Introduction
Myocardial infarction (MI) is the primary cause of
morbidity and mortality worldwide. Exercise training and
pharmacological treatments are the important strategies to reduce
the harmful effects following MI (1,2). When
MI occurs, the cardiomyocytes suffer irreversible damage and
necrosis due to hypoxia and a decreased supply of ATP (3,4). The
necrotic cells activate the autoimmune system and lead to a severe
inflammatory response after releasing their contents. On one hand,
the release of inflammatory mediators initiates the repair of
damaged tissues by the body (5,6).
Conversely, inflammatory cytokines could induce cardiomyocyte
apoptosis, which further promotes the increase of inflammatory
cytokines (7,8). Therefore, the use of anti-inflammatory
agents is an important facet of the treatment of patients with MI
(9).
Following MI, in addition to lifestyle
interventions, drug therapy is the cornerstone for improving
survival and decreasing the incidence of new cardiovascular events
(10). Compared with chemical drugs
and biopharmaceuticals, the multi-component and multi-target nature
of the compounds, and less adverse reactions, are the unique
advantages of Traditional Chinese Medicine in treating diseases.
Ginsenoside Rg3 (Rg3) is an active component isolated from ginseng.
It is a tetracyclic triterpenoid saponin, which can inhibit
neovascularization, induce tumor cell apoptosis, inhibit
inflammation and enhance immune function (11,12).
Previous studies have suggested that Rg3 not only inhibits the
inflammatory response caused by lipopolysaccharide (13), but also improves cardiac function
following MI/ (reperfusion) R via attenuating apoptosis and
inflammation (14), and these
effects are all associated with an Rg3-mediated inhibition of the
NF-κB pathway (13,14). In addition, a number of studies have
identified that Rg3 is an activator of sirtuin 1 (SIRT1) in rats
(15,16). SIRT1 is a histone deacetylase widely
expressed in human cells. It serves an important biological
function by deacetylating multiple transcription factors (17,18).
Transcription factor p65 (p65) is an important marker of the
activation of NF-κB signaling, which only functions following its
acetylation. In inflammatory responses, SIRT1 deacetylates p65,
thereby inhibiting the transcription of tumor necrosis factor-α
(TNF-α), interleukin (IL)-6 and other inflammatory genes downstream
of NF-κB (19). However, whether
Rg3 can inhibit the NF-κB pathway by activating SIRT1, and thereby
inhibiting the inflammatory response caused by MI, remains
unknown.
In the present study, a MI rat model was established
by coronary artery ligation and then treated with Rg3. It was
identified that Rg3 could attenuate inflammation in myocardial
tissue and serum of rat MI rats by inhibiting the SIRT1/NF-κB
pathway, thereby protecting cardiac function in MI rats.
Materials and methods
Animals and treatment
A total of 28 male SD rats (5-6 weeks old) weighing
165-200 g were used to generate the animal model in the present
study (18). The animals were
offered access to food and water ad libitum and housed in
the animal experimental center of Capital Medical University in a
12: 12 h light: Dark cycle at 22±2˚C. Rats were randomly assigned
to 4 groups: Sham; RG3; MI; and RG3 + MI. The rats in the sham and
RG3 groups had no ligation of coronary artery, and the rats in the
MI and RG3 + MI groups were used to establish the MI model by
ligation of coronary arteries.
At 7 days prior to and 28 days after coronary artery
ligation, the rats in the Rg3 and Rg3 + MI groups were administered
30 mg/kg/day Rg3 (cat. no., 64139; Sigma-Aldrich; Merck KGaA)
intragastrically for 7 days, and an equal amount of saline was used
in the sham and MI groups. All animal protocols were approved by
the Animal Care and Use Committee in Beijing Anzhen Hospital,
Capital Medical University, and conformed with the guidelines of
the National Institutes of Health (20).
Myocardial infarction rat model
Coronary artery ligation was used to establish a rat
model of MI, as described previously (21). Briefly, the rats were anesthetized,
and the depth of anesthesia was evaluated by observing the
conjunctival reflex, tail pinch and eyelid reflexes (20). Then, a normal II lead
electrocardiogram (ECG) was performed. For the surgical procedure,
the skin on the left side of the sternum was prepared, disinfected,
and a scalpel incision was made using a 10-0 purse string suture to
quickly close the chest following surgery. The intercostal muscles
between the 3rd to 4th ribs were separated, revealing the heart.
The coronary veins associated with the left coronary artery were
identified between the left atrial appendage and the pulmonary
artery cone. They were ligated with 6-0 non-invasive sutures at 2-3
mm below the left atrial appendage. The depth of the needle was
maintained at 0.1 cm and the width was 0.2-0.3 cm. The heart was
placed back into the chest cavity through the intercostal space.
The gas in the chest cavity was quickly squeezed to tighten the
purse string suture for ligation. Then the incision was sutured.
The sham operation and the RG3 groups were only threaded and not
ligated. Significant elevation of the S-T segment or widening of
the QRS complex was a sign of successful model establishment.
Post-surgery intramuscular penicillin was administered continuously
for 3 days to prevent infection. The rats were considered to be
healthy by observing the skin, activity, food intake, excretion,
abdominal respiration, external genitalia, and eyes following
surgery. Concomitantly, the cardiac function of the rats was also
evaluated by echocardiography and serum myocardial enzymes, and
serum inflammatory factors were measured to assess levels of
inflammation.
Hematoxylin & eosin (H&E)
staining
On the 28th day after the MI model was constructed,
the experimental rats were anesthetized by intraperitoneal
injection of sodium pentobarbital (30 mg/kg), and the depth of
anesthesia in the rats was evaluated by observing conjunctival
reflex, tail pinch and eyelid reflexes. Following euthanasia by
cervical dislocation, the chest cavity was opened to obtain the
heart tissue and to collect aneurysm specimens. A portion of the
specimen was dehydrated and paraffin-embedded, and the specimen was
cut into 5 µm slices using the CUT4062 serial slicer (SLEE medical
GmbH). Paraffin sections were routinely dewaxed and washed with
H&E staining kits for H&E staining (cat. no., G1120;
Beijing Solarbio Science & Technology Co., Ltd.) as follows:
The samples were dyed with hematoxylin for 5-10 min at room
temperature, washed for 3-5 min, soaked with 0.5-1% hydrochloric
acid in alcoholic solution for several seconds at room temperature,
washed for 3-5 min, and then colored with alkaline aqueous solution
for 1 min, followed by washing with water for 10 min, and
examination using an optical microscope (LSM800; Olympus
Corporation). Following staining, the slides were washed 1 or 2
times, stained with 0.5% eosin for 1-2 min at room temperature,
dehydrated with alcohol (80% ethanol for 30 sec, 95% ethanol for 1
min, 95% ethanol for 1 min, absolute ethanol for 3 min and absolute
ethanol for 3 min), washed with xylene, and sealed using a neutral
resin. An LSM800 optical microscope (x200 magnification; Olympus
Corporation) was used to observe the pathological changes in each
slice following H&E staining.
ELISA assay
Blood samples were collected from ~1 cm capillaries
underneath the eyelids, and the sera were obtained following
centrifugation at 1,000 x g for 5 min at room temperature. Creatine
kinase myocardial band (CK-MB; cat. no., XF2852b, Shanghai Xinfan
Biotechnology Co., Ltd.), lactate dehydrogenase (LDH; cat. no.
BC0685; Beijing Solarbio Science & Technology Co., Ltd.),
cardiac troponin-1 (cTnI; cat. no., ab246529; Abcam), IL-1β (cat.
no., ab100768; Abcam), TNF-α (cat. no., ab100785; Abcam), IL-6
(cat. no., ab100772; Abcam) and IL-10 (cat. no., ab100765; Abcam)
ELISA kits were used to detect the CK-MB, LDH, cTnI, IL-1β, TNF-α,
IL-6 and IL-10 levels in serum, respectively.
TUNEL assay
Tissues were fixed with 10% formalin solution at
room temperature for 24 h, and slices were prepared.
Paraffin-embedded cardiac tissue sections were first dewaxed in
xylene for 5-10 min at room temperature. Finally, they were
immersed in 90% ethanol for 2 min, 70% ethanol for 2 min and
distilled water for 2 min. Then, the one-step TUNEL Apoptosis
Detection kit (cat. no., C1088; Beyotime Institute of
Biotechnology) was used to detect the levels of apoptosis in the
heart tissue following the manufacturer's protocol. Nuclear
counterstaining was performed for 5 min using DAPI (5 µg/ml;
Sigma-Aldrich; Merck KGaA) at room temperature. An LSM800 optical
microscope (x200 magnification; Olympus Corporation) was used to
observe apoptotic cells.
Reverse transcription-quantitative PCR
(RT-qPCR)
Levels of TNF-α, IL-1β, L-6 and IL-10 in the rats
were measured as described previously (22) with RT-qPCR using gene-specific
TaqMan primer/probe sets in an ABI prism 7000 Sequence Detection
System (Applied Biosystems). The total RNA was reverse transcribed
into cDNA using a reverse transcription kit (cat. no., RR036B;
Takara Bio, Inc.) according to the manufacturer's protocol.
Subsequently, a 20-µl RT-qPCR system was prepared using the GoTaq
qPCR Master Mix kit according to the manufacturer's protocol (cat.
no., 638320; Takara Biotechnology, Co., Ltd.). GAPDH mRNA
transcription was used as the internal loading control. The PCR
primer sequences were as follows: TNF-α forward (F),
5'-CTGAACTTCGGGGTGATCGG-3'; TNF-α reverse (R):
5'-GGCTTGTCACTCGAATTTTGAGA-3'; IL-1β F,
5'-GAAATGCCACCTTTTGACAGTG-3'; IL-1β R, 5'-TGGATGCTCTCATCAGGACAG-3';
IL-6 F, 5'-TCTATACCACTTCACAAGTCGGA-3'; IL-6 R,
5'-GAATTGCCATTGCACAACTCTTT-3'; IL-10 F, 5'-GCTATCGCCCGGTATAG-3';
and IL-10 R, 5'-GTTTCGCGTCGATATTAGC-3'; GAPDH F,
5'-AGGTCGGTGTGAACGGATTTG-3' and GAPDH R, 5'-GGGGTCGTTGATGGCAACA-3'.
The thermocycling conditions for the qPCR were as follows: Initial
denaturation (94˚C for 2 min); 40 cycles of denaturation (95˚C for
30 sec), annealing (90˚C for 5 sec) and elongation (65˚C for 30
sec); and a final extension (72˚C for 60 sec). The
2-ΔΔCq method was used to quantify gene expression
levels (23).
Western blot analysis
Protein levels were analyzed by western blot
analysis, as described previously (22). GAPDH protein transcription was used
as the internal loading control. RIPA Lysis Buffer (cat. no.
P0013K; Beyotime Institute of Biotechnology) was used to lyse
fibroblast-like synoviocytes and synovial tissue, and the BCA
Protein Assay kit (cat. no. P0010S; Beyotime Institute of
Biotechnology) was used to measure lysate protein concentration. A
total of 50 µg protein/lane in cell lysates were separated by 10%
SDS-PAGE and then transferred to PVDF membranes, which were then
blocked with 5% skim milk powder for 1 h at room temperature. The
primary antibodies in the present study were as follows for
overnight incubation at 4˚C: Anti-p65 phosphorylated (p)-S636 (cat.
no. ab86299; 1:5,000; Abcam) and anti-GAPDH (cat. no. ab9484;
1:3,000; Abcam). The following secondary antibodies were used for 1
h incubation at room temperature: Horseradish peroxidase
(HRP)-conjugated goat anti-rabbit immunoglobulin G (IgG) H&L
(cat. no. ab6721; 1:3,000; Abcam) and HRP-conjugated goat
anti-mouse IgG H&L (cat. no. ab6789; 1:3,000; Abcam. ImageJ
v1.8.0 (National Institutes of Health) was used to analyze protein
gray value.
Statistical analysis
Data were presented as the mean ± SD. SPSS 20.0 (IBM
Corp) software was used to analysis the data in the present study.
A Student's t-test was used to compare differences between two
groups, and multiple groups were compared using one-way ANOVA
followed by a Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Rg3 enhances cardiac function in rats
following MI
A rat MI model was established by coronary artery
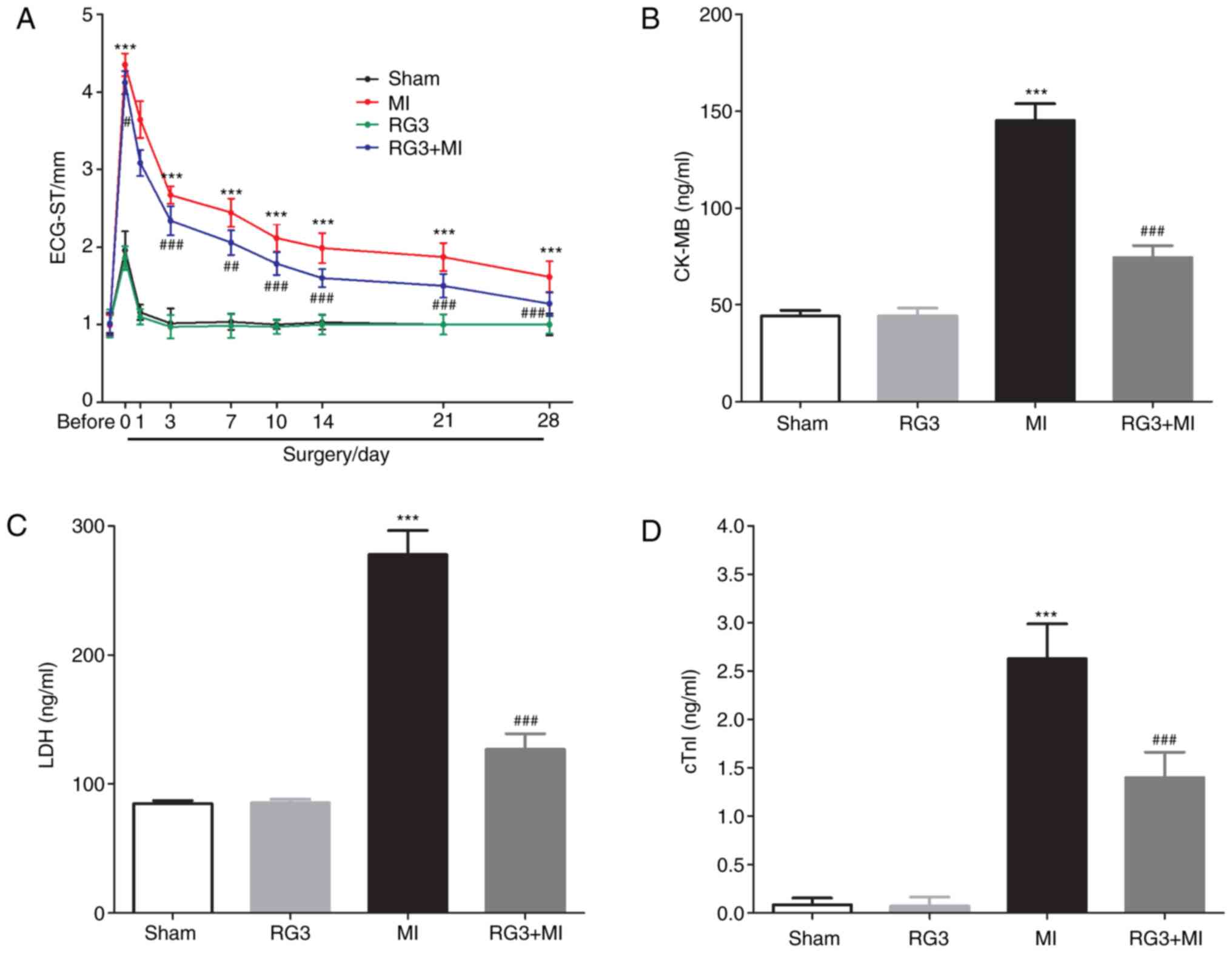
ligation, and as shown in Fig. 1A,
the S-T segment of the ECG in the rats was significantly increased
following coronary artery ligation compared with the sham group.
This indicated that the MI model was successfully established. It
was also identified that Rg3 could significantly attenuate the
increase in rat electrocardiogram S-T values caused by coronary
artery ligation. On 28th day post-MI, the serum levels of CK-MB,
LDH and cTnI in the RG3 + MI rats were significantly decreased
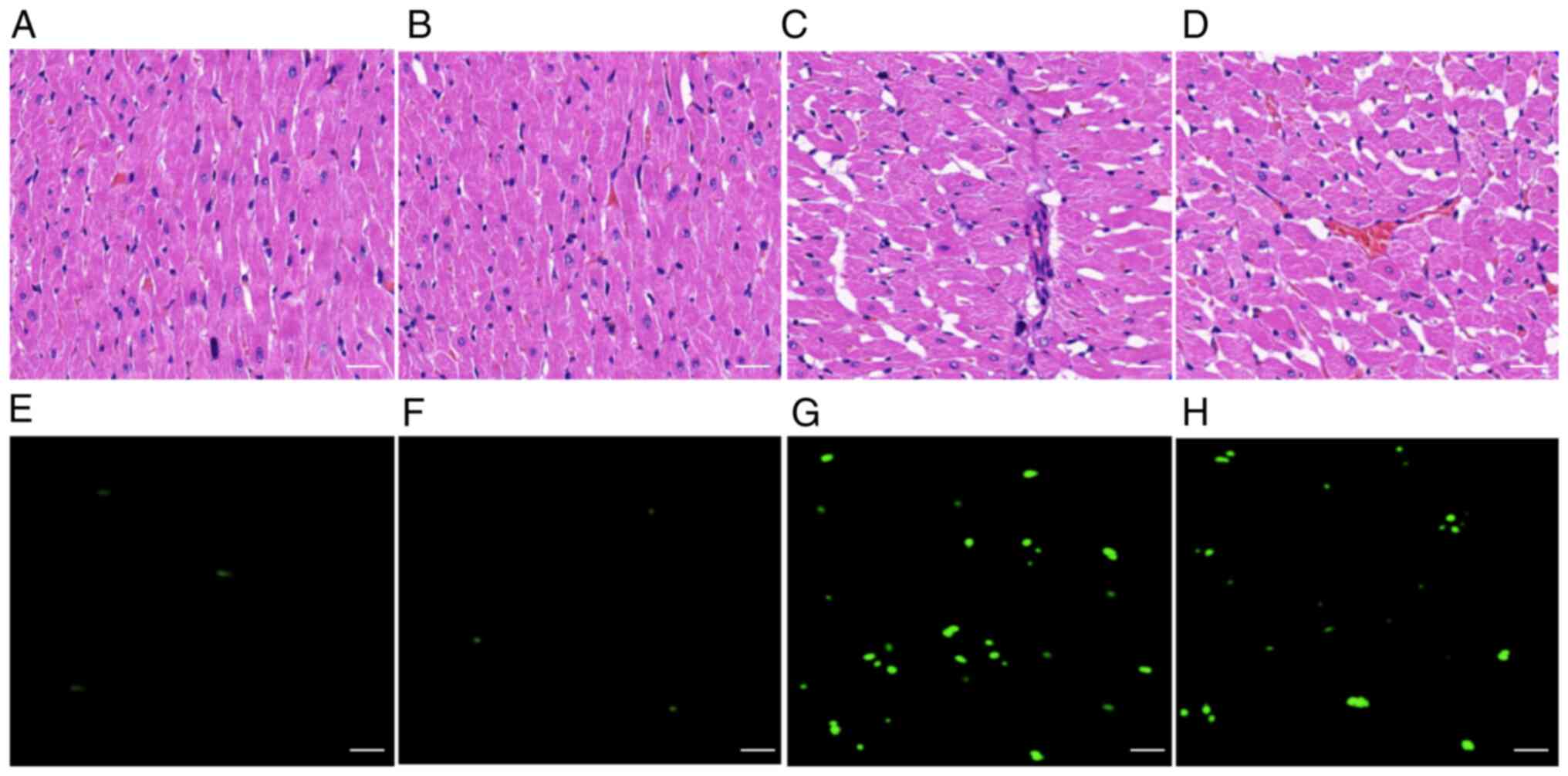
compared with those in the MI group (Fig. 1B-D). In addition, H&E staining
was used to detect pathological changes in rat heart tissue 28 days
following MI. It was observed that there were a number of
pathological changes in the heart tissue of the MI group (Fig. 2C) compared with the sham group
(Fig. 2A) and the RG3 group
(Fig. 2B), such as myocardial
tissue injury, and disordered cell and muscle bundle arrangement.
There were a large number of infiltrating inflammatory cells, and
the nuclei were dissolved, disappeared or necrotic. However, Rg3
was demonstrated to significantly alter the extent of pathological
damage in the heart tissue caused by MI. In the RG3 + MI group
(Fig. 2D), the muscle bundles were
arranged neatly, the cell morphology was intact, and the level of
inflammatory cell infiltration was decreased. It was also observed
that the level of cell apoptosis in the RG3 + MI group was
significantly decreased compared with that in the MI group
(Fig. 2E-H). These data suggested
that Rg3 protected cardiac function in rats following MI.
Rg3 attenuates inflammation of heart
tissue in rats following MI
The results of the H&E staining protocol
demonstrated that Rg3 attenuated MI-induced inflammatory cell
infiltration in the heart tissues of rats. On the 28th day post-MI,
the expression of inflammatory factor in the heart tissue in rats
was also detected, and it was identified that the mRNA and protein
expression levels of TNF-α, IL-1β and IL-6 in the heart tissues of
the MI rats were significantly increased compared with that in RG3
+ MI group (Fig. 3). The expression
of IL-10 was demonstrated to be the opposite. This indicated that
Rg3 attenuated inflammation of heart tissue in rats after MI.
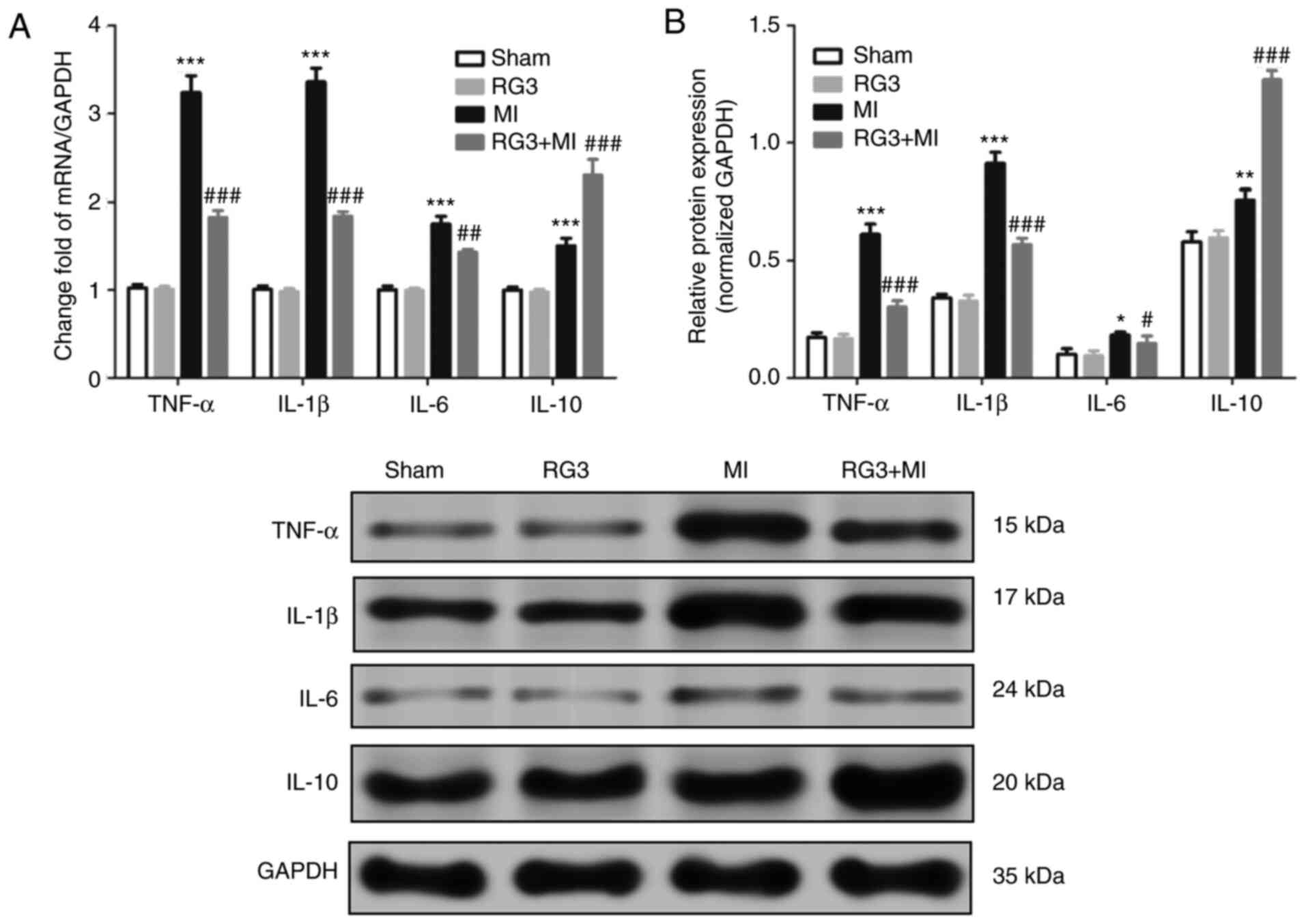 | Figure 3Effect of Rg3 on inflammatory factors
in heart tissues in rats following MI. (A and B) At 28 days
post-MI, the expression levels of TNF-α, IL-1β, IL-6 and IL-10 mRNA
in the heart tissues of rats were measured by RT-qPCR, and proteins
were measured by western blot. There were 7 rats per group and 3
independent repetitions per measurement. *P<0.05,
**P<0.01 and ***P<0.001 vs. sham group.
#P<0.05, ##P<0.01 and
###P<0.001 vs. MI group. Rg3, Ginsenoside Rg3; MI,
myocardial infarction; TNF-α, tumor necrosis factor α; IL,
interleukin. |
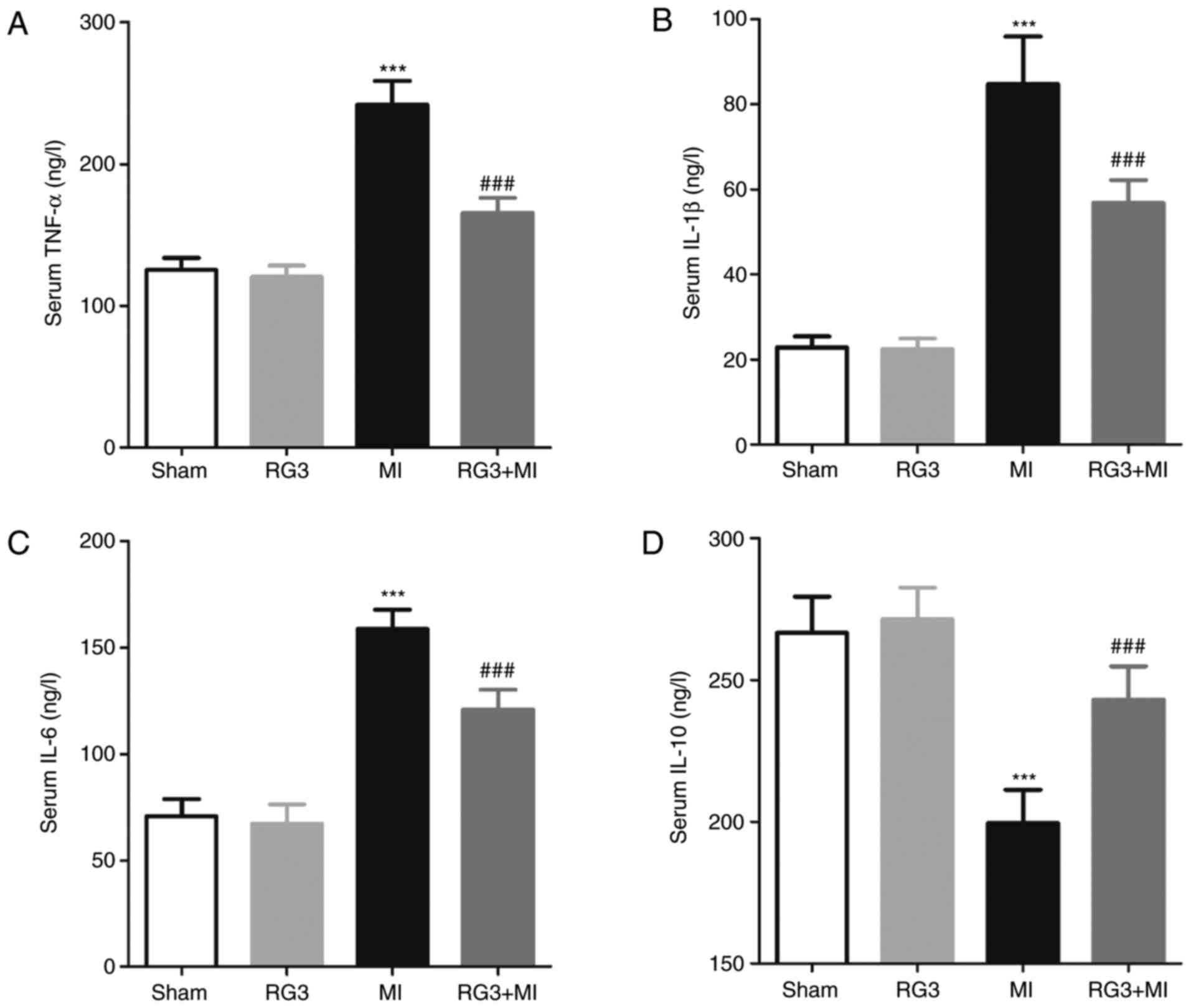
Rg3 attenuates the levels of serum
inflammatory markers in rats following MI
On the 28th day post-MI, the levels of TNF-α, IL-1β,
IL-6 and IL-10 in the serum of rats were measured, and it was
identified that the serum levels of TNF-α, IL-1β and IL-6 in the
RG3 + MI group were significantly decreased compared with those in
the MI group, and the serum levels of IL-10 were significantly
increased compared with the MI group (Fig. 4).
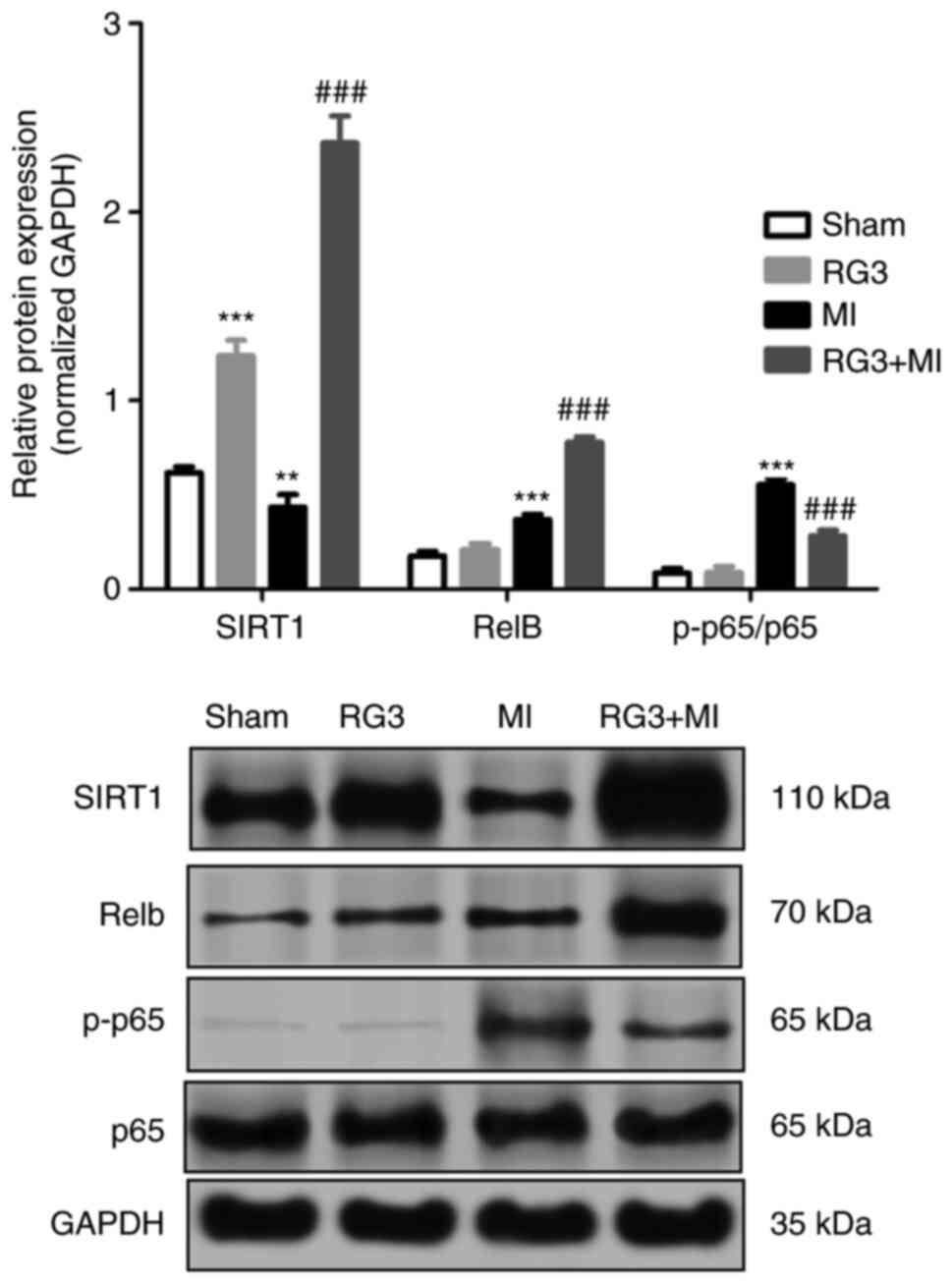
Rg3 inhibits the SIRT1/NF-κB pathway
in rat heart tissue following MI
Western blot analysis was used to measure the
expression levels of proteins in the heart tissues, and the results
are shown in Fig. 5. The expression
level of SIRT1 protein was significantly increased following Rg3
treatment in the RG3 group compared with the sham group, and in the
RG3 + MI group compared with the MI group, but the expression level
of SIRT1 protein in the MI group was significantly compared with
that in the sham group. Compared with the MI group, the expression
of transcription factor RelB (RelB) was significantly increased,
and the expression of p-p65/p65 was significantly decreased.
Discussion
Coronary artery ligation is a standardized and
universal model for the establishment of animal MI rat models to
assess the protective/preventive effects of various
cardioprotective plant components (21). Therefore, a MI rat model was
established by coronary artery ligation in the present study. In
cardiac ischemia, different myocardial enzymes become dysregulated,
and the most commonly dysregulated enzymes are LDH, CKMB and cTnI
(24), accompanied by abnormal
changes in ECG readings, such as an elevated S-T segment (25). Therefore, both myocardial enzymes
and ECG can be used as specific indicators of MI. In the present
study, it was identified that following coronary artery ligation,
the ECG-ST value of the MI model rats was significantly increased
compared with that of the sham group rats, indicating that the MI
model was successfully established. In addition, it was observed
that the serum LDH, CKMB and cTnI levels in the MI group were
significantly increased compared with that in sham group, and Rg3
treatment could decrease them. The same effect was observed in the
ECG-ST value. Compared with the MI group, H&E staining
indicated that the level of inflammatory cell infiltration of the
myocardial tissue was decreased in the RG3 + MI group, the cell
morphology integrity was improved, and the muscle bundles were
arranged neatly. This indicated that Rg3 may decrease CKMB, LDH and
cTnI leakage due to myocardial ischemia and reduce cardiomyocyte
damage to some extent.
Inflammatory reactions are closely associated with
the occurrence and development of acute MI. On one hand, the
release of inflammatory mediators initiates the repair of the
damaged tissues (26). Conversely,
inflammatory reactions continuously induce matrix degradation and
apoptosis of cardiomyocytes (27).
Following MI, the inflammatory regulatory system is activated by
factors including ischemia and hypoxia, inducing myocardial cell
apoptosis and myocardial fibrosis. In order to remove necrotic
cells, the body further activates the inflammatory response,
leading to compensatory deterioration of cardiac function, such as
myocardial remodeling (27).
Therefore, inhibition of inflammation in the body following MI is
one of the methods used to treat MI. Rg3 is one of the main active
ingredients extracted from ginseng. Recent studies have suggested
that Rg3 has the effect of inhibiting inflammation of cells in
vitro (28,29), and also has anti-inflammatory
effects in animal models of MI/R (14) or lipopolysaccharide-induced learning
and memory impairment in rats (13).
In the present study, it was identified that Rg3 not
only decreased the serum levels of TNF-α, IL-1β and IL-6 and
increased the serum IL-10 levels, but also decreased the expression
levels of TNF-α, IL-1β and IL-6 gene and increased the serum IL-10
mRNA expression in the heart tissues of MI rats. IL-6 is a
glycoprotein produced by both lymphocytes and non-lymphocyte cells,
which regulates the synthesis of hepatic C-reactive protein. It
increases the expression of adhesion molecules between
cardiomyocytes, promoting platelet aggregation, and promoting
smooth muscle cells proliferation and release of inflammatory
factors such as IL-1β and TNF-α (30), while IL-1β and TNF-α enhance
leukocyte chemotaxis and directly damage endothelial cells
(31), which participate in the
whole process response of MI inflammation. It was demonstrated that
IL-10 is firstly synthesized and secreted by murine CD4+
T helper type 2 cells and had the function of inhibiting IFN-γ
synthesis by T helper type 1 T cells (32). As the study progressed (33,34),
the immunological characteristics of IL-10 were gradually revealed.
The main cells for synthesizing IL-10 were monocytes and
macrophages, but a number of other cells can also synthesize IL-10,
including CD4+ and CD8+ T cells, and B cells.
In the immune system, IL-10 has the ability to inhibit the
proliferation of antigen-specific T cells, the formation of
antigen-presenting cells, and the synthesis and expression of
inflammatory cytokines and inflammatory mediators. Taken together,
IL-10 is a cytokine that inhibits the inflammatory response and
elicits immunosuppressive effects in the body (35). Therefore, IL-10 is widely used in
the treatment of autoimmune diseases as a natural immunosuppressive
agent (36,37).
The present study also identified that Rg3 not only
increased the expression levels of SIRT1 and RelB proteins, but
also decreased the expression of p-p65 protein. SIRT1 is a histone
deacetylase that was widely expressed in human cells. It performs
important biological functions by deacetylating multiple
transcription factors, such as p53(38), mitochondrial uncoupling protein
2(39), histone acetyltransferase
p300(40) and NF-κB (41). p65 is an important component of
NF-κB, which only functions following its acetylation. In
inflammatory responses, SIRT1-deacetylated p65 inhibits the
transcription of TNF-α, IL-6 and other inflammatory genes
downstream of NF-κB (41), which is
an important protein in the Toll-like receptors/NF-κB signaling
pathway. Its phosphorylation-mediated translocation from the
cytoplasm to the nucleus is an important marker of activation of
NF-κB signaling pathway.
NF-κB is a key transcription factor that mediates
the release of inflammatory factors. It exists in vascular
endothelial cells, vascular smooth muscle cells and cardiomyocytes,
and participates in the occurrence and development of
cardiovascular diseases (42,43).
Under normal physiological conditions, NF-κB binds to the
inhibitory protein IκB in the form of a homodimer and is inactive.
When stimulated by various pathological factors, NF-κB and IκB are
activated by phosphorylation and transferred to the nucleus, where
they bind to specific target genes, regulate target gene
transcription and release associated inflammatory factors, such as
IL-1β, IL-6 and TNF-α. The release of IL-1β, TNF-α and IL-6 also
serves as a feedback signal for NF-κB and maintains the activity of
NF-κB, which causes a feedback cycle of myocardial injury (41,42).
SIRT1 inhibits NF-κB activation by inhibiting NF-κB entry into the
nucleus.
Therefore, in the present study, it was concluded
that Rg3, as an activator of SIRT1, inhibited the activation of
NF-κB pathway by activating the expression of SIRT1 in rats,
thereby inhibiting the inflammatory response in MI rats.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ designed and performed the study and wrote the
article. CT and BW performed the data collection and analysis. All
authors read and approved the final manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
This study was reviewed and approved by the Ethics
Committee of Beijing Anzhen Hospital, Capital Medical
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mangion K, Gao H, Husmeier D, Luo X and
Berry C: Advances in computational modelling for personalised
medicine after myocardial infarction. Heart. 104:550–557.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Heusch G and Gersh BJ: The pathophysiology
of acute myocardial infarction and strategies of protection beyond
reperfusion: A continual challenge. Eur Heart J. 38:774–784.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Boersma E, Mercado N, Poldermans D,
Gardien M, Vos J and Simoons ML: Acute myocardial infarction.
Lancet. 361:847–858. 2003.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Reed GW, Rossi JE and Cannon CP: Acute
myocardial infarction. Lancet. 389:197–210. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Michaels AD, Gibson CM and Barron HV:
Microvascular dysfunction in acute myocardial infarction: Focus on
the roles of platelet and inflammatory mediators in the no-reflow
phenomenon. Am J Cardiol. 85:50B–60B. 2000.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Maier W, Altwegg LA, Corti R, Gay S,
Hersberger M, Maly FE, Sütsch G, Roffi M, Neidhart M, Eberli FR, et
al: Inflammatory markers at the site of ruptured plaque in acute
myocardial infarction: Locally increased interleukin-6 and serum
amyloid A but decreased C-reactive protein. Circulation.
111:1355–1361. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pop M, Qi X, Barry J, Strauss BH, Wright
GA and Ghugre NR: Hemorrhage promotes inflammation and myocardial
damage following acute myocardial infarction. J Cardiovasc Magn
Reson. 16 (Suppl 1)(O72)2014.
|
|
8
|
Westman PC, Lipinski MJ, Luger D, Waksman
R, Bonow RO, Wu E and Epstein SE: Inflammation as a driver of
adverse left ventricular remodeling after acute myocardial
infarction. J Am Coll Cardiol. 67:2050–2060. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ong SB, Hernández-Reséndiz S,
Crespo-Avilan GE, Mukhametshina RT, Kwek XY, Cabrera-Fuentes HA and
Hausenloy DJ: Inflammation following acute myocardial infarction:
Multiple players, dynamic roles, and novel therapeutic
opportunities. Pharmacol Ther. 186:73–87. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Eindhoven DC, Hilt AD, Zwaan TC, Schalij
MJ and Borleffs CJW: Age and gender differences in medical
adherence after myocardial infarction: Women do not receive optimal
treatment - The Netherlands claims database. Eur J Prev Cardiol.
25(2047487317744363)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sun HY, Lee JH, Han YS, Yoon YM, Yun CW,
Kim JH, Song YS and Lee SH: Pivotal roles of ginsenoside Rg3 in
tumor apoptosis through regulation of reactive oxygen species.
Anticancer Res. 36:4647–4654. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tang YC, Yan Z, Jin Z, Zhi Q, Wu MY, Gong
FR, Shen M, Liu L, Tao M, Shen B, et al: Ginsenoside Rg3 targets
cancer stem cells and tumor angiogenesis to inhibit colorectal
cancer progression in vivo. Int J Oncol. 52:127–138.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lee B, Sur B, Park J, Kim SH, Kwon S, Yeom
M, Shim I, Lee H and Hahm DH: Ginsenoside Rg3 alleviates
lipopolysaccharide-induced learning and memory impairments by
anti-inflammatory activity in rats. Biomol Ther (Seoul).
21:381–390. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang LP, Jiang YC, Yu XF, Xu HL, Li M,
Zhao XZ and Sui DY: Ginsenoside Rg3 improves cardiac function after
myocardial ischemia/reperfusion via attenuating apoptosis and
inflammation. Evid Based Complement Alternat Med.
2016(6967853)2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yang JL, Ha TK, Dhodary B, Kim KH, Park J,
Lee CH, Kim YC and Oh WK: Dammarane triterpenes as potential SIRT1
activators from the leaves of Panax ginseng. J Nat Prod.
77:1615–1623. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang Y, Chen Y, Wang H, Cheng Y and Zhao
X: Specific turn-on fluorescent probe with aggregation-induced
emission characteristics for SIRT1 modulator screening and
living-cell imaging. Anal Chem. 87:5046–5049. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kauppinen A, Suuronen T, Ojala J, Kai K
and Salminen A: Antagonistic crosstalk between NF-κB and SIRT1 in
the regulation of inflammation and metabolic disorders. Cell
Signal. 25:1939–1948. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jankowski M, Bissonauth V, Gao L, Gangal
M, Wang D, Danalache B, Wang Y, Stoyanova E, Cloutier G, Blaise G
and Gutkowska J: Anti-inflammatory effect of oxytocin in rat
myocardial infarction. Basic Res Cardiol. 105:205–218.
2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chen GD, Yu WD and Chen XP: SirT1
activator represses the transcription of TNF-α in THP-1 cells of a
sepsis model via deacetylation of H4K16. Mol Med Rep. 14:5544–5550.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
National Research Council. Guide for the
care and use of laboratory animals. National Academies Press,
2010.
|
|
21
|
Sun Q, Kang Z, Cai J, Liu W, Liu Y, Zhang
JH, Denoble PJ, Tao H and Sun X: Hydrogen-rich saline protects
myocardium against ischemia/reperfusion injury in rats. Exp Biol
Med (Maywood). 234:1212–1219. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tao J, Zhang J, Ling Y, McCall CE and Liu
TF: Mitochondrial sirtuin 4 resolves immune tolerance in monocytes
by rebalancing glycolysis and glucose oxidation homeostasis. Front
Immunol. 9(419)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mehta LS, Beckie TM, Devon HA, Grines CL,
Krumholz HM, Johnson MN, Lindley KJ, Vaccarino V, Wang TY, Watson
KE, et al: Acute myocardial infarction in women: A scientific
statement from the American Heart Association. Circulation.
133(916)2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Toggweiler S, Sabti Z and Cuculi F:
Prehospital ticagrelor in ST-segment elevation myocardial
infarction. South China J Cardiol. 9:657–659. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ruparelia N, Godec J, Lee R, Chai JT,
Dall'Armellina E, McAndrew D, Digby JE, Forfar JC, Prendergast BD,
Kharbanda RK, et al: Acute myocardial infarction activates distinct
inflammation and proliferation pathways in circulating monocytes,
prior to recruitment, and identified through conserved
transcriptional responses in mice and humans. Eur Heart J.
36:1923–1934. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Prabhu SD and Frangogiannis NG: The
biological basis for cardiac repair after myocardial infarction:
From inflammation to fibrosis. Circ Res. 119:91–112.
2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Shin YM, Jung HJ, Choi WY and Lim CJ:
Antioxidative, anti-inflammatory, and matrix metalloproteinase
inhibitory activities of 20(S)-ginsenoside Rg3 in cultured
mammalian cell lines. Mol Biol Rep. 40:269–279. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lee IS, Uh IJ, Kim KS, Kim KH, Park J, Kim
Y, Jung JH, Jung HJ and Jang HJ: Anti-inflammatory effects of
ginsenoside Rg3 via NF-κB pathway in A549 cells and human asthmatic
lung tissue. J Immunol Res. 2016(7521601)2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Brasier AR: The nuclear
factor-kappaB-interleukin-6 signalling pathway mediating vascular
inflammation. Cardiovasc Res. 86:211–218. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Khanna AK, Jianping X and Mehra MR:
Antioxidant N-acetyl cysteine reverses cigarette smoke-induced
myocardial infarction by inhibiting inflammation and oxidative
stress in a rat model. Lab Invest. 92:224–235. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Annacker O, Pimenta-Araujo R,
Burlen-Defranoux O, Barbosa TC, Cumano A and Bandeira A:
CD25+ CD4+ T cells regulate the expansion of
peripheral CD4 T cells through the production of IL-10. J Immunol.
166:3008–3018. 2001.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Dardalhon V, Awasthi A, Kwon H, Galileos
G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, et
al: IL-4 inhibits TGF-beta-induced Foxp3+ T cells and,
together with TGF-beta, generates IL-9+
IL-10+ Foxp3(-) effector T cells. Nat Immunol.
131:1347–1355. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
34
|
Timóteo RP, Silva AF, Micheli DC, Candido
Murta EF, Freire M, Teodoro RB, Lima FM, Martins Tavares Murta B
and Bertoncello D: Increased flexibility, pain reduction and
unaltered levels of IL-10 and CD11b + lymphocytes in patients with
systemic lupus erythematosus were associated with kinesiotherapy.
Lupus. 27:1159–1168. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Fujii S and Lotze MT: Interleukin-10
(IL-10). Cancer Drug Discov Dev: 165-179, 2007.
|
|
36
|
Dambuza IM, He C, Choi JK, Yu CR, Wang R,
Mattapallil MJ, Wingfield PT, Caspi RR and Egwuagu CE: IL-12p35
induces expansion of IL-10 and IL-35-expressing regulatory B cells
and ameliorates autoimmune disease. Nat Commun.
8(719)2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Groux H and Cottrez F: The complex role of
interleukin-10 in autoimmunity. J Autoimmun. 20:281–285.
2003.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lai M, Du G, Shi R, Yao J, Yang G, Wei Y,
Zhang D, Xu Z, Zhang R, Li Y, et al: miR-34a inhibits migration and
invasion by regulating the SIRT1/p53 pathway in human SW480 cells.
Mol Med Rep. 11:3301–3307. 2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
LI X and Jiang W: GW26-e0454 renalase
protects the cardiomyocytes of Sprague-Dawley rats against ischemia
and reperfusion injury by reducing myocardial cell necrosis and
apoptosis. J Am Coll Cardiol. 66:C43. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kim EK and Choi EJ: Compromised MAPK
signaling in human diseases: An update. Arc Toxicol. 89:867–882.
2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wagner SA, Satpathy S, Beli P and
Choudhary C: SPATA2 links CYLD to the TNF-α receptor signaling
complex and modulates the receptor signaling outcomes. EMBO J.
35:1868–1884. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Baker R, Hayden M and Ghosh S: NF-κB,
inflammation, and metabolic disease. Cell Metab. 13:11–22.
2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ghosh S and Hayden MS: New regulators of
NF-kappaB in inflammation. Nat Rev Immunol. 8:837–848.
2008.PubMed/NCBI View
Article : Google Scholar
|