Introduction
Fragility fracture generally occurs due to an injury
that would be insufficient to cause fracture in a normal bone
(1). It is a common disease in the
elderly, leading to huge burdens on health and the economy,
worldwide (2). Fragility fracture
has become a global public health threat, and ~2,000,000 new cases
occur in the USA annually (3). In
general, fractures lead to acute pain, decreased mobility and loss
of independence, and almost all patients require long-term fixation
(4). However, fracture healing is a
slow recovery process, leading to a significant reduction in
patient quality of life (5). Thus,
the mechanisms underlying fracture healing urgently need to be
explored further, which may help to reduce the time for fracture
healing. Fracture healing involves multiple growth factors and
cytokines that are associated with the proliferation, apoptosis and
differentiation of osteoblasts (6).
To accelerate bone regeneration, strategies to facilitate
osteoblastic activation are necessary.
MicroRNAs (miRNAs) are a group of small non-coding
RNAs with important regulatory roles in various cellular processes,
such as cell proliferation, migration, invasion, differentiation
and apoptosis (7). They can
directly bind the 3'-unstranlated region (3'-UTR) of target
messenger RNAs (mRNAs), leading to the inhibition in target gene
expression at post-transcriptional levels (8). Emerging studies have reported the
roles of miRNAs in the regulation of osteoblastic activity. For
example, Zhang et al (9)
observed that miR-146a suppressed osteoblastic proliferation and
enhanced apoptosis. Li et al (10) revealed that miR-342-5p could inhibit
osteoblastic cell proliferation, migration and differentiation by
targeting Bmp7. Differentially expressed miR-125a-3p in the
osteoblastic differentiation process could significantly regulate
osteoblastic cell proliferation (11). The decreased expression levels of
miR-491-3p in postmenopausal osteoporosis regulates osteoblastic
viability and differentiation (12). miR-328-3p has also been demonstrated
to regulate the proliferation of different types of cell, such as
colorectal cancer (13) and
osteosarcoma cells (14). Weilner
et al (15) investigated the
differentially expressed circulating miRNAs that may be associated
with osteoporotic fracture, the results of which revealed that
miR-328-3p was associated with osteogenic differentiation, and was
downregulated in patients with osteoporosis who developed
fractures. However, whether miR-328-3p is involved in fracture
healing processes remains unclear.
The present study assessed the expression of
miR-328-3p during healing in patients with fragility fracture and
the potential regulatory effect of miR-328-3p on osteoblastic
viability. In addition, the target gene of miR-328-3p was also
predicted and explored, which may provide a novel insight into the
pathogenesis of fracture healing.
Materials and methods
Patients and sample collection
The present study included 80 patients with
fragility fracture (34 males, 46 females; average age, 68.94±11.30
years; age range, 48-87 years), who underwent fixation therapy at
the Traditional Chinese Medicine Hospital of Weifang from January
2016 to December 2018. All patients suffered from fragility
fracture without trauma or due to low-energy trauma. Before
fixation therapy, blood samples were collected from the patients
within 24 h of the fracture. After fixation, blood samples were
obtained on day 7, 14 and 21. In addition, the blood samples of 40
healthy volunteers were also collected during the same time period.
All blood samples were immediately centrifuged at 1,500 x g for 10
min at 4˚C for serum extraction and stored at -80˚C for subsequent
use. The healthy individuals included in the current study
comprised 19 males and 21 females, with an average age of 68.10 ±
12.05 years (range of 46-86 years). All the participants signed
written informed consent for clinical sample collection and
analysis, and the experimental procedures were approved by the
Ethics Committee of Traditional Chinese Medicine Hospital of
Weifang.
Cell culture and transfection
A human osteoblast cell line hFOB1.19 was purchased
from the Chinese Academy of Sciences Cell Bank. The cells were
cultured in RPMI-1640 (HyClone; Cytiva) medium supplemented with
10% FBS (Invitrogen; Thermo Fisher Scientific, Inc.), 1,000 U/ml
penicillin and 100 µg/ml streptomycin in an incubator with 5%
CO2 at 37˚C.
To overexpress miR-328-3p in hFOB1.19, 50 nM
miR-328-3p mimic (5'-CUGGCCCUCUCUGCCCUUCCGU-3') was synthesized by
Shanghai GenePharma Co., Ltd. and transfected into cells using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) following the manufacturer's instructions. The
negative control for the mimic (mimic NC; 50 nM; Shanghai
GenePharma Co., Ltd.; 5'-UUCUCCGAACGUGUCACGU-3') was used and
transfected into hFOB1.19 to serve as a negative control group.
Additionally, the overexpression vector of PTEN, pcDNA3.1-PTEN
(Shanghai GenePharma Co., Ltd.), was synthesized and transfected
into hFOB1.19 to overexpress PTEN in osteoblasts. The cells
transfected with only transfection reagent were set as the mock
group. Cell transfection was performed at 37˚C for 6 h, the
transfection regent was removed and fresh RPMI-1640 medium was
added. After 48 h of cell transfection, cells were used for
subsequent experimentation. All experiments were repeated in
triplicate.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to extract total RNA from serum
and cell samples, and the obtained RNA was reversed transcribed
into cDNA using a PrimeScript RT reagent kit (Takara Bio, Inc.)
according to the manufacturer's protocol. The expression of
miR-328-3p and the mRNA expression of PTEN was quantified via qPCR,
which was performed using SYBR-Green I Master Mix kit (Invitrogen;
Thermo Fisher Scientific, Inc.) on a 7500 Real-Time PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The
thermocycling conditions were set as follows: 95˚C for 10 min,
followed by 40 cycles of 95˚C for 20 sec, 58˚C for 10 sec and 72˚C
for 20 sec. The expression levels of miR-328-3p and PTEN were
normalized to U6 and GAPDH, respectively, and calculated using the
2-ΔΔCq method (16). The following primer sequences were
used in the reactions: miR-328-3p forward,
5'-GCCGAGCTGGCCCTCUCTGC-3' and reverse, 5'-CTCAACTGGTGTCGTGGA-3';
PTEN forward, 5'-TCCCAGACATGACAGCCATC-3' and reverse,
5'-TGCTTTGAATCCAAAAACCTTACT-3'; U6 forward, 5'-CTCGCTTCGGCAGCACA-3'
and reverse, 5'-AACGCTTCACGAATTTGCGT-3'; GAPDH forward,
5'-CAATGACCCCTTCATTGACC-3' and reverse,
5'-TTGATTTTGGAGGGATCTCG-3'.
Cell proliferation assay
After 48 h of cell transfection, the proliferation
of hFOB1.19 was estimated using an MTT assay. Cells
(3x103 cells/well) were seeded into 96-well plates and
cultured at 37˚C. The proliferation of cells at 0, 24, 48 and 72 h
was measured by incubating cells with 5 mg/ml MTT solution
(Sigma-Aldrich; Merck KGaA) for 4 h at 37˚C. Subsequently, 150 µl
DMSO (Sigma-Aldrich; Merck KGaA) was added into the wells to
dissolve formazan for 30 min at 37˚C. The optical density of
samples at 490 nm was measured using a microplate reader (Bio-Rad
Laboratories, Inc.) to reflect cell proliferation.
Apoptosis assay
The apoptosis of hFOB1.19 was evaluated using an
Annexin V-FITC Apoptosis Detection kit (Nanjing KeyGen Biotech Co.,
Ltd.). Cells (1x105) were harvested at 48 h
post-transcription and washed with PBS three times. After
suspending with Annexin V binding buffer, cells were stained with 5
µl V-FITC and 10 µl propidium iodide for 20 min in the dark at room
temperature. Apoptosis was measured using a flow cytometer (Attune
NxT; BD Biosciences) and analyzed using the CellQuest Pro analysis
software (version 5.1; BD Biosciences). Apoptotic rate, including
early and late apoptosis, was calculated.
Bioinformatics prediction and
luciferase reporter assay
The targets of miR-328-3p were predicted using
TargetScan (http://www.targetscan.org/vert_72/), the results of
which predicted that PTEN was a potential target gene of
miR-328-3p. The wild-type (WT) and mutant type (MUT) 3'-UTR
sequences of PTEN with a concentration of 100 nM were synthesized
by Shanghai GenePharma Co., Ltd., and were combined into the
luciferase reporter vector PMIR-RB-REPORT (Guangzhou RiboBio Co.,
Ltd.) to perform the luciferase reporter assay. The recombinant
vectors were co-transfected with miR-328-3p mimic or mimic NC using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.). After 48 h of transfection, the relative
luciferase activity was detected using a Luciferase Reporter System
(Promega Corporation) and normalized to Renilla luciferase
activity.
Western blotting
Total protein was extracted from hFOB1.19 cells
using RIPA buffer (Beyotime Institute of Biotechnology), and the
concentration of proteins was determined using a bicinchoninic acid
kit (Beyotime Institute of Biotechnology). Protein were separated
by 10% SDS-PAGE and transferred to PVDF membranes (EMD Millipore).
After blocking with 5% skimmed milk at 37˚C for 1 h, membranes were
incubated with the following primary antibodies at 4˚C overnight:
Anti-AKT (1:1,000; cat. no. 4691), anti-phosphorylated (p)-AKT
(1:2,000; cat. no. 4060), anti-PTEN (1:1,000; cat. no. 9188) and
anti-GAPDH (1:1,000; cat. no. 5174; all purchased from Cell
Signaling Technology, Inc.). Membranes were then incubated with
horseradish peroxide-labelled anti-rabbit IgG secondary antibodies
(1:1,000; cat. no. 7074; Cell Signaling Technology, Inc.). The
protein bands were visualized by enhanced chemiluminescence (Thermo
Fisher Scientific, Inc.) and GAPDH was used as a loading control.
Protein bands were quantified using ImageJ software (1.46; National
Institutes of Health).
Statistical analysis
The data analyzed in the present study were
presented as the mean ± SD. Each experiment was repeated at least
three times. Differences between two groups were analyzed using
unpaired Student's t-test or χ2 test, and comparison
between multiple groups was assessed using one-way ANOVA followed
by Tukey's post hoc test. The Pearson correlation coefficient test
was used to evaluate correlation. SPSS 22.0 (IBM Corp.) and
GraphPad Prism 7.0 software (GraphPad Software, Inc.) were used to
perform analyses. P<0.05 was considered to indicate a
statistically significant difference.
Results
Baseline characteristics of the
participants
The age, sex, body mass index (BMI) and
25-hydroxyvitamine D (25OHD) of patients with a fracture and
healthy controls were listed and compared in Table I. The 80 patients with a fracture
included 38 (47.5%) vertebral fracture cases, 22 (27.5%) hip
fracture cases and 20 (25.0%) wrist fracture cases. No significant
differences were observed between the age, sex and BMI of the two
groups (P>0.05). Patients with a fracture had significantly
lower 25OHD levels and higher incidence rates of osteoporosis
compared with healthy controls (P<0.01).
 | Table IBaseline characteristics of the
participants in this study. |
Table I
Baseline characteristics of the
participants in this study.
| Variables | Healthy controls
(n=40) | Patients with a
fracture (n=80) | P-value |
|---|
| Age, years | 68.10±12.05 | 68.94±11.30 | 0.682 |
| Sex | | | 0.603 |
|
Female, n
(%) | 21 (52.5) | 46 (57.5) | |
|
Male, n | 19 (47.5) | 34 (42.5) | |
| BMI
(kg/m2) | 23.03±2.81 | 22.04±2.74 | 0.068 |
| 25OHD (ng/ml) | 30.56±7.74 | 25.91±6.64 | 0.002a |
| Osteoporosis, n
(%) | 16 (40.0) | 63 (78.8) |
<0.001a |
| Fracture sites, n
(%) | | | - |
|
Vertebrae | - | 38 (47.5) | |
|
Hip | - | 22 (27.5) | |
|
Wrist | - | 20 (25.0) | |
Dynamic expression patterns of
miR-328-3p in patients with fracture
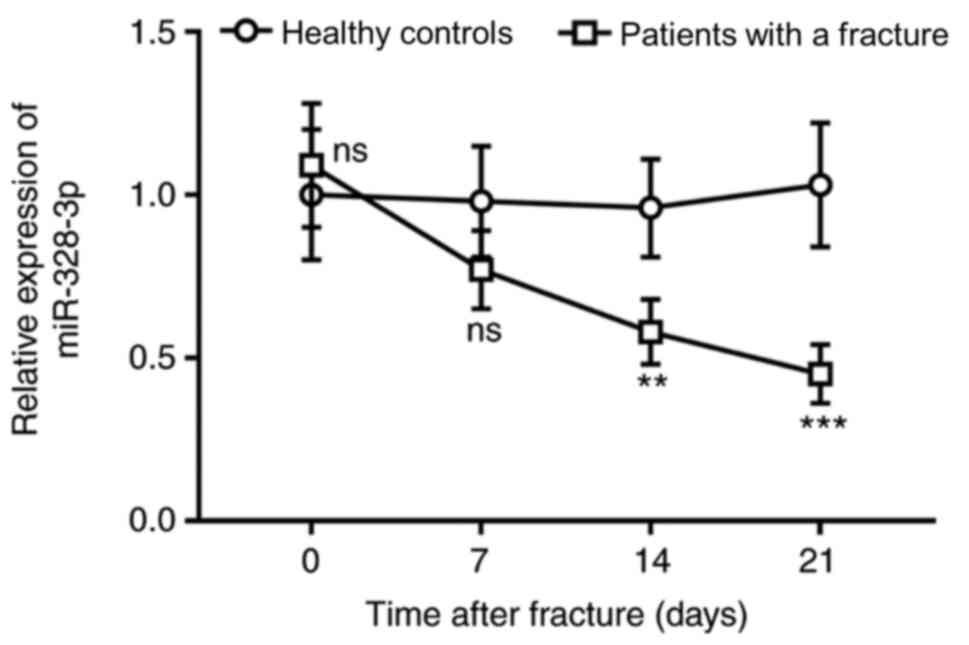
The serum expression levels of miR-328-3p were
slightly higher in patients with fracture compared with those of
healthy controls ≤24 h post fracture and before fixation therapy
(P>0.05; Fig. 1). After
fixation, no significant differences were observed in the serum
expression levels of miR-328-3p between the patients with a
fracture and healthy controls for 7 days (P>0.05). After that,
miR-328-3p expression levels gradually decreased and were
significantly downregulated on day 14 and 21 in patients with
fracture compared with healthy individuals (P<0.01; Fig. 1). The results suggested that
miR-328-3p may be involved in the progression of fracture
healing.
Effects of miR-328-3p on osteoblastic
proliferation and apoptosis
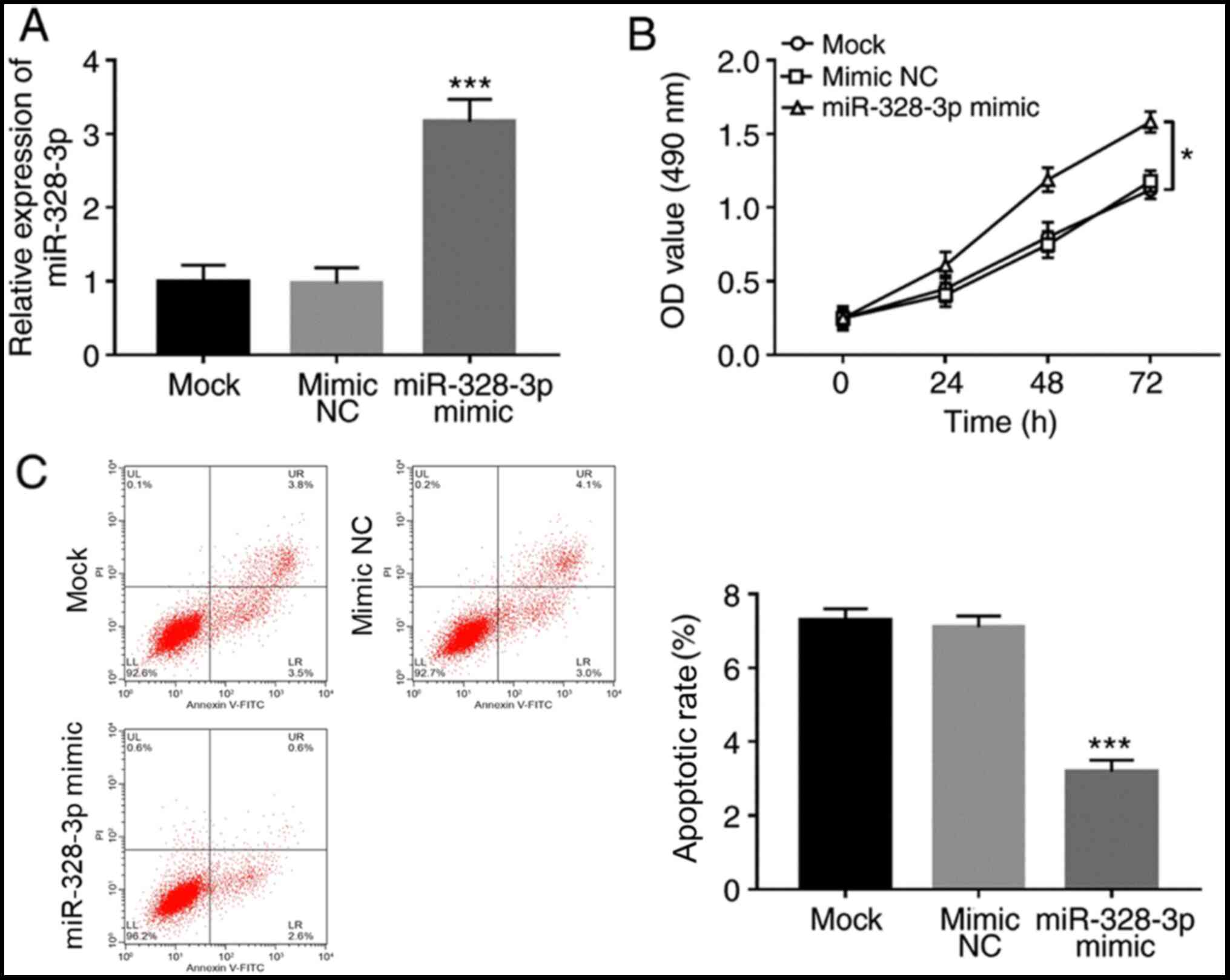
To understand the mechanisms underlying the aberrant
expression of miR-328-3p during the healing process of patients
with fracture, the effect of miR-328-3p on osteoblastic viability
was investigated. The expression levels of miR-328-3p in hFOB1.19
were successfully overexpressed by the miR-328-3p mimic
(P<0.001; Fig. 2A). The
proliferation of osteoblasts was significantly increased by the
overexpression of miR-328-3p (P<0.05; Fig. 2B). Additionally, apoptotic rate was
significantly reduced in cells transfected with miR-328-3p mimic
compared with cells transfected with mimic NC (P<0.001; Fig. 2C).
miR-328-3p directly regulates PTEN in
osteoblasts
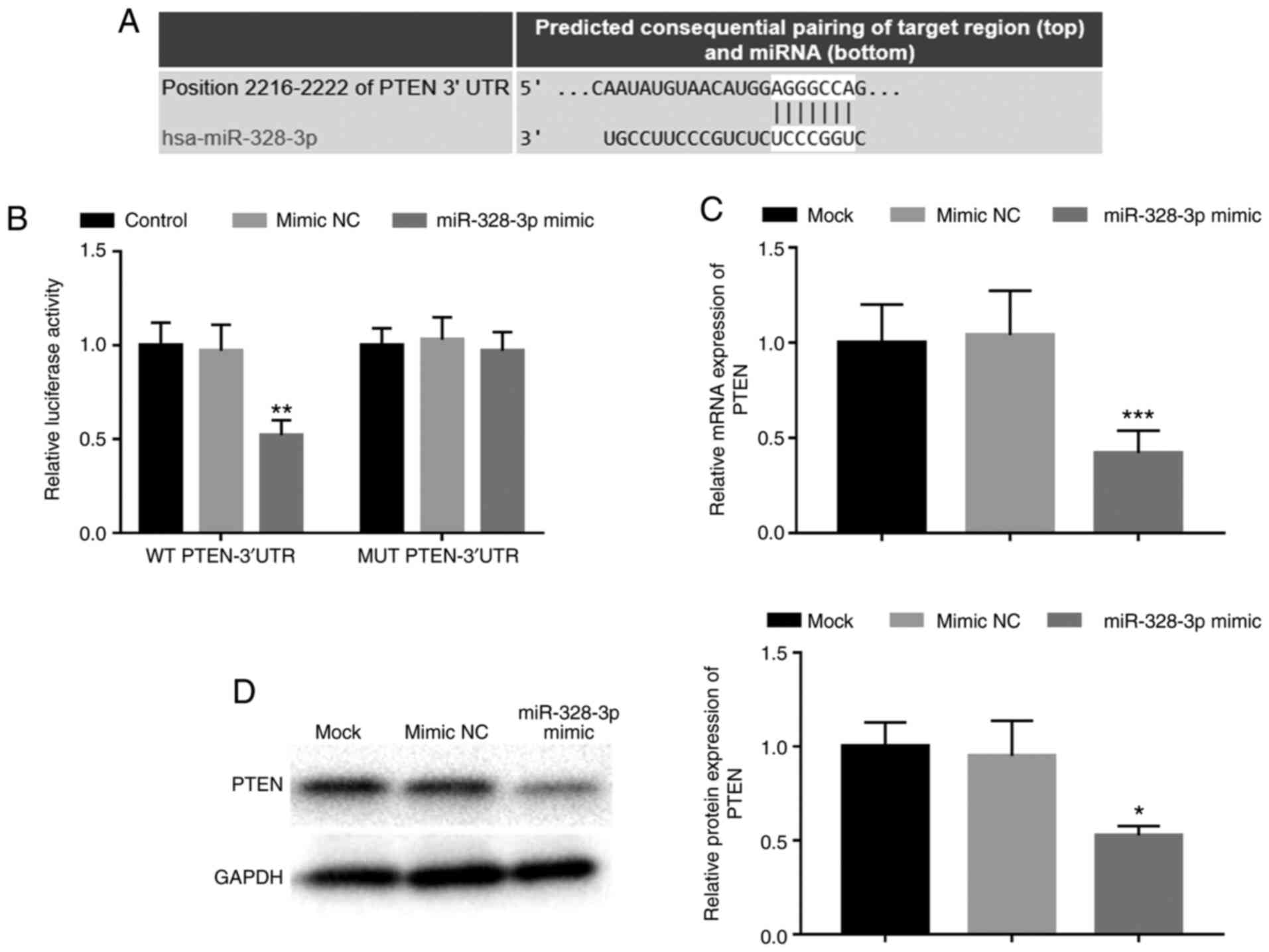
Bioinformatics prediction revealed a putative
binding site of miR-328-3p at the 3'-UTR of PTEN (Fig. 3A). Thus, a luciferase reporter assay
was performed to confirm the relationship between miR-328-3p and
PTEN. The results demonstrated that the relative luciferase
activity in the WT PTEN-3'UTR group was significantly inhibited by
the overexpression of miR-328-3p when compared with cells
co-transfected with mimic NC (P<0.01; Fig. 3B), whereas no significant
differences were observed in the MUT PTEN-3'UTR group (P>0.05;
Fig. 3B). Additionally, the
regulatory effect of miR-328-3p on PTEN in osteoblasts was
examined. The results demonstrated that overexpression of
miR-328-3p expression significantly decreased the mRNA and protein
expression levels of PTEN compared with those of the mimic NC group
in hFOB1.19 cells (P<0.05; Fig.
3B and C).
Correlation between miR-328-3p and
PTEN in patients with fracture
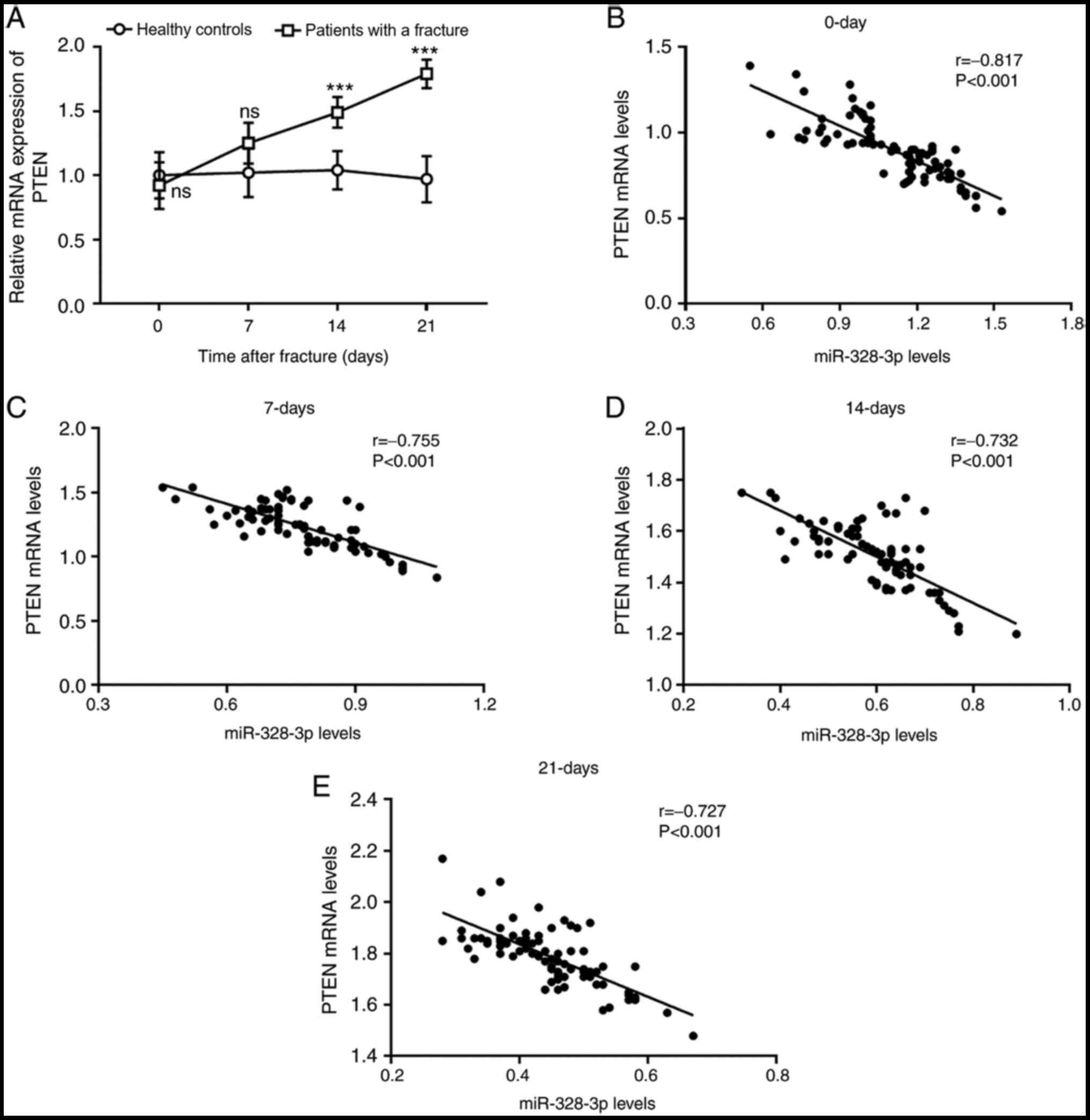
The mRNA expression levels of PTEN in patients with
fracture were evaluated. No significant differences were observed
in the expression levels of PTEN between patients with fracture and
healthy controls before fixation therapy and within 7 days post
therapy (P>0.05). However, the expression levels PTEN were
significantly increased on days 14 and 21 day in patients with
fracture compared with healthy controls (P<0.001; Fig. 4A). Additionally, the correlation of
serum miR-328-3p and PTEN levels was analyzed; at each time point,
a negative correlation was observed between serum levels of
miR-328-3p and PTEN (P<0.001; Fig.
4B-E).
PTEN mediates the regulatory effects
of miR-328-3p on osteoblastic viability
PTEN is known to serve pivotal roles in the
regulation of a cells biological function (17), and the inhibition of PTEN has been
reported to promote long non-coding RNA GHET1 on osteoblast
proliferation and differentiation (18). Thus, the effect of miR-328-3p on
osteoblastic viability may be mediated by PTEN. pcDNA3.1-PTEN was
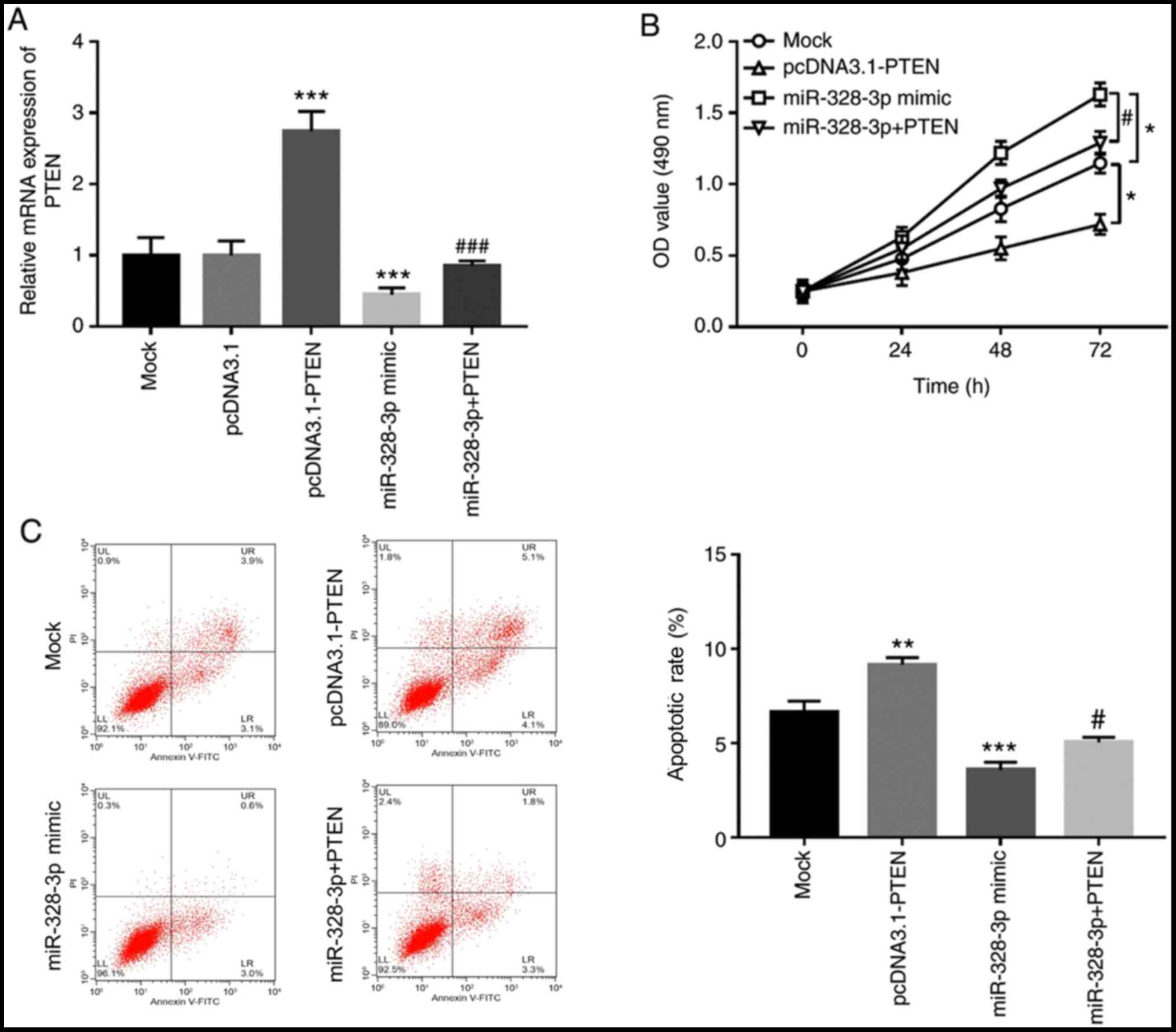
used to overexpress PTEN in hFOB1.19 cells (Fig. 5A). Overexpression of PTEN
significantly inhibited osteoblastic proliferation, but
significantly increased osteoblastic apoptosis (P<0.05; Fig. 5B and C). Additionally, in cells co-transfected
with miR-328-3p mimic and pcDNA3.1-PTEN, the expression levels of
PTEN were significantly reduced compared with those of cells
transfected with pcDNA3.1-PTEN alone (P<0.001; Fig. 5A). Notably, overexpression of PTEN
by pcDNA3.1-PTEN significantly reversed the effect of miR-328-3p on
osteoblastic viability, which was demonstrated by the inhibited
cell proliferation and enhanced apoptosis in hFOB1.19 (P<0.05;
Fig. 5B and C).
Regulatory effect of miR-328-3p on the
PI3K/AKT signaling pathway
PTEN is a negative regulator of the PI3K/AKT
signaling pathway, which is one of the most important pathways
during the processes of cell proliferation and apoptosis (19). Thus, the present study further
explored the effect of miR-328-3p on the activity of PI3K/AKT
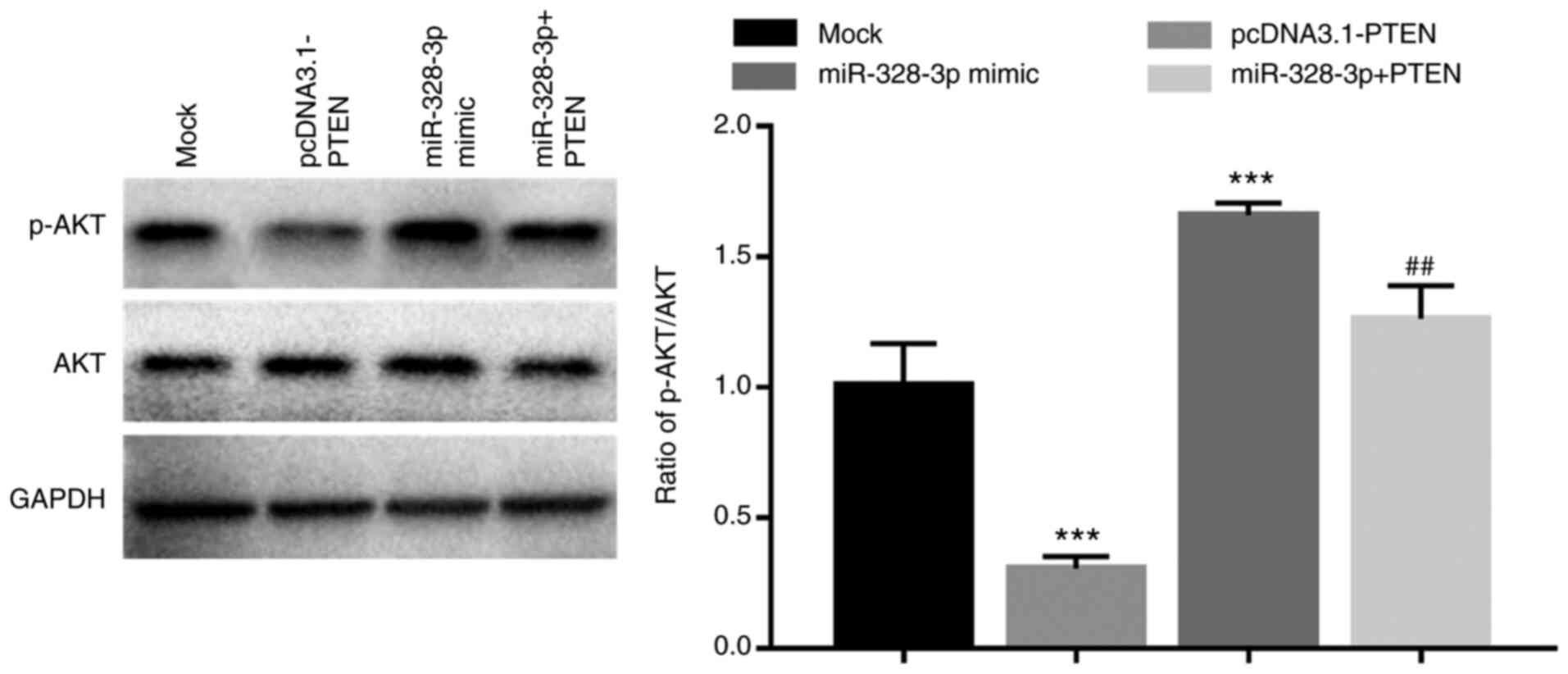
signaling. The results demonstrated that the ratio of p-AKT/AKT was
significantly increased in hFOB1.19 cells with overexpression of
miR-328-3p when compared with the mock group (P<0.001; Fig. 6), which suggested that the activity
of the AKT signaling pathway was enhanced by the overexpression of
miR-328-3p. Additionally, in the cells overexpressing PTEN, the
increase in p-AKT/AKT ratio induced by miR-328-3p overexpression
was significantly attenuated (P<0.01; Fig. 6), suggesting that miR-328-3p may
activate the PI3K/AKT signaling by inhibiting PTEN.
Discussion
Bone regeneration is the key to fracture healing,
which involves the proliferation, apoptosis and differentiation of
osteoblasts (20), implying that
methods that regulate osteoblastic activity may assist fracture
healing. miRNAs have important regulatory effects on various
cellular processes, such as cell proliferation, migration,
invasion, differentiation and apoptosis (21). Emerging studies have focused on
deregulated miRNAs during fracture healing, which are expected to
promote the development of novel strategies to accelerate healing
by improving osteoblastic viability. For example, Lang et al
(22) provided evidence for the use
of miR-25 to promote fracture healing by enhancing osteoblastic
proliferation and differentiation through activation of the Wnt
signaling pathway. Mi et al (23) demonstrated that decreased
miR-7223-5p during fracture healing regulated osteoblastic
proliferation and apoptosis by targeting PI3KR1, mediating the
promoting effect of circRNA AFF4 on fracture healing. The
expression of miR-203 in patients with fractures was also aberrant
during healing and could inhibit the proliferation and promote the
apoptosis of osteoblast cells by targeting prostate and breast
cancer overexpressed 1(24). These
data indicate the important roles of miRNAs, which have regulatory
effects on osteoblastic viability in fracture healing.
A previous study on differentially expressed miRNAs
in patients with osteoporosis reported that patients with
osteoporosis who develop fractures had significantly decreased
miR-328-3p levels (15), suggesting
that miR-328-3p may be associated with bone regeneration and
progression of osteoporosis. There is some evidence that miR-328-3p
regulates cell proliferation and apoptosis; the decreased
expression of miR-328-3p in patients with osteosarcoma has been
reported to be involved in tumor progression by regulating
osteosarcoma cell proliferation, migration and apoptosis (25). The expression of miR-328-3p in
hepatocellular carcinoma cells was also demonstrated to be reduced
and could decrease tumor cell proliferation and increase apoptosis
(26). However, the relationship
between miR-328-3p and osteoblastic viability has rarely been
reported. In the present study, the serum expression levels of
miR-328-3p was demonstrated to be slightly higher in patients with
fracture before fixation treatment and exhibited no significant
changes 7 days post fixation. However, its expression was markedly
decreased 14 and 21 days after fixation. The abnormal expression
patterns of miR-328-3p suggested its role during fracture healing.
Osteoporosis is the most frequent underlying cause of fragility
fracture (27). A total of 78.8% of
the patients with fracture enrolled in the current study had
osteoporosis, and the majority of female patients were
postmenopausal women, who have high risk of osteoporosis (28). Circulating miR-328-3p has been
identified as a miRNA associated with osteoblast differentiation in
osteoporosis (29), which also
indicated the crucial role of miR-328-3p in osteoporosis
pathogenesis and progression. The present study demonstrated that
the overexpression of miR-328-3p markedly increased osteoblastic
proliferation but decreased cell apoptosis, indicating the
important regulatory effect of miR-328-3p on osteoblastic
viability. Considering the strong demand for enough osteoblasts for
fracture healing, the present study hypothesized that the slight
increase in miR-328-3p at an early time of fracture is required to
obtain a sufficient number of osteoblasts. After the accumulation
of osteoblasts, the expression of miR-328-3p was downregulated, the
proliferation of osteoblasts was decreased and the inhibition of
apoptosis was attenuated. At the later stage of fracture healing,
miR-328-3p expression was significantly reduced, leading to the
osteoblasts proliferating slowly but further differentiating into
mature bone cells and chondrocytes, thereby achieving bone
regeneration. Therefore, the methods to increase miR-328-3p may
reduce the time spent in the early stage of fracture healing by
promoting osteoblastic viability.
According to bioinformatics prediction, PTEN was
identified as a target gene of miR-328-3p, and its interaction with
miR-328-3p was demonstrated using a luciferase reporter assay. PTEN
is a key molecule involved in various cellular processes (30,31).
For example, PTEN acted as a target gene of miR-29a-3p and could
mediate the regulatory effects of miR-29a-39 on vascular smooth
muscle cell proliferation and apoptosis (30). Another example demonstrated that the
regulatory role of methyltransferase-like protein 3 (METTL3)
regulated retinal pigment epithelium cell proliferation, apoptosis
and pyroptosis by inhibiting PTEN (31). In addition, Ge et al
(32) reported that PTEN inhibited
fracture healing by suppressing osteogenic differentiation of
mesenchymal stem cells. Mice with PTEN knockdown in osteoblasts
demonstrated significantly improved fracture healing (33). The present study demonstrated lower
PTEN expression levels at the early stage of fracture, but its
expression was significantly increased 14 days post fixation, which
was negatively correlated with serum miR-328-3p levels in patients
with fracture. The results suggested that the inhibition of PTEN
was required for the rapid proliferation of osteoblasts for
fracture healing. Furthermore, the overexpression of miR-328-3p
inhibited PTEN expression in osteoblasts, and PTEN overexpression
reversed the effect of miR-328-3p on osteoblastic proliferation and
apoptosis, which suggested that miR-328-3p may promote osteoblastic
viability by targeting PTEN.
The PI3K/AKT pathway is a well-known signaling
pathway that is important in regulating cell proliferation, cell
cycle progression and apoptosis, and its beneficial role in
fracture healing has been well reported (34,35).
PTEN acts as a major inhibitor of this signaling pathway, thereby
participating in various cellular processes (36). In the osteoblasts overexpressing
miR-328-3p, the expression of p-AKT was elevated, suggesting that
the overexpression of miR-328-3p promoted the activation of the
PI3K/AKT signaling pathway. Furthermore, elevated p-AKT induced by
miR-328-3p was abolished by the overexpression of PTEN, which
implied that the regulatory effect of miR-328-3p on the PI3K/AKT
signaling may be mediated by PTEN. Collectively, miR-328-3p may
facilitate osteoblastic viability by activating the PI3K/AKT
signaling pathway via PTEN.
The present study may provide novel insights into
the pathogenesis of fracture healing, and the methods to increase
miR-328-3p expression may be used to shorten healing time in
patients with fracture. However, the present study lacks animal
experiments, and further in vivo studies are required.
Another limitation of the present study is that the osteoblastic
viability under the inhibition of miR-328-3p was not assessed.
Thus, future studies should include in vitro functional
experiments using both miR-328-3p mimics and inhibitors. In
addition, fracture healing involves complex biological processes
and although the pesent study demonstrated the regulatory effect of
miR-328-3p on osteoblastic viability, further studies are necessary
to confirm the role of miR-328-3p in fracture healing and the
underlying mechanisms by considering other aspects that are
associated with bone repair, such as osteoblast differentiation,
bone matrix protein production and mineralization. In conclusion,
the present study suggested that the aberrant expression of
miR-328-3p in patients with a fracture may promote fracture healing
by accelerating osteoblastic viability through the PTEN/PI3K/AKT
pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
WX and ZZ contributed to the study design, the data
collection, data interpretation and manuscript preparation. ZW and
YZ contributed to data collection, data analysis and statistical
analysis. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Traditional Chinese Medicine Hospital of Weifang and
written informed consent was obtained from each patient.
Patient consent for publication
Written informed consent for publication was
obtained from each participant.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dreinhofer KE, Mitchell PJ, Bégué T,
Cooper C, Costa ML, Falaschi P, Hertz K, Marsh D, Maggi S, Nana A,
et al: A global call to action to improve the care of people with
fragility fractures. Injury. 49:1393–1397. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Horowitz JA, Puvanesarajah V, Jain A, Raad
M, Gjolaj JP, Shen FH and Hassanzadeh H: Fragility fracture risk in
elderly patients with cervical myelopathy. Spine (Phila Pa 1976).
44:96–102. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Friedman SM and Mendelson DA: Epidemiology
of fragility fractures. Clin Geriatr Med. 30:175–181.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wasserman H, Backeljauw PF, Khoury JC,
Kalkwarf HJ and Gordon CM: Bone fragility in Turner syndrome:
Fracture prevalence and risk factors determined by a national
patient survey. Clin Endocrinol (Oxf). 89:46–55. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Oryan A, Monazzah S and Bigham-Sadegh A:
Bone injury and fracture healing biology. Biomed Environ Sci.
28:57–71. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cheng C and Shoback D: Mechanisms
underlying normal fracture healing and risk factors for delayed
healing. Curr Osteoporos Rep. 17:36–47. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mohr AM and Mott JL: Overview of microRNA
biology. Semin Liver Dis. 35:3–11. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang CZ, Deng F, Li H, Wang DD, Zhang W,
Ding L and Tang JH: MiR-101: A potential therapeutic target of
cancers. Am J Transl Res. 10:3310–3321. 2018.PubMed/NCBI
|
|
9
|
Zhang B, Yi J, Zhang CL, Zhang QH, Xu JF,
Shen HQ and Ge DW: MiR-146a inhibits proliferation and induces
apoptosis in murine osteoblastic MC3T3-E1 by regulating Bcl2. Eur
Rev Med Pharmacol Sci. 21:3754–3762. 2017.PubMed/NCBI
|
|
10
|
Li X, Li K, Yu G, Yu C and Liu C:
miR-342-5p inhibits expression of Bmp7 to regulate proliferation,
differentiation and migration of osteoblasts. Mol Immunol.
114:251–259. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tu XM, Gu YL and Ren GQ: miR-125a-3p
targetedly regulates GIT1 expression to inhibit osteoblastic
proliferation and differentiation. Exp Ther Med. 12:4099–4106.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hu WX, Li H and Jiang JZ: MiR-491-3p is
down-regulated in postmenopausal osteoporosis and affects growth,
differentiation and apoptosis of hFOB1.19 cells through targeting
CTSS. Folia Histochem Cytobiol. 1:9–16. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pan S, Ren F, Li L, Liu D, Li Y, Wang A,
Li W, Dong Y and Guo W: MiR-328-3p inhibits cell proliferation and
metastasis in colorectal cancer by targeting Girdin and inhibiting
the PI3K/Akt signaling pathway. Exp Cell Res.
390(111939)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yi W, Tu MJ, Liu Z, Zhang C, Batra N, Yu
AX and Yu AM: Bioengineered miR-328-3p modulates GLUT1-mediated
glucose uptake and metabolism to exert synergistic
antiproliferative effects with chemotherapeutics. Acta Pharm Sin B.
10:159–170. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Weilner S, Skalicky S, Salzer B, Keider V,
Wagner M, Hildner F, Gabriel C, Dovjak P, Pietschmann P,
Grillari-Voglauer R, et al: Differentially circulating miRNAs after
recent osteoporotic fractures can influence osteogenic
differentiation. Bone. 79:43–51. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Malaney P, Uversky VN and Dave V: PTEN
proteoforms in biology and disease. Cell Mol Life Sci.
74:2783–2794. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li D, Li L, Chen X, Gao Y, Cao Y and Hao
B: LncRNA GHET1 promotes osteoblast proliferation and
differentiation by inhibiting PTEN. Panminerva Med Jul 29, 2019
(Online ahead of print).
|
|
19
|
Xu W, Yang Z, Xie C, Zhu Y, Shu X, Zhang
Z, Li N, Chai N, Zhang S, Wu K, et al: PTEN lipid phosphatase
inactivation links the hippo and PI3K/Akt pathways to induce
gastric tumorigenesis. J Exp Clin Cancer Res.
37(198)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Einhorn TA and Gerstenfeld LC: Fracture
healing: Mechanisms and interventions. Nat Rev Rheumatol. 11:45–54.
2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhao C, Zhou Y, Ran Q, Yao Y, Zhang H, Ju
J, Yang T, Zhang W, Yu X and He S: MicroRNA-381-3p functions as a
dual suppressor of apoptosis and necroptosis and promotes
proliferation of renal cancer cells. Front Cell Dev Biol.
8(290)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lang Y, Sun Q, Zhu LM, Qiu XD, Hu BS, Yang
J and Zhang JD: MiR-25 overexpression promotes fracture healing by
activating the Wnt signaling pathway. Eur Rev Med Pharmacol Sci.
23:7200–7208. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mi B, Xiong Y, Chen L, Yan C, Endo Y, Liu
Y, Liu J, Hu L, Hu Y, Sun Y, et al: CircRNA AFF4 promotes
osteoblast cells proliferation and inhibits apoptosis via the
Mir-7223-5p/PIK3R1 axis. Aging (Albany NY). 11:11988–12001.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang SY, Gao F, Peng CG, Zheng CJ and Wu
MF: Hsa-miR-203 inhibits fracture healing via targeting PBOV1. Eur
Rev Med Pharmacol Sci. 22:5797–5803. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shi J, An G, Guan Y, Wei T, Peng Z, Liang
M and Wang Y: miR-328-3p mediates the anti-tumor effect in
osteosarcoma via directly targeting MMP-16. Cancer Cell Int.
19(104)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li JZ, Li J and Liu BZ: MicroRNA-328-3p
inhibits malignant progression of hepatocellular carcinoma by
regulating MMP-9 level. Eur Rev Med Pharmacol Sci. 23:9331–9340.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Rommens PM, Wagner D and Hofmann A:
Fragility fractures of the pelvis. JBJS Rev.
5:01874474–201703000-00004. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Rossi LMM, Copes RM, Dal Osto LC, Flores
C, Comim FV and Premaor MO: Factors related with osteoporosis
treatment in postmenopausal women. Medicine (Baltimore).
97(e11524)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chen R, Liao X, Chen F, Wang B, Huang J,
Jian G, Huang Z, Yin G, Liu H and Jin D: Circulating microRNAs,
miR-10b-5p, miR-328-3p, miR-100 and let-7, are associated with
osteoblast differentiation in osteoporosis. Int J Clin Exp Pathol.
11:1383–1390. 2018.PubMed/NCBI
|
|
30
|
Zhou Y, Wang M, Zhang J, Xu P and Wang H:
MicroRNA-29a-3p regulates abdominal aortic aneurysm development and
progression via direct interaction with PTEN. J Cell Physiol.
235:9414–9423. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zha X, Xi X, Fan X, Ma M, Zhang Y and Yang
Y: Overexpression of METTL3 attenuates high-glucose induced RPE
cell pyroptosis by regulating miR-25-3p/PTEN/Akt signaling cascade
through DGCR8. Aging (Albany NY). 12:8137–8150. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ge JB, Lin JT, Hong HY, Sun YJ, Li Y and
Zhang CM: MiR-374b promotes osteogenic differentiation of MSCs by
degrading PTEN and promoting fracture healing. Eur Rev Med
Pharmacol Sci. 22:3303–3310. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Burgers TA, Hoffmann MF, Collins CJ,
Zahatnansky J, Alvarado MA, Morris MR, Sietsema DL, Mason JJ, Jones
CB, Ploeg HL and Williams BO: Mice lacking pten in osteoblasts have
improved intramembranous and late endochondral fracture healing.
PLoS One. 8(e63857)2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu Y, Liu J, Xia T, Mi BB, Xiong Y, Hu
LC, Ruan TY, Zhou W and Liu GH: MiR-21 promotes fracture healing by
activating the PI3K/Akt signaling pathway. Eur Rev Med Pharmacol
Sci. 23:2727–2733. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sun Z, Liu F, Cai X, Yu W, Xu L and Yang
B: MiR-126 affects femoral fracture healing in rats through
PI3K/AKT signaling pathway. Panminerva Med Jun 28, 2019.
|
|
36
|
Yao LZ, Zhu YL and Liu JJ: Inhibition of
PTEN gene expression by small interfering RNA on PI3K/Akt/FoxO3a
signaling pathway in human nasopharyngeal carcinoma. Technol Cancer
Res Treat 19: 1533033820917959, 2020.
|