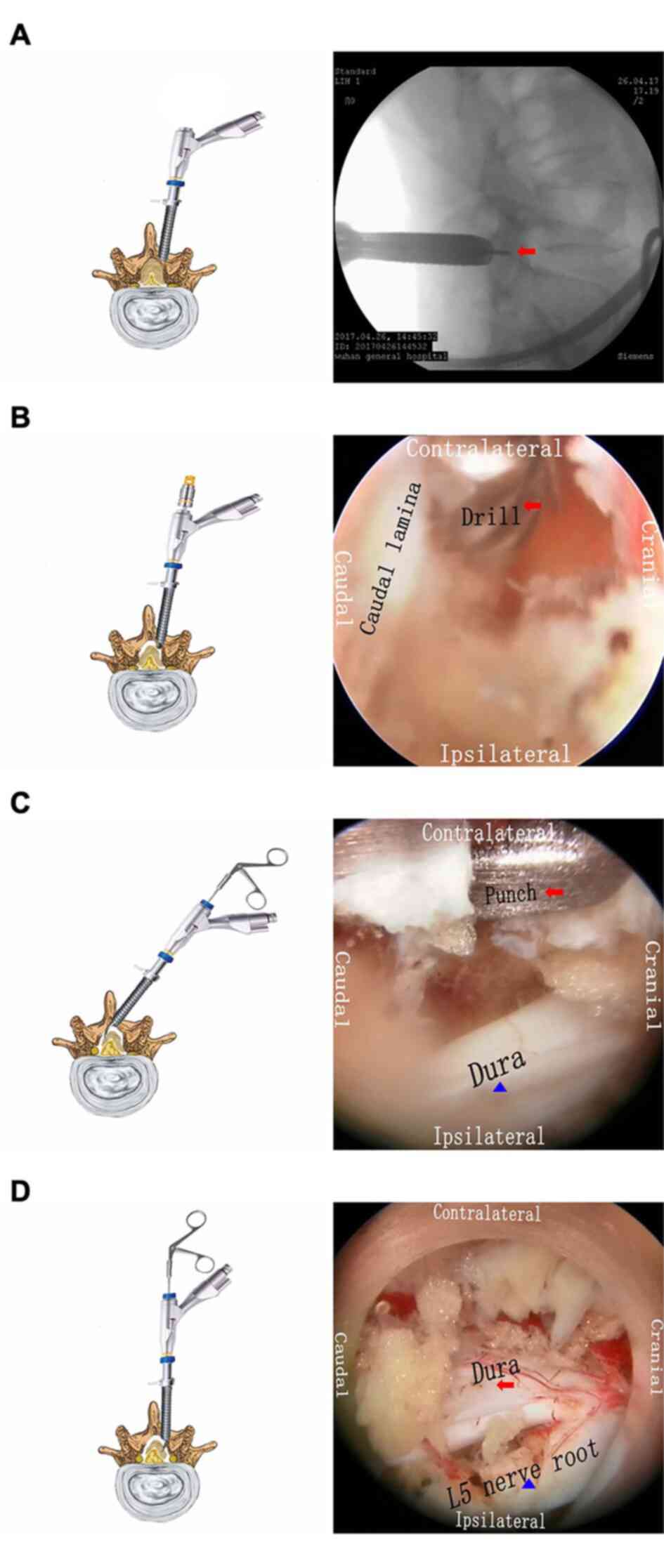
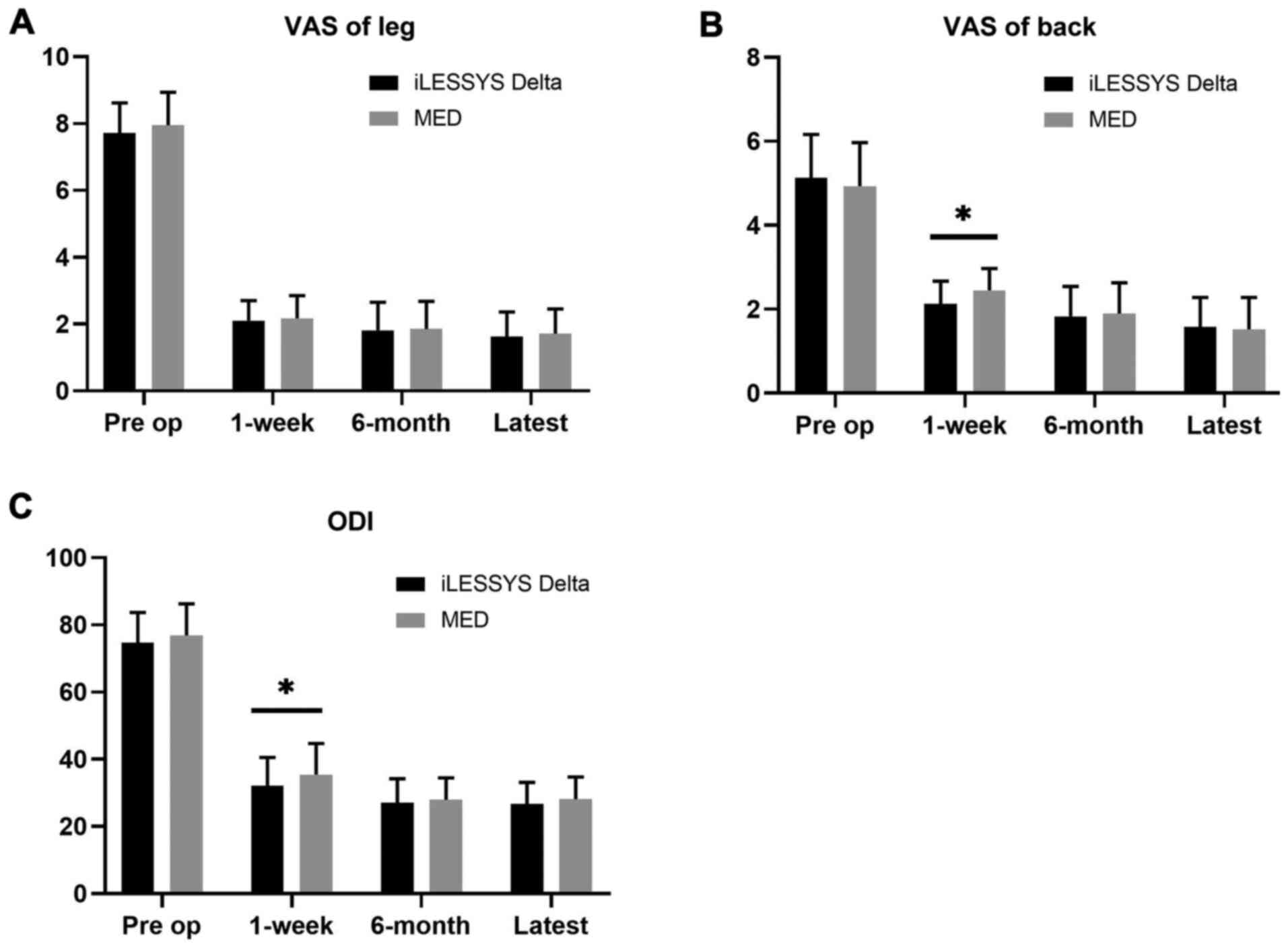
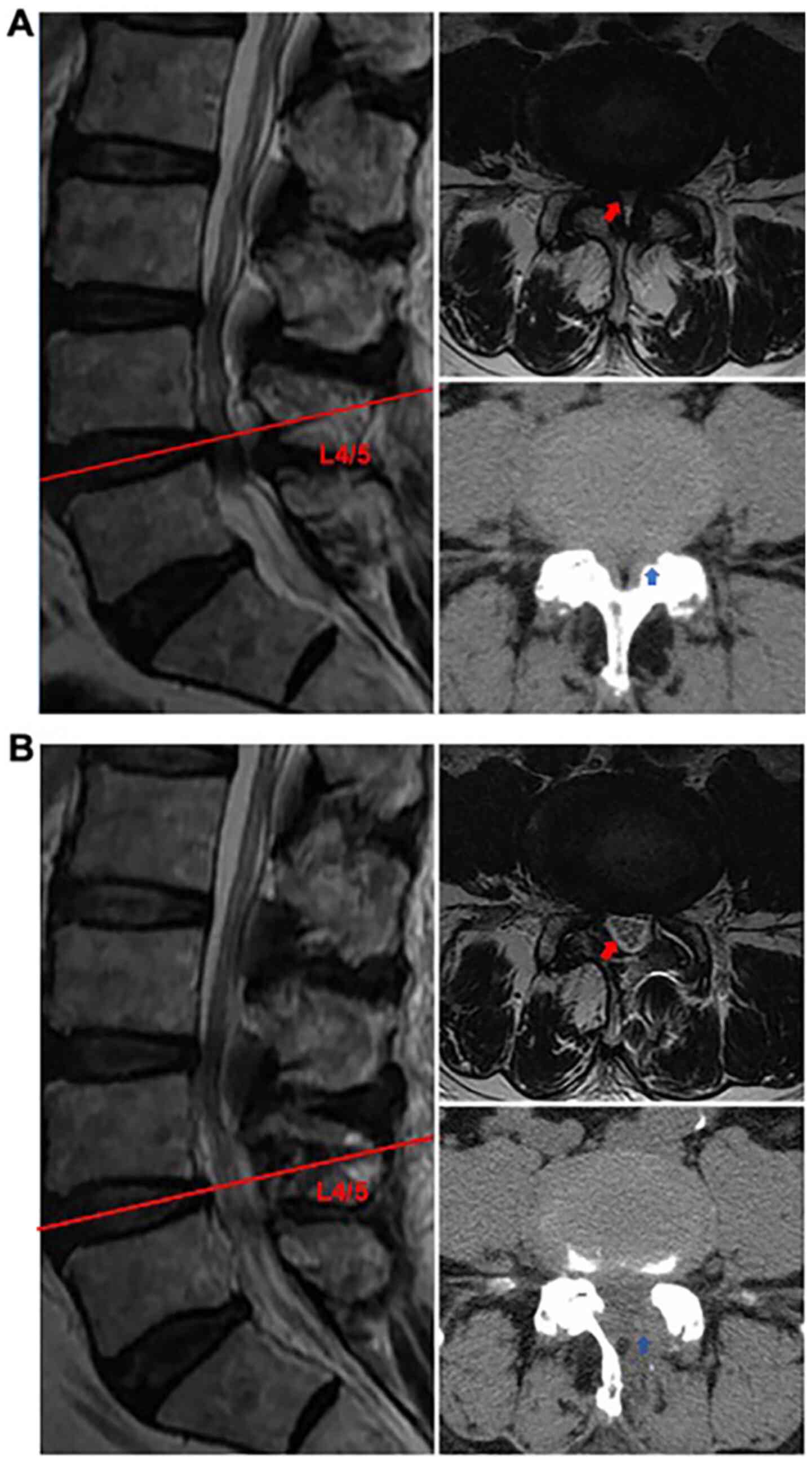
|
1
|
Ahn Y: Percutaneous endoscopic
decompression for lumbar spinal stenosis. Expert Rev Med Devic.
11:605–616. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Porter RW: Spinal stenosis and neurogenic
claudication. Spine (Phila Pa 1976). 21:2046–2052. 1996.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kreiner DS, Shaffer WO, Baisden JL,
Gilbert TJ, Summers JT, Toton JF, Hwang SW, Mendel RC and Reitman
CA: North American Spine Society: An evidence-based clinical
guideline for the diagnosis and treatment of degenerative lumbar
spinal stenosis (update). Spine J. 13:734–743. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tosteson AN, Lurie JD, Tosteson TD,
Skinner JS, Herkowitz H, Albert T, Boden SD, Bridwell K, Longley M,
Andersson GB, et al: Surgical treatment of spinal stenosis with and
without degenerative spondylolisthesis: Cost-effectiveness after 2
years. Ann Intern Med. 149:845–853. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Weinstein JN, Tosteson TD, Lurie JD,
Tosteson AN, Blood E, Hanscom B, Herkowitz H, Cammisa F, Albert T,
Boden SD, et al: Surgical versus nonsurgical therapy for lumbar
spinal stenosis. New Engl J Med. 358:794–810. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Thomé C, Zevgaridis D, Leheta O, Bäzner H,
Pöckler-Schöniger C, Wöhrle J and Schmiedek P: Outcome after
less-invasive decompression of lumbar spinal stenosis: A randomized
comparison of unilateral laminotomy, bilateral laminotomy, and
laminectomy. J Neurosurg Spine. 3:129–141. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Deyo RA, Mirza SK, Martin BI, Kreuter W,
Goodman DC and Jarvik JG: Trends, major medical complications, and
charges associated with surgery for lumbar spinal stenosis in older
adults. JAMA. 303:1259–1265. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lee C, Yoon K and Ha S: Comparative
analysis between three different lumbar decompression techniques
(microscopic, tubular, and endoscopic) in lumbar canal and lateral
recess stenosis: Preliminary report. Biomed Res Int.
2019(6078469)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
McGrath LB, White-Dzuro GA and Hofstetter
CP: Comparison of clinical outcomes following minimally invasive or
lumbar endoscopic unilateral laminotomy for bilateral
decompression. J Neurosurg Spine. 1–9. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xu BS, Tan QS Xia Q, Ji N and Hu YC:
Bilateral decompression via unilateral fenestration using mobile
microendoscopic discectomy technique for lumbar spinal stenosis.
Orthop Surg. 2:106–110. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yagi M, Okada E, Ninomiya K and Kihara M:
Postoperative outcome after modified unilateral-approach
microendoscopic midline decompression for degenerative spinal
stenosis. J Neurosurg Spine. 10:293–299. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ikuta K, Arima J, Tanaka T, Oga M, Nakano
S, Sasaki K, Goshi K, Yo M and Fukagawa S: Short-term results of
microendoscopic posterior decompression for lumbar spinal stenosis.
J Neurosurg Spine. 2:624–633. 2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Minamide A, Yoshida M, Yamada H, Nakagawa
Y, Kawai M, Maio K, Hashizume H, Iwasaki H and Tsutsui S:
Endoscope-assisted spinal decompression surgery for lumbar spinal
stenosis. J Neurosurg Spine. 19:664–671. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Youn MS, Shin JK, Goh TS, Son SM and Lee
JS: Endoscopic posterior decompression under local anesthesia for
degenerative lumbar spinal stenosis. J Neurosurg Spine. 29:661–666.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Komp M, Hahn P, Oezdemir S, Giannakopoulos
A, Heikenfeld R, Kasch R, Merk H, Godolias G and Ruetten S:
Bilateral spinal decompression of lumbar central stenosis with the
full-endoscopic interlaminar versus microsurgical laminotomy
technique: A prospective, randomized, controlled study. Pain
Physician. 18:61–70. 2015.PubMed/NCBI
|
|
16
|
Lee C, Yoon K and Jun J: Percutaneous
endoscopic laminotomy with flavectomy by uniportal, unilateral
approach for the lumbar canal or lateral recess stenosis. World
Neurosurg. 113:e129–e137. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Middleton SD, Wagner R and Gibson JA:
Multi-level spine endoscopy: A review of available evidence and
case report. EFORT Open Rev. 2:317–323. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Schizas C, Theumann N, Burn A, Tansey R,
Wardlaw D, Smith FW and Kulik G: Qualitative grading of severity of
lumbar spinal stenosis based on the morphology of the dural sac on
magnetic resonance images. Spine (Phila Pa 1976). 35:1919–1924.
2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Meyerding HW: Spondylolisthesis: Surgical
treatment and results. Surg Gynec Obst. 54:371–377. 1932.
|
|
20
|
DeVine J, Norvell DC, Ecker E, Fourney DR,
Vaccaro A, Wang J and Andersson G: Evaluating the correlation and
responsiveness of patient-reported pain with function and
quality-of-life outcomes after spine surgery. Spine(Phila Pa 1976).
36 (Suppl 21):S69–S74. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xiong C, Li T, Kang H, Hu H, Han J and Xu
F: Early outcomes of 270-degree spinal canal decompression by using
TESSYS-ISEE technique in patients with lumbar spinal stenosis
combined with disk herniation. Eur Spine J. 28:78–86.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Siepe CJ, Sauer D and Michael Mayer H:
Full endoscopic, bilateral over-the-top decompression for lumbar
spinal stenosis. Eur Spine J. 27 (Suppl 27):S563–S565.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Choi G, Lee S, Raiturker PP, Lee S and
Chae Y: Percutaneous endoscopic interlaminar discectomy for
intracanalicular disc herniations at L5-S1 using a rigid working
channel endoscope. Oper Neurosurg. 58 (Suppl 1):ONS59–68;
discussion ONS59-68. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ruetten S, Komp M and Godolias G: A new
full-endoscopic technique for the interlaminar operation of lumbar
disc herniations using 6-mm endoscopes: Prospective 2-year results
of 331 patients. Minim Invasive Neurosurg. 49:80–87.
2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ruan W, Feng F, Liu Z, Xie J, Cai L and
Ping A: Comparison of percutaneous endoscopic lumbar discectomy
versus open lumbar microdiscectomy for lumbar disc herniation: A
meta-analysis. Int J Surg. 31:86–92. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liu X, Yuan S, Tian Y, Wang L, Gong L,
Zheng Y and Li J: Comparison of percutaneous endoscopic
transforaminal discectomy, microendoscopic discectomy, and
microdiscectomy for symptomatic lumbar disc herniation: Minimum
2-year follow-up results. J Neurosurg Spine. 28:317–325.
2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ahn Y: Endoscopic spine discectomy:
Indications and outcomes. Int Orthop. 43:909–916. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li H, Jiang C, Mu X, Lan W, Zhou Y and Li
C: Comparison of MED and PELD in the treatment of adolescent lumbar
disc herniation: A 5-year retrospective follow-up. World Neurosurg.
112:e255–e260. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Taylor H, McGregor AH, Medhi-Zadeh S,
Richards S, Kahn N, Zadeh JA and Hughes SP: The impact of
self-retaining retractors on the paraspinal muscles during
posterior spinal surgery. Spine (Phila Pa 1976). 27:2758–2762.
2002.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Minamide A, Yoshida M, Yamada H, Nakagawa
Y, Hashizume H, Iwasaki H and Tsutsui S: Clinical outcomes after
microendoscopic laminotomy for lumbar spinal stenosis: A 5-year
follow-up study. Eur Spine J. 24:396–403. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Panjabi MM: Clinical spinal instability
and low back pain. J Electromyogr Kines. 13:371–379.
2003.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Casal-Moro R, Castro-Menéndez M,
Hernández-Blanco M, Bravo-Ricoy JA and Jorge-Barreiro FJ: Long-term
outcome after microendoscopic diskectomy for lumbar disk
herniation: A prospective clinical study with a 5-year follow-up.
Neurosurgery. 68:1568–1575. 2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Liu H, Tao H and Luo Z: Validation of the
simplified Chinese version of the Oswestry Disability Index. Spine
(Phila Pa 1976). 34:1211–1216. 2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Schonstrom NS, Bolender N and Spengler DM:
The pathomorphology of spinal stenosis as seen on CT scans of the
lumbar spine. Spine (Phila Pa 1976). 10:806–811. 1985.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yang JS, Chu L, Chen CM, Wang XF, Xie PG,
Deng R, Yu KX, Shi L, Zhang ZX, Rong LM, et al: Foraminoplasty at
the tip or base of the superior articular process for lateral
recess stenosis in percutaneous endoscopic lumbar discectomy: A
multicenter, retrospective, controlled study with 2-year follow-up.
Biomed Res Int. 2018(7692794)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yang J, Wu H, Kong Q, Wang Y, Peng Z,
Zhang L, Yan Y, Guo C and Zhang D: Full endoscopic transforaminal
decompression surgery for symptomatic lumbar spinal stenosis in
geriatric patients. World Neurosurg. 127:e449–e459. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Heo DH, Quillo-Olvera J and Park CK: Can
percutaneous biportal endoscopic surgery achieve enough canal
decompression for degenerative lumbarstenosis? Prospective
case-control study. World Neurosurg. 120:e684–e689. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Fritsch EW, Heisel J and Rupp S: The
failed back surgery syndrome: Reasons, intraoperative findings, and
long-term results: A report of 182 operative treatments. Spine
(Phila Pa 1976). 21:626–633. 1996.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Gerling MC, Leven D, Passias PG, Lafage V,
Bianco K, Lee A, Lurie JD, Tosteson TD, Zhao W, Spratt KF, et al:
Risk factors for reoperation in patients treated surgically for
lumbar stenosis: A subanalysis of the 8 year data from the SPORT
trial. Spine (Phila Pa 1976). 41:901–909. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Schöller K, Steingrüber T, Stein M, Vogt
N, Müller T, Pons-Kühnemann J and Uhl E: Microsurgical unilateral
laminotomy for decompression of lumbar spinal stenosis: Long-term
results and predictive factors. Acta Neurochir (Wien).
158:1103–1113. 2016.PubMed/NCBI View Article : Google Scholar
|