Introduction
Biochanin A (BA; chemical formula,
C16H12O5), is a phytochemical and
a flavonoid present in natural produce such as Trifolium
pratense and Arachis hypogaea (1). BA exhibits various protective effects
including anti-inflammatory, glucose and lipid metabolism
modulatory activity, antitumor capacity, and neuroprotective
activity (2-4).
BA can inhibit the lipopolysaccharide (LPS)-induced activation of
microglia (5). The
anti-inflammatory activity of BA has been demonstrated in a variety
of cells such as endothelial cells, various cancer cells and
macrophages (6,7). Studies in vitro have shown that
the proliferation of preosteoclast cells was markedly inhibited in
a dose-dependent manner in the presence of BA. Furthermore, BA
promoted the differentiation of primary osteoblasts into
osteoblasts, and significantly enhanced the expression and activity
of alkaline phosphatase (ALP) (4).
Mature osteoblast migration and the number of mineralized nodules,
consisting of phosphorus and calcium are significantly increased by
BA (4). Furthermore, it has been
reported that BA decreased the H2O2-induced
production of TNF-α, interleukin (IL)-6 and nitric oxide (NO) in
osteoblasts (8). The
anti-inflammatory effects of BA are mediated by inhibiting the
inflammatory mediators, includeing IL-1β, IL-6, TNF-α,
cyclooxygenase-2 (COX-2), matrix metalloprotein-9 (MMP-9) and NO in
primary rat chondrocytes (9). BA
decreases the expression of proinflammatory cytokines by inhibiting
IκB kinase activity, resulting in NF-κB-driven inhibition of gene
transcription (8,10,11).
Furthermore, BA can exert the antioxidative activity by scavenging
reactive oxygen species (ROS) and enhancing the glutathione
peroxidase and superoxide dismutase activity (12). A previous study conducted using
animal models with acute and chronic inflammation demonstrated a
marked anti-inflammatory and antioxidative activity of BA against
organ injury (13). BA-mediated
inhibition of bone loss in postmenopausal women has also been
reported in a previous study (14).
These findings suggested that BA treatment can potentially regulate
bone cell proliferation, apoptosis and migration, leading to
reduced bone loss and bone turnover.
Periodontitis is a prevalent chronic disease
worldwide. The 1990-2016 Global Burden of Disease Study indicated
that periodontitis and dental caries in permanent teeth were the
leading and the 11th most prevalent diseases worldwide in 2016.
Periodontal diseases had a high prevalence among all human diseases
(751 million; 95% uncertainty interval, 534-874 million) (15). Periodontitis is characterized by the
breakdown of connective tissues and alveolar bone resorption
eventually leading to mobility and loss of teeth (16). An inappropriate host immune
inflammatory response and high levels of ROS are considered major
factors associated with the destruction of periodontal tissues
(17). Elevated levels of
proinflammatory cytokines, such as IL-1β and TNF-α, trigger soft
and hard tissue breakdown in periodontitis (18). Moreover, previous studies have
suggested a dual role of ROS in periodontitis (19,20).
At a cellular level, ROS are required for physiological processes
and contribute to the oxidative killing of periodontal pathogens.
However, excessive production of ROS may trigger an oxidative
stress response resulting in periodontal destruction. In addition,
oxidative stress response can reinforce immune response through
redox-sensitive gene transcription factors such as NF-κB, leading
to periodontal tissue breakdown (21). Therefore, anti-inflammatory and
anti-oxidative responses are essential for the treatment of
periodontitis.
The biological effects of BA and the mechanisms
involved in the pathogenesis of periodontitis suggest that BA may
serve as an adjunctive treatment option for periodontitis via the
anti-inflammatory and anti-oxidative effects. Therefore, the aim of
the present study was to evaluate the influence of BA on gingival
inflammation and tissue destruction in rat periodontitis. In
addition, the effects of BA supplementation on the expression
levels of periodontal inflammatory markers and oxidative stress
were explored.
Materials and methods
Ethics approval
The present study was approved by Ethics Committee
of the State Key Laboratory of Oral Diseases, West China Hospital
of Stomatology, Sichuan University (approval no.
WCHSIRB-D-2018-148).
Animals
The animal experimental study was conducted using 26
male Wistar rats (age, 8 weeks; weight, 280-300 g) purchased from
the Chengdu Dashuo Experimental Animal Co., Ltd. (license no.
510109000176387). The rats were housed under 12-h light/dark cycles
and standard conditions of humidity (60±5%) and room temperature
(24±1˚C). All animals had free access to food and water. The rats
adapted to the environment for 7 days prior to further experiments.
One rat was used for isolation of gingival fibroblasts and 25 were
used to construct animal models, as described below.
Primary culture of rat gingival
fibroblasts
To isolate primary cultures of rat gingival
fibroblasts, one Wistar rat was anesthetized with isoflurane (5%
induction, 2% maintenance; inhaled until euthanized). Gingival
tissues were subsequently excised from the buccal area, submersed
in sterile PBS with 1% penicillin/streptomycin (Sigma Aldrich;
Merck KGaA), and then sectioned into small fragments of ~1
mm3. The gingival fragments were digested with 2 ml type
I collagenase solution in PBS (25 U/ml; Sigma Aldrich; Merck KGaA)
at 37˚C for 1 h, followed with a 15-min incubation at 37˚C in
trypsin-EDTA solution (0.25%; Thermo Fisher Scientific, Inc.).
Subsequently, the fragments were incubated in DMEM with 10% FBS
(both Sigma Aldrich; Merck KGaA) and 1% penicillin/streptomycin at
37˚C (22). The cultured cells from
gingiva were tested by immunocytochemical analysis. These cells
were tested negative for anti-keratin staining whilst staining
positive for vimentin, suggesting these cells to be gingival
fibroblasts (23). Cells between
sixth and ninth passages were used for the assessment of cell
cytotoxicity.
Cell cytotoxicity assay
The cytotoxicity of BA (purity, 99.9%; Sigma
Aldrich; Merck KGaA) on rat gingival fibroblasts was evaluated
using an MTT assay (Sigma Aldrich; Merck KGaA) according to
manufacturer's instructions. Briefly, rat gingival fibroblast cells
at a density of 2x104/well were cultured in 96-well
plates at 37˚C for 24 and 48 h and then treated with 0, 50, 100 and
150 µM BA for 24 h. Subsequently, 20 µl MTT was added into each
well and incubated for another 4 h before the supernatant was
removed. Finally, 150 µl DMSO was added to each well and the
absorbance values at a wavelength of 570 nm were measured using a
microplate reader (24).
Study design
A total of 25 Wistar rats were randomly assigned to
five study groups: i) Healthy control (control); ii) experimental
periodontitis (ligation); iii) ligation + BA low dose (12.5
mg/kg/day); iv) ligation + BA medium dose (25 mg/kg/day); and v)
ligation + BA high dose (50 mg/kg/day). The control group received
sham-ligation. For the ligation groups, periodontitis was induced
by inserting a nylon ligature around maxillary molars for 14 days
as described previously (25). The
ligatures were placed sub-gingivally, knotted at the mesial site
and checked on a daily basis. Rats in the ligation + BA group were
administered with BA (purity, 99.9%; Sigma Aldrich; Merck KGaA) 2
weeks after establishment of the experimental periodontitis model
intraperitoneally at low, medium and high doses, respectively.
Control and ligation groups' rats were injected 0.9% sodium
chloride instead of BA. An automatic electronic balance [cat. no.
PX4202 OHAUS Instruments (Shanghai) Co., Ltd.] was used to monitor
and record rat body weights on a weekly basis. After treatment with
BA for 4 weeks, all rats were anesthetized with isoflurane. After
deep anesthesia of the rats, blood was drawn by cardiac puncture
with non-anticoagulant vacuum tubes and 23G1 needle. The anesthesia
was checked by lack of spontaneous movements, slow breathing rate
and lack of response to stimuli. The rats were euthanized
immediately at the end of cardiac puncture. The maxillary jaws were
harvested and fixed in 10% neutral-buffered formalin at room
temperature (24±1˚C) for 24 h. The gingival tissues were collected
for subsequent analysis. All samples were stored at -80˚C.
Determination of inflammatory
cytokines, ROS and osteocalcin (OCN)
In order to isolate serum, the clotted blood samples
were centrifuged (10 min; 2432 x g). Gingival tissues were
homogenized with a glass pestle in PBS supplemented with RIPA lysis
buffer (Sigma Aldrich; Merck KGaA) and protease inhibitor (Thermo
Fisher Scientific, Inc.). Bicinchoninic acid assay kit (Thermo
Fisher Scientific, Inc.) was used for protein quantitation. Protein
levels of IL-1β and TNF-α in gingiva were measured by ELISA
kits (cat. nos. BMS630 and ERA57RB; Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. The
present study used TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) to extract total RNA from the gingival
tissues around the maxillary first molars. The PrimeScript Reagent
kit with gDNA Eraser (cat. no. RR047A, Takara Bio, Inc.) was used
for the reverse transcription of total RNA into cDNA (temperature
protocol: 37˚C for 15 min followed by 85˚C for 5 sec). Quantitative
PCR analysis were performed by use of SYBR® Premix Ex
Taq™ II Kit (cat. no. DRR820A, Takara Bio, Inc.) with
the CFX96 Touch™ Real-time PCR system (Bio-Rad Laboratories, Inc.)
using the following thermocycling conditions: Initial denaturation
at 95˚C for 30 sec, followed by 40 cycles of 95˚C for 5 sec and
60˚C for 30 sec. IL-1β and TNF-α expression levels in gingival
tissues were evaluated using GAPDH internal control and relative
quantitative analysis was performed using the 2-ΔΔCq
method (26). Primers for the qPCR
are provided in Table SI.
Histological analysis
For histological analysis, the alveolar bone tissue
were decalcified using 10% EDTA at room temperature for 8 days,
embedded in paraffin and cut into 4 µm-thick sections. After
heating at 60˚C for 30 min, xylene was used to deparaffinize the
sections (4-µm thick) and gradient ethanol solutions (100, 95, 80
and 70%) were used in a descending order to rehydrate the samples,
followed by rinsing with deionized water. Sections were stained
using hematoxylin and eosin at room temperature for 3 min and
histopathological analysis of six randomly selected fields of view
(100x100 µm) for each specimen was performed as reported previously
(27).
Micro-computed tomography (micro-CT)
analysis
A high-resolution micro-CT system (µCT 50; SCANCO
Medical AG) was used to scan maxillary jaw samples isolated from
the rats, which were harvested and fixed in 10% neutral-buffered
formalin at room temperature for 24 h. Fixed parameters (image
size, 2,048x2,048 pixels; voltage, 100 kV; electrical current, 0.1
mA; resolution, 10 µm) were used for scanning. The scanned image
data and 3D reconstructions were analyzed by CT-Analyser 1.13
software (SCANCO Medical AG). The vertical loss of bone in the
maxillary first molar was assessed by calculating distance (µm)
between cementoenamel junction and alveolar bone crest (CEJ-ABC) as
described previously (28). In
addition, bone volume fraction, represented as bone volume/tissue
volume, of the region of interest (ROI) in the first molar beneath
the furcation area were evaluated using the CT-Analyser 1.13
software (SCANCO Medical AG). The alveolar bone loss was evaluated
by a single examiner who was blinded to the experimental
design.
Leukocyte acid phosphatase (TRAP)
staining
For the quantification of osteoclasts, a TRAP kit
(Sigma Aldrich; Merck KGaA) was used to stain sections according to
the manufacturer's protocol. The results were assessed by a light
microscope with a camera connected to a computer (IX71; Olympus
Corporation). TRAP-positive multinucleated (≥2) cells at the
alveolar bone surface were defined as osteoclasts (29). The present study used Image-Pro Plus
6.0 software (Media Cybernetics, Inc.) to analyze three fields of
each section. Results are reported as the number of positively
stained cells per unit area of alveolar bone
(number/mm2).
Immunohistochemical analysis of OCN
and Nrf2
After the rats' maxilla were decalcified in 10% EDTA
solution for 1 month, the samples were dehydrated, embedded into
paraffin blocks and sliced into 4-μm-thick sections for
immunohistochemical analysis. The slides were washed twice at 5 min
each in TBS plus 0.025% Triton X-100 and blocked in 10% normal goat
serum (cat. no. 31873; Invitrogen; Thermo Fisher Scientific, Inc.)
with 1% BSA (cat. no. A2153; Sigma Aldrich; Merck KGaA) solution in
TBS for 2 h at room temperature. Primary antibodies of OCN (1:200;
cat. no. ab13420; Abcam) and Nrf2 (1:300; cat. no. ab137550; Abcam)
were diluted in TBS plus 1% BSA. The slides were incubated with the
primary antibodies overnight at 4˚C. After 2X TBS wash at 5 min
each, slides were incubated in horseradish peroxidase-linked
antibodies (cat. nos. 7076 and 7074, Cell Signaling Technology,
Inc.) at room temperature for 15 min. The sections were examined
for immunohistochemical analyses three times by the same examiner
using the aforementioned IX71 microscope with a camera connected to
a computer. Images of three visual fields from each rat were
captured. The total count of immuno-positive cells was performed in
the complete area of all photographs using Image-Pro Plus 6.0
software.
Statistical analysis
Data are presented as the mean ± SD and were
analyzed using the SPSS software (version 17.0; SPSS, Inc.).
One-way ANOVA and Tukey's test was used for statistical analysis
and P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of BA on gingival fibroblast
viability
The MTT assay results indicated that BA at 50 to 150
µM induced no significant cytotoxicity toward gingival fibroblasts
(Fig. S1). Cytotoxicity was
represented as the concentration of BA inhibiting cell viability by
50%.
Effects of BA on rat body weight
Body weight monitoring on a weekly basis for six
weeks revealed that the body weight of rats was increased by
2.9±0.1 and 2.4±0.2 g in the control and ligation group,
respectively. BA treatment groups showed a higher gain in the body
weight compared with the ligation group (Table I); however, the differences were not
statistically significant.
 | Table IChanges in the BW of rats during the
6-week experimental period. |
Table I
Changes in the BW of rats during the
6-week experimental period.
| Study group | Initial BW, g | Average BW BW,
g | Terminal gain/day,
g |
|---|
| Control | 291±5 | 371±2.6 | 2.9±0.1 |
| Ligation | 293±4 | 359±2.6 | 2.4±0.2 |
| Ligation +
BA12.5 | 290±7 | 366±6.1 | 2.7±0.1 |
| Ligation +
BA25 | 286±4 | 365±2.3 | 2.8±0.2 |
| Ligation +
BA50 | 289±9 | 364±6.4 | 2.7±0.5 |
Gingival inflammation and oxidative
stress levels are attenuated by BA
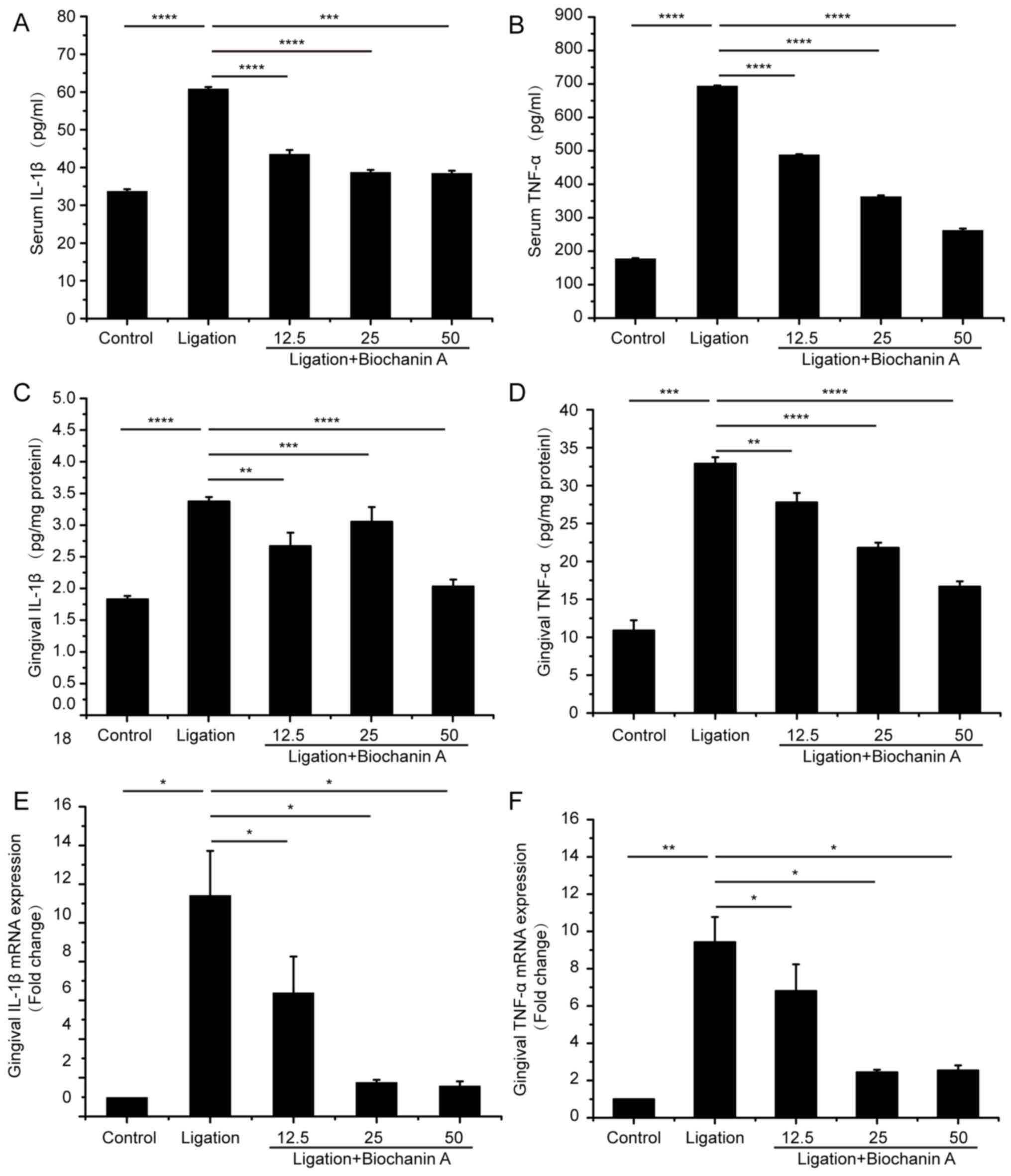
Serum and gingival IL-1β and TNF-α levels were
significantly increased in the ligation group compared with the
control group (Fig. 1A-D).
Treatment with BA significantly inhibited the serum TNF-α and IL-1β
levels induced by experimental periodontitis. Moreover, gingival
mRNA levels of TNF-α and IL-1β were also alleviated with BA
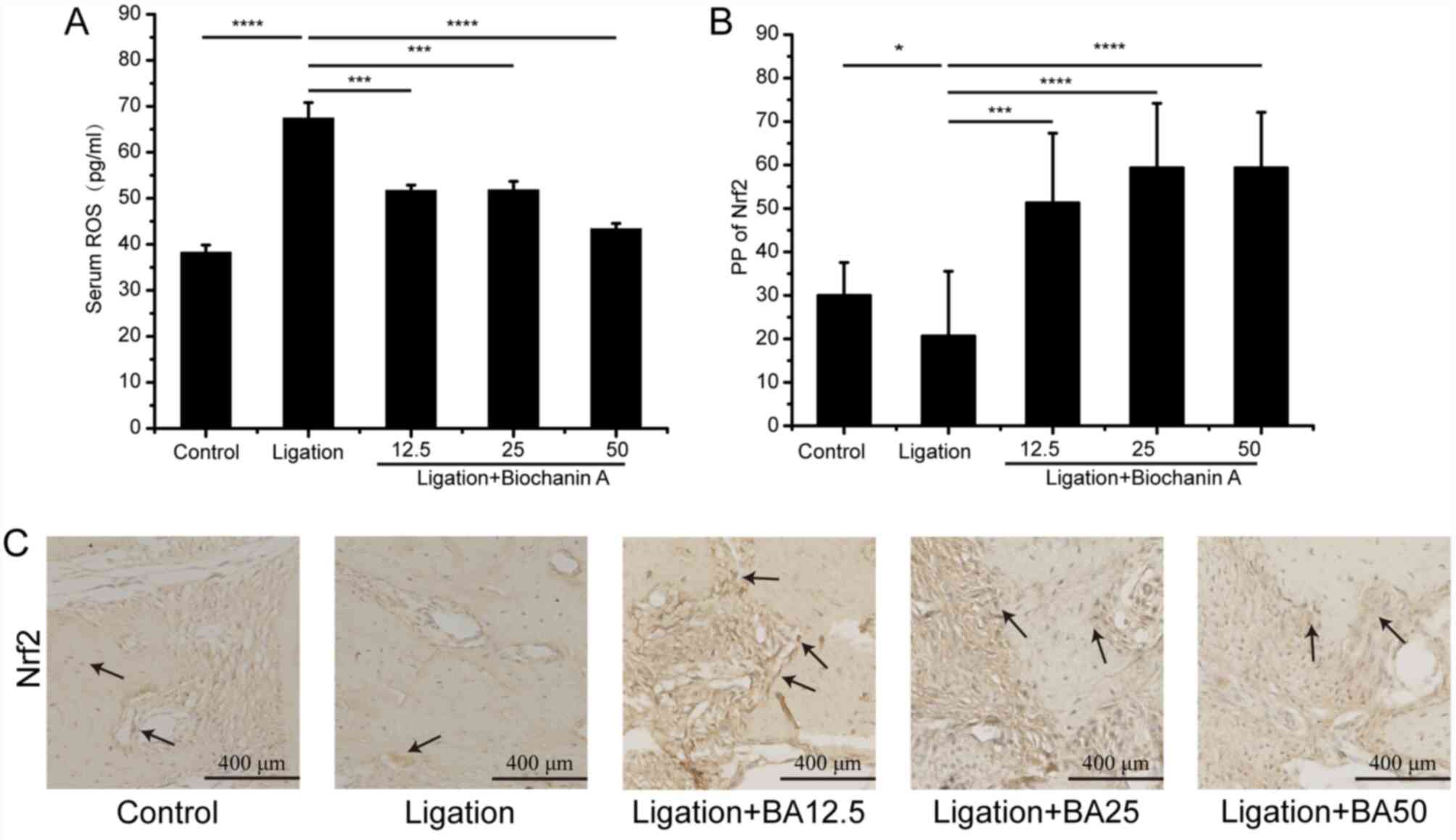
treatment (Fig. 1E and F). In addition, serum ROS level was
significantly higher in the ligation group compared with the
control group. However, treatment with BA inhibited
ligation-induced ROS levels (Fig.
2A). The analysis of effects of BA on Nrf2 expression by
immunohistochemistry showed that the expression of Nfr2 in gingival
tissues was increased following BA treatment (Fig. 2B and C).
BA alleviates bone loss
Histological analysis of control group specimens
showed normal physiological periodontium including clearly defined
gingiva, periodontal ligament, cementum and alveolar bone (Fig. 3A). By contrast, the ligation group
showed absorption in the alveolar bone between first and second
molars. The gingival epithelial spikes hyperplasia, thickened
stratum spinosum and marked breakdown of collagen fibers could also
be observed (Fig. 3A). The
treatment with BA apparently recovered tissue degradation in the
ligation group (Fig. 3A). The
micro-CT analyses indicated that the medium and high dose of
BA-treated groups showed shorter distances of CEJ-ABC compared with
those in the ligation group (Fig.
3B and C). The bone volume
fraction beneath the furcation area of maxillary first molar
increased in all three BA-treated groups compared with that in the
ligation group (Fig. 3D); however
the levels observed in the control group have not been fully
restored (Fig. 3B-D).
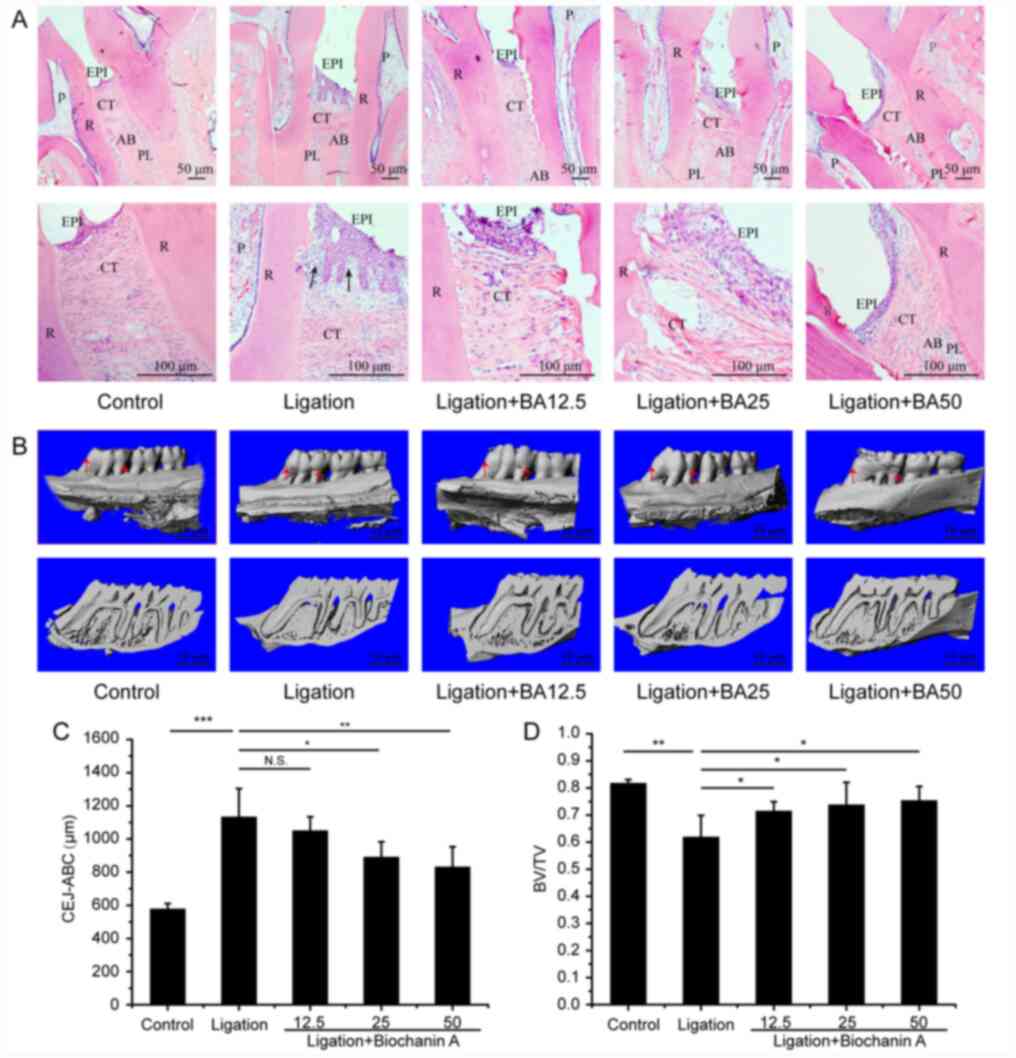 | Figure 3Role of biochanin A in ameliorating
the alveolar bone loss in rats with periodontitis. (A) Hematoxylin
and eosin staining indicated periodontal morphology. Images on the
bottom row magnifies 2.5 times of the regions of interest on the
top row. PL, periodontal ligament; R, root; AB, alveolar bone; EPI,
oral epithelium; CT, connective tissue; P, pulp. The black arrow
denotes the gingival epithelial spikes hyperplasia. (B)
Micro-computed tomography reconstruction shows alveolar bone loss
of maxillary first molars around mesial/distal aspect (buccal
view). (C) Quantitative analysis of bone loss around mesial/distal
aspect. (D) Quantitative analysis of BV of the region of interest
in the first molar beneath the furcation area. Data are presented
as the mean ± SD, n=5 rats per group. *P<0.05,
**P<0.01, ***P<0.001 and N.S. means no
significant difference. CEJ-ABC, cemento-enamel junction and
alveolar bone crest; BA12.5, 12.5 mg/kg/day biochanin A; BA25, 25
mg/kg/day biochanin A; BA50, 50 mg/kg/day biochanin A; BV/TV, bone
volume/tissue volume. |
BA affects bone turnover and
metabolism
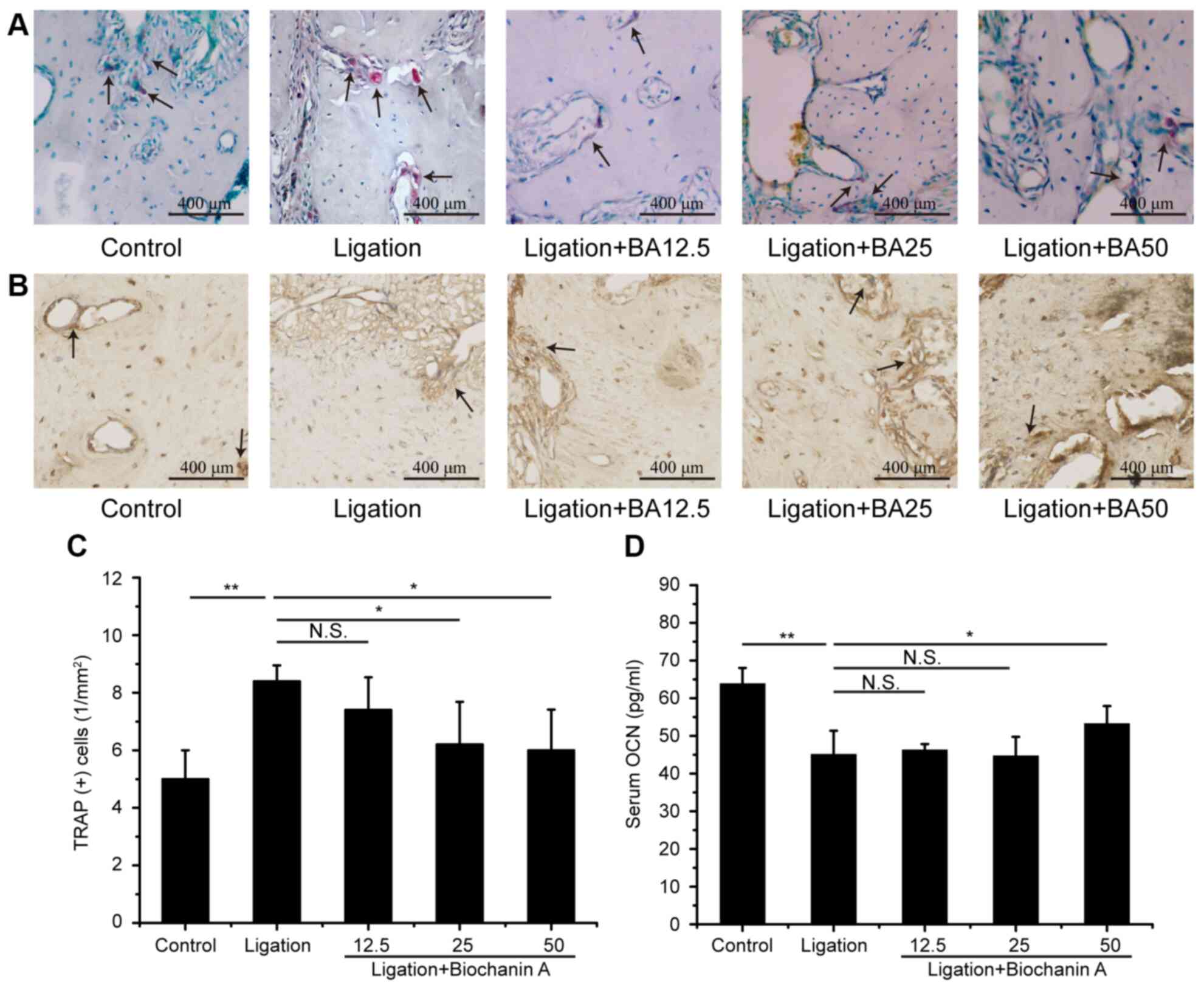
The ligation group showed a greater number of
TRAP-positive osteoclasts compared with the control group (Fig. 4A). There was also marked difference
in the OCN level between the control and the ligation group
(Fig. 4B). Medium and high doses of
BA treatment significantly decreased the TRAP-positive osteoclast
numbers in the alveolar bone of rats with experimental
periodontitis (Fig. 4C). Moreover,
treatment with a high dose of BA increased the serum OCN level
compared with the ligation group (Fig.
4D).
Discussion
The present study investigated the role of BA
administration for the management of gingival inflammation and
alveolar bone destruction associated with periodontitis. For this
purpose, effects of BA supplementation on oxidative stress levels,
various biomarkers associated with periodontitis, including IL-1β,
TNF-α and OCN, and bone loss were analyzed.
Ligature-induced periodontitis model is commonly
used and can induce soft tissue trauma, masticatory discomfort or
periodontal infection. A stress response, resulting in
postoperative weight loss, may also be observed in rat with
experimental periodontitis induced by LPS (30). A previous study has shown that under
stress, obese animals exhibit a more severe weight loss compared
with normal weight control groups, and it is difficult to recover
weight loss after repeated stress (31). However, in the present study,
ligation placed around the first molars did not significantly
influence the weight gain compared to that in the control group. A
possible explanation is that compared with injection of LPS,
ligation alone for 6 weeks can only gradually cause local
periodontal destruction by mechanical injurywithout affecting food
intake or body weight, consistent with a previous study (32).
Inflammatory response and oxidative stresses play an
important role in the pathogenesis of periodontitis (16,33);
therefore, host immune regulatory drugs may be considered as an
auxiliary treatment option for periodontitis management. The
current study used BA, an isoflavone compound which acts as a
competitive substrate for various enzymes and has been demonstrated
to bind to proteins and DNA (34,35).
In addition, a number of studies showed that BA played an
anti-inflammatory and antioxidative role in various conditions
including cancer, heart disease, perimenopausal syndrome and
osteoporosis (2-4).
These findings suggested that BA can be a potential
anti-inflammatory and anti-oxidative agent for protecting the
periodontal tissues against inflammatory destruction. However,
these results require further investigation. Biomarkers such as
IL-1β and TNF-α are considered osteoresorptive factors promoting
bone loss, osteoclastogenesis and bone resorption through
stimulation of osteoclast maturation and receptor activator of
nuclear factor-κB (RANK) ligand (RANKL) in osteoblasts (36). The present study revealed that TNF-α
and IL-1β levels were reduced in serum and gingival tissues of rats
in groups treated with BA. Therefore, the inhibitory role of BA on
bone resorption may be associated with its anti-inflammatory
effects. The tissue destruction in periodontitis is mainly caused
by an imbalance between periodontal pathogens and the host defense,
which leads to an aberrant inflammatory response and release of
enzymes by neutrophils and ROS for a prolonged period of time
(37). In the present study,
oxidative stress was measured via ROS levels in serum. In the
ligation group, the mean ROS level was increased, confirming that
periodontitis was accompanied by ROS activity. However, compared
with the ligation group, a significantly lower level of serum ROS
was observed following BA supplementation. These findings suggested
that treatment with BA is likely to attenuate the overproduction of
ROS.
The redox-sensitive transcription factor Nrf2 plays
an important role in the antioxidant signaling pathway through
inducing the expression of a number of cytoprotective proteins,
including heme-oxygenasae-1, glutamate-cysteine ligase catalytic
subunit, quinone oxidoreductase-1 and nicotinamide adenine
dinucleotide phosphate via an antioxidant response element (ARE)
(38). Since BA exhibits
antioxidative characteristics to inhibit oxidative stresses in the
pathogenesis of periodontitis, the present study examined the
Nrf2-positive cell counts in the alveolar bone of rats determined
by immunohistochemical staining to further explore the underlying
mechanisms at the molecular level. The current study revealed that
BA treatment significantly improved the number of Nrf2-positive
cells in the alveolar bone. These results are consistent with a
recent study by Liang et al (39), where BA was an effective Nfr2
activator. In addition, BA has the capability to counteract
oxidative damages induced by LPS (33,40),
high-fat diet (12) and
D-galactosamine (41). A previous
in vitro study reported that BA promoted the nuclear
accumulation of Nrf2 and enhanced its binding activity with ARE,
hence upregulating the expression of antioxidative and
cytoprotective enzymes (42). To
the best of our knowledge, the current study is the first to report
that BA protected periodontal tissues from oxidative damage via
enhancing the expression of Nfr2 in rats with experimental
periodontitis.
Alveolar bone resorption is another characteristic
feature of periodontitis (43). The
present study used micro-CT to evaluate the effectiveness of BA in
preventing bone loss in rats with periodontitis. It was observed
that the alveolar bone volume beneath the furcation area was
increased in all rats treated with BA compared with the ligation
group. The protective effect of high dose of BA was manifested by
an increased level of OCN. Furthermore, it was found that medium
and high dose BA markedly attenuated the number of TRAP-positive
cells, which indicate that BA may suppress osteoclastic growth and
activity resulting in a reduced bone turnover. BA administered at a
dose of 50 mg/kg per/day exerted the greatest therapeutic effect.
These results are consistent with a previous study were BA had
positive effects on bone loss in ovariectomized rats (4). Alveolar bone resorption is driven by
osteoclasts (44). As a key
osteoclastogenic cytokine receptor activator, RANKL can be
recognized by receptor activator of RANK, thus inducing the
differentiation of bone marrow macrophages into osteoclasts
(45). IL-1β and TNF-α have been
implicated in stimulating osteoblasts to express RANKL, which in
turn induces the differentiation of osteoclasts (44,46).
Furthermore, ROS acts as an intracellular signaling
molecule, which can active NF-κB and cause bone destruction via
osteoclastogenesis (47). BA had a
significant effect on the production of IL-1β, TNF-α and ROS in the
current study. Considering the association between proinflammatory
cytokines and RANKL as well as ROS and RANKL, BA may attenuate
alveolar bone resorption by reducing oxidative stress. The possible
effect of BA on markers of alveolar bone turnover and metabolism,
including RANKL/osteoprotegerin, osterix, transforming growth
factor β and ALP, should be further studied.
In conclusion, the present study used an animal
model to investigate the effects of BA against experimental
periodontitis induced by ligation. BA can inhibit inflammation and
regulate unbalanced oxidative stress response and ameliorate the
alveolar bone loss. Therefore, BA represents a promising adjunctive
therapy and can be used to modulate host response to periodontitis.
Although the present study provided an insight into the potential
applications of BA for the treatment of periodontitis, further
molecular studies and clinical trials are required before it can be
used in clinical practice.
Supplementary Material
Figure S1. Effects of BA on the
viability of gingival fibroblasts. Gingival fibroblasts were
treated with 0, 50, 100 and 150 ìM BA for 24 and 48 h,
respectively. Cell cytotoxicity of BA was assessed by an MTT assay.
Data are presented as the mean ± SD. Con, concentration; BA,
biochanin A.
Table SI. Primers used in the present
study.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China (grant no. 81600871) and Fundamental
Research Funds for the Central Universities (2019SCU12062).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SZ performed the majority of the experiments and
produced the figures. YN analyzed majority of the data and wrote
the first draft of the manuscript. ZY constructed the animal
models. YZ completed the ELISA experiments. QG collected and
analyzed micro-CT data. YY and XZ contributed to the conception and
design of the work and revised the manuscript critically for
important intellectual content and contributed to the discussion.
YD and CL contributed to conception, design, data acquisition,
analysis, and interpretation, drafted and critically revised the
manuscript for important intellectual content. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by Ethics Committee
of the State Key Laboratory of Oral Diseases, West China Hospital
of Stomatology, Sichuan University (approval no.
WCHSIRB-D-2018-148).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Renda G, Yalçın FN, Nemutlu E, Akkol EK,
Süntar İ, Keleş H, Ina H, Çalış İ and Ersöz T: Comparative
assessment of dermal wound healing potentials of various Trifolium
L. Extracts and determination of their isoflavone contents as
potential active ingredients. J Ethnopharmacol. 148:423–432.
2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Srinivas NR: Biochanin A: Understanding
the complexities in the paradoxical drug-drug interaction
potential. Eur J Drug Metab Pharmacokinet. 40:119–125.
2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Puthli A, Tiwari R and Mishra KP:
Biochanin a enhances the radiotoxicity in colon tumor cells in
vitro. J Environ Pathol Toxicol Oncol. 32:189–203. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Su SJ, Yeh YT and Shyu HW: The preventive
effect of biochanin a on bone loss in ovariectomized rats:
Involvement in regulation of growth and activity of osteoblasts and
osteoclasts. Evid Based Complement Alternat Med.
2013(594857)2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chen HQ, Jin ZY and Li GH: Biochanin A
protects dopaminergic neurons against lipopolysaccharide-induced
damage through inhibition of microglia activation and
proinflammatory factors generation. Neurosci Lett. 417:112–117.
2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kole L, Giri B, Manna SK, Pal B and Ghosh
S: Biochanin-A, an isoflavon, showed anti-proliferative and
anti-inflammatory activities through the inhibition of iNOS
expression, p38-MAPK and ATF-2 phosphorylation and blocking NFκB
nuclear translocation. Eur J Pharmacol. 653:8–15. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ming X, Ding M, Zhai B, Xiao L, Piao T and
Liu M: Biochanin A inhibits lipopolysaccharide-induced inflammation
in human umbilical vein endothelial cells. Life Sci. 136:36–41.
2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lee KH and Choi EM: Biochanin A stimulates
osteoblastic differentiation and inhibits hydrogen peroxide-induced
production of inflammatory mediators in MC3T3-E1 cells. Biol Pharm
Bull. 28:1948–1953. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Oh JS, Cho IA, Kang KR, You JS, Yu SJ, Lee
GJ, Seo YS, Kim CS, Kim DK, Kim G, et al: Biochanin-A antagonizes
the interleukin-1β-induced catabolic inflammation through the
modulation of NFκB cellular signaling in primary rat chondrocytes.
Biochem Biophys Res Commun. 477:723–730. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Vanden Berghe W, Dijsselbloem N, Vermeulen
L, Ndlovu MN, Boone E and Haegeman G: Attenuation of mitogen- and
stress-activated protein kinase-1-driven nuclear factor-kappaB gene
expression by soy isoflavones does not require estrogenic activity.
Cancer Res. 66:4852–4862. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wu LY, Ye ZN, Zhuang Z, Gao Y, Tang C,
Zhou CH, Wang CX, Zhang XS, Xie GB, Liu JP, et al: Biochanin A
Reduces inflammatory injury and neuronal apoptosis following
subarachnoid hemorrhage via suppression of the
TLRs/TIRAP/MyD88/NF-κB pathway. Behavioural Neurology. 2018:1–10.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Xue Z, Zhang Q, Yu W, Wen H, Hou X, Li D
and Kou X: Potential lipid-lowering mechanisms of biochanin A. J
Agric Food Chem. 65:3842–3850. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ko WC, Lin LH, Shen HY, Lai CY, Chen CM
and Shih CH: Biochanin a, a phytoestrogenic isoflavone with
selective inhibition of phosphodiesterase 4, suppresses
ovalbumin-induced airway hyperresponsiveness. Evid Based Complement
Alternat Med. 2011(635058)2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Beck V, Rohr U and Jungbauer A:
Phytoestrogens derived from red clover: An alternative to estrogen
replacement therapy? J Steroid Biochem Mol Biol. 94:499–518.
2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
GBD 2016 Disease and Injury Incidence and
Prevalence Collaborators: Global, regional, and national incidence,
prevalence, and years lived with disability for 328 diseases and
injuries for 195 countries, 1990-2016: A systematic analysis for
the Global Burden of Disease Study 2016. Lancet 390: 1211-1259,
2017.
|
|
16
|
Darveau RP: Periodontitis: A polymicrobial
disruption of host homeostasis. Nat Rev Microbiol. 8:481–490.
2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liu C, Mo L, Niu Y, Li X, Zhou X and Xu X:
The role of reactive oxygen species and autophagy in periodontitis
and their potential linkage. Front Physiol. 8(439)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hanazawa S, Nakada K, Ohmori Y, Miyoshi T,
Amano S and Kitano S: Functional role of interleukin 1 in
periodontal disease: Induction of interleukin 1 production by
Bacteroides gingivalis lipopolysaccharide in peritoneal macrophages
from C3H/HeN and C3H/HeJ mice. Infect Immun. 50:262–270.
1985.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nibali L and Donos N: Periodontitis and
redox status: A review. Curr Pharm Des. 19:2687–2697.
2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chapple IL and Matthews JB: The role of
reactive oxygen and antioxidant species in periodontal tissue
destruction. Periodontol. 43:160–232. 2010.
|
|
21
|
Dursun E, Akalin FA, Genc T, Cinar N, Erel
O and Yildiz BO: Oxidative stress and periodontal disease in
obesity. Medicine (Baltimore). 95(e3136)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Soares ASLS, Scelza MZ, Spoladore J,
Gallito MA, Oliveira F, Moraes RCM and Alves GG: Comparison of
primary human gingival fibroblasts from an older and a young donor
on the evaluation of cytotoxicity of denture adhesives. J Appl Oral
Sci. 26(e20160594)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chen H, Shi Q, Qing Y, Yao YC and Cao YG:
Cytotoxicity of modified nonequilibrium plasma with chlorhexidine
digluconate on primary cultured human gingival fibroblasts. J
Huazhong Univ Sci Technolog Med Sci. 36:137–141. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wu DQ, Zhong HM, Ding QH and Ba L:
Protective effects of biochanin A on articular cartilage: In vitro
and in vivo studies. BMC Complement Altern Med.
14(444)2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Abe T and Hajishengallis G: Optimization
of the ligature-induced periodontitis model in mice. J Immunol
Methods. 394:49–54. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bhattarai G, Kook SH, Kim JH, Poudel SB,
Lim SS, Seo YK and Lee JC: COMP-Ang1 prevents periodontitic damages
and enhances mandible bone growth in an experimental animal model.
Bone. 92:168–179. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Papathanasiou E, Kantarci A,
Konstantinidis A, Gao H and Van Dyke TE: SOCS-3 regulates alveolar
bone loss in experimental periodontitis. J Dent Res. 95:1018–1025.
2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li CH and Amar S: Morphometric,
histomorphometric, and microcomputed tomographic analysis of
periodontal inflammatory lesions in a murine model. J Periodontol.
78:1120–1128. 2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Dumitrescu AL, Abd-El-Aleem S, Morales-Aza
B and Donaldson LF: A model of periodontitis in the rat: Effect of
lipopolysaccharide on bone resorption, osteoclast activity, and
local peptidergic innervation. J Clin Periodontol. 31:596–603.
2004.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lawrence CB, Brough D and Knight EM: Obese
mice exhibit an altered behavioural and inflammatory response to
lipopolysaccharide. Dis Model Mech. 5:649–659. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Rettori E, De Laurentiis A, Zorrilla
Zubilete M, Rettori V and Elverdin JC: Anti-inflammatory effect of
the endocannabinoid anandamide in experimental periodontitis and
stress in the rat. Neuroimmunomodulation. 19:293–303.
2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang J, He C, Wu WY, Chen F, Wu YY, Li WZ,
Chen HQ and Yin YY: Biochanin A protects dopaminergic neurons
against lipopolysaccharide-induced damage and oxidative stress in a
rat model of Parkinson's disease. Pharmacol Biochem Behav.
138:96–103. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Luo Q, Shi X, Ding J, Ma Z, Chen X, Leng
Y, Zhang X and Liu Y: Network pharmacology integrated molecular
docking reveals the antiosteosarcoma mechanism of biochanin A. Evid
Based Complement Alternat Med. 2019(1410495)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Nestel P, Cehun M, Chronopoulos A, DaSilva
L, Teede H and McGrath B: A biochanin-enriched isoflavone from red
clover lowers LDL cholesterol in men. Eur J Clin Nutr. 58:403–408.
2004.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tanaka Y, Nakayamada S and Okada Y:
Osteoblasts and osteoclasts in bone remodeling and inflammation.
Curr Drug Targets Inflamm Allergy. 4:325–328. 2005.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Brock GR, Butterworth CJ, Matthews JB and
Chapple IL: Local and systemic total antioxidant capacity in
periodontitis and health. J Clin Periodontol. 31:515–521. 2010.
|
|
38
|
Liu Q, Hu Y, Cao Y, Song G, Liu Z and Liu
X: Chicoric acid ameliorates lipopolysaccharide-induced oxidative
stress via promoting the Keap1/Nrf2 transcriptional signaling
pathway in BV-2 microglial cells and mouse brain. J Agric Food
Chem. 65:338–347. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Liang F, Cao W, Huang Y, Fang Y, Cheng Y,
Pan S and Xu X: Isoflavone biochanin A, a novel nuclear factor
erythroid 2-related factor 2 (Nrf2)-antioxidant response element
activator, protects against oxidative damage in HepG2 cells.
Biofactors. 45:563–574. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wang J, Wu WY, Huang H, Li WZ, Chen HQ and
Yin YY: Biochanin a protects against lipopolysaccharide-induced
damage of dopaminergic neurons both in vivo and in vitro via
inhibition of microglial activation. Neurotox Res. 30:486–498.
2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Liu X, Wang T, Liu X, Cai L, Qi J, Zhang P
and Li Y: Biochanin A protects
lipopolysaccharide/D-galactosamine-induced acute liver injury in
mice by activating the Nrf2 pathway and inhibiting NLRP3
inflammasome activation. Int Immunopharmacol. 38:324–331.
2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kim J, Cha YN and Surh YJ: A protective
role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in
inflammatory disorders. Mutat Res. 690:12–23. 2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Bostanci N, Abe T, Belibasakis GN and
Hajishengallis G: TREM-1 Is upregulated in experimental
periodontitis, and its blockade inhibits IL-17A and RANKL
expression and suppresses bone loss. J Clin Med.
8(1579)2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Hienz SA, Sweta P and Saso I: Mechanisms
of bone resorption in periodontitis. J Immunol Res.
2015(615486)2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Teitelbaum SL: Bone resorption by
osteoclasts. Science. 289:1504–1508. 2000.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Feng W, Guo J and Li M: RANKL-independent
modulation of osteoclastogenesis. J Oral Biosci. 61:16–21.
2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Sağlam M, Köseoğlu S, Hatipoğlu M, Esen HH
and Köksal E: Effect of sumac extract on serum oxidative status,
RANKL/OPG system and alveolar bone loss in experimental
periodontitis in rats. J Appl Oral Sci. 23:33–41. 2015.PubMed/NCBI View Article : Google Scholar
|