treated symptomatically.
 | Table IVComparison of post-operative
complications between the two groups. |
Table IV
Comparison of post-operative
complications between the two groups.
| | Complications | |
|---|
| Group | Skin reddening and
swelling | Incision
infection | Malfusion | Loosening of
internal fixation | Incidence | χ2
statistic | P-value |
|---|
| A (n=24) | 3 (12.5) | 2 (8.3) | 1 (4.2) | 2 (8.3) | 8 (33.3) | 0.362 | 0.547 |
| B (n=29) | 4 (13.8) | 3 (10.3) | 2 (6.9) | 3 (10.3) | 2 (41.3) | | |
Discussion
With the rapid development of economy,
transportation and construction industry, the number of patients
with hand trauma is exhibiting yearly increases (27,28).
Studies indicate that hand injuries account for 25-30% of the total
cases of traumatic surgery (29-31).
Hand injuries are usually accompanied by bone and joint injuries.
Maximization of the efficiency of the repair of bone and joint
defects has been a major clinical challenge. Autografts, allografts
and synthetic bone graft substitutes have been indicated to be
effective in repairing bone defects (32). In the present study, autologous bone
grafts (n=29) and titanium alloy implants (n=24) were respectively
used to treat metacarpal bone defects in 53 patients who achieved
good bone fusion, as well as hand appearance and functional
recovery. The excellent and good recovery rate reached up to
91.6%.
Due to their osteoconductivity, osteoinductivity and
osteogenesis, autogenous bone grafts are able to integrate into the
host bone more rapidly and completely compared with synthetic bone
substitutes. Autogenous bone grafts are generally considered the
gold standard for repairing bone defects and are also the benchmark
for evaluating other bone grafts and bone substitutes (15). However, it is not always possible to
use autologous bone grafts for bone defect repair (32). Apart from the intrinsic limitations
of this procedure (restricted bone resources, longer operation
time, pain and infection of the donor site), population ageing and
prevalence of diabetes also bring a great challenge to autologous
bone grafting. In a word, autologous bone grafting cannot satisfy
clinical requirements (33). Bone
transplantation is gradually changing from natural grafts to
synthetic bone substitutes and biological factors. Hung et
al (34) performed a 13-year
follow-up of 24 patients receiving surgery for osteosarcoma of the
hand. The results indicated that synthetic bone graft substitute
was a safe and effective option for the treatment of hand
chondroma, with good functional and radiological effects and a low
complication rate (34).
In the present study, a retrospective cohort study
of patients undergoing autogenous bone transplantation and titanium
alloy implantation to repair metacarpal defects was performed, and
the safety and clinical outcomes of the two types of surgery were
compared. The titanium alloy implants used are made of
TiAl6V4. Customized joint prostheses are
usually manufactured by rapid prototyping and alloy casting
techniques. Porous titanium and titanium alloys have been indicated
to have excellent mechanical properties, enabling them to be used
as permanent orthopedic implants under load-bearing conditions
(35). Titanium alloy implants for
repairing metacarpal defects have a similar elastic modulus, high
tissue compatibility and the same anatomical structure as real
metacarpophalangeal joints. Therefore, it is an ideal option for
individualized repair and prosthesis replacement for traumatic hand
bone defects (36). The present
results suggested that, compared with autologous bone grafting as
the gold standard, the excellent and good recovery rate of the
titanium alloy implantation group at 16 weeks was comparable (91.7%
vs. 89.7%, P>0.05). There was also no significant difference in
the incidence of post-operative complications (33.3% vs. 41.3%,
P>0.05). Patients with post-operative complications received
symptomatic treatment and the symptoms were soon relieved without
causing any adverse impact on treatment and recovery. The present
study indicated that the two procedures were comparable in terms of
post-operative functional recovery and incidence of
complications.
Furthermore, the titanium alloy implant is superior
to autologous bone grafting in the following four aspects: i)
Avoidance of new defects and complications of the donor site, which
is of high importance for elderly and feeble patients, as well as
those with poor immunity (37); ii)
the operation time was shortened and there was a significant
difference in the operation time between the groups A and B
(82.08±6.64 min vs. 104.69±8.63 min, P<0.05); iii) the time to
bone fusion was shortened and the two groups exhibited a
significant difference (7.75±1.73 weeks vs. 9.17±2.78 weeks,
P<0.05); iv) the pain was relieved and there was a significant
difference in VAS scores at day 3 after surgery between the two
groups (4.75±1.32 vs. 5.51±1.58, P<0.05).
The hospitalization costs of the two groups were
compared, indicating that the costs in the group receiving titanium
alloy implantation to treat metacarpophalangeal bone defect were
significantly higher than those in the autogenous bone
transplantation group and the difference was statistically
significant. This points at the requirement to constantly improve
the proficiency and quality of surgery in future clinical practice,
shorten the hospitalization time of patients and avoid
complications, so as to reduce hospitalization costs of patients,
and better promote and popularize this technology.
To conclude, titanium alloy implantation and
autologous bone grafting may achieve similar clinical effects for
the repair of metacarpal bone defects and cause few complications.
The two procedures are reliable methods for the repair of
metacarpal bone defects. However, the present study has the
following limitations: The sample size was small, as this method
was only introduced recently and no long-term follow-up was
performed. Thus, a retrospective, non-randomized controlled study
was performed. It appears that titanium alloy implantation is a
good option, as it shortens the operation time and time to bone
fusion, relieves the pain, avoids injury to the donor site and
improves the appearance and functions of the hand. This procedure
is therefore worthy of wider application. As the biological
implants and surgical technique are improved and upgraded, this
procedure will have a broader application scope in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ was responsible for the conception and design of
the study. LZ and YZ prepared the manuscript and revised the final
draft of the manuscript. YZ, JW and BC performed the experiments
and analyzed the data. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The protocol was approved by the ethics committee of
The Third Hospital of Hebei Medical University (Shijiazhuang,
China). Written informed consent was obtained from all
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
WHO: Injuries and violence: The facts
2014. World Health Organization, Geneva, 2014.
|
|
2
|
Mauffrey C, Barlow BT and Smith W:
Management of segmental bone defects. J Am Acad Orthop Surg.
23:143–53. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lu H, Liu Y, Guo J, Wu H, Wang J and Wu G:
Biomaterials with antibacterial and osteoinductive properties to
repair infected bone defects. Int J Mol Sci. 17(334)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Güleç A, Özdemir A, Durgut F, Yildirim A
and Acar MA: Comparison of innervated digital artery perforator
flap versus homodigital reverse flow flap techniques for fingertip
reconstruction. J Hand Surg Am. 44:801.e1–801.e6. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
García-Gareta E, Coathup MJ and Blunn GW:
Osteoinduction of bone grafting materials for bone repair and
regeneration. Bone. 81:112–121. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Panattoni JB, De Ona IR and Ahmed MM:
Reconstruction of fingertip injuries: Surgical tips and avoiding
complications. J Hand Surg Am. 40:1016–1024. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Germann G, Rudolf KD, Levin SL and
Hrabowski M: Fingertip and thumb tip wounds: Changing algorithms
for sensation, aesthetics, and function. J Hand Surg Am.
42:274–284. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Özcanlı H, Bektaş G, Cavit A, Duymaz A and
Coşkunfırat OK: Reconstruction of fingertip defects with digital
artery perforator flap. Acta Orthop Traumatol Turc. 49:18–22.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Griffin KS, Davis KM, McKinley TO, Anglen
JO, Chu TMG, Boerckel JD and Kacena MA: Evolution of bone grafting:
Bone grafts and tissue engineering strategies for vascularized bone
regeneration. Clinic Rev Bone Miner Metab. 13:232–244. 2015.
|
|
10
|
Bhumiratana S, Bernhard JC, Alfi DM,
Yeager K, Eton RE, Bova J, Shah F, Gimble JM, Lopez MJ, Eisig SB
and Vunjak-Novakovic G: Tissue-engineered autologous grafts for
facial bone reconstruction. Sci Transl Med. 8:343ra83.
2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tang D, Tare RS, Yang LY, Williams DF, Ou
KL and Oreffo RO: Biofabrication of bone tissue: Approaches,
challenges and translation for bone regeneration. Biomaterials.
83:363–382. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Evans CH and Huard J: Gene therapy
approaches to regenerating the musculoskeletal system. Nat Rev
Rheumatol. 11:234–242. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
García JR, Clark AY and García AJ:
Integrin-specific hydrogels functionalized with VEGF for
vascularization and bone regeneration of critical-size bone
defects. J Biomed Mater Res A. 104:889–900. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Martino MM, Briquez PS, Maruyama K and
Hubbell JA: Extracellular matrix-inspired growth factor delivery
systems for bone regeneration. Adv Drug Deliv Rev. 94:41–52.
2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Egol KA, Nauth A, Lee M, Pape HC, Watson
JT and Borrelli J Jr: Bone grafting: Sourcing, timing, strategies,
and alternatives. J Orthop Trauma. 29 (Suppl 12):S10–S14.
2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
A.M.J.J.o.S.M. Ahmad Oryan, D. Studies, An
Overview on Bone Tissue Engineering and Regenerative Medicine:
Current Challenges, Future Directions and Strategies.
|
|
17
|
Campana V, Milano G, Pagano E, Barba M,
Cicione C, Salonna G, Lattanzi W and Logroscino G: Bone substitutes
in orthopaedic surgery: From basic science to clinical practice. J
Mater Sci Mater Med. 25:2445–2461. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gruskay JA, Basques BA, Bohl DD, Webb ML
and Grauer JN: Short-term adverse events, length of stay, and
readmission after iliac crest bone graft for spinal fusion. Spine
(Phila Pa 1976). 39:1718–1724. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Heneghan HM and McCabe JP: Use of
autologous bone graft in anterior cervical decompression: Morbidity
& quality of life analysis. BMC Musculoskelet Disord.
10(158)2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hernigou P: Bone transplantation and
tissue engineering, part III: Allografts, bone grafting and bone
banking in the twentieth century. Int Orthop. 39:577–587.
2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Agarwal R and García AJ: Biomaterial
strategies for engineering implants for enhanced osseointegration
and bone repair. Adv Drug Deliv Rev. 94:53–62. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Beuriat PA, Szathmari A, Grassiot B, Di
Rocco F and Mottolese C: Why a hydroxyapatite cranioplasty can be
used to repair a cranial bone defect in children: Experience of 19
cases. Neurochirurgie. 62:251–257. 2016.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
23
|
Lindsey RW, Gugala Z, Milne E, Sun M,
Gannon FH and Latta LL: The efficacy of cylindrical titanium mesh
cage for the reconstruction of a critical-size canine segmental
femoral diaphyseal defect. J Orthop Res. 24:1438–1453.
2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ryan GE, Pandit AS and Apatsidis DP:
Porous titanium scaffolds fabricated using a rapid prototyping and
powder metallurgy technique. Biomaterials. 29:3625–3635.
2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lin SY, Huang PJ, Huang HT, Chen CH, Cheng
YM and Fu YC: An alternative technique for the management of
phalangeal enchondromas with pathologic fractures. J Hand Surg Am.
38:104–109. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Soni A, Gulati A, Bassi JL, Singh D and
Saini UC: Outcome of closed ipsilateral metacarpal fractures
treated with mini fragment plates and screws: A prospective study.
Comparative Study. 13:29–33. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hsia RY, Nath JB and Baker LC: California
emergency department visit rates for medical conditions increased
while visit rates for injuries fell, 2005-11. Health Aff
(Millwood). 34:621–626. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
De Putter CE, van Beeck EF, Polinder S,
Panneman MJ, Burdorf A, Hovius SE and Selles RW: Healthcare costs
and productivity costs of hand and wrist injuries by external
cause: A population-based study in working-age adults in the period
2008-2012. Injury. 47:1478–1482. 2017.
|
|
29
|
Dias JJ and Garcia-Elias M: Hand injury
costs. Injury. 37:1071–1077. 2006.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Grivna M, Eid HO and Abu-Zidan FM:
Epidemiology of isolated hand injuries in the United Arab Emirates.
World J Orthop. 7:570–776. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chau N, Gauchard GC, Siegfried C,
Benamghar L, Dangelzer JL, Français M, Jacquin R, Sourdot A, Perrin
PP and Mur JM: Relationships of job, age, and life conditions with
the causes and severity of occupational injuries in construction
workers. Int Arch Occup Environ Health. 77:60–66. 2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Scheker LR and Becker GW: Distal finger
replantation. J Hand Surg Am. 36:521–528. 2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang W and Yeung KWK: Bone grafts and
biomaterials substitutes for bone defect repair: A review. Bioact
Mater. 2:224–247. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hung YW, Ko WS, Liu WH, Chow CS, Kwok YY,
Wong CW, Tse WL and Ho PC: Local review of treatment of hand
enchondroma (artificial bone substitute versus autologous bone
graft) in a tertiary referral centre: 13 years' experience. Hong
Kong Med J. 21:217–223. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Heinl P, Rottmair A, Koerner C and Singer
RF: Cellular Titanium by Selective Electron Beam Melting. Adv Eng
Mater. 9:360–364. 2010.
|
|
36
|
Saulacic N, Bosshardt DD, Bornstein MM,
Berner S and Buser D: Bone apposition to a titanium-zirconium alloy
implant, as compared to two other titanium-containing implants. Eur
Cell Mater. 23:273–288. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liodaki E, Kraemer R, Mailaender P and
Stang F: The use of bone graft substitute in hand surgery: A
prospective observational study. Medicine (Baltimore).
95(e3631)2016.PubMed/NCBI View Article : Google Scholar
|