Introduction
Pre-eclampsia (PE), a disorder peculiar to
pregnancy, is one of the main causes of health problems in pregnant
women and fetuses worldwide, featured with hypertension and
proteinuria after 20 weeks of pregnancy (1,2). PE is
divided into early-onset PE (before 34 weeks) and late-onset PE
(after 34 weeks). The two subtypes are caused by different factors
and are accompanied by different complications. Early-onset PE
accounts for 5-20% of all PE cases (3). Early-onset severe pre-eclampsia
(ES-PE), characterized as a serious condition with a sudden onset,
is the leading factor responsible for poor prognosis in pregnant
women and perinatal children, manifested as convulsion and coma
(4).
At present, the pathogenesis of ES-PE is not clear.
The expression of complement system's activator factors in the
maternal circulation of ES-PE pregnant women is higher than that of
healthy pregnant women (5,6). A previous in vitro study has
shown that activation of the complement system can mediate the
abnormal expression of angiogenic factors, which are related to the
pathogenesis of PE (7). Soluble
Fms-like tyrosine kinase-1 (sFlT-1) and placental growth factor
(PlGF) are major anti-angiogenic factors (8). Previous studies have shown that
increase of sFlT-1 levels and decrease of PlGF levels can break the
balance of placental angiogenesis, resulting in insufficient
invasion of trophoblast cells to the endometrium, ischemia and
hypoxia in the placenta, eventually leading to PE (9,10).
Thus, sFlT-1 and PlGF may play important roles in the pathogenesis
of ES-PE (11). Clinically, ES-PE
is mainly treated with decompression, spasmolysis and sedation
(12). Magnesium sulfate has a long
history of application in obstetrics. Magnesium sulfate is the
first-line treatment of PE patients and is the preferred drug
treatment for ES-PE (13). However,
despite the wide application of magnesium sulfate as a tocolytic,
and its ability to improve placental function, magnesium sulfate is
not ideal for treatment because of the increase of the blood
pressure caused after drug withdrawal (14). Labetalol is a β blocker that slows
sinus rhythm, reduces blood pressure and peripheral vascular
resistance, and is mainly used in the treatment of hypertension
(15). The use of magnesium sulfate
and labetalol in PE has been explored by a number of studies
(16-18);
however, little is known about the efficacy of their combination in
ES-PE treatment and their combined effect on sFlt-1/PlGF ratio.
In the present study, magnesium sulfate was combined
with labetalol in the treatment of ES-PE patients to explore the
efficacy of this regimen and its effect on sFlt-1/PlGF ratio.
Patients and methods
General information
A total of 164 ES-PE patients admitted to the
Maternity and Child Health Care Hospital of Hubei (Wuhan, China)
from April 2014 to January 2016 were assigned to this observational
study. Among them, 83 patients were enrolled in group A and treated
with magnesium sulfate combined with labetalol hydrochloride, and
81 patients were enrolled in group B and treated with magnesium
sulfate. Patients in group A were 22-37 years of age, with an
average age of 27.6±4.3 years, and the patients' gestational age
was 24-34 weeks, with an average of 28.7±2.6 weeks. In group B,
patients were 22-35 years of age, with an average age of 27.2±5.5
years, and the patients' gestational age was 23-35 weeks, with an
average of 28.3±2.4 weeks. The study was approved by the Ethics
Committee of the Maternity and Child Health Care Hospital of Hubei.
Patients who participated in this research had complete clinical
data. All research subjects had full knowledge of the study and
provided signed written informed consents.
Inclusion and exclusion criteria
The study followed the ‘Strengthening the Reporting
of Observational Studies in Epidemiology’ (STROBE) guidelines.
Inclusion criteria: Patients diagnosed with ES-PE according to the
diagnostic criteria issued by the International Society for the
Study of Hypertension in Pregnancy (ISSHP) (19); patients with varying degrees of
abdominal pain, dyspnea, headache, urinary protein excretion, and
edema; patients with a systolic blood pressure (SBP) of ~160 mmHg
and a diastolic blood pressure (DBP) of ~110 mmHg; patients with
complete clinical data. Exclusion criteria: Patients previously
treated with other antihypertensive drugs; patients with
contraindications to the drugs of the study; patients with chronic
hypertension before pregnancy; patients with liver and kidney
dysfunction, autoimmune diseases, connective tissue diseases,
diabetes, malignant tumors, cholestasis during pregnancy,
hematological diseases, abnormal blood coagulation, cognitive
dysfunction, or mental illness.
Treatment methods
After admission, the two groups of patients were
ordered to rest in bed and received supplementary oxygen,
anticonvulsant treatment, diuretic therapy, and fetal lung
maturation-promoting therapy. In group B, patients underwent an
intravenous infusion of 15 ml of 25% magnesium sulfate (CFDA
approval no. H12020994; Tianjin Kingyork Pharmaceuticals Co., Ltd.)
mixed with 20 ml of 10% glucose, followed by an intravenous drip of
60 ml of 25% magnesium sulfate and 1,000 ml of 5% glucose at a 1
g/h, once a day. In group A, in addition to the drugs used in group
B, an intravenous drip of 1,000 mg of labetalol hydrochloride (CFDA
approval no. H20052264; Hainan Lionco Pharmaceutical Co., Ltd.) and
5% glucose was administered at 1-4 mg/min, once a day. Both groups
were treated for 7 days.
Outcome measures and efficacy
evaluation
Meditech ABPM-05 dynamic blood pressure measuring
device (Shanghai Chuangxin Medical Instruments Co., Ltd.) was used
to measure the SBP and DBP in the two groups of patients, before
and after treatment. Mindray BS-820 automatic biochemical analyzer
(Shenzhen Mindray Bio-Medical Electronics Co., Ltd.) was used to
quantify the 24 h urine protein (24hUP) and 24 h urine volume
(24hUV). The detection process was carried out in strict accordance
with the manufacturer's instructions.
The clinical efficacy in the two groups was
evaluated according to the clinical symptoms, SBP and DBP. Marked
response: Clinical symptoms, such as headache, edema and
proteinuria, had almost disappeared, DBP was <90 mmHg and SBP
was <140 mmHg. Moderate response: Clinical symptoms, such as
headache, edema, and proteinuria, were alleviated, DBP was 90-110
mmHg and SBP was 140-160 mmHg. No response: Clinical symptoms and
blood pressure were not notably improved or even aggravated.
Treatment efficacy rate=(marked response + moderate
response)/(total no. of cases) x100%.
Detection method
Blood samples of 3 ml of elbow venous blood were
collected from all patients, before and after treatment, and were
placed in a coagulation tube. The samples were centrifugalized at
1,500 x g at 4℃ for 10 min by a DT5-3 low-speed tabletop centrifuge
(Beijing Era Beili Centrifuge Co., Ltd.). The upper supernatant was
collected for further experimentation. Serum sFlt-1 and PlGF
concentrations were measured, before and after treatment, by
enzyme-linked immunosorbent assay (ELISA) (20), in strict accordance with the
manufacturer's instructions of human sFlt-1 and PlGF ELISA kits
(ml038106, ml024102; Shanghai Enzyme-linked Biotechnology Co.,
Ltd.). The blank wells (without enzyme reagents and samples) and
the sample wells were set up. A total of 40 µl of sample dilution
and 10 µl of the sample (dilution ratio was 5 times) were added
into the sample well and sealed for incubation at 37˚C for 30 min.
The liquid in each well was then discarded and the well was dried
and washed 5 times. Enzyme-labeling reagent (50 µl) was added into
the sample well and incubated at 37˚C for 30 min. The liquid in
each well was discarded and the well was dried and washed 5 times.
Next, 50 µl of developer A and developer B were added into the well
and incubated at 37˚C for 15 min in the dark for color development.
Finally, 50 µl of stop solution were added into each well, and the
blue liquid turned yellow. The blank wells were adjusted to zero
value. The optical density of each well was detected at a
wavelength of 450 nm using a Model 680 automatic microplate reader
(Bio-Rad Laboratories, Inc.), and the concentrations of sFlt-1 and
PlGF were calculated. sFlt-1 sensitivity ranges from 12.5 to 400
pg/ml and PlGF sensitivity ranges from 2.5 to 80 pg/ml.
Statistical analysis
SPSS 22.0 software (IBM Corp.) was used for
statistical analysis and the data were visualized using GraphPad
Prism 6 software (GraphPad Software, Inc.). Measurement data were
expressed as the mean ± standard deviation (SD) and were compared
between the two groups by independent samples t-test. Count data
were expressed as the case number and percentage [n (%)], and were
compared between the two groups by Chi-square test (Fisher's exact
test was used when the minimum theoretical frequency in the
Chi-square test was <5). Intergroup comparisons between the
pre-treatment and the post-treatment data were analyzed by one-way
ANOVA, whereas the pairwise comparisons were analyzed by the
Bonferroni post hoc test. ROC curve analysis was carried out to
evaluate the predictive value of sFlt-1/PlGF ratio for clinical
efficacy. P<0.05 was considered to indicate a statistically
significant difference.
Results
General information of the two
groups
Groups A and B were not significantly different in
age, gestational age, maternal type, body mass index (BMI), mode of
delivery, smoking, drinking, place of residence, hemoglobin,
platelet count, white blood cell count, serum total protein, serum
albumin, alanine aminotransferase, serum creatinine, cholesterol
and triglycerides (P>0.05) (Table
I).
 | Table IGeneral information of patients in
groups A and B [n (%), mean ± SD]. |
Table I
General information of patients in
groups A and B [n (%), mean ± SD].
| Factors | Group A (n=83) | Group B (n=81) | t/χ2 | P-value |
|---|
| Age (years) | 27.6±4.3 | 27.2±5.5 | 0.520 | 0.604 |
| Gestational age
(weeks) | 28.7±2.6 | 28.3±2.4 | 1.023 | 0.308 |
| Maternal type | | | 0.236 | 0.627 |
|
Primipara | 44 (53.01) | 46 (56.79) | | |
|
Multipara | 39 (46.99) | 35 (43.21) | | |
| BMI
(kg/m2) | 22.86±2.26 | 22.35±2.43 | 1.392 | 0.166 |
| Mode of delivery | | | 0.195 | 0.658 |
|
Cesarean
section | 25 (30.12) | 27 (33.33) | | |
|
Natural
labor | 58 (69.88) | 54 (66.67) | | |
| Smoking | | | 0.316 | 0.574 |
|
Yes | 15 (18.07) | 12 (14.81) | | |
|
No | 68 (81.93) | 69 (85.19) | | |
| Drinking | | | 0.075 | 0.785 |
|
Yes | 16 (19.28) | 17 (20.99) | | |
|
No | 67 (80.72) | 64 (79.01) | | |
| Place of
residence | | | 1.005 | 0.316 |
|
Urban
area | 60 (72.29) | 64 (79.01) | | |
|
Rural
area | 23 (27.71) | 17 (20.99) | | |
| Hemoglobin | 126.15±24.43 | 119.58±22.07 | 1.806 | 0.073 |
| Platelet count
(x109/l) | 166.13±77.06 | 168.26±67.76 | 0.188 | 0.851 |
| White blood cell
count (x109/l) | 16.02±2.21 | 15.68±2.29 | 0.968 | 0.335 |
| Serum total protein
(g/l) | 54.31±7.02 | 54.73±6.95 | 0.385 | 0.701 |
| Serum albumin
(g/l) | 27.49±4.36 | 28.15±4.01 | 1.008 | 0.315 |
| Alanine
aminotransferase (U/l) | 36.43±22.19 | 33.84±20.57 | 0.775 | 0.440 |
| Serum creatinine
(µmol/l) | 77.82±7.56 | 76.25±7.32 | 1.351 | 0.179 |
| Cholesterol
(µmol/l) | 6.81±0.95 | 6.73±1.02 | 0.520 | 0.604 |
| Triglyceride
(mmol/l) | 4.08±0.92 | 3.97±0.89 | 0.778 | 0.438 |
Treatment efficacy, adverse reactions
and pregnancy outcomes in the two groups
The effective rate was 91.57% in group A,
significantly higher than that in group B (80.25%) (P<0.05).
Groups A and group B were not markedly different in the incidence
rate of adverse reactions (P>0.05). Group A had superior
pregnancy outcomes over group B. Details are shown in Tables
II-IV.
SBP, DBP, 24hUP and 24hUV before and
after treatment in the two groups
No significant difference in SBP, DBP, 24hUP and
24hUV was detected between groups A and B before treatment
(P>0.05). After treatment, SBP, DBP and 24hUP were markedly
decreased in both groups (P<0.001), whereas 24hUV was markedly
increased (P<0.001). After treatment, SBP, DBP and 24hUP in
group A were significantly lower than those in group B
(P<0.001), whereas 24hUV was significantly higher in group A
than in group B (P<0.001). The data are presented in Table V.
 | Table VComparison of SBP, DBP, 24hUP and
24hUV (mean ± SD). |
Table V
Comparison of SBP, DBP, 24hUP and
24hUV (mean ± SD).
| Factors | Group A (n=83) | Group B (n=81) | t | P-value |
|---|
| SBP (mmHg) |
|
Before
treatment | 176.35±15.28 | 174.69±15.63 | 0.688 | 0.493 |
|
After
treatment | 138.43±14.52 | 156.25±13.48 | 7.734 | <0.001 |
|
t | 16.560 | 7.955 | - | - |
|
P-value | <0.001 | <0.001 | - | - |
| DBP (mmHg) |
|
Before
treatment | 117.28±16.24 | 116.52±15.75 | 0.304 | 0.761 |
|
After
treatment | 95.13±11.68 | 108.52±12.36 | 6.058 | <0.001 |
|
t | 10.080 | 3.598 | - | - |
|
P-value | <0.001 | 0.002 | - | - |
| 24hUP (g/l) |
|
Before
treatment | 3.41±1.28 | 3.38±1.15 | 0.158 | 0.875 |
|
After
treatment | 1.25±0.67 | 1.89±1.02 | 3.886 | <0.001 |
|
t | 13.200 | 8.993 | - | - |
|
P-value | <0.001 | <0.001 | - | - |
| 24hUV (ml) |
|
Before
treatment | 946.28±46.42 | 951.53±43.61 | 0.746 | 0.457 |
|
After
treatment |
2,423.41±196.15 |
1,821.41±185.36 | 20.19 | <0.001 |
|
t | 66.760 | 41.110 | - | - |
|
P-value | <0.001 | <0.001 | - | - |
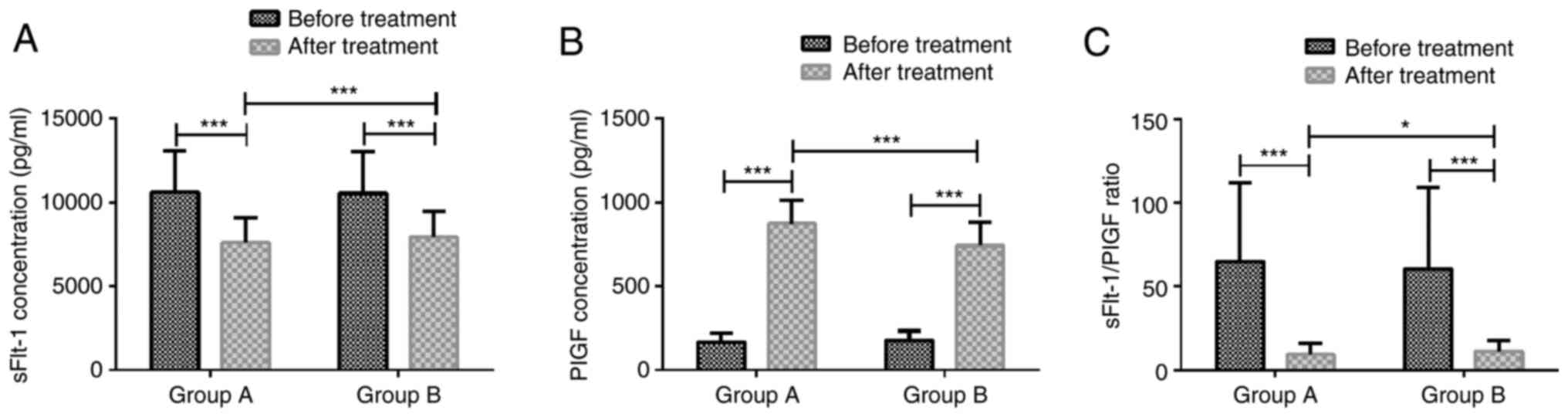
Serum sFlt-1 and PlGF concentrations,
and their ratio before and after treatment in the two groups
sFlt-1/PlGF ratio was 68.86±47.26 in group A and
64.56±48.35 in group B before treatment, and 9.32±6.69 in group A
and 11.37±6.56 in group B after treatment. Groups A and B were not
markedly different in serum sFlt-1 level, PlGF level, and
sFlt-1/PlGF ratio before treatment (P>0.05). After treatment,
serum sFlt-1 concentration and sFlt-1/PlGF ratio were significantly
lower than those before treatment in groups A and B (P<0.001),
whereas PlGF concentration was significantly higher than that
before treatment in both groups (P<0.001). After treatment,
group A had markedly lower serum sFlt-1 concentration (P<0.001),
lower sFlt-1/PlGF ratio (P<0.05), and markedly higher PlGF
concentration (P<0.001) than group B (Fig. 1).
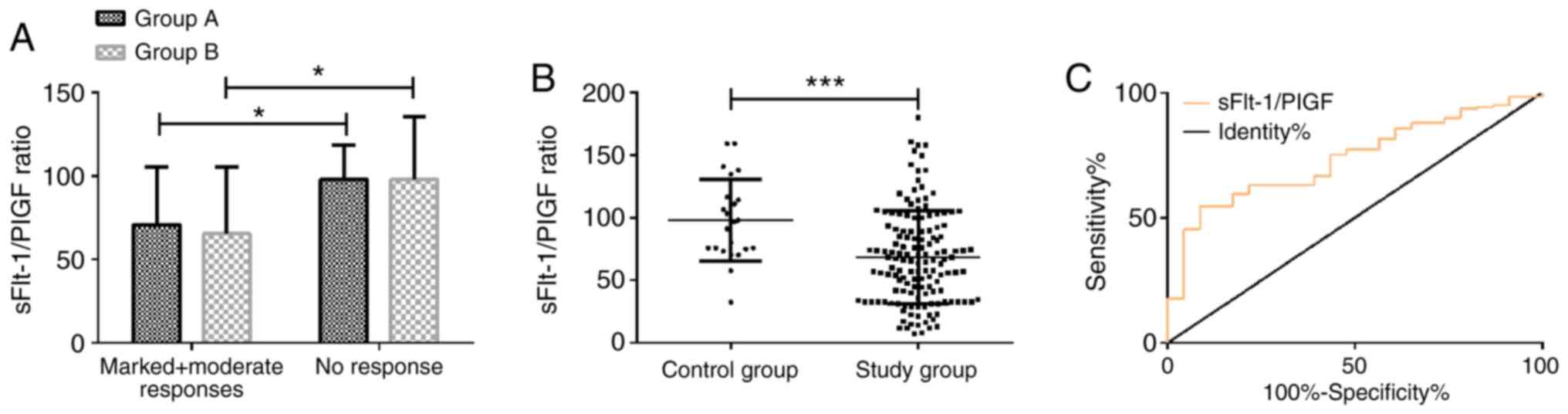
Predictive value of pre-treatment
serum sFlt-1/PlGF ratio for the treatment effect
In both groups A and B, the sFlt-1/PlGF ratio was
notably lower in patients with a marked or moderate response than
in patients with no response (P<0.05). Group A was not
significantly different from group B in reference to the patients
with a marked or moderate response to the treatment or the patients
with no response at all (P>0.05). Patients with marked or
moderate response (141 cases) were considered as the study group,
whereas patients with no response (23 cases) were considered as the
control group. The serum sFlt-1/PlGF ratio was 68.28±37.28 in the
study group and 98.03±32.80 in the control group. The serum
sFlt-1/PlGF ratio in the study group before treatment was
significantly lower than that in the control group (P<0.001).
ROC curve analysis of the serum sFlt-1/PlGF ratio before treatment
for predicting the treatment effect demonstrated that the area
under curve (AUC) was 0.737 (95% CI, 0.643-0.831), the sensitivity
was 54.61%, the specificity was 91.30%, and the best cut-off value
was 70.41 (Fig. 2).
Discussion
ES-PE is very dangerous for pregnant women with
hypertension. Characterized by quick onset and rapid progression,
ES-PE can cause organ failure, fetal distress and asphyxia
(4). ES-PE poses a big threat to
the safety of both the mother and the baby (21) and therefore, its treatment is a hot
topic in clinical research.
Magnesium sulfate is the preferred drug for ES-PE
treatment. Magnesium sulfate can promote peripheral blood vessel
relaxation, lower blood pressure, relieve skeletal muscle spasm,
increase uterine artery blood flow, and boost diuretic action
(22). Labetalol is a kind of
salicylamide derivative, which can block α and β receptors,
effectively dilate blood vessels, reduce peripheral blood vessel
resistance, decrease myocardial oxygen consumption and cardiac
preload, and exert antihypertensive effects (23). Numerous studies have been reported
on magnesium sulfate and labetalol in PE patients. In the study by
Das et al (24), a low
dosage of magnesium sulfate was proven to be safe and not toxic for
PE pregnant women and neonates. In the study by Sun et al
(25), CYP2D6 and CYP2C9 gene
polymorphisms were associated with the pathogenesis of ES-PE
patients, and the allele of G in CYP2D6 gene rs1065852 was
considered possibly related to the efficacy of labetalol treatment.
In the present study, the effective rate in group A was
significantly higher than that in group B. Blood pressure was
remarkably decreased to near the normal range in ES-PE patients
treated with magnesium sulfate combined with labetalol. Patients in
group A had markedly lower 24hUP and markedly higher 24hUV than
patients in group B, indicating that the combination of magnesium
sulfate and labetalol can promote urination, enhance the
permeability of the glomerulus and reduce the production of
proteinuria. These results suggest that magnesium sulfate combined
with labetalol is effective in treating ES-PE and can relieve the
clinical symptoms of patients. In the study by Abdelrahman et
al (26), 25 ES-PE patients
treated with magnesium sulfate combined with labetalol or
hydralazine presented good tolerance to the drug treatment, and
their blood pressure was effectively and safely controlled. In
addition, the fetal heart rate was not significantly changed after
treatment, which is similar to the results of the present study.
Magnesium sulfate can rarely threaten maternal life; however,
magnesium can promote peripheral vasodilation, leading to adverse
reactions, such as blushing, nausea, vomiting, and headache
(27). In the present study, group
A was not markedly different from group B in the incidence of
adverse reactions, and group A had notably superior pregnancy
outcomes over group B, indicating that magnesium sulfate combined
with labetalol is fairly safe and results in better pregnancy
outcomes. Xie et al (28)
reported that labetalol could inhibit the agglutination of
platelets and promote fetal lung maturation, without causing a
rapid decrease of blood pressure and palpitations. Thus, it can be
inferred that, although patients from groups A and B suffered from
certain adverse reactions, labetalol did not aggravate the adverse
reactions of patients and improved the perinatal outcomes.
Angiogenic markers are critical in the diagnosis and
subsequent prediction and treatment of PE and placental-related
diseases (29). The increase of
serum sFlt-1 levels and the decrease of PlGF levels result in an
increased sFlt-1/PlGF ratio. In women diagnosed with PE combined
with intrauterine fetal growth restriction or stillbirth
(placenta-related disease), an increase in sFlt-1/PlGF ratio can be
detected in the late pregnancy (30). In the study by Ohkuchi et al
(31), a cut-off value of 45 for
the sFlt-1/PlGF ratio resulted in the best sensitivity and
specificity for the diagnosis of pre-eclampsia (97 and 95%,
respectively), and for the diagnosis of early-onset pre-eclampsia
(100 and 95%, respectively). In the study by Schoofs et al
(32), repeated measurements of the
sFlt-1/PlGF ratio identified pathological pregnancy outcomes in
patients with intrauterine fetal growth restriction before
diagnosis. Thus, the importance of sFlt-1/PlGF ratio in ES-PE
diagnosis and prognosis is promising. However, little is known
about the changes of sFlt-1/PlGF ratio and its effects during
treatment. In the present study, the serum sFlt-1 concentration and
sFlt-1/PlGF ratio after treatment were significantly lower than
those before treatment in both groups A and B, whereas PlGF
concentration was significantly higher than that before treatment.
After treatment, group A had markedly lower serum sFlt-1
concentration and sFlt-1/PlGF ratio, and markedly higher PlGF
concentration, than group B. These results suggest that the effects
of magnesium sulfate combined with labetalol to improve the balance
of sFlt-1/PlGF may be one of the mechanisms of ES-PE treatment. Xu
et al (33) reported that
some antihypertensive drugs used during pregnancy could improve the
cellular interaction between trophoblasts and endothelial cells
exposed to TNF-α. For example, labetalol could act on sFlt-1 to
promote the integration of trophoblast cells. The possible reason
for this function may be the fact that labetalol can directly act
on vascular smooth muscle cells and improve the function of
vascular endothelium by regulating the secretion of inflammatory
factors in the blood vessels (34).
The present study further investigated the role of sFlt-1, PlGF,
and their ratio in the treatment of ES-PE patients. The sFlt-1/PlGF
ratio before treatment was markedly lower in patients with a marked
or moderate response to treatment than in patients with no response
in both groups A and B, and the AUC of sFlt-1/PlGF ratio for
predicting the treatment effect was 0.737, suggesting that
pre-treatment sFlt-1/PlGF ratio has a certain predictive value for
the treatment effect. Therefore, sFlt-1/PlGF ratio is a potential
predictor of the treatment effect of ES-PE patients. However, this
study failed to identify the contributing factors affecting the
treatment effect of ES-PE patients or to reveal the underlying
mechanism of magnesium sulfate and labetalol for treating ES-PE.
These issues will be the aim of our future research.
In conclusion, magnesium sulfate combined with
labetalol can be effectively used for the treatment of ES-PE and
can reduce the serum sFlt-1/PlGF ratio. The assessment of
sFlt-1/PlGF ratio before treatment has a certain predictive value
for the efficacy of ES-PE treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW wrote the manuscript, interpreted and analyzed
the patient data. JB designed the study and was responsible for the
patient treatment and the detection methods. MP was responsible for
the analysis and discussion of the data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Maternity and Child Health Care Hospital of Hubei
(Wuhan, China). Patients who participated in this research, had
complete clinical data. Signed written informed consents were
obtained from the patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pilliod RA, Feinberg BB and Burwick RM:
Maternal and Feto-placental phenotypes of early-onset severe
preeclampsia. J Matern Fetal Neonatal Med. 29:1209–1213.
2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fisher SJ: Why is placentation abnormal in
preeclampsia? Am J Obstet Gynecol. 213 (4 Suppl):S115–S122.
2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yang W, Wang A, Zhao C, Li Q, Pan Z, Han
X, Zhang C, Wang G, Ji C, Wang G, et al: miR-125b enhances IL-8
production in early-onset severe preeclampsia by targeting
Sphingosine-1-phosphate lyase 1. PLoS One.
11(e0166940)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
van Esch JJA, van Heijst AF, de Haan AFJ
and van der Heijden OWH: Early-onset preeclampsia is associated
with perinatal mortality and severe neonatal morbidity. J Matern
Fetal Neonatal Med. 30:2789–2794. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
He Y, Xu B, Song D, Yu F, Chen Q and Zhao
M: Correlations between complement system's activation factors and
anti-angiogenesis factors in plasma of patients with
early/late-onset severe preeclampsia. Hypertens Pregnanc.
35:499–509. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Müller A, Horvat V, Vulin M, Mandić S,
Šerić V and Vidosavljević D: The soluble Fms-like tyrosin kinase-1
(sFLT-1) to placental growth factor (PIGF) ratio as a possible
indicator for the severity of preeclampsia-single institution
experience. Med Glas (Zenica). 16:53–59. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
He Y, Xu B, Song D, Yu F, Chen Q and Zhao
M: Expression of the complement system's activation factors in
plasma of patients with early/late-onset severe pre-eclampsia. Am J
Reprod Immunol. 76:205–211. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yusuf AM, Kahane A and Ray JG: First and
second trimester serum sFlt-1/PlGF ratio and subsequent
preeclampsia: A systematic review. J Obstet Gynaecol Can.
40:618–626. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Herraiz I, Simón E, Gómez-Arriaga P,
Martínez-Moratalla JM, García-Burguillo A, Jiménez EA and Galindo
A: Angiogenesis-related biomarkers (sFlt-1/PLGF) in the prediction
and diagnosis of placental dysfunction: An approach for clinical
integration. Int J Mol Sci. 16:19009–19026. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Suzuki H, Hirashima C, Nagayama S,
Takahashi K, Yamamoto T, Matsubara S and Ohkuchi A: Increased serum
levels of sFlt-1/PlGF ratio in preeclamptic women with onset at
<32 weeks compared with ≥32 weeks. Pregnancy Hypertens.
12:96–103. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Caillon H, Tardif C, Dumontet E, Winer N
and Masson D: Evaluation of sFlt-1/PlGF ratio for predicting and
improving clinical management of pre-eclampsia: Experience in a
specialized perinatal care center. Ann Lab Med. 38:95–101.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yang K, Dong G, Tian Y and Li J: Effects
of compound Danshen injection combined with magnesium sulfate on
serum MPO and hs-CRP in patients with severe preeclampsia. Exp Ther
Med. 16:167–170. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Duley L, Gülmezoglu AM, Henderson-Smart DJ
and Chou D: Magnesium sulphate and other anticonvulsants for women
with Pre-eclampsia. Cochrane Database Syst Rev.
2010(CD000025)2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bain ES, Middleton PF and Crowther CA:
Maternal adverse effects of different antenatal magnesium sulphate
regimens for improving maternal and infant outcomes: A systematic
review. BMC Pregnancy Childbirth. 13(195)2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shekhar S, Sharma C, Thakur S and Verma S:
Oral nifedipine or intravenous labetalol for hypertensive emergency
in pregnancy: A randomized controlled trial. Obstet Gynecol.
122:1057–1063. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Duley L, Henderson-Smart DJ and Chou D:
Magnesium sulphate versus phenytoin for eclampsia. Cochrane
Database Syst Rev. (CD000128)2010.PubMed/NCBIdoi: 10.1002/14651858.CD000128.
|
|
17
|
Kassie GM, Negussie D and Ahmed JH:
Maternal outcomes of magnesium sulphate and diazepam use in women
with severe pre-eclampsia and eclampsia in Ethiopia. Pharm Pract
(Granada). 12(400)2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Giannubilo SR, Bezzeccheri V, Cecchi S,
Landi B, Battistoni GI, Vitali P, Cecchi L and Tranquilli AL:
Nifedipine versus labetalol in the treatment of hypertensive
disorders of pregnancy. Arch Gynecol Obstet. 286:637–642.
2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Abdel-Hady el-S, Fawzy M, El-Negeri M,
Nezar M, Ragab A and Helal AS: Is expectant management of
early-onset severe preeclampsia worthwhile in low-resource
settings? Arch Gynecol Obstet. 282:23–27. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chen Q, Sousa JD, Snowise S, Chamley L and
Stone P: Reduction in the severity of early onset severe
preeclampsia during gestation may be associated with changes in
endothelial cell activation: A pathological case report. Hypertens
Pregnancy. 35:32–41. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Romero R, Chaemsaithong P, Tarca AL,
Korzeniewski SJ, Maymon E, Pacora P, Panaitescu B, Chaiyasit N,
Dong Z, Erez O, et al: Maternal plasma-soluble ST2 concentrations
are elevated prior to the development of early and late onset
preeclampsia-a longitudinal study. J Matern Fetal Neonatal Med.
31:418–432. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wen J, Zhang X and Li C: Clinical effect
of low molecular weight heparin sodium combined with magnesium
sulfate in the treatment of patients with severe preeclampsia. J
Coll Physicians Surg Pak. 29:119–122. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Molvi SN, Mir S, Rana VS, Jabeen F and
Malik AR: Role of antihypertensive therapy in mild to moderate
pregnancy-induced hypertension: A prospective randomized study
comparing labetalol with alpha methyldopa. Arch Gynecol Obste.
285:1553–1562. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Das M, Chaudhuri PR, Mondal BC, Mitra S,
Bandyopadhyay D and Pramanik S: Assessment of serum magnesium
levels and its outcome in neonates of eclamptic mothers treated
with Low-dose magnesium sulfate regimen. Indian J Pharmacol.
47:502–508. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sun CJ, Li L, Li XY, Zhang WY and Liu XW:
Associations of polymorphisms of CYP2D6 and CYP2C9 with early onset
severe pre-eclampsia and response to labetalol therapy. Arch
Gynecol Obstet. 298:125–132. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Abdelrahman TN, Youssry MA, Radwan AM and
Ahmed A: Impact of intravenous infusion of labetalol combined with
magnesium sulfate versus hydralazine combined with magnesium
sulfate on fetomaternal hemodynamics in severe preeclampsia. Ain
Shams J Anesthesiol. 11(5)2019.
|
|
27
|
Lu JF and Nightingale CH: Magnesium
sulfate in eclampsia and pre-eclampsia. Clin Pharmacokin.
38:305–314. 2000.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Xie RH, Guo Y, Krewski D, Mattison D,
Walker MC, Nerenberg K and Wen SW: Association between labetalol
use for hypertension in pregnancy and adverse infant outcomes. Eur
J Obstet Gynecol Reprod Biol. 175:124–128. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Foidart JM, Schaaps JP, Chantraine F,
Munaut C and Lorquet S: Dysregulation of anti-angiogenic agents
(sFlt-1, PLGF, and sEndoglin) in preeclampsia-a step forward but
not the definitive answer. J Reprod Immunol. 82:106–111.
2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Stepan H, Herraiz I, Schlembach D,
Verlohren S, Brennecke S, Chantraine F, Klein E, Lapaire O, Llurba
E, Ramoni A, et al: Implementation of the sFlt-1/PlGF ratio for
prediction and diagnosis of pre-eclampsia in singleton pregnancy:
Implications for clinical practice. Ultrasound Obstet Gynecol.
45:241–246. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ohkuchi A, Hirashima C, Suzuki H,
Takahashi K, Yoshida M, Matsubara S and Suzuki M: Evaluation of a
new and automated electrochemiluminescence immunoassay for plasma
sFlt-1 and PlGF levels in women with preeclampsia. Hypertens Res.
33:422–427. 2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Schoofs K, Grittner U, Engels T, Pape J,
Denk B, Henrich W and Verlohren S: The importance of repeated
measurements of the sFlt-1/PlGF ratio for the prediction of
preeclampsia and intrauterine growth restriction. J Perinat Med.
42:61–68. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Xu B, Charlton F, Makris A and Hennessy A:
Antihypertensive drugs methyldopa, labetalol, hydralazine, and
clonidine improve trophoblast interaction with endothelial cellular
networks in vitro. J Hypertens. 32:1075–1083. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Xu B, Bobek G, Makris A and Hennessy A:
Antihypertensive methyldopa, labetalol, hydralazine, and clonidine
reversed tumour necrosis factor-α inhibited endothelial nitric
oxide synthase expression in Endothelial-trophoblast cellular
networks. Clin Exp Pharmacol Physiol. 44:421–427. 2017.PubMed/NCBI View Article : Google Scholar
|