Introduction
The intervertebral disc is primarily divided into
the peripheral fibrous annulus, the central nucleus pulposus (NP)
and the upper and lower cartilage endplates. The surrounding
fibrous rings are arranged according to concentric circles by
multiple layers of fibrous cartilage and are supplied by vascular
nutrients at the surface of the rings (1). The intervertebral disc is composed of
glycoprotein, proteoglycan, collagen and elastic fibers. The
proteoglycan in the intervertebral disc tissue can form a stable
structure with elastin and collagen and adjust the pressure of the
disc. The hydrating function of proteoglycan can maintain normal
ion concentration and osmotic pressure in the intervertebral disc,
so as to ensure the maintenance of the ion concentration and
osmotic pressure gradient and the normal function of the
intervertebral disc (2).
Lower back pain is a common problem in middle-aged
and elderly people, and intervertebral disc degeneration (IVDD) is
often the main cause. The prevalence of low back pain caused by
IVDD reaches 20% worldwide, and 60% of people over 70 years old
have been indicated to suffer from intervertebral disc disease
(3). IVDD is caused by multiple
factors, in which inflammation and oxidative stress play an
important role (3). Studies have
suggested that pro-inflammatory factors (such as TNF-α and IL-1β)
play an important role in the process of IVDD (4,5). A
significant increase in the expression of TNF-α and IL-1β was
detected in the degenerated intervertebral disc of patients
(6). These inflammatory factors can
stimulate nerve growth and angiogenesis of NP cells by inducing the
release of nerve growth factors, such as brain derived neurotrophic
factor and vascular endothelial growth factor (7). In NP cells, TNF-α and IL-1β can also
upregulate the expression of matrix metalloproteinases (MMPs) that
degrade the extracellular matrix of NP cells (8). In addition, during the process NP cell
degeneration, the balance of oxygen free radical production and
antioxidant defense is disrupted, leading to the accumulation of
reactive oxygen species (ROS), which further leads to cell damage
and apoptosis (8). Therefore,
inhibiting inflammation and apoptosis of NP cells is the key to
preventing and treating IVDD.
Dimethyl fumarate (DMF) has been used in Europe to
treat severe psoriasis. In 2012, the US Food and Drug
Administration approved DMF application in the clinical treatment
of relapse-remitting multiple sclerosis (9). In clinical statistical analysis, DMF
has a good safety record so far in diseases such as psoriasis
(10). DMF was superior to placebo
in terms of the proportion of patients achieving a ≥75% improvement
from baseline according to the Psoriasis Area and Severity Index
(10). Studies have shown that DMF
has immunomodulatory and anti-inflammatory effects in the treatment
of neurological diseases, such as neuritis and spinal cord injury
(11,12). Therefore, some studies have applied
DMF to different organs and different diseases. For example, in
breast cancer cells, DMF can inhibit the NF-κB pathway, thereby
reducing the inflammatory response (13). After applying DMF to lung
fibroblasts, it was reported that DMF inhibits the TNF-β1 signaling
pathway and ultimately exerts anti-fibrotic effects (14). It is noteworthy that numerous
studies on the central nervous system have demonstrated that DMF
can effectively activate nuclear factor erythroid 2-related factor
(Nrf)2 and promote the activation of its downstream antioxidant
stress pathway. Therefore, DMF has a good application prospect in
terms of diseases associated with inflammation and oxidative damage
(15). However, to the best of our
knowledge, there is still no relevant study investigating whether
DMF has anti-inflammatory and antioxidative effects in IVDD.
The present study used C57BL5 mice to construct an
IVDD model, with the aim to study the effect of DMF on IVDD, and
cultured human nucleus pulposus (NP) cells to study the molecular
mechanisms underlying DMF function. The present results highlight
the potential of DMF treatment for clinical IVDD.
Materials and methods
Animals and grouping
A total of 60 C57/BL6 male mice (Charles River
Laboratories, Inc.) were used for the present study. The mice were
8 weeks old, weighed 20-25 g and were housed in standard barrier
facilities (25˚C; 55-65% humidity; alternating light rhythm of 24
h). The mice had free access to lab mouse food and distilled water,
which were supplemented daily. The mice were randomly divided into
three experimental groups (20 mice each): Control group,
degenerative group and treatment group. Mice in the control group
were routinely housed, while mice in the degenerative and treatment
groups were modeled for IVDD. The mice in the treatment group were
given a daily intraperitoneal injection of 10 mg/kg DMF
(Sigma-Aldrich; Merck KGaA) when the IVDD model was performed
(16). The study was approved by
The Animal Ethics Committee of Qinghai Provincial People's Hospital
Animal Center (Xining, China; approval no. GS-XNH-17-A-0832).
IVDD model procedure and
treatment
After anesthetizing the degenerative and treatment
group mice with pentobarbital sodium at a dose of 40 mg/kg, the
tail of the mouse was wrapped with a medical tape and it was
suspended on the top pulley in a special squirrel cage. The hind
limbs of the mice are >1-cm above the ground and the forelimbs
were able to crawl freely in the cages. The front paws could
support the mice to move and feed (17). The health and behavior of the mice
were observed twice a day. After 1 month of hanging the tail of the
mouse, X-rays were used to observe the condition of the lumbar
intervertebral disc. Signs of severe pain, including abnormal
movement and sound, were considered humane endpoints requiring
immediate euthanasia. All mice then received euthanasia via
cervical dislocation (after being anesthetized using
intraperitoneal administration of pentobarbital sodium at a dose of
40 mg/kg). Death was confirmed by observing breathing and the
heartbeat.
X-ray
After 1 month of hanging, the mice were anesthetized
and placed in a tray in the right lateral position. Small animal
X-ray instruments were purchased from the American Faxitron and
model number was MX-20. After obtaining X-ray images of each mouse,
the intervertebral space height of the lumbar vertebrae of each
mouse was measured, and the intervertebral disc height index (DHI)
was calculated. DHI is the ratio of the sum of the heights of two
adjacent vertebral bodies to the height of the intervertebral disc
(18).
Cell culture and treatment
Human primary intervertebral disc nucleus cells were
cultured to study the effect of DMF on IVDD. Human NP cells (cat.
no. CP-H097) were purchased from Procell Life Science &
Technology Co., Ltd. NP cells were cultured using Dulbecco's
modified eagle medium/F12 medium and added 10% fetal bovine serum
(with 1% penicillin plus streptomycin) to the medium (all Gibco;
Thermo Fisher Scientific, Inc.). The conditions of the cell culture
incubator were set to 37˚C and 5% CO2. When the cell
density reached 90%, recombinant human IL-1β (50 ng/ml;
Sigma-Aldrich; Merck KGaA) was used to stimulate NP cell
degeneration. DMSO was used to dissolve the DMF powder and stored
as a 10 µmol/ml stock solution. In total, 70 µmol/l of DMF was used
to treat NP cells for 24 h at 37˚C.
Western blotting
At the end of the mouse modeling, the mice in all
groups were sacrificed as aforementioned and the spine was
extracted. Then, the intervertebral discs of the lumbar spine were
scraped using a sterile blade and collected to extract the proteins
using RIPA lysis buffer (Invitrogen; Thermo Fisher Scientific,
Inc.). A BCA kit (Pierce; Thermo Fisher Scientific, Inc.) was used
to measure protein concentration. An equal amount of protein (30
µg) was added to each well of a 10% SDS-PAGE gel. After gel
electrophoresis the proteins were transferred to polyvinylidene
fluoride (PVDF) membranes (Roche Diagnostics). Then the membranes
were blocked with a non-specific antigen with 5% skimmed milk for 1
h at room temperature. The following primary antibodies were
incubated with the PVDF membranes: Collagen II (1:3,000; cat. no.
ab34712) aggrecan (1:5,000; cat. no. ab3778), superoxide dismutase
(SOD)1 (1:2,000; cat. no. ab183881), SOD2 (1:1,000; cat. no.
ab68155), Peroxiredoxin 1 (Prdx1) (1:3,000; cat. no. ab41906) Prdx4
(1:4,000; cat. no. ab184167), MMP3 (1:5,000; cat. no. ab52915),
MMP13 (1:3,000; cat. no. ab219620), C/EBP homologous protein (CHOP;
1:5,000; cat. no. ab179823), endoplasmic reticulum chaperone BiP
(GRP-78; 1:2,000; cat. no. ab21685), caspase-12 (1:2,000; cat. no.
ab62484), Nrf2 (1:3,000; cat. no. ab92946), HO-1, (1:2,000; cat.
no. ab68477), Akt (1:1,000; cat. no. ab18785), phosphorylated Akt
(1:3,000; cat. no. ab38449) and β-actin (1:5,000; cat. no. ab6276)
(all rabbit antibodies; Abcam) were used to incubate the PVDF
membranes. CHOP, GRP-78 and caspase-12 are involved in the ER
stress pathway (19). The PVDF
membranes were incubated overnight at 4˚C. After washing the PVDF
membranes with TBS-0.1% Tween-20, the PVDF membranes were incubated
with secondary HRP-goat anti-rabbit (1:3,000; cat. no. ab205718;
Abcam) for 2 h at room temperature. Finally, the amount of protein
expression was detected using ECL (Invitrogen; Thermo Fisher
Scientific, Inc.). Protein extraction and detection from NP cells
was also performed using this methodology.
RNA isolation and reverse
transcription-quantitative (RT-q)PCR
After obtaining mouse intervertebral disc tissue,
total RNA was extracted using TRIzol® (Invitrogen;
Thermo Fisher Scientific, Inc.). RNase-free water was used to
dissolve RNA and the RNA concentration was detected using a
spectrophotometer [Unico (Shanghai) Instrument Co., Ltd.;
http://www.unicosh17.com.cn/].
SuperScript® IV Reverse Transcriptase (Invitrogen;
Thermo Fisher Scientific, Inc.) was used to reverse transcribe the
mRNA to cDNA (10 min at 55˚C; 10 min at 80˚C; 30 min at 4˚C)
according to the manufacturer's protocol. Primers used to amplify
cDNA were designed by Shanghai Jierui Biotechnology Co., Ltd. cDNA
was amplified using PowerUp™ SYBR® Green
Master Mix (Invitrogen; Thermo Fisher Scientific, Inc.). The
thermocycling conditions used were as follows: 2 min at 50˚C; 2 min
at 95˚C; and 30 cycles of 3 sec at 95˚C and 30 sec at 60˚C,
according to the manufacturer's protocol. GAPDH expression was used
as the control and the 2-ΔΔCq
method was used to calculate relative RNA expression (20). The extraction and detection of RNA
from NP cells was also performed using this methodology. The
primers sequences are shown in Table
I.
 | Table IReverse transcription-quantitative
PCR primer sequences. |
Table I
Reverse transcription-quantitative
PCR primer sequences.
| A, Human |
|---|
| Name | Sequence,
5'-3' |
|---|
| IL-6 |
|
Forward |
ACTCACCTCTTCAGAACGAATTG |
|
Reverse |
CCATCTTTGGAAGGTTCAGGTTG |
| IL-8 |
|
Forward |
CAGTTGAAGTTGCCATCAGC |
|
Reverse |
CAGTTGAAGTTACCATCAGC |
| MMP3 |
|
Forward |
GGTCACCATCAACGCTGACTGACTG |
|
Reverse |
AGTCCGATCGACTACTAGCACTGACT |
| MMP13 |
|
Forward |
CGTACGTACTACTACGACGGCTAT |
|
Reverse |
GTGCACTGACGAGCACTGTGCGTTG |
| SOD1 |
|
Forward |
ATGGTGTTTGCAACCACATGGCATG |
|
Reverse |
GACAACAGTTGGTTGCAACGGTCATG |
| SOD2 |
|
Forward |
ACTGAGCTCTGCATGACGTAGTC |
|
Reverse |
CGACTACGTACGACGCGACCTGCA |
| Prdx1 |
|
Forward |
CGCTGAGCTATGTTGCACACAGT |
|
Reverse |
GCGTATGCGACTACACATCAGT |
| Prdx4 |
|
Forward |
GGTTGGTACACACATCAGCTACAC |
|
Reverse |
GGTGTATCGACACTGTGTGACA |
| CHOP |
|
Forward |
AGTCTTGGTTGTGTACGTTGTG |
|
Reverse |
CGTGTGTGCACATACTGTGAC |
| GRP-78 |
|
Forward |
GTGTGCACGACGTACAGCT |
|
Reverse |
AGCTATCAGCACACGTTGTGT |
| Caspase-12 |
|
Forward |
GGTGTGCAGCACACGTACTGACG |
|
Reverse |
CGACTACACTGCACTGACTGAC |
| Nrf2 |
|
Forward |
ACTGACGACTGACGTACGACGG |
|
Reverse |
GCTACGTACTGACTGATCCATGCA |
| HO-1 |
|
Forward |
AGCATCGTACTGACTGATGGTCAC |
|
Reverse |
GTGCTGACGTACTGACGACCATGC |
| Akt |
|
Forward |
CCACAACAGCTATCGACCGTAC |
|
Reverse |
GTCATCTACTGACTGACACTA |
| GAPDH |
|
Forward |
ACAACTTTGGTATCGTGGAAGG |
|
Reverse |
GCCATCACGCCACAGTTTC |
| B, Mouse |
| Collagen II |
|
Forward |
ACGTGCGTATCACGGACGA |
|
Reverse |
CGTACGTAGCTGGTGTTTGACC |
| Aggrecan |
|
Forward |
GGTGTTGACACACCGCGCGTATA |
|
Reverse |
GTGCTCTATGAGATCTGATCG |
| MMP3 |
|
Forward |
CGCTATTAGCGTCATCAGTC |
|
Reverse |
GGTCTAGACTTAAAGCTACGT |
| MMP13 |
|
Forward |
CAAGCTCGTTGTGCACGTGTA |
|
Reverse |
CGTGTTGACACGTATGGTTCA |
| GAPDH |
|
Forward |
GCATCACGTACTGACACCATG |
|
Reverse |
CGACTGACTGACACTGCGA |
Cell Counting Kit (CCK)-8 assay
The optimal concentration of DMF and IL-1β was
determined by detecting the viability of NP cells using CCK-8
assay, according to the manufacturer's protocol. The NP cells were
plated onto 96-well plates, and then NP cells were stimulated with
10, 30, 50, 70 or 100 µmol/l DMF and 10, 30, 50, 70 or 100 ng/ml
IL-1β. After 3 days of stimulation, 10 µl CCK-8 reagent (Dojindo
Molecular Technologies, Inc.) was added to each well and the cells
were placed in the incubator for a further 4 h. Then microplate
reader was used to measure the absorbance at 450 nm of each
well.
ELISA
After the density of NP cells reached 70%, NP cells
were stimulated with IL-1β (50 ng/ml) alone of IL-1β (50 ng/ml) +
DMF (70 µmol/l) for 3 days. At the end of the treatment, the
supernatant of the cells was taken and the precipitate was removed
using centrifugation (10,500 x g, 15 min, 4˚C). ELISA kits
[Hangzhou Multi Sciences (Lianke) Biotech Co., Ltd.; http://lianke.lnxysf.com/introduce/]
were used to detect the activity of IL-6 (cat. no. 70-EK106/2-96),
IL-8 (cat. no. 70-EK108-96), MMP3 (cat. no. HA-ET1705-98-100) and
MMP13 (cat. no. RK-KOA0275).
Detection of catalase (CAT) and SOD
activity
The level of oxidative stress in NP cells was
determined by detecting the activity of CAT and SOD in NP cells.
After treating the NP cells for 1 day, the precipitate from the
culture supernatant of the NP cells was removed using
centrifugation. Then CAT (cat. no. 70-EK1306-24) and SOD (cat. no.
2A-706002-480) activity kits [both Hangzhou Multi Sciences (Lianke)
Biotech Co., Ltd.] were used to measure the content of CAT and
SOD.
Immunofluorescence (IF) staining
Plates (24-well) were used to culture NP cells.
After the cell density reached 70%, the cells were stimulated with
IL-1β (50 ng/ml) alone or IL-1β (50 ng/ml) + DMF (70 µmol/l) for 1
day at 37˚C. Then, the medium was discarded, cells removed from the
24-well plates and treated with 4% paraformaldehyde and 0.5%
Triton-PBS. Skimmed milk (5%) was used to block non-specific
antigens for 1 h at room temperature. Cells were incubated with the
aforementioned primary antibodies at 4˚C overnight. After washing
the cells with PBS, the cells were incubated with fluorescent
secondary antibody dilution for 1 h. DAPI was used to stain the
nucleus for 10 min at room temperature. Finally, the staining
results were observed using a fluorescence microscope
(magnification, x400).
Statistical analysis
SPSS version 21.0 (IBM Corp) and GraphPad Prism
version 7.0 (GraphPad Software) were used analyze the data. The
data are presented as the mean ± SD. All experiments were repeated
more than three times. Comparisons between multiple groups
performed using one-way ANOVA test followed by Tukey's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
DMF relieves tail suspension-induced
IVDD in mice
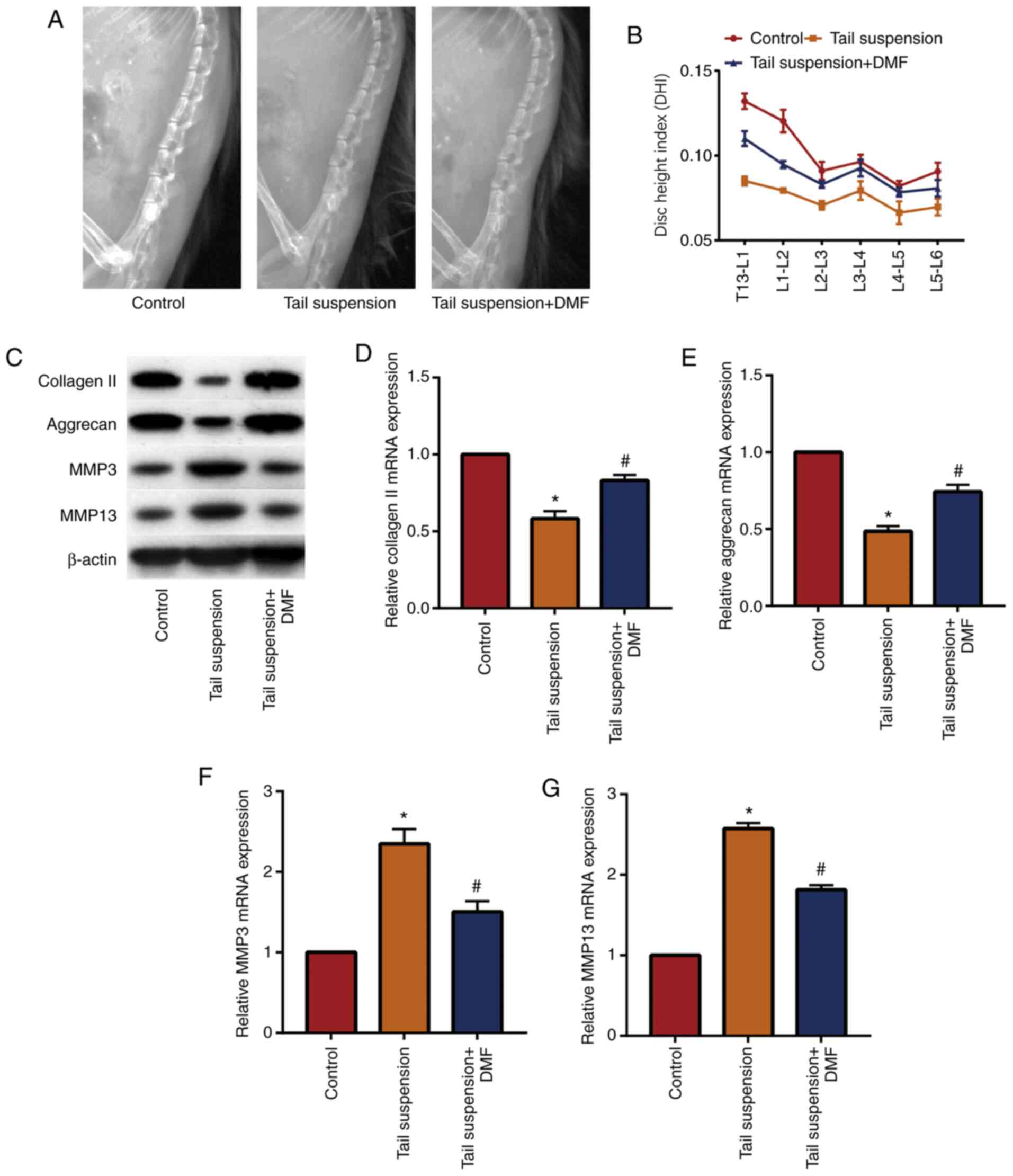
A mouse model of IVDD was established and DMF was
administered to determine the effect of DMF on IVDD. X-ray results
(Fig. 1A and B) showed that the DHI of the lumbar
vertebrae of the tail-suspended mice was lower compared with that
of the control group, while the DHI of the mice in the DMF group
was significantly increased compared with the tail suspension
group. These data indicated that DMF improved the intervertebral
disc height of the lumbar vertebrae of the mice. Western blotting
(Fig. 1C) and RT-qPCR (Fig. 1D-1G) showed that DMF significantly
increased extracellular matrix collagen II and aggrecan in NP
tissue, and significantly decreased MMP3 and MMP13 compared with
the tail suspension group. These results indicated that the IVDD
model was successfully established and that DMF alleviated IVDD in
mice, likely due to the decreased degradation of the extracellular
matrix of the NP tissue.
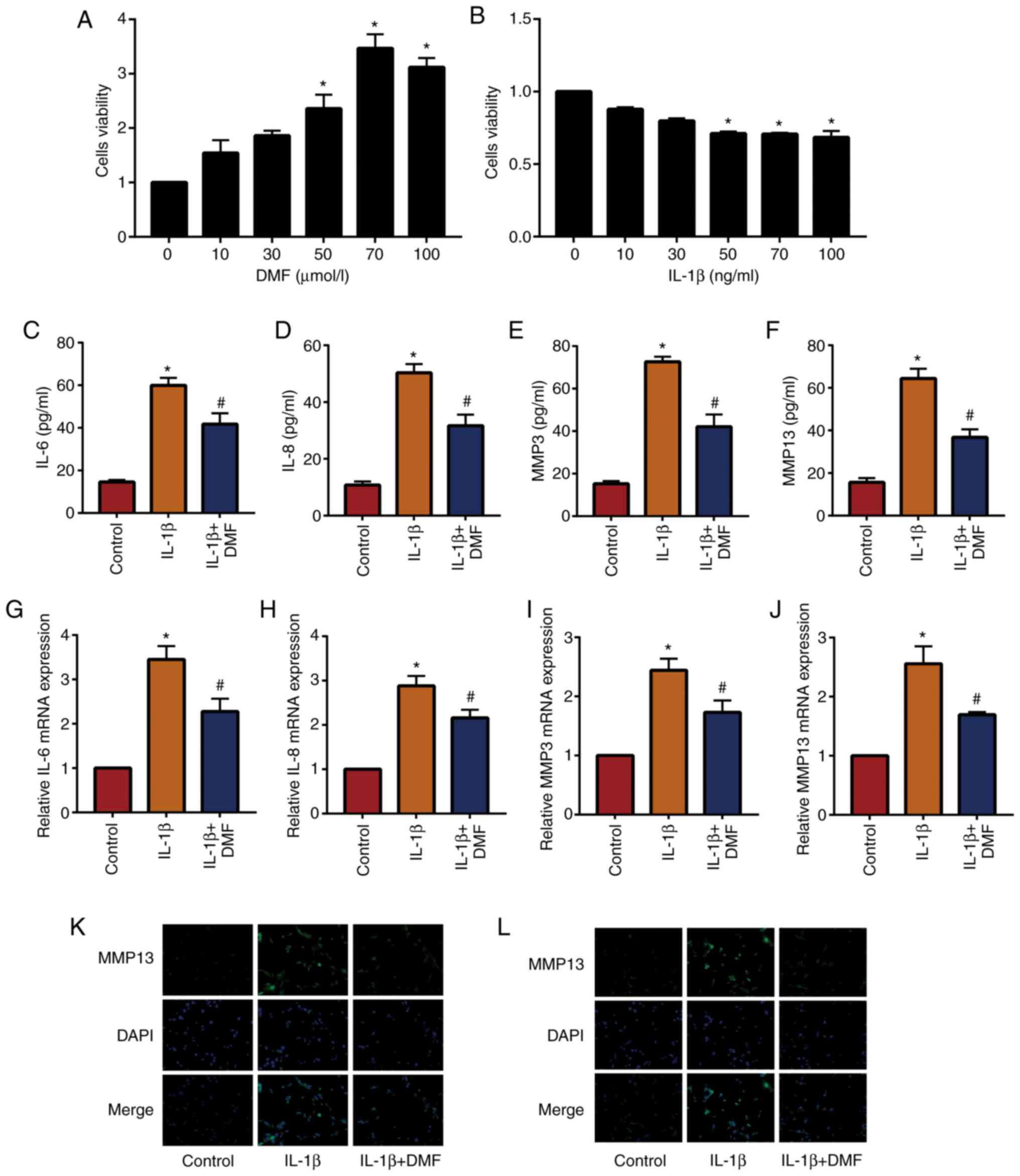
DMF decreases IL-1β-induced
inflammatory levels in NP cells
In total, 10, 30, 50, 70 and 100 µmol/l DMF was used
to stimulate NP cells and analyze cell viability using a CCK-8
assay (Fig. 2A). The results showed
that cells stimulated with 70 µmol/l DMF reached the highest cell
viability, therefore 70 µmol/l DMF was used to treat NP cells in
subsequent experiments. In addition, 50 ng/ml IL-1β was the lowest
dose to induce a significant difference compared with the untreated
group (Fig. 2B). The results of
ELISA (Fig. 2C-2F) and RT-qPCR
(Fig. 2G-2J) showed that IL-1β
increased the expression of inflammatory factors (IL-6, IL-8, MMP3
and MMP13) in NP cells compared with the control group, while IL-1β
+ DMF decreased these levels in NP cells compared with the IL-1β
treatment group. IF staining (Fig.
2K and L) also demonstrated
that IL-1β + DMF decreased the expression of MMP3 and MMP13
compared with IL-1β only treatment.
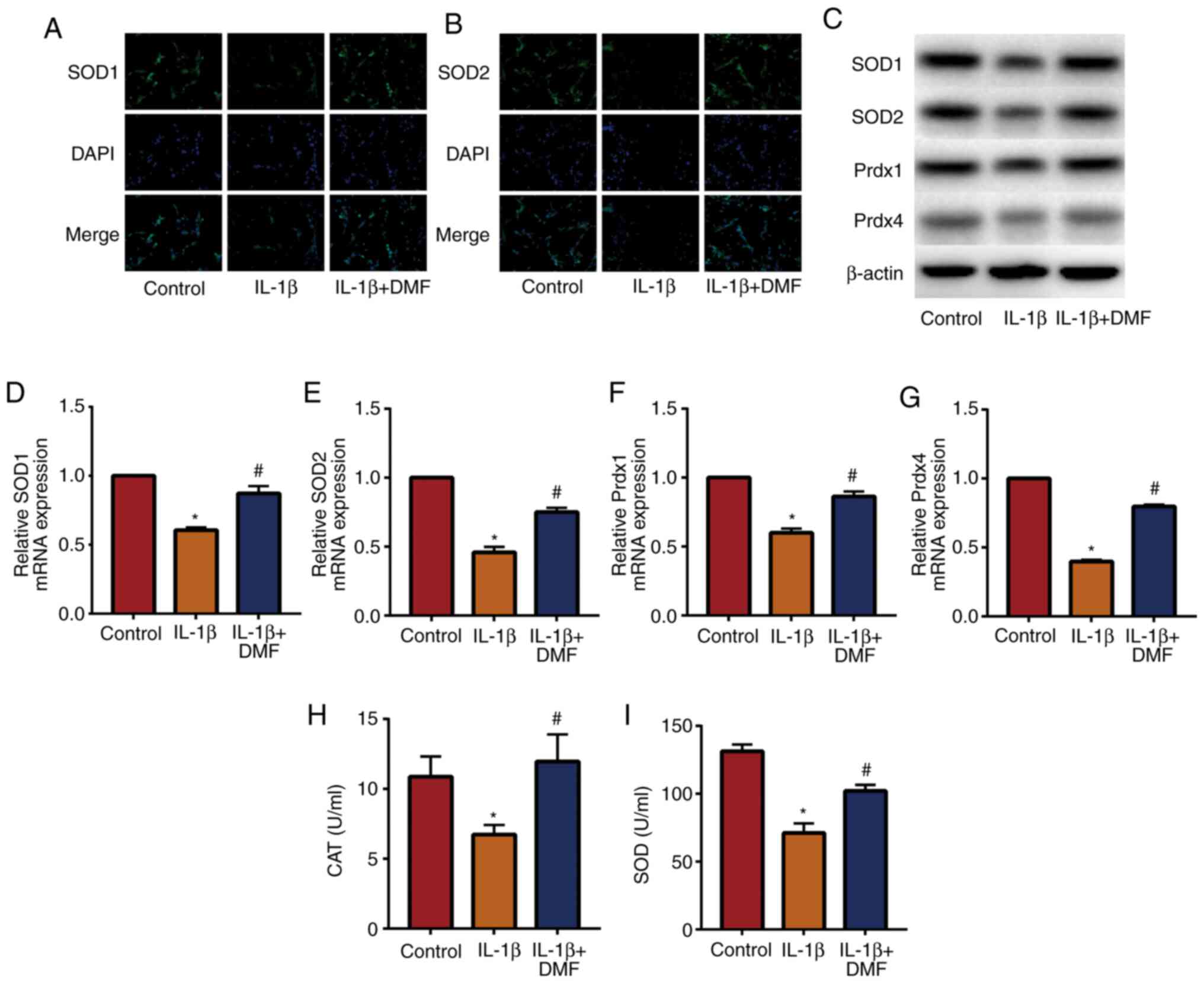
DMF inhibits oxidative stress in NP
cells
Oxidative stress is an important factor in IVDD
(21), therefore the effect of DMF
on oxidative stress in NP cells was analyzed. IF staining (Fig. 3A and B) detected the expression of SOD1 and
SOD2, indicating that IL-1β + DMF treatment increased SOD1 and SOD2
expression compared with IL-1β treatment. The results of western
blotting (Fig. 3C) and RT-qPCR
(Fig. 3D-G) also showed that the
expression of SOD1, SOD2, Prdx1 and Prdx4 was significantly
decreased in IL-1β-stimulated NP cells compared with the control
group, while DMF attenuated IL-1β-induced oxidative stress. In
addition, the activity of CAT (Fig.
3H) and SOD (Fig. 3I) were
examined in NP cells, and the results showed that IL-1β + DMF
increased the activity of CAT and SOD compared with the IL-1β
group.
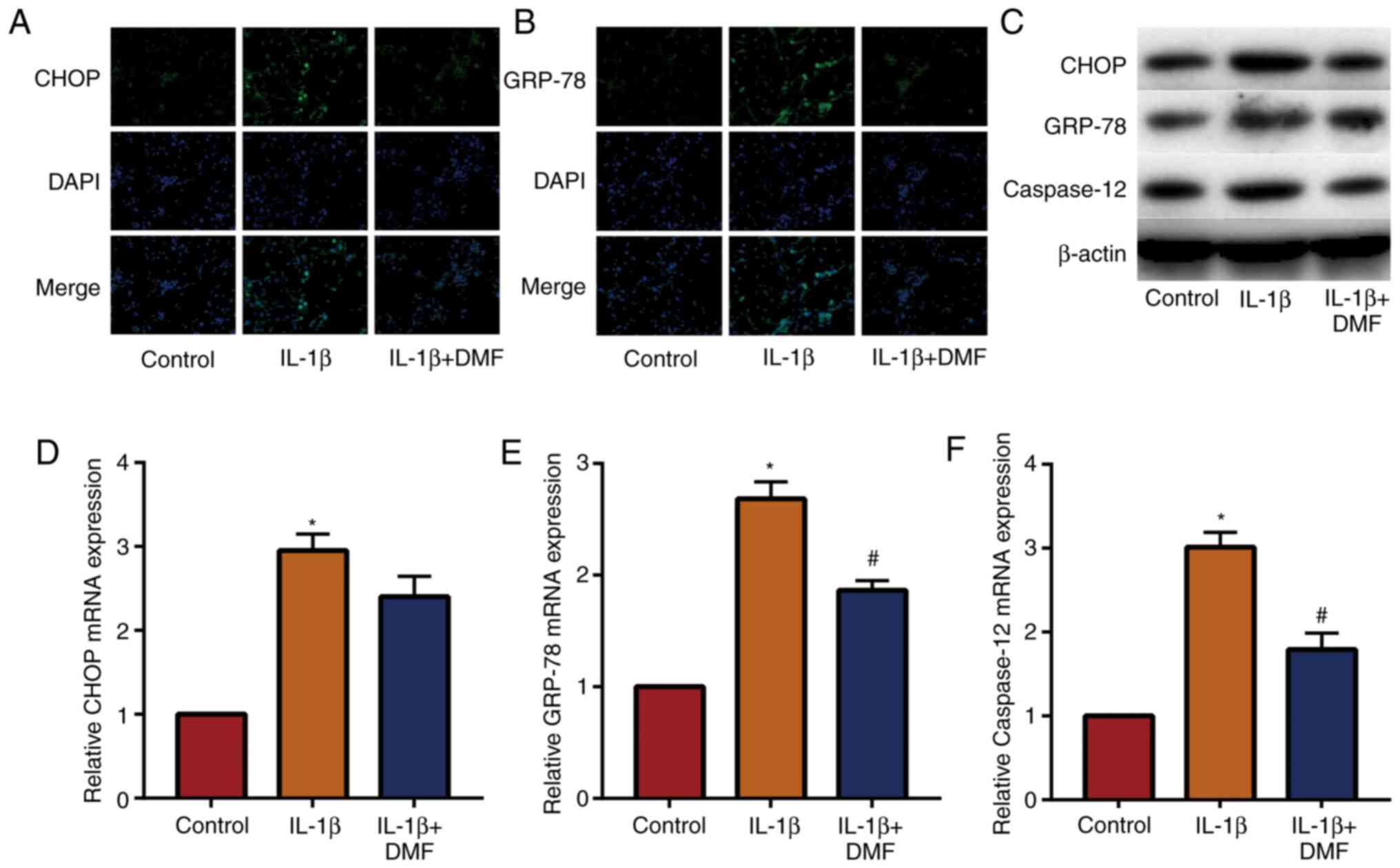
DMF decreases IL-1β-induced
endoplasmic reticulum stress in NP cells
The expression of signaling molecules in the
endoplasmic reticulum stress-related pathway was examined in NP
cells. The results of IF staining (Fig.
4A and B) showed that the
expression of CHOP and GRP-78 was increased in IL-1β induced NP
cells and decreased by DMF. The results of western blotting
(Fig. 4C) and RT-qPCR (Fig. 4D-4F) also showed that DMF decreased
the expression of CHOP, GRP-78 and caspase-12. These results
indicated that DMF decreased endoplasmic reticulum stress levels in
NP cells.
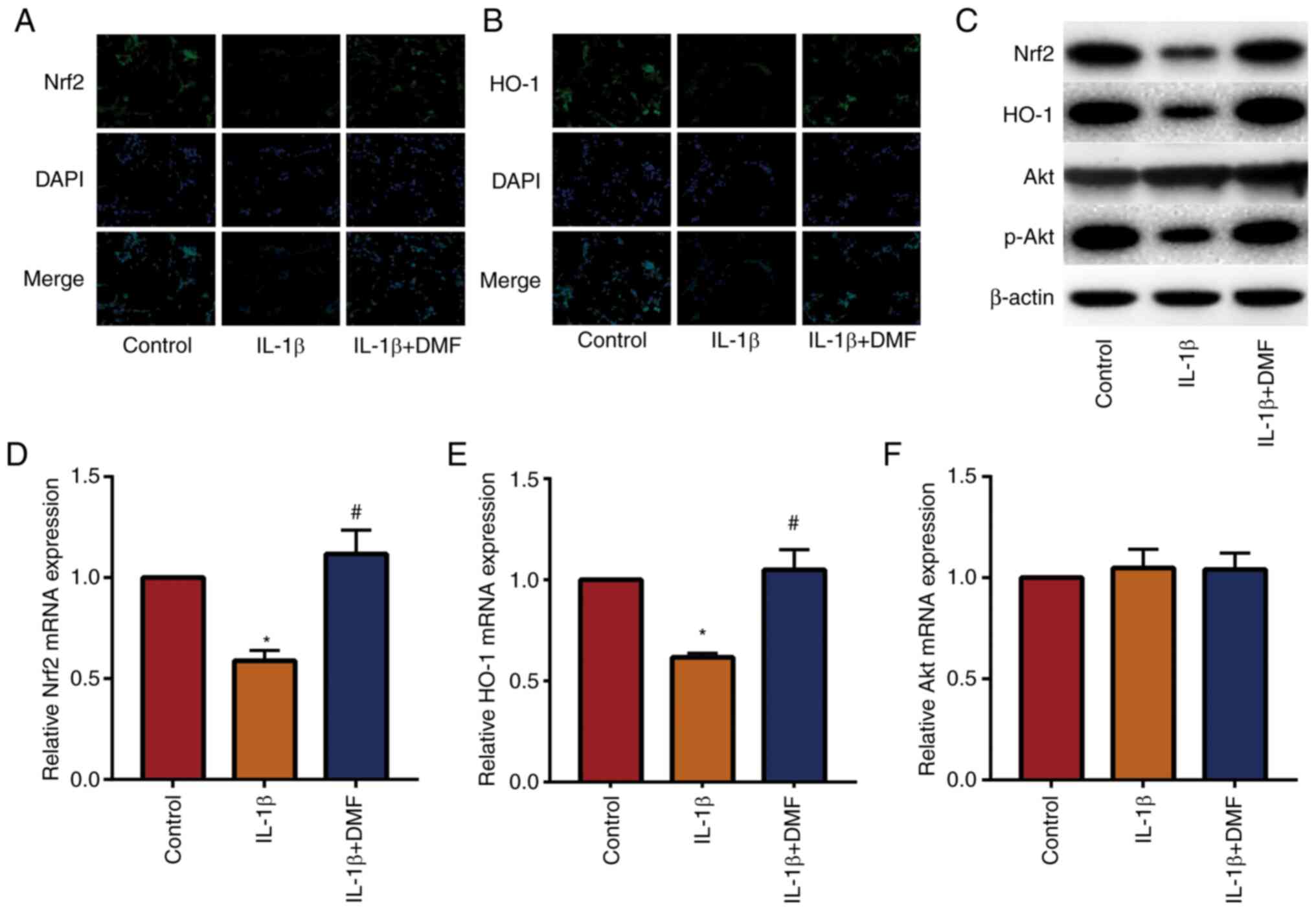
DMF promotes the Nrf2/HO-1 and
PI3K/Akt signaling pathways in NP cells
The Nrf2/HO-1 and PI3K/AKT signaling pathways are
important antioxidant and anti-inflammatory pathways (22). The results of IF staining (Fig. 5A and B) showed that DMF increased the expression
of Nrf2 and HO-1 in NP cells compared with the control. Western
blotting (Fig. 5C) and RT-qPCR
(Fig. 5D-5F) also showed that DMF
increased Nrf2 and HO-1 expression and promoted the phosphorylation
of Akt compared with IL-1β treatment. These data indicated that DMF
increased the activity of the Nrf2/HO-1 and PI3K/Akt signaling
pathways.
Discussion
At present, the molecular mechanisms underlying
degenerative disc disease are not fully understood. The
pathological basis primarily involves inflammatory infiltration,
decreased NP cell number and functional decline, NP dehydration,
proteoglycan reduction and the change of collagen type and
distribution (23). Gruber et
al (24) reported that the
number of aged and apoptotic NP cells in degenerated intervertebral
disc tissue were increased, causing elevated inflammatory factors
and abnormal metabolism of extracellular matrix (via increased MMP
activity, and decreased quantity of collagen and proteoglycan).
These changes are closely related to ROS. ROS are normal aerobic
metabolites, including hydrogen peroxide, superoxide anion
(O2-) and hydroxyl radicals (OH). Under
normal circumstances, the production and elimination of ROS are in
a dynamic equilibrium, and low concentrations of ROS function as
signaling molecules in normal physiological activities, such as
cell proliferation and differentiation, body damage repair and
inflammatory immunity. When the ROS production in the body
increases due to various reasons (such as injury and senescence) or
the body's ability to resist ROS decreases, the body enters an
oxidative stress state (25). At
this time, ROS can directly cause the oxidative damage to DNA,
proteins and lipids through the NP cell membrane, thereby causing
destruction of these cells (26).
High concentrations of ROS can also cause apoptosis in NP cells by
disrupting the membrane permeability of mitochondria (27). The oxidative respiratory chain on
the mitochondrial inner membrane is the primary site of ROS
production, and it is also the most sensitive part of the ROS
generation pathway (27). During
the aerobic metabolism electron transfer process, some electrons
will leak from the electron transport chain and combine with oxygen
molecules to form O2- (27). Importantly, mitochondria have a
two-layer membrane structure; the outer membrane has good
permeability, while the inner membrane has poor permeability, which
prevents the transport of protons, most ions and water molecules
(27). When the ROS content in the
body exceeds the normal requirement, the mitochondrial membrane
permeability transition pore is activated, increasing the
permeability of the mitochondrial inner membrane, and the water
molecules diffuse into the mitochondrial cytoplasm where there is a
lower osmotic pressure (28). The
increasing osmotic pressure of the mitochondria causes it to swell,
leading to rupture of the mitochondrial outer membrane (29). Cytochrome c located in the
mitochondrial membrane gap is released into the cytosol to activate
the downstream caspases 3, 6 and 7, causing apoptosis of the NP
cells (29). In addition, ROS can
also participate in the inflammatory response, regulate NP cell
apoptosis and promote the synthesis and release of intracellular
MMPs (MMPs 1, 3 and 13) and aggrecanase (ADAM-TS) by activating
various pathways, such as JNK, p38/MAPK and NF-κB (29). MMPs and ADAM-TS are the main enzymes
for extracellular matrix and proteoglycan and collagen II, which
can induce the degradation of the intervertebral disc extracellular
matrix, ADAM-TS-dependent proteoglycan and collagen II (30). This is the pathophysiological basis
of the IVDD (30). It has been
demonstrated that ROS participates in the occurrence and
development of IVDD (31,32). In the present study, DMF-treated
mice expressed less MMP3 and MMP13 in the intervertebral disc
tissue, while the increased expression of extracellular matrix
components (collagen II and aggrecan) indicated that DMF protected
the extracellular matrix of NP cells from degradation of MMPs. In
addition, DMF stimulated NP cells and significantly decreased
inflammation and oxidative stress in NP cells, as shown by the
decrease of inflammatory factor expression and increase of SOD
activity. This indicated that the anti-inflammatory and
antioxidative effects of DMF can protect NP cells. DMF was also
reported to reduce IL-1β-induced cell nucleus endoplasmic reticulum
stress, which is further evidence of the protective effect of DMF
on cell nucleus.
When various external factors, such as injury and
infection among others, cause the balance between ROS production
and the antioxidant defense system to be disrupted, the body enters
an oxidative stress state, activating a series of antioxidant
stress defense responses and antioxidant transcription factors,
including Nrf2 and AP-1. Activation of these transcription factors
ultimately increases the expression and synthesis of antioxidant
enzymes, such as SOD, CAT and HO-1, which protects the body from
oxidative stress (33). Nrf2 is an
important antioxidant defense factor in the body, expressed in
almost all cells. When the body's ROS levels increase, the Nrf2
located in the cytosol is dissociates from its inhibitor Keap1 and
is activated (34). Free Nrf2
enters the nucleus and binds to the Maf protein to form a dimer and
binds to the antioxidant response element, thereby initiating the
transcriptional expression of a series of antioxidant enzymes
(35). The Nrf2/HO-1 signaling
pathway is a crucial protective pathway against oxidative stress
caused by various external causes, such as injury and infection.
Studies have confirmed that Nrf2/HO-1 signaling pathway can
regulate >200 antioxidant proteases, phase II detoxification
enzymes and anti-inflammatory factors (36,37).
The transcriptional expression of endogenous protective genes plays
an irreplaceable role in antioxidative stress, anti-inflammatory,
anti-apoptosis and antitumor responses (38). Studies have confirmed that numerous
diseases are related to the increase of oxygen free radicals in the
body, such as Alzheimer's disease, Parkinson's disease and
pulmonary fibrosis, and there are several changes in the expression
of Nrf2 at different stages of such diseases (39,40).
The degenerative disease of the intervertebral disc also has an
inflammatory reaction accompanied by the pathological increase of
oxygen radicals in the body (41).
The role of Nrf2 in antioxidation and anti-inflammation has been
recognized in a number of diseases, such as myocardial infarction
and inflammatory bowel disease (42,43);
however, and whether these functions of Nrf2 are also closely
associated with degenerative diseases of intervertebral disc is
unclear. The PI3K/Akt pathway regulates apoptosis during oxidative
stress. Studies have shown that activation of the PI3K/Akt pathway
protects nerve cells from oxidative stress (44,45).
Inhibition of Akt activity makes cells more sensitive to ROS and
more susceptible to oxidative damage (46). The PI3K/Akt signaling pathway plays
an important role in the expression of antioxidant enzymes,
including HO-1 and glutamate cysteine ligase. Therefore,
Akt-related signaling pathways are important for the body to resist
oxidative stress (47). In the
present study, the activity of the Nrf2/HO-1 signaling pathway and
the PI3K/Akt signaling pathway in the NP was significantly
decreased after stimulation of the NP cells with IL-1β. In
DMF-stimulated NP cells, the expression of Nrf2 and HO-1 and the
level of phosphorylated Akt was increased, indicating that DMF
promoted the activity of these two signaling pathways in NP cells.
This may be the mechanism of the anti-inflammatory and antioxidant
effects of DMF in NP cells.
In DMF-treated mice, DHI was significantly increased
and extracellular matrix degradation was also inhibited, indicating
that DMF relieved IVDD in mice. In cell experiments, DMF increased
the viability of NP cells and inhibited IL-1β-induced inflammation,
oxidative stress and endoplasmic reticulum stress. In addition, DMF
also promoted the activity of the Nrf2/HO-1 and PI3K/Akt signaling
pathways in NP cells, which helps clarify the mechanism of DMF in
protecting intervertebral discs. Overall, DMF demonstrated good
efficacy in protecting against IVDD. To the best of our knowledge,
the present study is the first to report that DMF improves IVDD,
and these results may aid in the development of DMF treatment to
clinically prevent IVDD.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HZ, GC and NS designed the study and performed the
experiments. HZ and YW established the animal models. GC and XL
collected the data. JZ and ZW analyzed the data. HZ, GC and NS
prepared the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by The Animal Ethics
Committee of Qinghai Provincial People's Hospital Animal Center
(Xining, China; approval no. GS-XNH-17-A-0832).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wu CZ, Ou DQ, Rong LM, Xu YC, Dong JW, Fan
L and Wang QY: Expression of lamin A/C protein in degenerated human
intervertebral disc. Eur Rev Med Pharmacol Sci. 22:7607–7613.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
González Martínez E, García-Cosamalón J,
Cosamalón-Gan I, Esteban Blanco M, García-Suarez O and Vega JA:
Biology and mechanobiology of the intervertebral disc. Neurocirugia
(Astur). 28:135–140. 2017.(In Spanish). PubMed/NCBI View Article : Google Scholar
|
|
3
|
Navone SE, Marfia G, Giannoni A, Beretta
M, Guarnaccia L, Gualtierotti R, Nicoli D, Rampini P and Campanella
R: Inflammatory mediators and signalling pathways controlling
intervertebral disc degeneration. Histol Histopathol. 32:523–542.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lin X and Lin Q: MiRNA-495-3p attenuates
TNF-α induced apoptosis and inflammation in human nucleus pulposus
cells by targeting IL5RA. Inflammation. 43:1797–1805.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
He M, Pang J, Sun H, Zheng G, Lin Y and Ge
W: P14ARF inhibits regional inflammation and vascularization in
intervertebral disc degeneration by upregulating TIMP3. Am J
Physiol Cell Physiol. 318:C751–C761. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang Y, Che M, Xin J, Zheng Z, Li J and
Zhang S: The role of IL-1β and TNF-α in intervertebral disc
degeneration. Biomed Pharmacother. 131(110660): Aug 24.
2020.(Online ahead of print). PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dowdell J, Erwin M, Choma T, Vaccaro A,
Iatridis J and Cho SK: Intervertebral disk degeneration and repair.
Neurosurgery. 80:S46–S54. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jiang JY and Lu XH: Biological treatment
for intervertebral disc degeneration. Zhongguo Gu Shang.
29:576–580. 2016.(In Chinese). PubMed/NCBI
|
|
9
|
Kalincik T, Kubala Havrdova E, Horakova D,
Izquierdo G, Prat A, Girard M, Duquette P, Grammond P, Onofrj M,
Lugaresi A, et al: Comparison of fingolimod, dimethyl fumarate and
teriflunomide for multiple sclerosis. J Neurol Neurosurg
Psychiatry. 90:458–468. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Blair HA: Dimethyl fumarate: A review in
moderate to severe plaque psoriasis. Drugs. 78:123–130.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hayashi G, Jasoliya M, Sahdeo S, Saccà F,
Pane C, Filla A, Marsili A, Puorro G, Lanzillo R, Brescia MV and
Cortopassi G: Dimethyl fumarate mediates Nrf2-dependent
mitochondrial biogenesis in mice and humans. Hum Mol Genet.
26:2864–2873. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Oey O, Rao P, Luciuk M, Mannix C, Rogers
NM, Sagar P, Wong A and Rangan G: Effect of dimethyl fumarate on
renal disease progression in a genetic ortholog of
nephronophthisis. Exp Biol Med (Maywood). 243:428–436.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Al-Jaderi Z and Maghazachi AA: Utilization
of dimethyl fumarate and related molecules for treatment of
multiple sclerosis, cancer, and other diseases. Front Immunol.
7(278)2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Saidu NE, Noé G, Cerles O, Cabel L,
Kavian-Tessler N, Chouzenoux S, Bahuaud M, Chéreau C, Nicco C,
Leroy K, et al: Dimethyl fumarate controls the NRF2/DJ-1 axis in
cancer cells: Therapeutic applications. Mol Cancer Ther.
16:529–539. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Krishnamoorthy S, Pace B, Gupta D,
Sturtevant S, Li B, Makala L, Brittain J, Moore N, Vieira BF,
Thullen T, et al: Dimethyl fumarate increases fetal hemoglobin,
provides heme detoxification, and corrects anemia in sickle cell
disease. JCI Insight. 2(96409)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Iniaghe LO, Krafft PR, Klebe DW, Omogbai
EKI, Zhang JH and Tang J: Dimethyl fumarate confers neuroprotection
by casein kinase 2 phosphorylation of Nrf2 in murine intracerebral
hemorrhage. Neurobiol Dis. 82:349–358. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Che H, Li J, Li Y, Ma C, Liu H, Qin J,
Dong J, Zhang Z, Xian CJ, Miao D, et al: p16 deficiency attenuates
intervertebral disc degeneration by adjusting oxidative stress and
nucleus pulposus cell cycle. Elife. 9(e52570)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Urquhart DM, Kurniadi I, Triangto K, Wang
Y, Wluka AE, OʼSullivan R, Jones G and Cicuttini FM: Obesity is
associated with reduced disc height in the lumbar spine but not at
the lumbosacral junction. Spine (Phila Pa 1976). 39:E962–E966.
2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rana SVS: Endoplasmic reticulum stress
induced by toxic elements-a review of recent developments. Biol
Trace Elem Res. 196:10–19. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xu WN, Zheng HL, Yang RZ, Liu T, Yu W,
Zheng XF, Li B, Jiang SD and Jiang LS: Mitochondrial NDUFA4L2
attenuates the apoptosis of nucleus pulposus cells induced by
oxidative stress via the inhibition of mitophagy. Exp Mol Med.
51:1–16. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Loboda A, Damulewicz M, Pyza E, Jozkowicz
A and Dulak J: Role of Nrf2/HO-1 system in development, oxidative
stress response and diseases: An evolutionarily conserved
mechanism. Cell Mol Life Sci. 73:3221–3247. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Centeno C, Markle J, Dodson E, Stemper I,
Williams CJ, Hyzy M, Ichim T and Freeman M: Treatment of lumbar
degenerative disc disease-associated radicular pain with
culture-expanded autologous mesenchymal stem cells: A pilot study
on safety and efficacy. J Transl Med. 15(197)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gruber HE, Hoelscher GL, Ingram JA, Bethea
S and Hanley EN Jr: Autophagy in the degenerating human
intervertebral disc: In vivo molecular and morphological evidence,
and induction of autophagy in cultured annulus cells exposed to
proinflammatory cytokines-implications for disc degeneration. Spine
(Phila Pa 1976). 40:773–782. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yang S and Lian G: ROS and diseases: Role
in metabolism and energy supply. Mol Cell Biochem. 467:1–12.
2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Feng C, Yang M, Lan M, Liu C, Zhang Y,
Huang B, Liu H and Zhou Y: ROS: Crucial intermediators in the
pathogenesis of intervertebral disc degeneration. Oxid Med Cell
Longev. 2017(5601593)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Nakamura T, Naguro I and Ichijo H: Iron
homeostasis and iron-regulated ROS in cell death, senescence and
human diseases. Biochim Biophys Acta Gen Subj. 1863:1398–1409.
2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nasto LA, Robinson AR, Ngo K, Clauson CL,
Dong Q, St CC, Sowa G, Pola E, Robbins PD, Kang J, et al:
Mitochondrial-derived reactive oxygen species (ROS) play a causal
role in aging-related intervertebral disc degeneration. J Orthop
Res. 31:1150–1157. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Jiang LB, Cao L, Ma YQ, Chen Q, Liang Y,
Yuan FL, Li XL, Dong J and Chen N: TIGAR mediates the inhibitory
role of hypoxia on ROS production and apoptosis in rat nucleus
pulposus cells. Osteoarthritis Cartilage. 26:138–148.
2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ma KG, Shao ZW, Yang SH, Wang J, Wang BC,
Xiong LM, Wu Q and Chen SF: Autophagy is activated in
compression-induced cell degeneration and is mediated by reactive
oxygen species in nucleus pulposus cells exposed to compression.
Osteoarthritis Cartilage. 21:2030–2038. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Song Y, Wang Z, Liu L, Zhang S, Zhang H
and Qian Y: 1,4-Dihydropyridine (DHP) suppresses against oxidative
stress in nucleus pulposus via activating sirtuin-1. Biomed
Pharmacother. 121(109592)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
He R, Cui M, Lin H, Zhao L, Wang J, Chen S
and Shao Z: Melatonin resists oxidative stress-induced apoptosis in
nucleus pulposus cells. Life Sci. 199:122–130. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Fang W, Zhou X, Wang J, Xu L, Zhou L, Yu
W, Tao Y, Zhu J, Hu B, Liang C, et al: Wogonin mitigates
intervertebral disc degeneration through the Nrf2/ARE and MAPK
signaling pathways. Int Immunopharmacol. 65:539–549.
2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Guo Z and Mo Z: Keap1-Nrf2 signaling
pathway in angiogenesis and vascular diseases. J Tissue Eng Regen
Med. 14:869–883. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Dimozi A, Mavrogonatou E, Sklirou A and
Kletsas D: Oxidative stress inhibits the proliferation, induces
premature senescence and promotes a catabolic phenotype in human
nucleus pulposus intervertebral disc cells. Eur Cell Mater.
30:89–102. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kasai S, Shimizu S, Tatara Y, Mimura J and
Itoh K: Regulation of Nrf2 by mitochondrial reactive oxygen species
in physiology and pathology. Biomolecules. 10(320)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sajadimajd S and Khazaei M: Oxidative
stress and cancer: The role of Nrf2. Curr Cancer Drug Targets.
18:538–557. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Panieri E, Buha A, Telkoparan-Akillilar P,
Cevik D, Kouretas D, Veskoukis A, Skaperda Z, Tsatsakis A, Wallace
D, Suzen S and Saso L: Potential applications of NRF2 modulators in
cancer therapy. Antioxidants (Basel). 9(193)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Cherif H, Bisson DG, Jarzem P, Weber M,
Ouellet JA and Haglund L: Curcumin and o-vanillin exhibit evidence
of senolytic activity in human IVD cells in vitro. J Clin Med.
8(433)2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ungvari Z, Tarantini S, Nyúl-Tóth Á, Kiss
T, Yabluchanskiy A, Csipo T, Balasubramanian P, Lipecz A, Benyo Z
and Csiszar A: Nrf2 dysfunction and impaired cellular resilience to
oxidative stressors in the aged vasculature: From increased
cellular senescence to the pathogenesis of age-related vascular
diseases. Geroscience. 41:727–738. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Feng C, Liu H, Yang M, Zhang Y, Huang B
and Zhou Y: Disc cell senescence in intervertebral disc
degeneration: Causes and molecular pathways. Cell Cycle.
15:1674–1684. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Strom J and Chen QM: Loss of Nrf2 promotes
rapid progression to heart failure following myocardial infarction.
Toxicol Appl Pharmacol. 327:52–58. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Pompili S, Sferra R, Gaudio E, Viscido A,
Frieri G, Vetuschi A and Latella G: Can Nrf2 modulate the
development of intestinal fibrosis and cancer in inflammatory bowel
disease? Int J Mol Sci. 20(4061)2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Cai L, Tu L, Li T, Yang X, Ren Y, Gu R,
Zhang Q, Yao H, Qu X, Wang Q and Tian J: Downregulation of lncRNA
UCA1 ameliorates the damage of dopaminergic neurons, reduces
oxidative stress and inflammation in Parkinson's disease through
the inhibition of the PI3K/Akt signaling pathway. Int
Immunopharmacol. 75(105734)2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Liu AH, Chu M and Wang YP: Up-regulation
of Trem2 inhibits hippocampal neuronal apoptosis and alleviates
oxidative stress in epilepsy via the PI3K/Akt pathway in mice.
Neurosci Bull. 35:471–485. 2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ouyang ZH, Wang WJ, Yan YG, Wang B and Lv
GH: The PI3K/Akt pathway: A critical player in intervertebral disc
degeneration. Oncotarget. 8:57870–57881. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Meierjohann S: Oxidative stress in
melanocyte senescence and melanoma transformation. Eur J Cell Biol.
93:36–41. 2014.PubMed/NCBI View Article : Google Scholar
|