Introduction
Peripheral arterial disease (PAD), caused by the
occlusion of the arteries extending to the lower extremities, is an
increasing medical problem that affects ~20% of the global
population >55 years of age (1-4).
Delivery of oxygen, nutrients and other mediators to ischemic sites
in patients with PAD is dependent on the growth of new blood
vessels, known as angiogenesis (5-8).
However, at present, there are no known medications that are
available to induce functional neovascularization and thereby treat
patients with PAD (9-11).
MicroRNAs (miRs) are a class of small non-coding
RNAs 17-25 nucleotides in length that bind to the 3'-untranslated
region (UTR) of specific mRNAs and induce mRNA degradation or
suppress protein translation (12).
In patients with PAD and animal models, miRs have been reported to
serve important functions in tissue recovery and angiogenesis
(12). Previous evidence has
demonstrated that the expression of miRs are profoundly altered
under hypoxic conditions; among these miRs, miR-210 is robustly
upregulated following ischemia (13-15).
In addition, circulating miR-210 levels have been reported to be
significantly higher in patients with PAD when compared with
healthy controls (16). However,
whether miR-210 modulates angiogenesis in PAD remains elusive. The
present study was designed to investigate the function of miR-210
in angiogenesis following PAD, and further elucidate the underlying
molecular mechanisms.
Materials and methods
Murine hind limb ischemia (HLI)
Unilateral HLI was generated via surgical ligation
and excision of the left femoral artery in mice to create an
experimental PAD model, as previously described (17). In order to understand the function
of miR-210 on perfusion recovery, miR-210 was overexpressed in the
ischemic limb using an intramuscular injection of 5 nmol miR-210
mimic (cat. no. MC10516; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) 1 day prior, and 1, 3 and 5 days (4 doses) subsequent to
HLI in BALB/c mice. Scrambled RNA was used as the negative control.
In the present study, male BALB/c mice (aged 14-16 weeks; 20-25 g
in weight, n=18/group; 6 mice were sacrificed in each group on day
7 for biochemical studies, the rest of the mice were maintained
until the end of the experiments) were included and anesthetized
with 3% isoflurane inhalation during the operations. Subsequent to
HLI, analgesics (buprenorphine, 0.2 mg/kg) were administered
subcutaneously twice a day in the first 5 days after surgery. The
health and behavior of the mice were monitored once a day.
All procedures in the present study followed the
Guide for the Care and Use of Laboratory Animals published by the
US National Institutes of Health (publication no. 85-23, revised
1996). The experimental protocol was ethically approved by the
Committee on Animal Experiments of Wuhan University School of
Medicine (Wuhan, China). The mice were obtained from the
Experimental Animal Center of Wuhan University (Wuhan, China), and
were housed in a specific pathogen-free laboratory environment with
ad libitum access to food and water under a 12-h light/dark
cycle, and a constant humidity of 50±10% at a temperature of 25˚C.
No mice succumbed to mortality during the experiment except by
euthanization. For the tissue harvest, animals were euthanized by
CO2 suffocation, followed by cervical dislocation 7 and
28 days after HLI.
Perfusion recovery
Mice were anesthetized and subjected to a
non-invasive assessment of ischemic and non-ischemic limb perfusion
using a laser Doppler perfusion imaging system (LDPI; Perimed AB,
Järfälla, Sweden) at 0, 7, 14, 21 and 28 days after HLI, as
previously described (18).
Briefly, with this system, the perfusion was determined based on
the concentration of red blood cells x their velocity in the lower
limb, using a beam of laser light carried by a fiber-optic probe.
Perfusion of the ischemic limb was quantified and normalized to the
non-surgical limb, and the results are presented as a percentage of
the values in the non-ischemic side.
Immunofluorescence
Mice were sacrificed in a CO2 chamber 28
days after HLI, and fresh gastrocnemius anterior muscles from the
ischemic side were imbedded in optimal cutting temperature
(Tissue-Tek® O.C.T.™; Sakura Finetek USA, Inc.) compound
in liquid nitrogen overnight, and then cryo-sectioned into 6-µm
thick sections. The sections were blocked with 5% goat serum
(Sigma-Aldrich; Merck KGaA) for 1 h at room temperature, then
anti-CD31 antibody (rat anti-mouse CD31; 1:100; cat. no. 550274; BD
Pharmingen; BD Biosciences) was applied to acetone-fixed (-20˚C for
10 min) sections of gastrocnemius muscle tissue at 4˚C overnight,
followed by 1 h incubation at room temperature with an Alexa Fluor
555 anti-rabbit secondary antibody (1:400; cat. no. BM2004; Boster
Biological Technology). Images were captured using an Olympus IX71
high-magnification microscope (Olympus Corporation), and four
images were randomly selected from each sample. Capillary densities
were analyzed by counting four randomly selected high-power fields
(magnification, x100) and expressed as the number of
CD31+ cells per field.
Biochemical assay
Malondialdehyde (MDA) in the mouse ischemic muscle
homogenates was measured using a thiobarbituric acid kit (Nanjing
Jiancheng Bioengineering Institute), as previously described
(19). Briefly, tissue homogenates
or a series of standard dilutions (1.063, 3.125, 6.25, 12.5, 25, 50
and 100 mmol/l) were incubated with thiobarbituric acid at 95˚C for
60 min, and then centrifuged at 2,000 x g and 4˚C for 10 min. The
supernatant was collected and extracted using butanol for
spectrophotometric measurement at 532 nm. The absolute quantity of
MDA was read from a standard curve prepared from serial dilutions
of the primary standard using an MDA Parameter Assay kit (R&D
Systems, Inc.).
Endothelial isolation from muscle
tissue, RNA isolation and reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
In order to quantify miR-210 levels in the
endothelium, endothelial cells were isolated from the ischemic and
non-ischemic hind limb muscles using CD31 antibody-bound
immunobeads (cat. no. 11155D; Thermo Fisher, Scientific, Inc.) and
separated using magnets. Total RNA was isolated from tissues or
cells using a PureLink® RNA Mini kit (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. A
TaqManTM MicroRNA assay for specifically miR-210 (assay
ID no. 000512; Thermo Fisher Scientific, Inc.) was used for the
RT-qPCR according to the manufacturer's protocol; briefly, this
assay contains the primers and reagent for miR-210 reverse
transcriptions, and the temperature for reverse transcription were
16˚C for 30 min, 42˚C for 30 min and 85˚C for 5 min. Small
nucleolar RNA U43 (assay no. 001095; Thermo Fisher Scientific,
Inc.) served as an internal control for miR quantification. The
amplification temperatures for RT-PCR were 95˚C for 10 min,
followed by 40 cycles of 95˚C for 15 sec and 60˚C for 1 min. The
quantification cycle (Cq) value obtained for each gene was
normalized to that of the respective internal control (ΔCq). Each
gene was then further normalized to the mean ΔCq value of its
control group (ΔΔCq). The final fold expression changes were
calculated using the 2-ΔΔCq equation (20).
Cell culture and in vitro
transfection
Human umbilical vein endothelial cells (HUVECs) were
purchased from Cyagen Biosciences Inc., and grown in standard
endothelial cell growth medium (Cell Applications, Inc.) with 10%
fetal bovine serum (Boster Biological Technology). To mimic
endothelial cells under ischemic conditions as a model for PAD,
HUVECs were subjected to hypoxia (3% oxygen; BioSpherix Medical)
and serum starvation (HSS). The reason why the present study used
combined HSS to mimic in vivo ischemia is that there is no
oxygen or nutrition supply to the ischemic tissue without a blood
supply. In vitro transfection of miRNA inhibitors or mimics
at a concentration of 10 nM was used to knockdown miR-210
expression in HUVECs, as previously described (17). Briefly, a reverse transfection
protocol using neofx transfection agent (Ambion; Thermo Fisher
Scientific, Inc.) was used to transfect the miR-210 mimic (assay ID
no. MH10516, Thermo Fisher Scientific, Inc.), miR-210 inhibitor
(assay ID no. MH10516; Thermo Fisher Scientific, Inc.) or
mirVanaTM negative control miRNA (cat no. 4464060;
Thermo Fisher Scientific, Inc.) into HUVECs for 48 h at 37˚C in
cell culture incubator, the concentration used for miR mimic or
inhibitor transfection was 10 nM.
Western blotting
Subsequent to harvesting, HUVECs were homogenized in
RIPA lysis buffer containing 1% protease inhibitor cocktail
(Sigma-Aldrich; Merck KGaA) as previously described (19). Protein concentrations were
quantified using a bicinchoninic acid protein assay kit (cat. no.
PICPI23223; Thermo Fisher Scientific, Inc.). Equal amounts (30 µg)
of protein in homogenate samples were separated by 16.5% SDS-PAGE
gel (Bio-Rad Laboratories, Inc.), transferred to nitrocellulose
membranes, blocked with 5% bovine serum albumin (Sigma-Aldrich;
Merck KGaA) at room temperature for 1 h, and then blotted with
primary antibodies at 4˚C overnight and the corresponding
peroxidase-conjugated secondary antibodies at room temperature for
1 h. Primary antibodies against polypyrimidine tract binding
protein 3 (PTBP3; cat. no. sc-100845; 1:1,000), prolyl
4-hydroxylase subunit β (P4HB; cat. no. sc-136230; 1:1,000) and
β-actin (cat. no. sc-47778; 1:5,000) and horseradish peroxidase
(HRP) conjugated mouse anti-rabbit secondary antibodies (cat. no.
sc-2357; 1:10,000) were obtained from Santa Cruz Biotechnology,
Inc. The bound antibody signal was visualized using an Immun-Star
HRP chemiluminescent kit (Tanon Science and Technology Co., Ltd.).
The western blotting images were obtained using a Tanon 5200
Chemiluminescence Imaging System (Tanon Science and Technology Co.,
Ltd.). Semi-quantitative analyses of the immunoblots were performed
using Image J software v1.8 (National Institutes of Health).
Intracellular reactive oxygen species
(ROS) assay
Intracellular ROS production was detected using the
cell permeating compound, 2',7'-dichlorodihydrofluorescein
diacetate (DCFH-DA; Sigma-Aldrich; Merck KGaA) as previously
described (19). Briefly, DCFH-DA
was hydrolyzed to the fluorescent product in the presence of ROS.
Following incubation with 10 µM DCFHDA for 45 min at 37˚C, the
fluorescence intensity of the HUVECs was read at 525 nm emission
when excited at 488 nm in a 96 well plate reader.
Cellular viability and angiogenesis
assay
For assessing cellular viability, HUVECs were seeded
into a 96-well plate at a density of 1x104 cells/well
(n=8 per group), and then cultured under HSS conditions for 48 h.
Subsequently, the cell viability was assessed using tetrazolium dye
incorporation (BioVision, Inc.) using a 96-well plate reader at a
wavelength of 450 nm (OD450), and the viability values
were presented as a ratio to the cells transfected with the
negative control. In vitro angiogenesis assays were
performed as previously described (21), under HSS conditions. In brief,
HUVECs were plated in 96-well plates coated with growth
factor-reduced Matrigel (BD Biosciences) at a density of
1x104 cells/well, and then exposed to HSS conditions for
24 h to assess tube formation. This time point was selected based
on the results of a previous study (17). The experiment was repeated in 6
replicates. The degree of tube formation was determined by
measuring the number of loops under magnification x40, using a
light microscope (CKX53; Olympus Corporation) using Image J
software 1.15K (National Institutes of Health). With four images
captured at random for each well, the mean number of loops in each
well were used for statistical analysis. Each experiment was
repeated using at least two different batches of HUVECs.
Luciferase assay
In order to investigate the interaction between the
miR-210 and its targeting site(s) on the untranslated region
(3'UTR) of P4HB mRNA, 1 mg/l pEZX-MT06 plasmid containing P4HB
3'UTR (product ID, HmiT012109-MT06; GeneCopoeia, Inc.) or nonsense
sequences (cat. no. CmiT000001-MT06; GeneCopoeia, Inc.) were
transfected with 10 nM miR-210 mimic or negative control RNA using
Lipofectamine® 3000 (Thermo Fisher Scientific, Inc.) and
luciferase activities were measured with the Luc-Pair™
Duo-Luciferase HS Assay kit (GeneCopoeia, Inc.) according to the
manufacturer's protocol, using a microplate reader (cat. no.
GM3000; Promega Corporation) 48 h after transfection and normalized
to Renilla luciferase activity.
Statistical analysis
All data were presented as the mean ± SEM.
Statistical analysis was performed with GraphPad Prism software
(v7.0; GraphPad Software, Inc.). An unpaired Student's t-test was
used for comparisons between two groups, and comparisons between ≥3
groups were performed using a one-way analysis of variance and
Tukey's post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
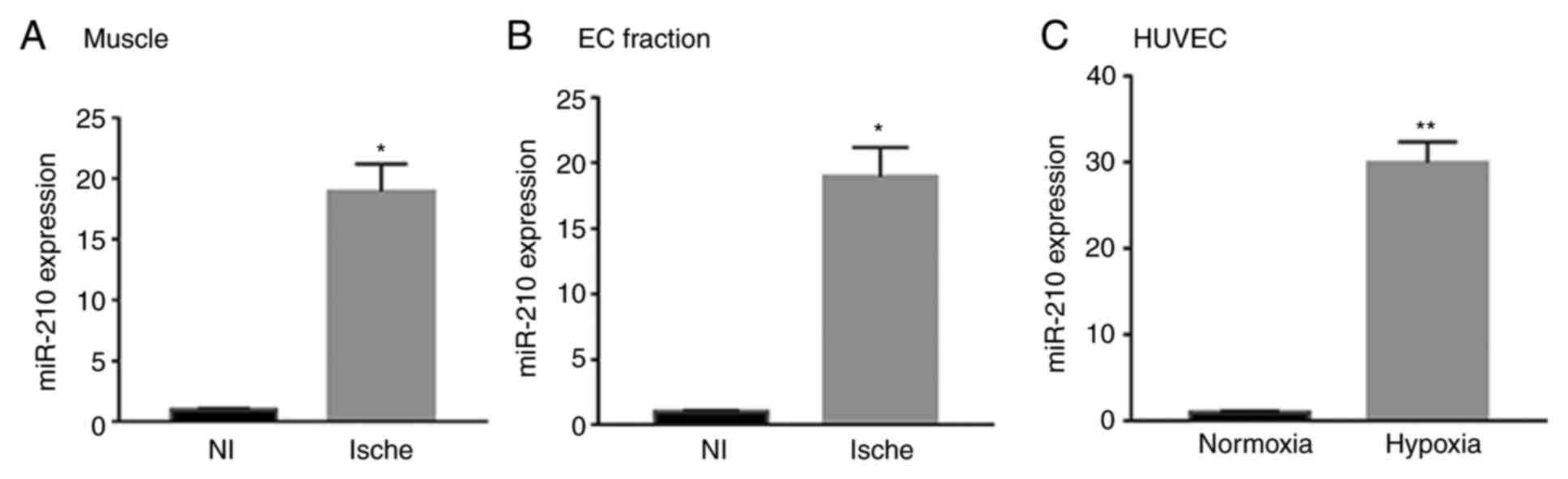
miR-210 is upregulated in experimental
PAD
Using RT-qPCR, the present study revealed that the
miR-210 expression levels in the muscle from ischemic hind limbs
were significantly higher compared with the non-ischemic limb 7
days after HLI (P<0.05; Fig.
1A). The present study then sought to determine whether
endothelial cells contribute to the miR-210 elevation following
HLI; therefore, CD31+ cells were isolated from mice hind
limbs using immunobeads, and a significantly higher miR-210
expression level was revealed compared with the non-ischemic limbs
(P<0.05; Fig. 1B) in the
ischemic side. The present study then analyzed the expression of
miR-210 in HUVECs under normoxic or HSS conditions for 48 h.
Consistent with what was observed in mouse muscles, miR-210
expression levels in HUVECs cultured under HSS were significantly
higher compared with in HUVECs under normoxic conditions
(P<0.01; Fig. 1C).
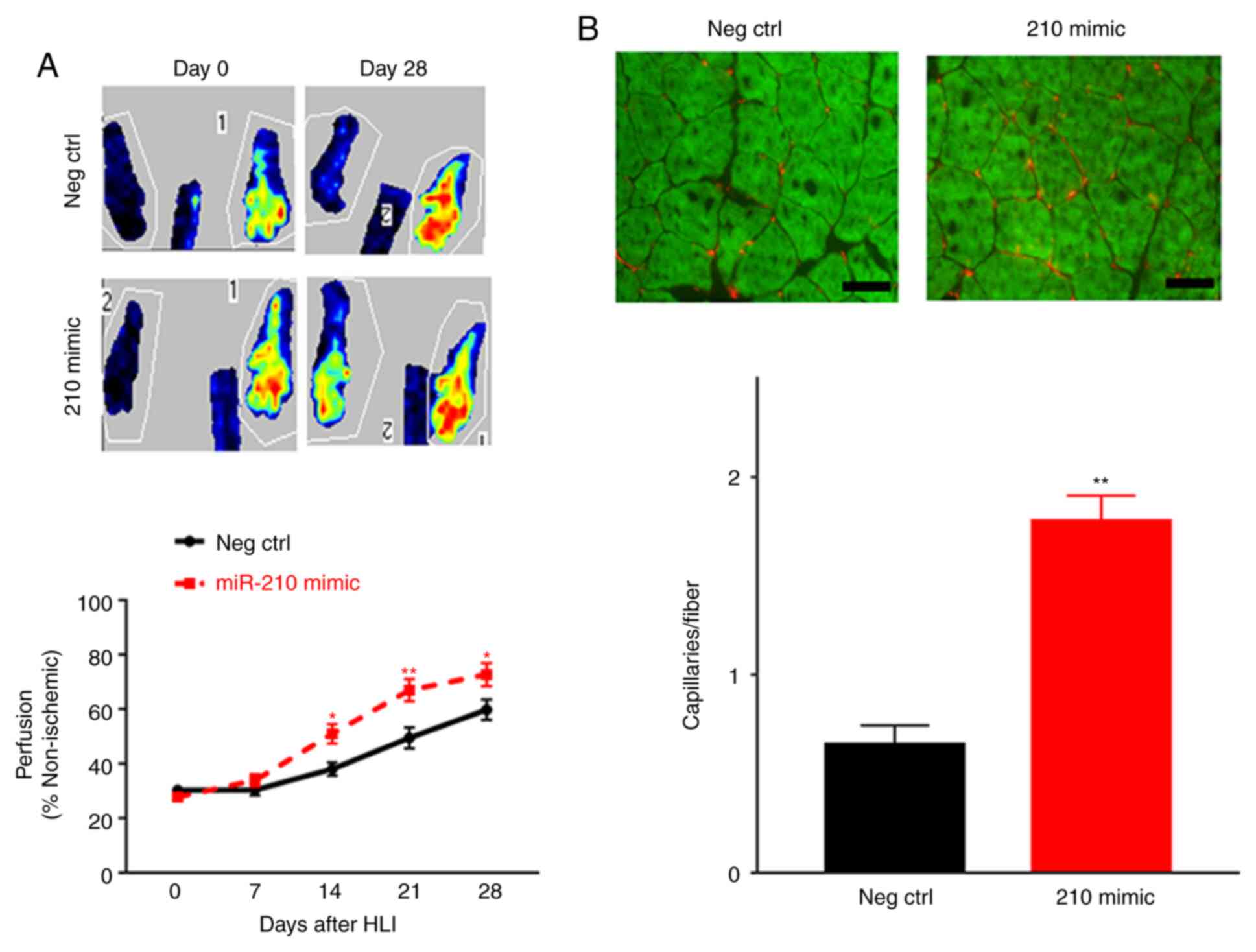
miR-210 mimic improves perfusion
recovery and angiogenesis following HLI
In order to understand the impact of elevated
miR-210 expression on perfusion recovery, the present study
overexpressed miR-210 using a miRNA mimic in the left hind limb 3
days prior to HLI in BALB/c mice. The expression levels of miR-210
in the ischemic muscle were assessed 7 days subsequent to
transfection, and miR-210 mimics exhibited a ~90-fold higher
expression of miR-210 compared with mice receiving control RNA
(data not shown). At days 14, 21 and 28 post-HLI, miR-210
overexpression resulted in significantly improved perfusion
recovery compared with those receiving scrambled RNA as indicated
by LDPI (n=10 per group; P<0.05; Fig. 2A). The present study then determined
the capillary density in the ischemic muscle using immunostaining
with CD31; mice receiving the miR-210 mimic exhibited a
significantly higher capillary density compared with those
receiving control RNA (0.65±0.05 vs. 1.79±0.08 capillaries/fiber;
P<0.01) in the ischemic gastrocnemius muscle 28 days subsequent
to HLI (Fig. 2B).
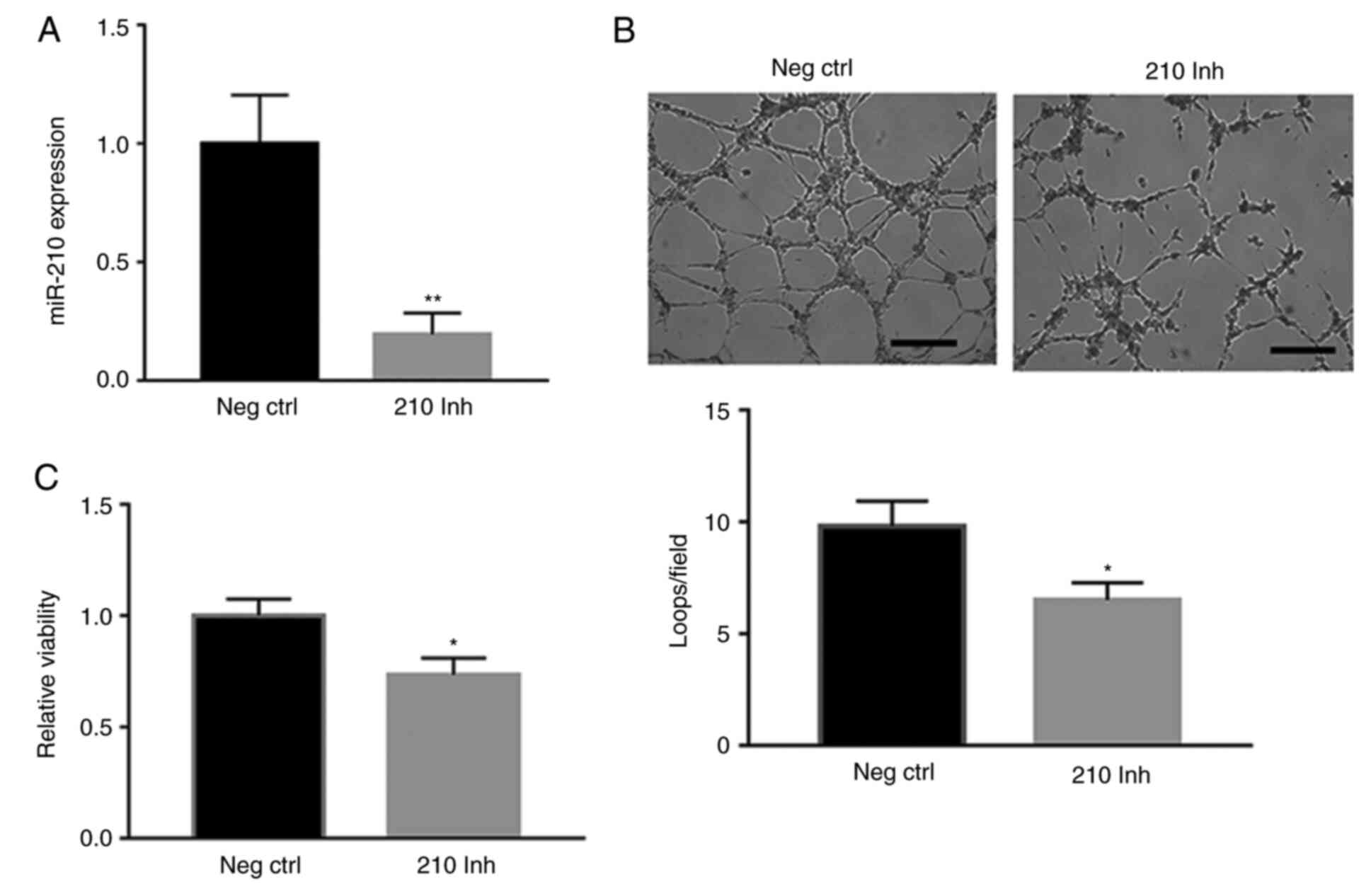
miR-210 modulated angiogenesis in
vitro
The present study next investigated the effects of
miR-210 on angiogenesis in vitro using HUVECs. Transfection
of an miR-210 inhibitor significantly decreased intracellular
miR-210 levels compared with the negative control (P<0.01;
Fig. 3A), and, similar to what was
observed in the ischemic muscle, miR-210 antagonism in HUVECs under
HSS conditions significantly decreased cell survival (P<0.05;
Fig. 3B) and tube formation
(P<0.05; Fig. 3C) compared with
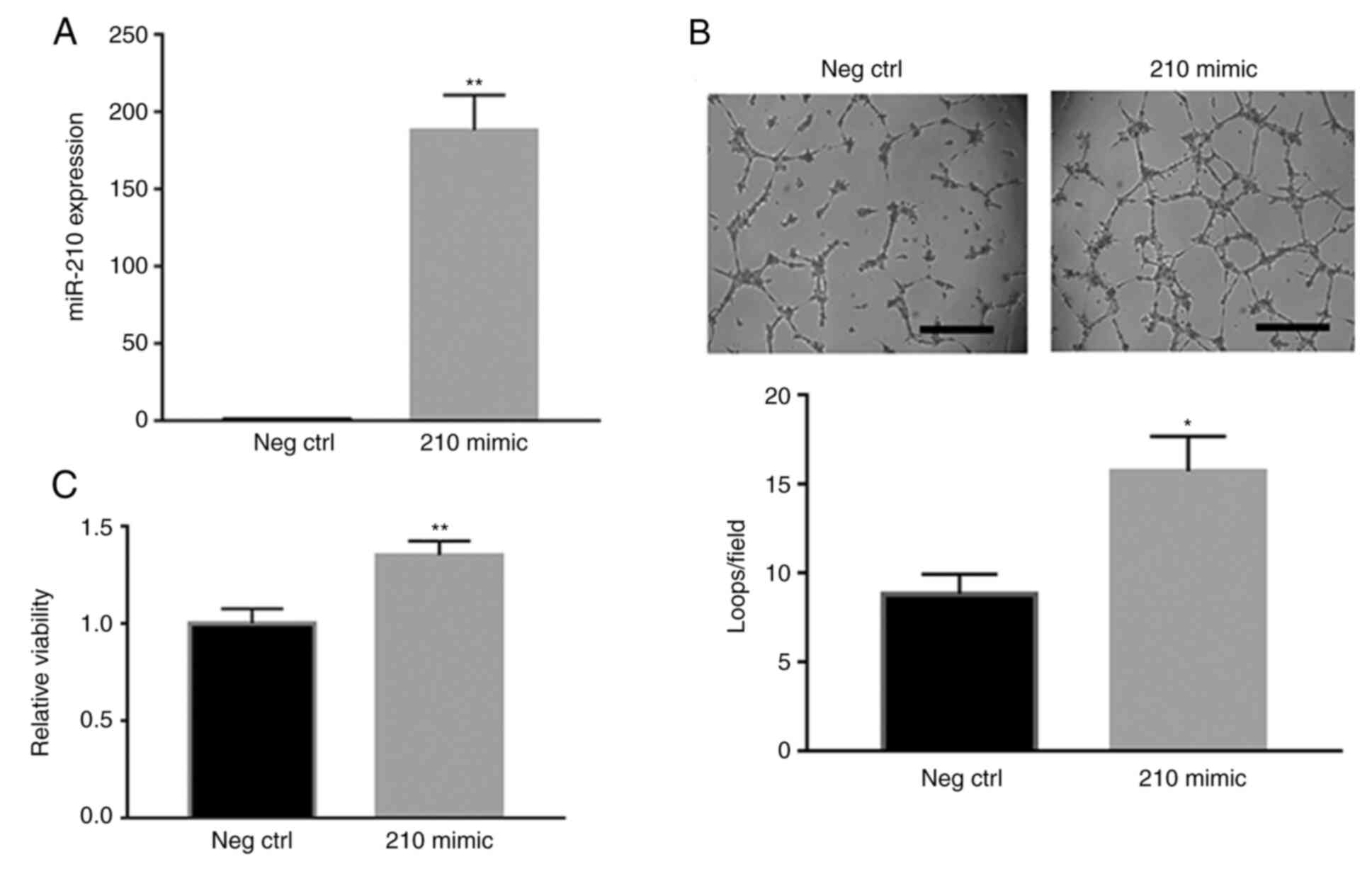
the negative control. In contrast, miR-210 overexpression using
miRNA mimic transfection in HUVECs under HSS conditions
significantly increased miR-210 expression (P<0.01; Fig. 4A), in addition to cell survival
(P<0.01; Fig. 4B) and tube
formation (P<0.05; Fig. 4C),
compared with the negative control.
miR-210 increases ROS in experimental
PAD
PTBP3 and P4HB have previously been reported as the
targets of miR-210 in ischemic tissue (13). Here, P4HB was significantly
upregulated compared with the negative control (P<0.01), but
PTBP3 was not altered in the HUVECs when miR-210 was antagonized
under HSS conditions (Fig. 5A). To
assess the interactions between miR-210 and P4HB mRNA, the
cotransfection of miR-210 mimic and reporter plasmid with a P4HB
3'UTR region was performed and revealed to decrease P4HB expression
levels, as indicated by significantly decreased luciferase activity
(P<0.01; Fig. 5B). P4HB is a
strong regulator of ROS (22),
thus, the present study assessed ROS levels in HUVECs using an
intracellular ROS assay. It was revealed that the miR-210 mimic
significantly decreased ROS levels compared with the negative
control (P<0.01; Fig. 5C), while
the miR-210 inhibitor significantly increased ROS levels compared
with the negative control (P<0.01; Fig. 5D). The present study then measured
the ischemic muscle tissue levels of MDA, which have widely been
used to reflect tissue ROS bioactivity (23). miR-210 mimic significantly decreased
MDA levels in the ischemic muscle 7 days following HLI compared
with the negative control (P<0.01; Fig. 5E).
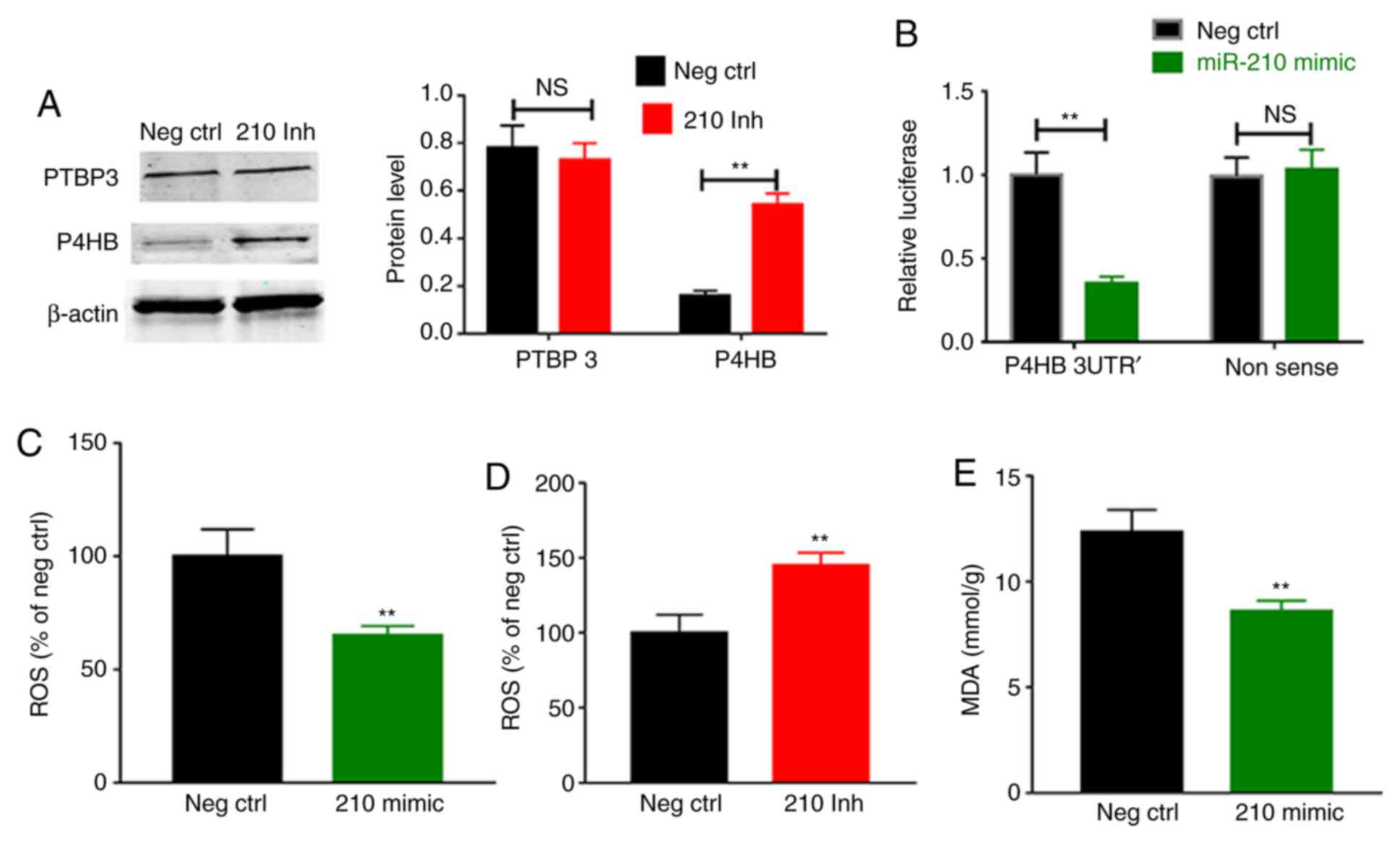 | Figure 5(A) Transfection of an miR-210
inhibitor in HUVECs did not alter the protein levels of PTBP3, but
decreased P4HB protein levels. (B) Transfection of an miR-210 mimic
decreased the luciferase activity in cells transfected with a P4BH
3'UTR plasmid, but not those with the negative control plasmid,
which contains the luciferase sequence with a nonsense sequence in
the 3'UTR region. (C) miR-210 mimic decreased the ROS levels in
HUVECs cultured under hypoxia. (D) miR-210 inhibitor increased ROS
levels in HUVECs. (E) Transfection of miR-210 mimic decreased MDA
levels in ischemic muscle 7 days subsequent to hind limb ischemia.
Scale bar, 100 µM. Data are presented as the mean ± standard error
of the mean. ROS, reactive oxygen species; NS, non-significant; Neg
Ctrl, negative control; PTBP3, polypyrimidine tract binding protein
3; P4HB, prolyl 4-hydroxylase subunit β; 210 mimic, miR-210 mimic;
210 Inh, miR-210 inhibitor; MDA, malondialdehyde; HUVECs, human
umbilical vein endothelial cells; 3'UTR, 3'untranslated region.
**P<0.01 vs. negative control. |
Discussion
To the best of our knowledge, the present study is
the first to demonstrate that miR-210 improves angiogenesis and
perfusion recovery in experimental PAD. It was also revealed that
miR-210 decreased ROS levels in ischemic muscle tissue and
endothelial cells, and targeted P4HB via decreasing P4HB protein
levels in endothelial cells under hypoxic conditions. These results
may partially explain the molecular mechanisms underlying the
angiogenesis and perfusion recovery induced by miR-210.
Endothelial cells are one of the key cells involved
in vascular stability and angiogenesis; however, this cell type is
vulnerable to ischemia-induced damage, ischemia-induced generation
of ROS resulting in decreased synthesis and increased degradation
of nitric oxide (24). In the
present study, miR-210 overexpression resulted in lower ROS levels,
and miR-210 knockdown resulted in higher ROS levels in ischemic
muscle and cultured endothelial cells. It is of note that the
HUVECs with miR-210 inhibitor transfection appeared unhealthy,
which may be partially due to the miR-210 inhibition-induced
endothelial cell apoptosis via ROS. A previous study reported that
miR-210 modulates P4HB and PTBP protein levels and thus decreases
ROS levels in acute ischemic muscles (25); in the present study, it was revealed
that only P4HB was regulated by miR-210 in endothelial cells. P4HB
is the β-subunit of prolyl 4-hydroxylase, which functions as an
endoplasmic reticulum chaperone to suppress the aggregation of
misfolded proteins. A previous study in colon cancer suggested that
P4HB increases ROS production through the activation of nuclear
factor (erythroid-derived 2)-like 2(22). Although PTBP3 has been reported as a
target of miR-210 in a previous study (25), there is no miR-210 seed sequence in
PTBP3 mRNA, and thus, miR-210 regulates PTBP3 only in certain
tissues, including prostate cancer and cardiovascular tissues,
whereas elsewhere PTBP3 is regulated by miR-499(26). These may be the reasons why PTBP3 is
not regulated by miR-210 in endothelial cells.
A notable result of the present study is that
miR-210 was higher in the ischemic limb and endothelial cells
exposed to hypoxic conditions. Hypoxia-inducible factor 1-α
(HIF-1-α) is the most well-known factor that is activated under
hypoxic conditions. Following activation, HIF-1-α translocates to
the cell nucleus and binds to the promoter, activating the
transcription of miR-210 upon low oxygen exposure (25). This may explain why miR-210 is
upregulated in ischemic limb endothelial cells; however, this was
not investigated in the present study.
In the present study, it has been demonstrated that
miR-210 upregulation in endothelial cells under ischemic conditions
is protective and induces angiogenesis in the ischemic tissue.
However, these results are primarily based on mice studies, and
results from larger animals including primates are necessary to
clarify whether the results from the present study may be applied
to human patients with PAD.
In conclusion, miR-210 is upregulated in endothelial
cells under ischemic conditions, and this upregulation may well be
adaptive as miR-210 induces angiogenesis in ischemic limb tissue,
potentially via regulating oxidative stress.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ and QW conceived the project and designed the
experiments. JZ, QW and RH wrote and revised the manuscript. JZ,
QW, GR, JQ and RH performed the experiments, read and approved the
final manuscript.
Ethical approval and consent to
participate
The experimental animal protocol was ethically
approved by the Committee on Animal Experiments of Wuhan University
School of Medicine (Wuhan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Criqui MH and Aboyans V: Epidemiology of
peripheral artery disease. Circ Res. 116:1509–1526. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Willey J, Mentias A, Vaughan-Sarrazin M,
McCoy K, Rosenthal G and Girotra S: Epidemiology of lower extremity
peripheral artery disease in veterans. J Vasc Surg. 68:527–535.e5.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fowkes FG, Aboyans V, Fowkes FJ, McDermott
MM, Sampson UK and Criqui MH: Peripheral artery disease:
Epidemiology and global perspectives. Nat Rev Cardiol. 14:156–170.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Thiruvoipati T, Kielhorn CE and Armstrong
EJ: Peripheral artery disease in patients with diabetes:
Epidemiology, mechanisms, and outcomes. World J Diabetes.
6:961–969. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Espinola-Klein C and Savvidis S:
Peripheral arterial disease. Epidemiology, symptoms and diagnosis.
Internist (Berl). 50:919–926. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Joh JH, Joo SH and Park HC: Simultaneous
hybrid revascularization for symptomatic lower extremity arterial
occlusive disease. Exp Ther Med. 7:804–810. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tanaka M, Taketomi K and Yonemitsu Y:
Therapeutic angiogenesis: Recent and future prospects of gene
therapy in peripheral artery disease. Curr Gene Ther. 14:300–308.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Grochot-Przeczek A, Dulak J and Jozkowicz
A: Therapeutic angiogenesis for revascularization in peripheral
artery disease. Gene. 525:220–228. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Aviles RJ, Annex BH and Lederman RJ:
Testing clinical therapeutic angiogenesis using basic fibroblast
growth factor (FGF-2). Brit J Pharmacol. 140:637–646.
2003.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Owens CD and Conte MS: Medical management
of peripheral arterial disease bridging the ‘Gap’? Circulation.
126:1319–1321. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Annex BH: Therapeutic angiogenesis for
critical limb ischaemia. Nat Rev Cardiol. 10:387–396.
2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Stather PW, Sylvius N, Wild JB, Choke E,
Sayers RD and Bown MJ: Differential MicroRNA expression profiles in
peripheral arterial disease. Circ Cardiovasc Genet. 6:490–497.
2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Guo S, Bai R, Liu W, Zhao A, Zhao Z, Wang
Y, Wang Y, Zhao W and Wang W: MicroRNA-210 is upregulated by
hypoxia-inducible factor-1α in the stromal cells of giant cell
tumors of bone. Mol Med Rep. 12:6185–6192. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ikeda S, Kitadate A, Abe F, Saitoh H,
Michishita Y, Hatano Y, Kawabata Y, Kitabayashi A, Teshima K, Kume
M, et al: Hypoxia-inducible microRNA-210 regulates the DIMT1-IRF4
oncogenic axis in multiple myeloma. Cancer Sci. 108:641–652.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pulkkinen K, Malm T, Turunen M, Koistinaho
J and Ylä-Herttuala S: Hypoxia induces microRNA miR-210 in vitro
and in vivo ephrin-A3 and neuronal pentraxin 1 are potentially
regulated by miR-210. FEBS Lett. 582:2397–2401. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Signorelli SS, Volsi GL, Pitruzzella A,
Fiore V, Mangiafico M, Vanella L, Parenti R, Rizzo M and Volti GL:
Circulating miR-130a, miR-27b, and miR-210 in patients with
peripheral artery disease and their potential relationship with
oxidative stress. Angiology. 67:945–950. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang J, Wang Q, Rao G, Qiu J and He R:
Curcumin improves perfusion recovery in experimental peripheral
arterial disease by upregulating microRNA-93 expression. Exp Ther
Med. 17:798–802. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Albadawi H, Oklu R, Cormier NR, O'Keefe
RM, Heaton JT, Kobler JB, Austen WG and Watkins MT: Hind limb
ischemia-reperfusion injury in diet-induced obese mice. J Surg Res.
190:683–691. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chen L, Liu C, Sun D, Wang T, Zhao L, Chen
W, Yuan M, Wang J and Lu W: MicroRNA-133a impairs perfusion
recovery after hindlimb ischemia in diabetic mice. Biosci Rep.
38(BSR20180346)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang T, Cunningham A, Dokun AO, Hazarika
S, Houston K, Chen L, Lye RJ, Spolski R, Leonard WJ and Annex BH:
Loss of interleukin-21 receptor activation in hypoxic endothelial
cells impairs perfusion recovery after hindlimb ischemia.
Arterioscler Thromb Vasc Bio. 35:1218–1225. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhou Y, Yang J, Zhang Q, Xu Q, Lu L, Wang
J and Xia W: P4HB knockdown induces human HT29 colon cancer cell
apoptosis through the generation of reactive oxygen species and
inactivation of STAT3 signaling. Mol Med Rep. 19:231–237.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Pirinccioglu AG, Gökalp D, Pirinccioglu M,
Kizil G and Kizil M: Malondialdehyde (MDA) and protein carbonyl
(PCO) levels as biomarkers of oxidative stress in subjects with
familial hypercholesterolemia. Clin Biochem. 43:1220–1224.
2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Steven S, Daiber A, Dopheide JF, Munzel T
and Espinola-Klein C: Peripheral artery disease, redox signaling,
oxidative stress-Basic and clinical aspects. Redox Biol.
12:787–797. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zaccagnini G, Maimone B, Di Stefano V,
Fasanaro P, Greco S, Perfetti A, Capogrossi MC, Gaetano C and
Martelli F: Hypoxia-induced miR-210 modulates tissue response to
acute peripheral ischemia. Antioxid Redox Signal. 21:1177–1188.
2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hosoda T, Zheng H, Cabral-da-Silva M,
Sanada F, Ide-Iwata N, Ogórek B, Ferreira-Martins J, Arranto C,
D'Amario D, del Monte F, et al: Human cardiac stem cell
differentiation is regulated by a mircrine mechanism. Circulation.
123:1287–1296. 2011.PubMed/NCBI View Article : Google Scholar
|