Introduction
Postoperative cognitive dysfunction (POCD) is one of
the most common complications in patients undergoing general
anesthesia (1), whose clinical
manifestations are usually progressive weakening of cognitive
function, decline in self-care ability, increased length of
hospitalization and delayed recovery (2). POCD may also develop postoperative
delirium with a risk of long-term cognitive impairment (3). However, the pathogenesis and effective
treatment for POCD remain unclear (4). Previous studies have shown that the
expression levels of pro-inflammatory cytokines, such as
interleukin (IL)-1β, IL-6 and TNF-α, are increased in patients with
POCD (5-7).
Therefore, systemic inflammation induced by surgery may be the
cause of POCD (8). IL-17A is the
first member of the IL-17 protein family, which can induce the
secretion of pro-inflammatory cytokines to exacerbate inflammatory
response (9). S-100β protein is
secreted by astrocytes in the central nervous system (CNS)
(10). The high concentration of
S-100β in the serum suggests the damage of the brain and therefore
can be used as a biomarker to assess the severity of brain damage
(11).
Dexmedetomidine is a highly selective α-2 adrenalin
receptor with dose-dependent sedative and hypnotic effects
(12). Dexmedetomidine binds to the
nicotinic acetylcholine receptor 7 subunit to promote the release
of acetylcholine, thereby suppressing the secretion of inflammatory
cytokines (13,14). Dexmedetomidine has been reported to
exert neuroprotective effects in early POCD animal models (2), thereby preventing POCD (15). It has been shown (16) that dexmedetomidine could improve the
neuronal apoptosis caused by isoflurane in neonatal rats. In
addition, Etomidate is a type of drug used for general anesthesia
and sedation (17), which has the
advantages of quick effect, short acting time and little effect on
circulation. According to Liu et al (18), etomidate inhibited the production of
pro-inflammatory cytokines in rat macrophages.
In the present study, a POCD rat model was
constructed by partial hepatectomy in aged rats to observe the
effect of dexmedetomidine combined with etomidate on the expression
levels of IL-17A and S-100β, and evaluate the therapeutic effect of
dexmedetomidine combined with etomidate, in order to provide future
reference for clinical application.
Materials and methods
Main reagents and animals
Dexmedetomidine hydrochloride injection was
purchased from Sichuan Guorui Pharmaceutical Co., Ltd. (approval
no. H20110097; 2 ml/0.2 mg). Etomidate fat emulsion injection was
purchased from Jiangsu Nhwa Pharmaceutical Co., Ltd. (approval no.
H20020511; 10 ml/20 mg). Rat IL-17A (ELISA) kit was purchased from
Abcam (item no. ab214028). Rat S-100β protein (ELISA) kit was
purchased from Abcam (item no. ab234573). TNF-α, IL-6 and IL-1β
(ELISA) kits were all purchased from Abcam (items no. ab236712,
ab234570, ab255730). Rat tissue protein extract was purchased from
Best Biotechnology Co., Ltd. (batch no. BB18011). BCA protein assay
kit was purchased from Beyotime Institute of Biotechnology (batch
no. P0012). NF-κB Pathway Sampler kit, goat anti-mouse IgG (H+L)
and β-actin were purchased from Shanghai Abcam. Morris water maze
system was purchased from Beijing Zhongshi Dichuang Science and
Technology Development Co., Ltd.
A total of 50 healthy male SD rats, 19-23 months of
age and weighing 510-695 g, were acquired from Hunan Silaike Jingda
Experimental Animal Co., Ltd. The study was approved by the Ethics
Committee of Jiangxi Provincial People's Hospital Affiliated to
Nanchang University (Nanchang, China) and all procedures were
carried out in strict accordance to the Guidelines of the Nursing
and Use of Laboratory Animals (published by the National Institutes
of Health and revised in 1996; no. 85-23) (19). It was confirmed that there was no
obvious abnormal behavior in all the rats enrolled and the rats
were randomly allocated to the control group, model group,
etomidate group (Eto group), dexmedetomidine group (Dex group) and
dexmedetomidine combined with etomidate group (Dex-Eto group), with
10 rats in each group.
POCD model construction
All rats were maintained at 22-23˚C, with a 12-h
light/12-h dark cycle. All rats were fasted overnight before
surgery with free access to water. After anesthesia with 2%
sevoflurane, the rats in the five groups were ventilated and
intubated with 1.5-2% sevoflurane continuously. Dose conversion
calculations between laboratory animals and humans were based on
the FDA recommendation to use surface area standardization
(20). Since the surface area per
unit body area of rats is ~6 times that of a human body, the
commonly used regimen for normal clinical dexmedetomidine sedation
is load dose 1.0 g/kg (for ≥10 min), and thus, the rats were given
6.0 µg/kg (for ≥10 min); etomidate induced dose is 0.2 mg/kg, so
the rats were given 1.2 mg/kg. The rats of the model group received
no intravenous anesthesia, except general anesthesia with
intubation, and no treatment was given in the control group. In the
Dex group, 6 µg/kg dexmedetomidine were injected intravenously for
10 min. In the Eto group, 1.2 mg/kg etomidate were injected
intravenously for 10 min. In the Dex-Eto group, 6 µg/kg
dexmedetomidine with 1.2 mg/kg etomidate were injected
intravenously for 10 min. Except for the control group, partial
lobectomy was performed after 30 min in the rest of the four groups
in order to construct the rat POCD model. The procedures were as
follows: the rats were placed on a sterile pad. After sterilizing
and removing part of the hair, the rats were subjected to
longitudinal incision along the lower edge of the xiphoid process.
The left lobe of the liver was separated, and part of the left lobe
of the liver was removed. The incision was then soaked with 2%
lidocaine and then sutured with thread so that the surgical
procedure should be aseptically controlled. All these procedures
should be completed within 30 min. Ventilation and appropriate
temperature should be maintained at the end of the operation. After
the operation, the rats were placed in the previous feeding room
and fed separately, and anti-infection measures were taken.
Morris water maze test
Cognitive evaluation was performed using Morris
water maze system 24 h after anesthesia. Morris water maze was a
cylindrical pool, which was separated into four quadrants, all
containing water. A quadrant platform was selected randomly.
Place navigation test: one day before the
experiment, the aged rats became familiar with the environment in
Morris system, so that they could learn to swim for a platform
within 120 sec. The rats that did not find the platform in 120 sec
were guided to the platform to stay for 30 sec. Only rats that
could swim were included in this experiment. Morris water maze test
was carried out 24 h after anesthesia. The aged rats were randomly
placed into water with their backs to the wall of the pool. The
time spent in finding a fixed platform is called escape latency,
and the escape latency and swimming distance of the rats were
recorded. If the platform was not found within 2 min, it was
recorded as 120 sec.
Spatial probe test: the platform was removed from
the water and the rats were placed in the water from any point in
the corresponding quadrant of the platform area. The target
quadrant residence time and the number of times of accurately
crossing the platform within 2 min were recorded. The poorer the
cognitive function, the longer the escape latency and the swimming
distance, the less target quadrant residence time and times of
crossing the platform.
ELISA detection
At the end of T3, tail vein blood samples
(1 ml) of the rats in all five groups were collected and placed
into anticoagulant tubes. The samples were centrifuged at 4˚C at
3x103 rpm for 30 min to collect the supernatant. The
expression levels of IL-17A, S-100β, TNF-α, IL-6 and IL-1β in the
supernatant were detected by the corresponding kit. All operations
were strictly carried out according to the manufacturer's
instructions of the respective kit.
Detection of NF-κB p65 protein
expression by western blot analysis
At the end of T3, pentobarbital sodium (150 mg/kg
body weight) was injected intraperitoneally until breathing and
heartbeat stopped. The right hippocampus tissues (20 mg) were
isolated from the rats, mixed with 100-200 µl lysates and
homogenized in a glass homogenator at 4˚C for 15 min,
1.2x104 rpm. The supernatant was taken. We used the BCA
kit (Abcam) to detect protein concentration. SDS-PAGE
electrophoresis was used to distinguish the proteins before
transferring to the nitrocellulose membrane, and then the proteins
were blocked at room temperature for 1 h with 5% PBS solution.
Next, NF-κB p65 (1:1,000) was added and maintained overnight at
4˚C. Membranes were washed with PBS solution. This operation was
repeated 3 times before a secondary antibody (HRP cross-linking,
1:10,000) was added, and the mixture was allowed to stand at room
temperature for 1 h. The membrane was finally washed with PBS
solution and protein bands were visualized using an
electrochemiluminescent substrate kit (cat. no. ab133406; Abcam).
The internal reference protein was β-actin. The relative protein
expression of NF-κB p65 was calculated: Relative expression level
of protein=(gray value of protein band)/(gray value of β-actin
band). We used ImageJ (National Institutes of Health) to measure
the gray value.
Statistical analysis
The experimental data were processed by SPSS 20.0
software package (AsiaAnalytics; formerly SPSS China) for
statistical analysis. The measurement data were expressed as the
mean ± SD and one-way analysis of variance with Tukey's HSD post
hoc test were used for their comparison. Pearson's correlation
analysis was used to study the correlation of IL-17A and S-100β
with NF-κB p65, TNF-α, IL-6 and IL-1β. The confidence interval was
95%. P<0.05 was considered to indicate a statistically
significant difference.
Results
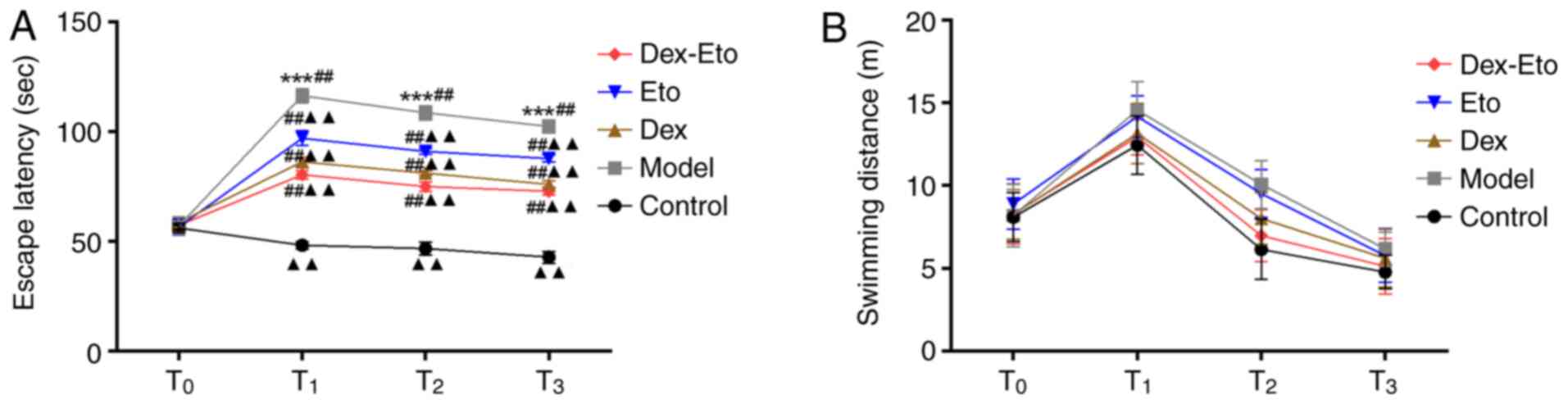
Place navigation test
In order to investigate the effect of
dexmedetomidine combined with etomidate on the cognitive function
in rats with POCD, the place navigation test was used to assess the
cognitive ability of the rats by recording the escape latency and
swimming distance (Fig. 1). The
shorter the escape latency or the longer the swimming distance, the
stronger the cognitive function.
In the model group, the escape latency in the other
four groups increased at T1, T2 and
T3 compared with that at T0, and the
difference was statistically significant (P<0.001). Compared
with the control group, the escape latency in the model group
increased during the same period, with statistical significance
(P<0.01). Compared with the model group, the escape latency was
reduced in the Dex, Eto and Dex-Eto groups during the same period,
among which the Dex-Eto group presented the shortest time, and the
difference was statistically significant (P<0.01) (Fig. 1A).
Compared with the control group, the swimming
distance in the model group increased during the same period, but
the difference was not statistically significant. Compared with the
model group, there was no significant difference in the swimming
distance in the Dex, Eto and Dex-Eto groups, but the Dex-Eto group
presented the shortest swimming distance (Fig. 1B).
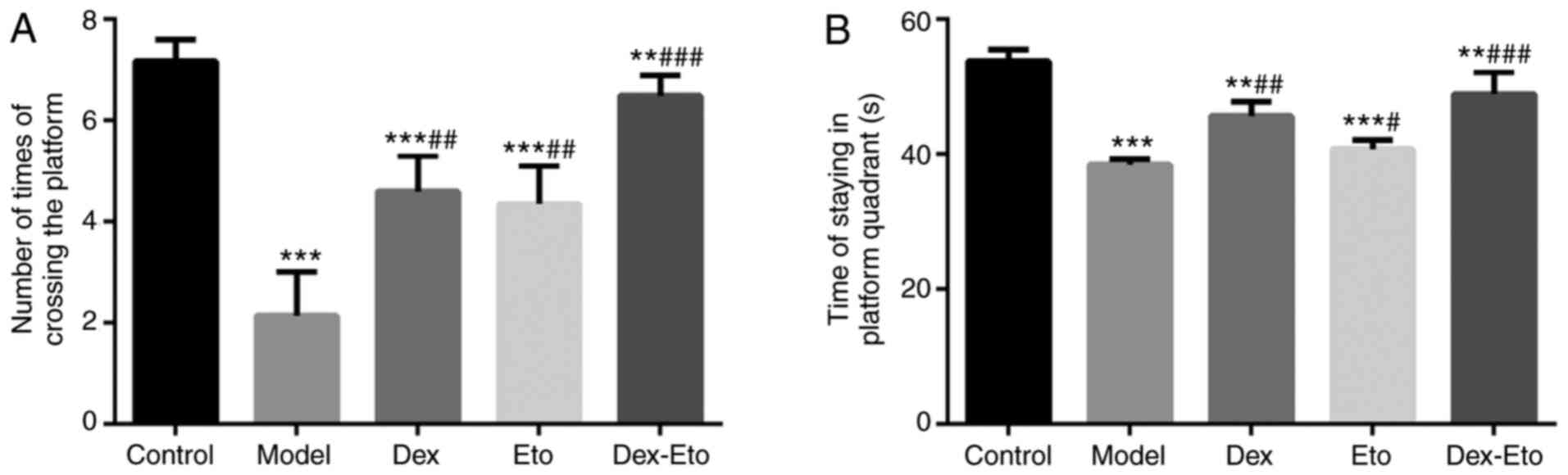
Spatial probe test
Spatial exploration experiments were performed to
evaluate the cognitive function of the rats by recording the times
the rats crossed the platform and the time of stay at the target
quadrant (Fig. 2). The more times
the rats crossed the platform or the longer the target quadrant
stays, the stronger the cognitive function of the rats.
Compared with the control group, the number of times
of crossing the platform and the target quadrant residence time in
the model group decreased, and the differences were statistically
significant (P<0.001). Compared with the model group, the number
of times of crossing the platform and the target quadrant residence
time increased in the Dex, Eto and Dex-Eto groups during the same
period, among which the Dex-Eto group presented the highest
frequency of crossing the platform and the longest residence time
in the target quadrant, and the differences were statistically
significant (P<0.05) (Fig.
2).
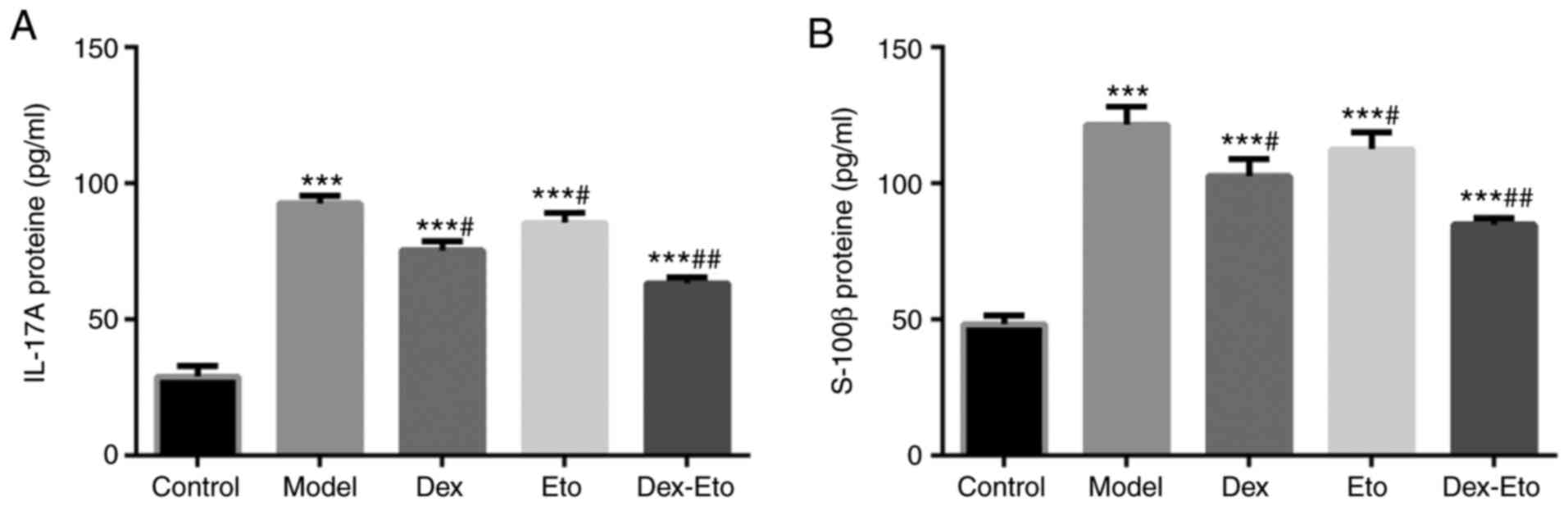
Expression levels of IL-17A and
S-100β
Since IL-17A and S-100β undergo significant changes
during the POCD process, the levels of IL-17A and S-100β in serum
and brain tissue of rats were detected. The higher the IL-17A, the
more obvious the inflammatory response in rats. The higher the
S-100β, the more severe the brain damage in rats.
Compared with the control group, the expression
levels of IL-17A and S-100β in the model group increased
significantly (P<0.001). Compared with the model group, the
expression levels of IL-17A and S-100β decreased in the Dex, Eto
and Dex-Eto groups, among which the IL-17A and S-100β expression
levels in the Dex-Eto group were the lowest, and the differences
were statistically significant (P<0.05) (Fig. 3).
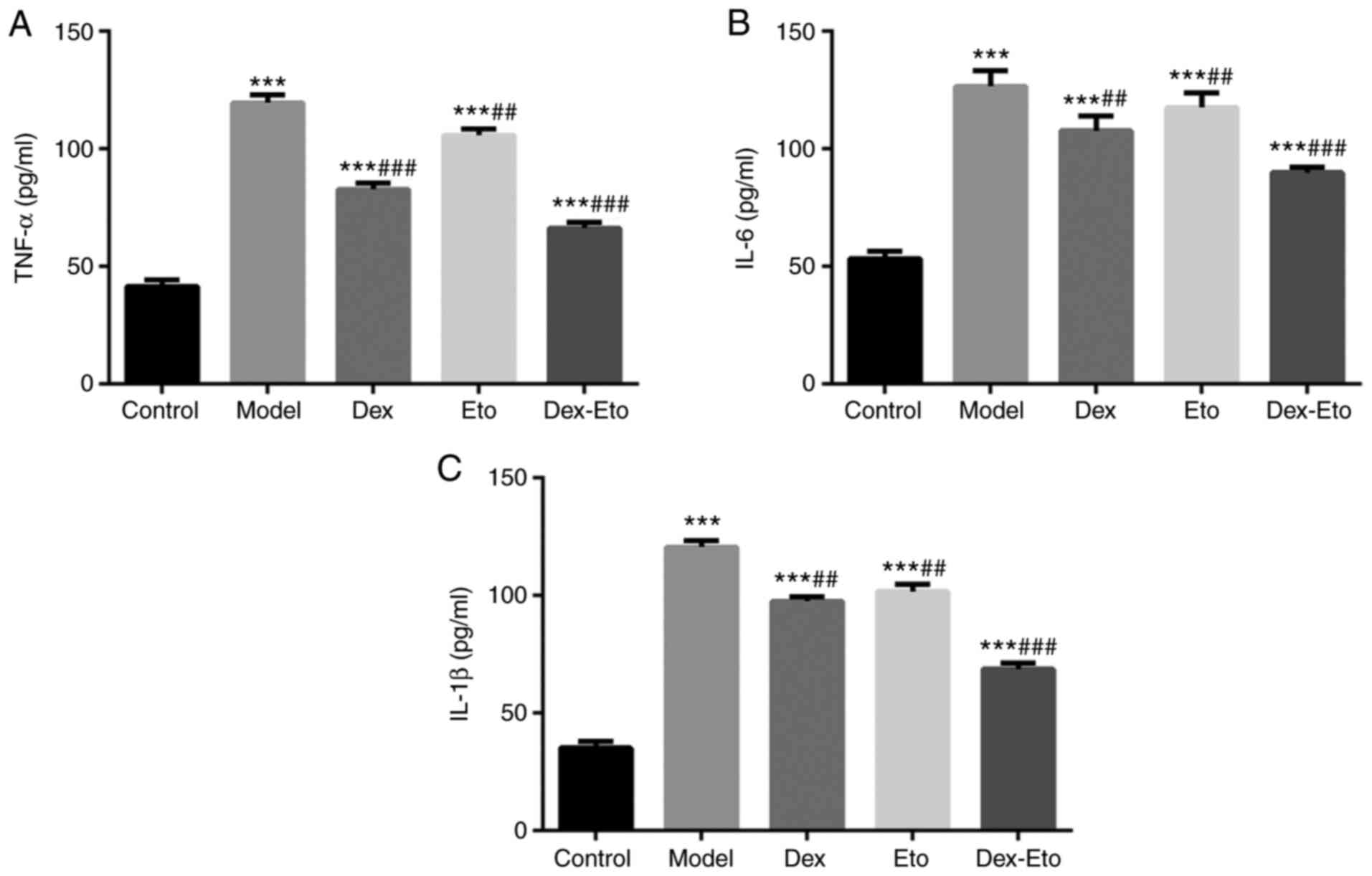
Expression levels of TNF-α, IL-6 and
IL-1β
In addition to assessing the inflammatory response
using IL-17A levels, the three common pro-inflammatory cytokines
TNF-α, IL-6 and IL-1β were also detected.
The expression levels of TNF-α, IL-6 and IL-1β
increased in the model group compared with those in the control
group, with statistically significant differences (P<0.001).
Compared with the model group, TNF-α, IL-6 and IL-1β expression
levels decreased in the Dex, Eto and Dex-Eto groups, of which the
expression levels in the Dex-Eto group were the lowest, and the
differences were statistically significant (P<0.01) (Fig. 4).
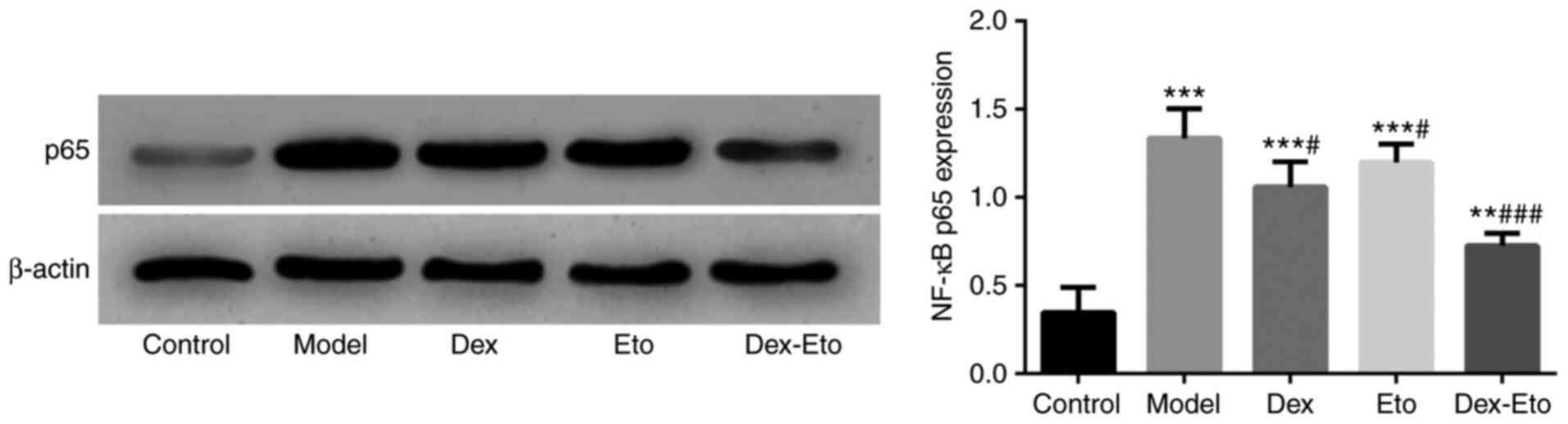
Expression of NF-κB p65
The NF-κB pathway is closely related to the
inflammatory response, so the detection of the expression of NF-κB
pathway-associated protein p65 is also helpful in understanding the
mechanism by which dexmedetomidine combined with etomidate affects
the inflammatory response.
Compared with the control group, the expression of
NF-κB p65 in the model group significantly increased (P<0.001).
Compared with the model group, the expression of NF-κB p65 in the
Dex, Eto and Dex-Eto groups significantly decreased during the same
period, with the lowest expression in the Dex-Eto group (P<0.01)
(Fig. 5).
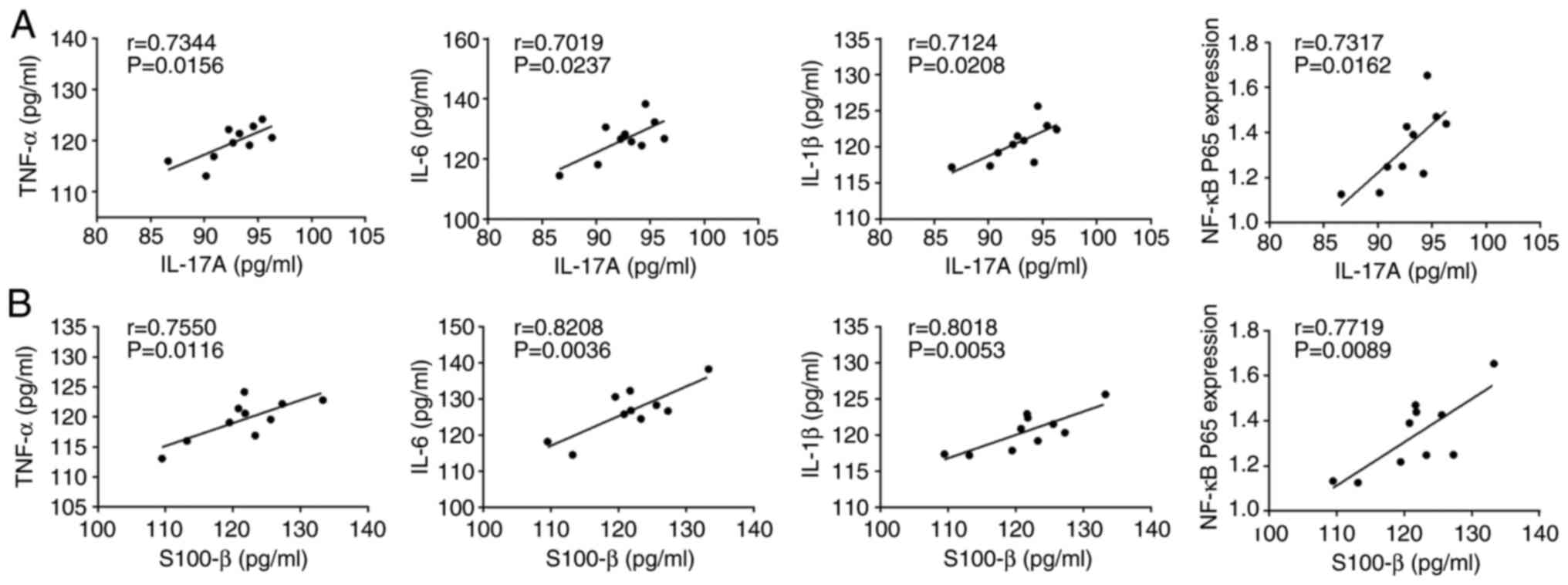
Correlation of IL-17A and S-100β
expression levels with NF-κB p65, TNF-α, IL-6 and IL-1β
Since IL-17A, S-100β, NF-κB p65, TNF-α, IL-6 and
IL-1β all changed during POCD, the correlation of IL-17A and S-100β
with p65, TNF-α, IL-6 and IL-1β in the model group was analyzed.
The results revealed that IL-17A and S-100β were positively
correlated with p65, TNF-α, IL-6 and IL-1β (Fig. 6), suggesting that a decrease in
IL-17A and S-100β may cause decrease in p65, TNF-α, IL-6 and IL-1β
expression levels.
Discussion
POCD is one of the most common postoperative
complications in elderly patients (21). Tissue damage during surgery
stimulates the peripheral immune system activation and causes
cytokine cascade and release of inflammatory mediators (22). IL-17 protein family coordinates
local tissue inflammation by inducing the release of
pro-inflammatory cytokines and neutrophil-mobilizing cytokines
(23). S-100β plays an important
role in the normal development and injury recovery of CNS (24). For patients with brain injury caused
by symptoms, the concentration of S-100β protein in blood and
cerebrospinal fluid increases (25). Mercier et al (11) have suggested that S-100β could be
used to assess the degree of brain injury and to determine the
long-term prognosis in patients with moderate and severe traumatic
brain injury. In the present study, dexmedetomidine combined with
etomidate was administered to aged rats with POCD and the
therapeutic effect of dexmedetomidine combined with etomidate was
evaluated by the expression levels of IL-17A and S-100β in aged
rats.
Morris water maze test revealed that the cognitive
function of aged rats with POCD was impaired, while restored to
some extent after dexmedetomidine treatment. Xiong et al
(26) studied the effect of
dexmedetomidine on POCD aged rats and showed that the rats treated
with dexmedetomidine presented a certain degree of cognitive
recovery in water maze test, which was consistent with the findings
presented in this study. It is worth mentioning that the present
study also explored the performance of dexmedetomidine alone and
dexmedetomidine combined with etomidate using water maze test, and
the results demonstrated that the combination of dexmedetomidine
and etomidate has a better therapeutic effect. This may be due to
the fact that both dexmedetomidine and etomidate can protect the
brain to some extent (27,28).
In addition, the test results revealed that the
expression levels of TNF-α, IL-6 and IL-1β were increased in aged
rats with POCD, whereas decreased after drug treatment. The lowest
expression levels in Dex-Eto group indicated that compared with the
simple treatment with dexmedetomidine alone, dexmedetomidine
combined with etomidate had more significant inhibition on
inflammatory cytokines. In the study of Wang et al (4), dexmedetomidine was reported to be able
to reduce TNF-α, IL-6, IL-1β and other expression levels, and thus
reduce inflammation; whereas Liu et al (18) reported that etomidate could inhibit
the production of pro-inflammatory cytokines in rat macrophages, so
dexmedetomidine and etomidate may play a joint role in
downregulating TNF-α, IL-6 and IL-1β without eliminating each
other.
Furthermore, as IL-17A may be involved in the
process of brain injury (29) and
S-100β can be used to evaluate the degree of brain injury (11), the expression levels of IL-17A and
S-100β in each group were compared in the present study. The
results revealed that the expression levels of IL-17A and S-100β in
aged rats with POCD increased, but decreased after drug treatment.
The IL-17A and S-100β expression levels in the Dex-Eto group were
the lowest, indicating that the inhibition effect of
dexmedetomidine combined with etomidate on IL-17A and S-100β was
the most obvious. Yang et al (29) reported that anti-IL-17A treatment
could improve neuroinflammation and oxidative stress, thereby
relieving cognitive dysfunction in aged rats, and it was also
believed that this mechanism was involved in the reduction of NF-κB
pathway. On this basis, the expression of NF-κB p65 in the
hippocampus of each group was studied, and the results indicated
that the expression of NF-κB p65 was increased in the hippocampus
of aged rats with POCD in Morris water maze test, but decreased
after drug treatment, with that of the Dex-Eto group being the
lowest. This may be due to the fact that dexmedetomidine and
etomidate co-inhibit NF-κB in order to alleviate inflammation
(18,30), with no elimination effect on each
other. In addition, by analyzing the correlation of the above
factors, it was shown that the decrease of IL-17A and S-100β may
cause the decrease of NF-κB p65, TNF-α, IL-6 and IL-1β. Therefore,
the treatment of IL-17A and S-100β with dexmedetomidine combined
with etomidate can alleviate the condition of rats with POCD.
In the present study, the combined treatment with
dexmedetomidine and etomidate in aged rats with POCD was
investigated, and its effects on IL-17A and S-100β were explored.
However, the test results concerning the expression levels of
IL-17A and S-100β were only collected at the end of T3,
without the reference results of T1 and T2.
Thus, the dynamic effects of dexmedetomidine combined with
etomidate on IL-17A and S-100β remain to be further investigated.
The purpose of this study was to explore the changes in IL-17A and
S-100β. Therefore, only the changes of NF-κB were detected by
western blot analysis, and the immunofluorescence analysis of NF-κB
was not considered. This is the limitation of the present study.
Since dexmedetomidine and etomidate can be used to regulate the
expression of p65, NF-κB will be systematically analyzed in our
future studies. The effect of NF-κB nuclear translocation will be
the aim of our future experiments.
In conclusion, compared with the simple treatment
with dexmedetomidine or etomidate, dexmedetomidine combined with
etomidate has a better therapeutic effect on aged rats with POCD,
which can effectively improve cognitive dysfunction and alleviate
stress inflammation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XY conceived, designed the study and drafted the
manuscript. XY and YX collected, analyzed and interpreted the
experimental data and revised the manuscript critically for
important intellectual content. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Jiangxi Provincial People's Hospital Affiliated to Nanchang
University (Nanchang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhou C, Zhu Y, Liu Z and Ruan L: Effect of
dexmedetomidine on postoperative cognitive dysfunction in elderly
patients after general anaesthesia: A meta-analysis. J Int Med Res.
44:1182–1190. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Qian XL, Zhang W, Liu MZ, Zhou YB, Zhang
JM, Han L, Peng YM, Jiang JH and Wang QD: Dexmedetomidine improves
early postoperative cognitive dysfunction in aged mice. Eur J
Pharmacol. 746:206–212. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sprung J, Roberts RO, Weingarten TN, Nunes
Cavalcante A, Knopman DS, Petersen RC, Hanson AC, Schroeder DR and
Warner DO: Postoperative delirium in elderly patients is associated
with subsequent cognitive impairment. Br J Anaesth. 119:316–323.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang WX, Wu Q, Liang SS, Zhang XK, Hu Q,
Chen QH, Huang HJ, Xu L and Lou FQ: Dexmedetomidine promotes the
recovery of neurogenesis in aged mouse with postoperative cognitive
dysfunction. Neurosci Lett. 677:110–116. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cibelli M, Fidalgo AR, Terrando N, Ma D,
Monaco C, Feldmann M, Takata M, Lever IJ, Nanchahal J, Fanselow MS
and Maze M: Role of interleukin-1beta in postoperative cognitive
dysfunction. Ann Neurol. 68:360–368. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wright CB, Sacco RL, Rundek T, Delman J,
Rabbani L and Elkind M: Interleukin-6 is associated with cognitive
function: The northern manhattan study. J Stroke Cerebrovasc Dis.
15:34–38. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Terrando N, Monaco C, Ma D, Foxwell BMJ,
Feldmann M and Maze M: Tumor necrosis factor-alpha triggers a
cytokine cascade yielding postoperative cognitive decline. Proc
Natl Acad Sci USA. 107:20518–20522. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Alam A, Hana Z, Jin Z, Suen KC and Ma D:
Surgery, neuroinflammation and cognitive impairment. EBioMedicine.
37:547–556. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ma X, Reynolds SL, Baker BJ, Li X,
Benveniste EN and Qin H: IL-17 enhancement of the IL-6 signaling
cascade in astrocytes. J Immunol. 184:4898–4906. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zuo Y, Zhao L, Zeng M, Yang Q, Chen X and
Yang T: The effects of vitamin-rich carbohydrate pretreatment on
the surgical stress response and S-100β after splenectomy in
elderly rats. BMC Anesthesiol. 19(77)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mercier E, Boutin A, Lauzier F, Fergusson
DA, Simard JF, Zarychanski R, Moore L, McIntyre LA, Archambault P,
Lamontagne F, et al: Predictive value of S-100β protein for
prognosis in patients with moderate and severe traumatic brain
injury: Systematic review and meta-analysis. BMJ.
346(f1757)2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li Y, He R, Chen S and Qu Y: Effect of
dexmedetomidine on early postoperative cognitive dysfunction and
peri-operative inflammation in elderly patients undergoing
laparoscopic cholecystectomy. Exp Ther Med. 10:1635–1642.
2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xiang H, Hu B, Li Z and Li J:
Dexmedetomidine controls systemic cytokine levels through the
cholinergic anti-inflammatory pathway. Inflammation. 37:1763–1770.
2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhu YJ, Peng K, Meng XW and Ji FH:
Attenuation of neuroinflammation by dexmedetomidine is associated
with activation of a cholinergic anti-inflammatory pathway in a rat
tibial fracture model. Brain Res. 1644:1–8. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Monk TG and Price CC: Postoperative
cognitive disorders. Curr Opin Crit Care. 17:376–381.
2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sanders RD, Xu J, Shu Y, Januszewski A,
Halder S, Fidalgo A, Sun P, Hossain M, Ma D and Maze M:
Dexmedetomidine attenuates isoflurane-induced neurocognitive
impairment in neonatal rats. Anesthesiology. 110:1077–1085.
2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li X, Lu F, Li W, Xu J, Sun XJ, Qin LZ,
Zhang QL, Yao Y, Yu QK and Liang XL: Underlying mechanisms of
memory deficits induced by etomidate anesthesia in aged rat model:
Critical role of immediate early genes. Chin Med J (Engl).
129:48–53. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu M, Zhang Y, Xiong JY, Wang Y and Lv S:
Etomidate mitigates lipopolysaccharide-induced CD14 and TREM-1
expression, NF-κB activation, and pro-inflammatory cytokine
production in rat macrophages. Inflammation. 39:327–335.
2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Clark JD, Gebhart GF, Gonder JC, Keeling
ME and Kohn DF: Special report: The 1996 guide for the care and use
of laboratory animals. ILAR J. 38:41–48. 1997.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kishikawa H, Kobayashi K, Takemori K,
Okabe T, Ito K and Sakamoto A: The effects of dexmedetomidine on
human neutrophil apoptosis. Biomed Res. 29:189–194. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yu H, Dong R, Lu Y, Yang X, Chen C, Zhang
Z and Peng M: Short-term postoperative cognitive dysfunction and
inflammatory response in patients undergoing cytoreductive surgery
and hyperthermic intraperitoneal chemotherapy: A pilot study.
Mediators Inflamm. 2017(3605350)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Giannoudis PV, Dinopoulos H, Chalidis B
and Hall GM: Surgical stress response. Injury. 37 (Suppl 5):S3–S9.
2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kolls JK and Lindén A: Interleukin-17
family members and inflammation. Immunity. 21:467–476.
2004.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Schaf DV, Tort AB, Fricke D, Schestatsky
P, Portela LVC, Souza DO and Rieder CRM: S100B and NSE serum levels
in patients with Parkinson's disease. Parkinsonism Relat Disord.
11:39–43. 2005.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yardan T, Cevik Y, Donderici O, Kavalci C,
Yilmaz FM, Yilmaz G, Vural K, Yuzbasioglu Y, Gunaydin YK and Sezer
AA: Elevated serum S100B protein and neuron-specific enolase levels
in carbon monoxide poisonin. Am J Emerg Med. 27:838–842.
2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Xiong B, Shi Q and Fang H: Dexmedetomidine
alleviates postoperative cognitive dysfunction by inhibiting neuron
excitation in aged rats. Am J Transl Res. 8:70–80. 2016.PubMed/NCBI
|
|
27
|
Chen J, Yan J and Han X: Dexmedetomidine
may benefit cognitive function after laparoscopic cholecystectomy
in elderly patients. Exp Ther Med. 5:489–494. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Baughman VL, Hoffman WE, Miletich DJ and
Albrecht RF: Cerebral metabolic depression and brain protection
produced by midazolam and etomidate in the rat. J Neurosurg
Anesthesiol. 1:22–28. 1989.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yang Z and Yuan C: IL-17A promotes the
neuroinflammation and cognitive function in sevoflurane
anesthetized aged rats via activation of NF-κB signaling pathway.
BMC Anesthesiol. 18(147)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tan J, Shen J, Gong Y and Zhu H:
Dexmedetomidine ameliorates lipopolysaccharide-induced endothelial
barrier disruption and inflammation by inhibiting NF-κB activity
and activating α2-adrenergic receptor. Int J Clin Exp Med.
10:532–539. 2017.
|