Introduction
The treatment of long-term bone defects represents a
clinical challenge for orthopedic specialists (1). Bone regeneration and healing processes
are regulated by three key factors: Scaffold materials, growth
factors (GFs) and seeding cells (2). GFs have an important biochemical role
in bone regeneration (3).
Platelet-rich plasma (PRP) is a concentration of
platelets from the peripheral blood, containing ~300 bioactive
cytokines that, when released, can promote tissue repair (4). PRP contains a number of GFs (4). These activated factors are essential
for tissue regeneration and wound healing (5). In 1987, PRP was first used for heart
surgery (6) and in 1998, it was
used in the initial stage of enhanced fracture healing (7). Since then, PRP has been widely used in
orthopedics to promote bone regeneration (8,9).
Usually, PRP is administered at a concentration 3-5x higher than
the baseline. It has been reported that concentrations of platelets
and available cytokines involved in stimulating and accelerating
the repair process are positively correlated (10). However, studies on PRP have yielded
contradictory results, which is probably due to different PRP
preparation techniques (11).
Currently, there is no international standard protocol for the
preparation of PRP (12). The
present study protocol was adapted from Landesberg et al
(13), which is able to maintain
the content of GFs, platelet-derived GF (PDGF) and transforming GF
(TGF)-β, as much as possible and has good osteogenic
properties.
Bone grafting materials, such as autografts,
allografts, xenografts and synthetic biomaterials, have been
extensively studied (2,14). Although bone substitute materials
exhibit good performance (biological function, mechanical
qualities, efficiency or safety), autologous bone is considered the
gold standard for grafting materials (15). Wang et al (16) indicated that particulate bone powder
grafts may accelerate bone defect healing, as compared with large
bone grafts, in a rat radial defect model. Furthermore, our
previous study suggested that autologous bone particle/titanium
fiber composites improved the mechanical strength to some extent in
a segmental bone defect model (17). Autologous bone particles (diameter,
300-500 µm) are able to maximize the number of bone cells that
release different types of GFs, thus enhancing the contact area and
the supplement of nutrients to the tissue. However, insufficient
strength and donor unavailability have been identified as
weaknesses (18,19). Bone mesenchymal stem cells (BMSCs)
are a type of pluripotent stem cell, which have been successfully
used to reconstruct bone defects in clinical trials (20,21).
The present study hypothesized that PRP may release
GFs and promote osteogenesis. The aim of the current study was to
explore a novel method of bone defect reconstruction and the
mechanism underlying the effects of PRP on supporting bone
formation.
Materials and methods
BMSC isolation and culture
New Zealand white rabbits (male; age, 6-9 months;
weight, 2.5-3.0 kg) were obtained from Beijing Vital River
Laboratory Animal Technology Co., Ltd. and were housed in the
Animal Laboratory of Xuanwu Hospital (Beijing, China). All animals
were kept in separate cages and housed under the following
conditions: Temperature, 22±1˚C; relative humidity, 50±1% and on a
12-h light/dark cycle. Food and water access was ad libitum.
All animal studies, including the rabbit euthanasia procedure, were
performed in compliance with the American Association for
Accreditation of Laboratory Animal Care and Institutional Animal
Care and Use Committee (IACUC) guidelines. All animal studies were
approved by the IACUC of Capital Medical University (Beijing,
China; ethics approval no. XWH2018070011). BMSCs were obtained as
previously described (17,22).
BMSCs were collected from the bilateral femurs of
rabbits (22). Under sterile
conditions, the bone marrow was collected and washed with
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.). To harvest a single cell suspension containing
BMSCs, the cell suspension was centrifuged for 5 min at 250 x g and
room temperature and resuspended with DMEM. Subsequently, the cell
suspension (5 ml) was slowly transferred to a 15 ml centrifuge tube
containing Ficoll-Paque (5 ml) (Sigma-Aldrich; Merck KGaA), and
then centrifuged at 400 x g at room temperature for 25 min.
Isolated BMSCs were collected from the interphase (cloud-like cell
layer) and washed with PBS. The cell-PBS mixture was centrifuged
for 5 min at 250 x g at room temperature and the supernatant was
removed to obtain the isolated BMSCs. Isolated BMSCs were then
cultured in DMEM containing 10% fetal bovine serum (FBS) (Gibco;
Thermo Fisher Scientific, Inc.) and 1% penicillin-streptomycin
solution (10 kU/ml 100X penicillin; 10 mg/ml streptomycin) in a
humidified atmosphere containing 5% CO2/95% air at 37˚C.
Cells were cultured at a concentration of 1x106/l in a
25-cm2 flask. The culture medium was changed three times
per week. All experiments were performed using cells at passages
3-5.
PRP preparation
A total of 10 ml whole blood was collected from each
animal (same rabbits used for BMSC extraction) ear arteries. The
blood then underwent a two-step centrifugation process [initially,
blood was centrifuged at 200 x g for 10 min at 20˚C to remove red
blood cells (bottom fraction), and then at 200 x g for 10 min at
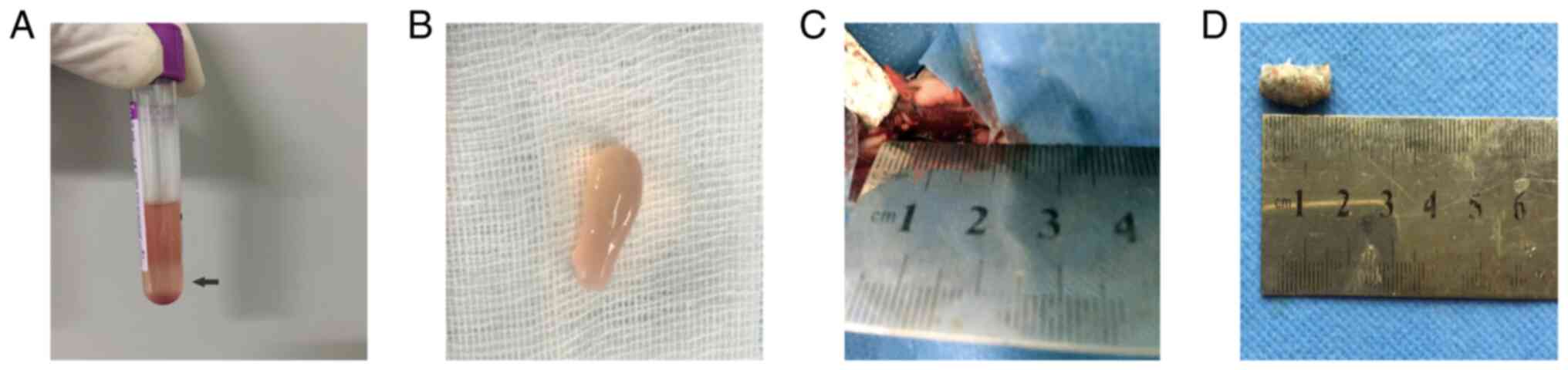
20˚C to obtain the PRP] [pre-activated PRP; (middle fraction, 1 ml;
Fig. 1A and B)]. The mean platelet number was
10.77x108/ml (SD 0.85x108) and that of whole
blood was 2.92x108/ml (SD 0.28x108). Thrombin
activators [500 units bovine thrombin; cat. no. 0219990701; MP
Biomedicals (Shanghai) Co., Ltd.in 1 ml 10% calcium chloride] were
added to the pre-activated PRP and gently mixed to activate PRP
(post-activated PRP). A total of 1 ml activated PRP was then mixed
with 1x107 BMSCs.
Animal model and grouping
A rabbit radial defect model was established
bilaterally, as previously described (1). Briefly, 45 New Zealand male rabbits
(male; age, 6-9 months; weight, 2.5-3.0 kg) were used. Rabbits
obtained from the same company and housed under the same conditions
as previously mentioned. Rabbits were anesthetized with intravenous
3% sodium pentobarbital (30 mg/kg and were euthanized after surgery
at 4, 8 and 12 weeks by intravenous injection of 3% sodium
pentobarbital sodium (100 mg/kg). The methods used to verify death
included the lack of a heartbeat, no autonomous respiration for 2-3
min and no blink reflex. The iliac bone (400 mg), stripped of the
periosteum and cartilage, was obtained from each rabbit. After
removing the bone marrow and endosteum, the iliac bone was ground
into bone powder grafts of 300-500 µm in diameter using a spherical
grinding drill in saline. Bone particles and PRP/BMSCs were mixed.
Subsequently, the autogenous bone particle/PRP/BMSC compound was
shaped and prepared for the next step (Fig. 1C and D). A 15-mm segment of the bilateral radius
defect was cut off in the middle of the radius. The defect site was
then washed with physiological saline and the graft was fixed into
the bone. Finally, muscles and skin were respectively sutured.
The rabbits were divided into the following groups:
Group A, empty bone defect (n=9); group B, bone defect filled with
PRP (n=9); group C, bone defect filled with autogenous bone
particles (200 mg) + BMSCs (1x107) on the left radius;
and group D, bone defect filled with autogenous bone particles (200
mg) + PRP (0.5 ml) + BMSCs (1x107) on the right radius.
There were 27 rabbits in group C (left) and D (right). After
surgery, the rabbits were maintained in individual cages and were
fed normally. Bone specimens were harvested 4, 8 and 12 weeks
post-surgery.
Macroscopic observations
After euthanasia, defective radius specimens were
dissected and images were captured to observe the following: i)
Bone formation in the bone defect area; ii) osteogenesis and
inflammatory reaction in the local area; and iii) the relationship
between the surrounding soft tissue and the bone defect (8).
X-ray examination and scoring
An X-ray device (45 kV, 100 mA, 0.12 sec; Siemens
1350; Siemens AG) was used to investigate the bone-healing process.
X-ray scoring was performed according to the Lane-Sandhu standards
(Table I) (23), including bone connection,
recanalization of the medullary cavity and bone formation in the
bone defect area, which were scored by three independent examiners.
The images were captured three per group at three time points. The
Lane-Sandhu score of each image was the sum of sub-parts of the
scoring standard. The mean of the scores given by the three
observers were accepted to be the final score (23).
 | Table ILane-Sandhu radiographic scoring
system. |
Table I
Lane-Sandhu radiographic scoring
system.
| Variable | Description | Score |
|---|
| Degree of bone
formation | No new bone
formed | 0 |
| | The area of new
bone accounts for 25% of the defect area | 1 |
| | The area of new
bone accounts for 50% of the defect area | 2 |
| | The area of new
bone accounts for 75% of the defect area | 3 |
| | The area of new
bone accounts for 100% of the defect area | 4 |
| Degree of
union | Fracture line is
fully visible | 0 |
| | Fracture line is
partially visible | 2 |
| | Fracture line is
not visible | 4 |
| Degree of medullary
cavity remodeling | No sign of
remodeling | 0 |
| | Recanalization of
medullary cavity | 2 |
| | Cortical bone
structure forms after recanalization of medullary cavity | 4 |
Histology and histomorphometry
Histological analysis of bone specimens was
conducted using ImagePro software (version 7.0; Media Cybernetics,
Inc.). Briefly, bone samples (15-mm new regenerated bone) were
fixed in a 4% formaldehyde solution at room temperature for 48 h
and rinsed with water. The samples were then dehydrated using
graded alcohol and embedded in methyl methacrylate resin.
Subsequently, sections (5 µm)were stained with hematoxylin and
eosin (H&E) at room temperature for 5 min and examined under a
light microscope (Nikon TE2000-U; Nikon Corporation). A single
section from each animal was analyzed and 3 images in total were
obtained from each group. Finally, the percentage of new bone in
the defect area was calculated by histomorphometry using ImagePro
image analysis software.
Immunohistochemistry
Sections prepared from groups C and D using the
aforementioned process were analyzed by immunohistochemical
staining. Sections (5 µm) were deparaffinized in xylene and
dehydrated with graded alcohol. Samples were then treated with 3%
hydrogen peroxide for 10 min and Tris/hydrochloric acid buffer for
5 min at room temperature, followed by incubation with an
anti-osteocalcin monoclonal antibody (cat. no. ab13418; Abcam;
1:200) at 4˚C overnight. Subsequently, sections were washed with
PBS and incubated at room temperature with biotin-labeled goat
anti-mouse IgG secondary antibodies (cat. no. A0286; Beyotime
Institute of Biotechnology; 1:200) for 30 min and counterstained
with hematoxylin (cat. no. H8070; Beijing Solarbio Science &
Technology Co., Ltd.) for 3 min at room temperature before
observation using a light microscope. A single section from each
animal was analyzed and 3 images in total were obtained from each
group.
Cell proliferation assay
Two groups of cells were analyzed: i) BMSCs treated
with DMEM + 10% FBS; and ii) BMSCs treated with DMEM + 10% PRP for
7 days. Briefly, BMSCs (100 µl/well, 2x103) were plated
in 96-well plates and incubated in an atmosphere containing 5%
CO2/90% air at 37˚C. At each time point (1-7 days), 10
µl Cell Counting Kit-8 (CCK-8) (cat. no. C0037; Beyotime Institute
of Biotechnology) solution was added to each well and incubated for
2 h at 37˚C. The absorbance at 450 nm was determined using a
microplate reader (SpectraMax 340PC; Molecular Devices, LLC).
ELISA
The concentrations of TGF-β1 and PDGF-AB, in whole
blood plasma, and pre- and post-activated PRP were detected using
an TGF-β1 ELISA kit (cat. no. SEKRT-0401; Beijing Solarbio Science
& Technology Co., Ltd.) and PDGF-AB ELISA kit (cat. no.
SEKRT-0030-96T; Beijing Solarbio Science & Technology Co.,
Ltd.), according to the manufacturer's protocol. Briefly, a 50 µl
sample was added to each well of 96-well microplates that were
coated with a monoclonal antibody against TGF-β1 or PDGF-AB for 2 h
at 37˚C. After washing with buffer, each sample was incubated with
horseradish peroxidase-conjugated cytokine for 1 h. Samples were
then incubated with substrate solution for 30 min at 37˚C and
stopped with termination solution. Results were obtained by
measuring absorbance at 450 nm using a microplate reader.
Osteogenic differentiation of
BMSCs
BMSCs were cultured in 24-well plates
(5x104/cm2). When the cell density reached
60-70%, cells were exposed to osteogenic induction medium (cat. no.
RBXMX-90021; Cyagen Biosciences, Inc.), including 100 U/ml
penicillin-streptomycin, 1% glutamine, 10 nmol/l dexamethasone, 0.2
mmol/l ascorbate and 10 mmol/l β-glycerophosphate. They were
divided into two groups, one group was treated with 10% FBS and the
other with 10% PRP at room temperature for 2 weeks. The medium was
changed every 3 days. After 2 weeks, mineralization was detected by
alkaline phosphatase (ALP) staining and Alizarin Red staining
(ARS).
ALP staining
After induction in osteogenic induction medium for 2
weeks, in vitro mineralization was assessed using an ALP staining
kit (cat. no. C3026; Beyotime Institute of Biotechnology). Briefly,
the cells were washed three times with PBS and fixed in 4%
paraformaldehyde for 10 min at room temperature. Subsequently, the
cells were embedded in ALP staining solution for 1 h at room
temperature in a 24-well plate, followed by three PBS washes. A
light microscope (Nikon TE2000-U) was then used to obtain the
image.
ARS
An ARS kit, provided with the differentiation medium
(cat. no. RBXMX-90021; Cyagen Biosciences, Inc.), was used to
detect matrix mineralization depositions after 2 weeks. After
washing three times with PBS, the cells were fixed with 4% formalin
for 10 min at room temperature, and then stained with ARS solution
for 30 min. Subsequently, the solution was removed, the cells were
rinsed three times with PBS and images (n=3 per group) were
captured under a light microscope (Nikon TE2000-U; Nikon
Corporation).
ALP activity assay
BMSCs were treated with osteogenic induction media
in 24-well plates for 2 weeks. They were then divided into the 10%
PRP and 10% FBS groups. The cells were collected and the level of
ALP activity was determined using an ALP activity kit (cat. no.
P0321; Beyotime Institute of Biotechnology) according to
manufacturer's protocol. The absorbance values of samples were
measured at 405 nm using a spectrophotometer. The total protein
content was determined via the BCA method using aliquots of the
same samples with the protein assay kit (cat. no. P0010; Beyotime
Institute of Biotechnology). ALP activity was normalized to total
protein concentration. The formula for the ALP activity was as
follows: ALP activity (U/g prot) = [(absorbance of the
determination tube/absorbance of the standard tube) x the amount of
nitrophenol in the standard tube]/grams of total protein.
Statistical analysis
For continuous variables, data are expressed as the
mean ± SD. The cell proliferation assay data were analyzed using a
two-way ANOVA followed by Bonferroni post hoc test. ELISA results
were analyzed using one-way ANOVA followed by the least significant
difference post hoc test. Histomorphometry and ALP activity were
analyzed using an independent samples t-test between the two
groups. Data analysis was performed using SPSS 22.0 software (IBM,
Corp.) For categorical variables, data are expressed as a median
(interquartile range). Lane-Sandhu score was analyzed using
Kruskal-Wallis test with Dunn's post hoc test. Data analysis was
performed using SAS 9.4 software (SAS, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
Macroscopic observations of bone
defects with and without treatment
After surgery, the incisions to the experimental
animals exhibited light swelling and there was no obvious secretion
around the incisions. After ~4 days, the swelling gradually
subsided, and the incision healed well in ~12 days. Images of the
gross specimen of each group are shown in Fig. 2A. Group A did not exhibit complete
repair of the bone defect after 4, 8 and 12 weeks. Surrounded by
soft tissue, there was a small number of calluses at the end of the
fracture at 4/8 weeks. At 12 weeks, bone sclerosis and enlargement
were observed, the defects could be seen, and they were replaced by
soft tissue. Group B was similar to group A. In groups C and D, the
defects were wrapped with dense fibrous tissue and new bone
processes were observed at the edge of the bone defect. Some callus
formation was identified. There was increased bone formation in
group D in comparison to group C. At 8 weeks, continuous new bone
was observed in the bone defect area of group C, with slight
depression at the radial side of the defect. The newly regenerated
bone in the middle of the defect area was fused with the ulna. Bone
in group D also formed continuously. The radial part of the defect
was less depressed than that of group C, and the new bone was also
connected partly with the ulna. The bone defect area of group C at
12 weeks displayed continuous new bone formation, less depression
in the radial part and nearly well-integrated shape. Group D formed
bony connections at 12 weeks and had a well-integrated shape.
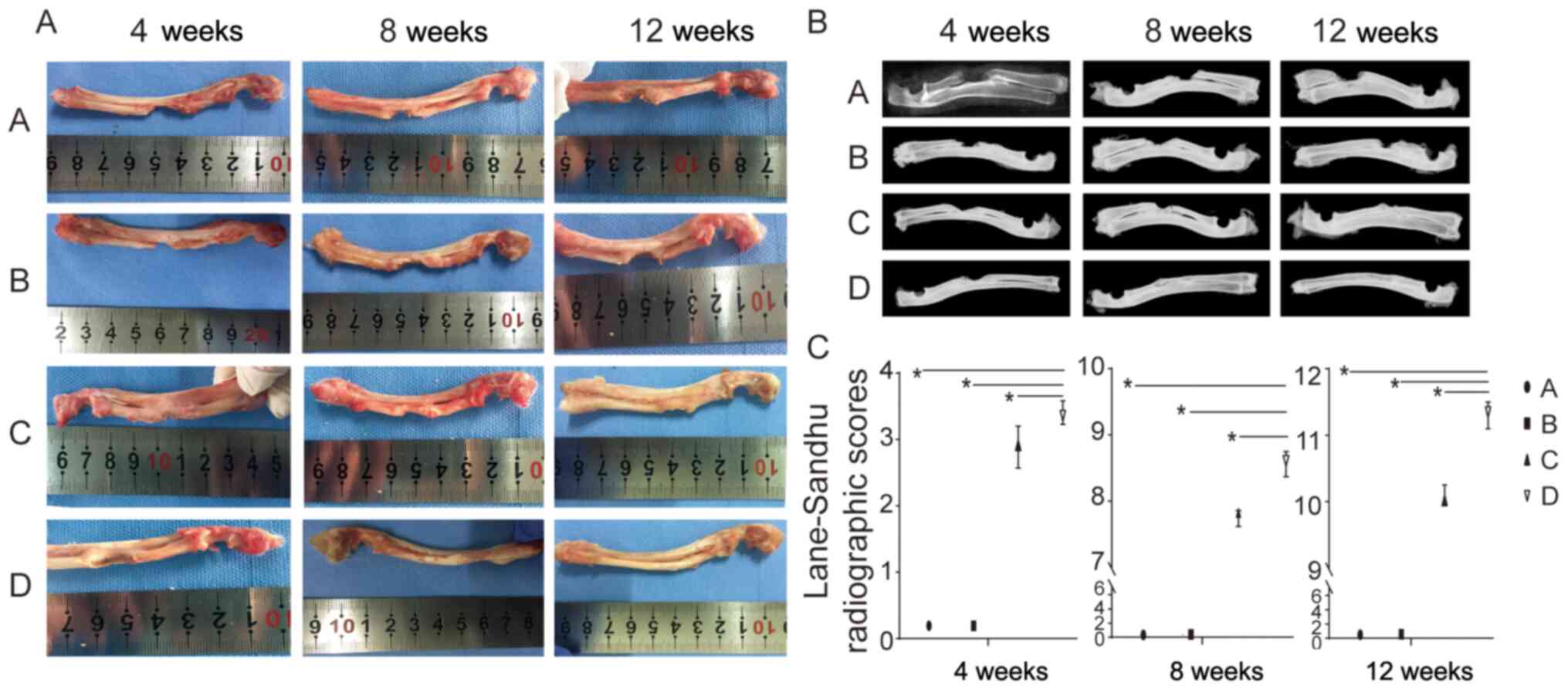 | Figure 2(A) In vivo macroscopic
observation of groups A, B, C and D during osteogenic repair at
three time points (4, 8 and 12 weeks). (B) Representative
radiograph from groups A, B, C and D at three time points (4, 8 and
12 weeks). (C) Lane-Sandhu radiographic score of the four groups
(n=3/group). Group A, empty bone defect; group B, PRP; group C,
autogenous bone particles + BMSCs on the left radius; group D,
autogenous bone particles + PRP + BMSCs on the right radius.
*P<0.05. PRP, platelet-rich plasma; BMSCs, bone
mesenchymal stem cells. |
Radiographic evaluation
As shown in Fig. 2B,
there was no bony bridge in groups C and D at 4, 8, or 12 weeks. A
few bone calluses were visible around the broken end in group A and
B at 4 weeks. The broken end was ossified with a large fissure in
the defect center and medullary cavities were blocked at 8 and 12
weeks. Bone healing in group D was superior to that in group C at
three time points. At week 4 after surgery, bone union had occurred
around the bone defect sites in groups C and D. At 8 weeks,
continuous bone callus formation and partial recanalization of the
medullary cavity segment was observed in groups C and D. Remodeling
was superior in group D to that in group C.
Lane-Sandhu radiographic score for the evaluation of
defect repair revealed the following mean scores: No statistically
significant differences were observed between groups A and B at
each time point (P>0.05). Furthermore, a higher score was found
in group D compared with the score in the other groups, and the
difference was statistically significant at each time point
(P<0.05; Fig. 2C; Table II).
 | Table IILane-Sandhu radiographic scoring
results. |
Table II
Lane-Sandhu radiographic scoring
results.
| Group | 4 weeks | 8 weeks | 12 weeks |
|---|
| A | 0.19
(0.19-0.20) | 0.34
(0.32-0.35) | 0.48
(0.47-0.49) |
| B | 0.19
(0.18-0.20) | 0.38
(0.34-0.39) | 0.56
(0.55-0.58) |
| C | 2.91
(2.57-3.20) | 7.83
(7.62-7.86) | 10.02
(9.95-10.25) |
| D | 3.36
(3.23-3.58)a | 8.61
(8.37-8.75)a | 11.35
(11.10-11.50)a |
Histology and histomorphometry
X-ray images revealed that there was no bridging and
repair in groups A and B. Thus, histological data and
histomorphometry were only obtained for groups C and D.
H&E staining revealed that trabecular bone was
disturbed at 4 weeks. The trabecular bone tended to be smooth, and
partial bone marrow cavity re-opening was observed at 8 weeks. At
12 weeks, good bone reconstruction and medullary cavity
recanalization was observed in groups C and D (Fig. 3A and B). The trabecular bone was mature and
intensive lamellar bone was generated. Furthermore, better bone
formation was observed in group D compared with in group C at the
three time points.
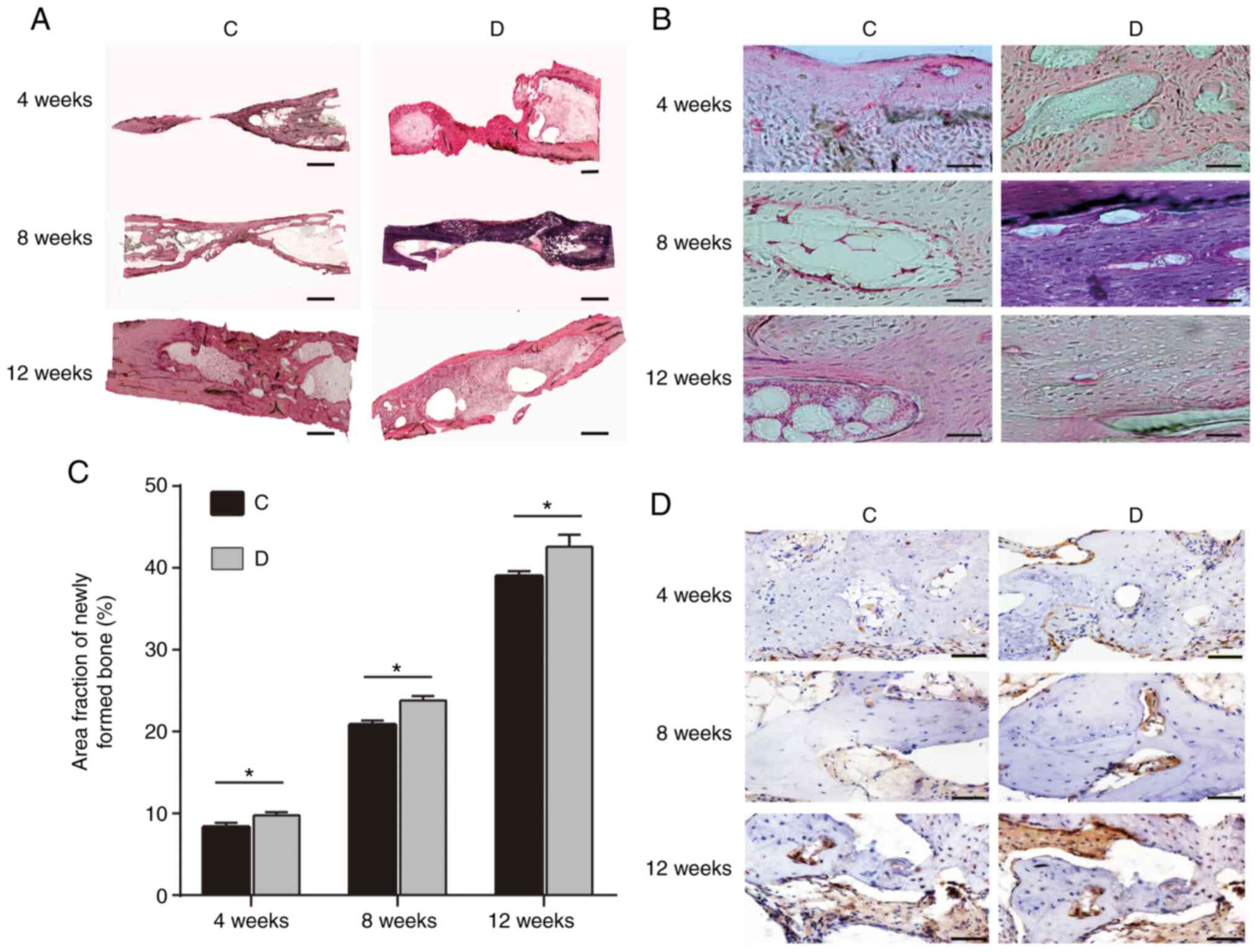 | Figure 3H&E staining of rabbit radius
samples at 4, 8 and 12 weeks from groups C and D. (A and B) H&E
staining. (A) Magnification, x4; scale bars, 3,000 µm. (B)
Magnification, x40; scale bars, 100 µm. (C) Fraction area of the
newly formed bone within the former defect as a percentage of total
defect area at 4, 8 and 12 weeks (n=3/group). (D)
Immunohistochemical observation of osteocalcin in groups C and D at
4, 8 and 12 weeks. Scale bar, 100 µm. Group C, autogenous bone
particles + BMSCs on the left radius; group D, autogenous bone
particles + PRP + BMSCs on the right radius. *P<0.05.
H&E, hematoxylin and eosin; PRP, platelet-rich plasma; BMSCs,
bone mesenchymal stem cells. |
Histomorphometry was performed using ImagePro
software; the results indicated that at 4 weeks the ratios of the
fraction area of the newly formed bone for groups C and D were
8.35±0.52 and 9.78±0.36, respectively, and the difference was
statistically significant (P<0.05; Fig. 3C). Similarly, at 8 and 12 weeks,
group D (23.83±0.54 and 42.60±1.47) exhibited a significantly
increased ratio compared with that in group C (20.85±0.50 and
39.02±0.58; P<0.05; Fig.
3C).
Immunohistochemistry
Immunohistochemical staining for osteocalcin was
used to detect new bone specimens at three time points following
surgery. Briefly, osteocalcin-positive cells were observed in the
regeneration bone area of groups C and D throughout newly formed
bone with lacunae. The area that stained positive for bone
osteocalcin in group D was larger that in group C at each time
point and bone formation in group D was increased compared to group
C (Fig. 3D).
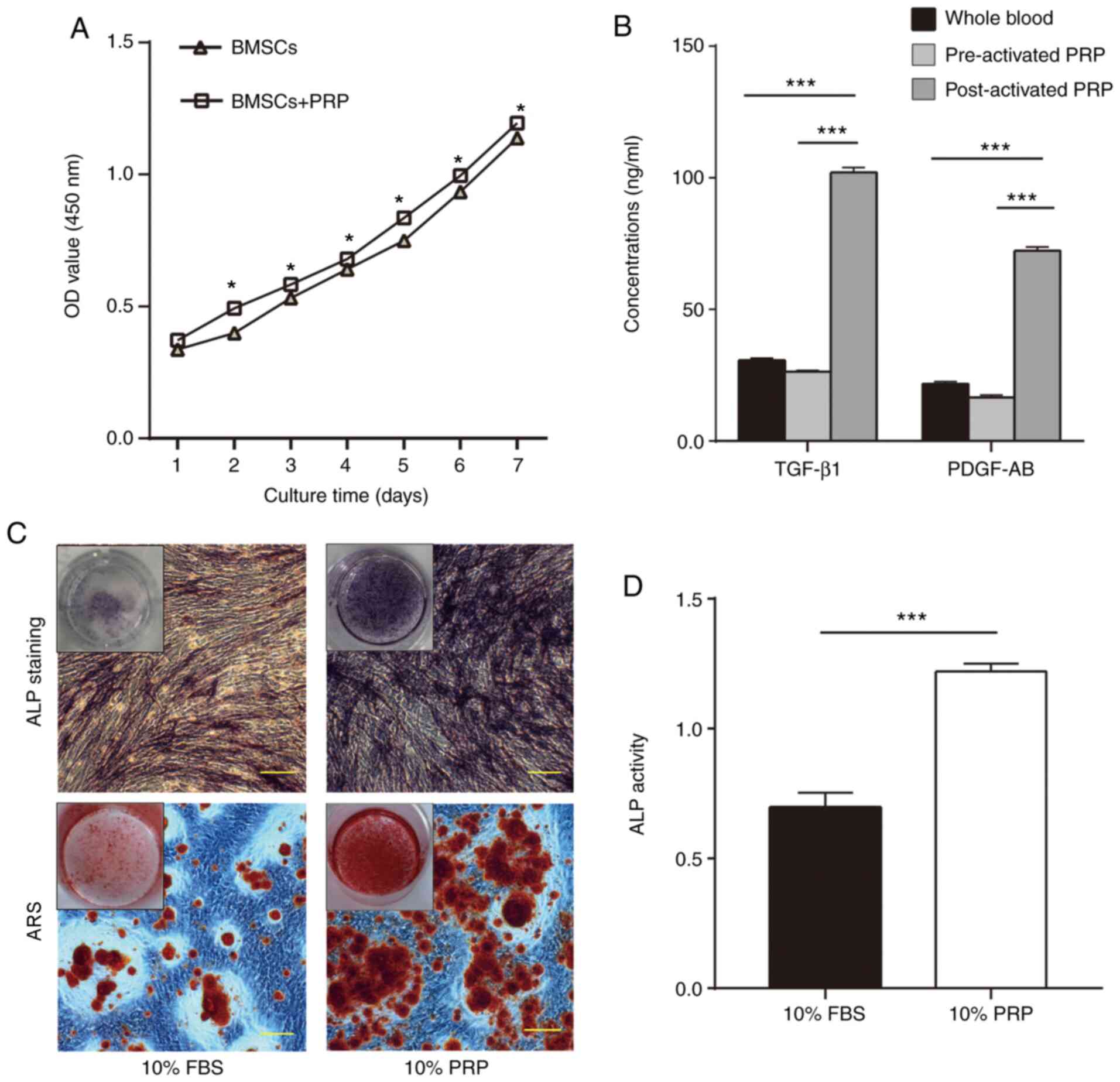
CCK-8 assay
To evaluate BMSC proliferation with or without PRP,
growth curves were established using a CCK-8 kit. The proliferative
activity of the PRP group was increased compared with that of the
control group (without PRP). The difference between the two groups
was significant (P<0.05; Fig.
4A).
GF levels in PRP
The concentrations of TGF-β1 and PDGF-AB in the
three groups (whole blood plasma, and pre-and post-activated PRP)
were measured using ELISA. As shown in Fig. 4B, the levels of the two GFs were
higher in post-activated PRP (102.11±1.56 and 72.27±1.37 ng/ml)
compared with those in pre-activated PRP (26.35±0.40 and 16.60±0.85
ng/ml) and whole blood plasma (30.57±0.78 and 21.70±0.86 ng/ml;
P<0.001).
Osteogenic effect of PRP on BMSCs
To detect the osteogenic potential of PRP, ALP and
ARS staining were performed after 14 days of osteogenic induction.
The results revealed that cells treated with 10% PRP exhibited more
matrix mineralization compared with in cells treated with 10% FBS
(Fig. 4C). ALP activity was
measured following osteogenic induction for 2 weeks. The ALP
activity of BMSCs in the 10% PRP group was higher compared with
that in the 10% FBS group (P<0.001; Fig. 4D).
Discussion
The present study revealed that the combination of
PRP, BMSCs and autogenous bone particles stimulated bone
regeneration. The results confirmed the hypothesis that PRP may be
beneficial to bone grafting, as it had positive effects on cell
proliferation and osteogenic differentiation, and, when activated,
it induced the expression of TGF-β1 and PDGF-AB. Fracture healing
may be divided into three stages: i) Inflammatory phase (hematoma
and granulation tissue), this stage lasts about 2-4 weeks. ii)
Reparative phase (callus formation), in areas closer to the
well-vascularized healthy bone tissue, osteogenic cells
differentiate into osteoblasts, which produce spongy bone
trabeculae, this stage lasts ~4-8 weeks. iii) Remodeling phase,
this is the final phase of healing, where compact bone replaces
spongy bone around the periphery of the fracture site, this stage
lasts ~8-12 weeks (23). Therefore,
4, 8 and 12 weeks were chosen as the time points assessed in the
present study; this is consistent with other studies (23,24).
Tajima et al (21) reported that adipose-derived stem
cells (ASCs) combined with PRP exhibited an augmentative effect on
bone regeneration in a rat calvarial defect model. Moreover,
Blaszczyk et al (25)
suggested that a combination of poly(L/DL-lactide) 80/20 and PRP
may promote bone regeneration in a large sheep calvarial defect.
Furthermore, Qi et al (26)
proposed the use of BMSC/PRP gel/calcium phosphate particles as a
treatment strategy for bone defect repair in patients. Notably, the
present study revealed that autogenous bone particles/PRP/BMSCs had
a good effect on connecting bone defects. Nevertheless, certain
studies have reported some contradictory results in the use of PRP
for bone defect healing. For example, Guerra et al (27) examined the femur defect-healing
effect after treating rabbits with NuOss™, a commercially available
self-expanding composite graft, combined with plasma rich in GFs
(PRGF), and NuOss™ with a resorbable collagen membrane (RCM). Their
data suggested that the percentage of bone tissue in direct contact
with the implant surface was significantly lower in the NuOss™ +
PRGF group compared with that in the NuOss™ + RCM group at 4 and 8
weeks after surgery. Another study demonstrated that there was no
statistical difference in the total new bone formation at an early
stage (<4 weeks post-surgery/treatment) in a rabbit calvarial
defect treated with PRP mixed with particulate autogenous bone and
autogenous bone alone (28). These
contradictory results could be explained by the use of different
methods for PRP preparation, as there is currently no universal
standard for it.
The methods for PRP activation may also indirectly
influence the concentration of GFs. Landesberg et al
(13) suggested that platelets
could be destroyed by centrifugation at 250 x g, thus causing a
further decrease in the release of GFs. In the previous study by
Guerra et al (27), low bone
formation was observed following centrifugation at 391 x g. The
protocol followed by Landesberg et al (13) is considered more conducive to
releasing osteogenic GFs and promoting osteogenesis. Therefore, the
present study adapted the protocol followed by Landesberg et
al (13). Currently, the hybrid
application of calcium chloride and thrombin in chitosan is the
most commonly used approach to activate PRP compared with calcium
chloride, thrombin or chitosan alone. The method of hybrid
application of calcium chloride and thrombin to activate PRP
followed by the Landesberg protocol was chosen in the present
study. Finally, the physiological state and living environment of
the animals varied, and different animals and species could have a
vital effect on the ability of PRP.
Recently, autologous PRP has been successfully
applied as 3-D scaffold material in rebuilding human cartilage
(29). In addition, Tajima et
al (21) transplanted a mixture
of ASCs and PRP into the calvarial defect of rats and found that
the mixture induced defect healing. Nevertheless, other studies
have suggested that PRP has no effect on osteochondral defects of
the talus (30). Guerra et
al (27) indicated that PRP
might induce slight bone formation in calvarial defects. The
present results were in line with data obtained by Guerra et
al (27). In the present study,
radial defects in rabbits were filled with BMSCs and PRP (group B)
and slight bone formation was observed, with no bridge formation
around the defect. Previous research examined bone defects, of
different types and sizes (21,27,29,30).
In small defects or cartilage injury, PRP alone performed well in
remodeling and repair, whereas in the large and segmental bone
defects, PRP scaffold had limitations in osteogenesis (21,27,29,30).
Following X-ray examination in vivo, as well
as H&E staining and histomorphometry ex vivo, it was revealed
that rabbits treated with autogenous bone particles combined with
PRP and BMSCs exhibited improved bone healing compared with that in
rabbits treated with autogenous bone particles and BMSCs. A
combination of autogenous bone particles with PRP and BMSCs was
conducive to bone bridge reconstruction through the proliferation
of BMSCs and the release of GFs by platelets in PRP. In addition,
the present study demonstrated that BMSCs treated with PRP had a
greater proliferative activity compared with that of BMSCs without
PRP. Certain studies had similar outcomes, indicating that PRP
could be used to induce MSC proliferation and promote bone
formation (31,32). The present results revealed that
treatment with 10% PRP indicated stronger ALP and Alizarin Red
staining compared with that in cells treated with 10% FBS. PRP
exhibited a good osteogenic differentiation ability. The present
study also showed that post-activated PRP contained higher
concentrations of GFs, TGF-β1 and PDGF-AB, than pre-activated PRP.
It has been reported that PRP could improve bone healing or bone
remodeling, due to the GFs release from PRP. PRP contains several
GFs, TGF-β1 and PDGF-AB being the most prominent (12). TGF-β1 may stimulate osteoblastic and
fibroblastic properties, and the mitogenic effects of other GFs,
and contribute to BMSC differentiation. PDGF-AB is responsible for
cell proliferation, and guiding cell migration, blood vessel repair
and regeneration (33,34). The release of GFs may be the reason
why PRP promotes cell proliferation and osteogenic differentiation,
as well as osteogenesis of bone defects in vivo. Autologous
bone particles can preserve BMSCs and enhance the bone contact
area, whereas PRP can increase cell proliferation and osteogenic
differentiation, and release GFs, thus supplying more nutrients to
the surrounding tissues.
There were two limitations to the present study.
Firstly, this study did not use various doses of PRP to verify
osteogenic capacity; only a recommended dose, according to the
literature (13), was used.
Secondly, it was shown that PRP could promote bone particle
formation; however, the mechanism underlying bone regeneration
remains unclear. Future studies should focus on estimating the
ability of osteogenesis for different preparation methods, doses
and centrifugation speeds of PRP, and should explore the osteogenic
potential of BMSCs.
In conclusion, PRP itself could not bridge the
defect. The combination of particulate autologous bone grafts and
PRP could further improve bone healing in a diaphyseal rabbit model
compared with the particulate autologous bone group. The release of
cytokines by platelets in PRP may have an important role in bone
repair. This study supported the autologous use of PRP for bone
healing as a treatment option forlong-term bone defects.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HX and LC carried out the experiments and drafted
the manuscript. WS, LY, CJ and JH performed the statistical
analysis and participated in the study design. GS and JD helped to
collect data and performed the statistical analysis. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All animal studies, including the rabbit euthanasia
procedure, were approved by the IACUC of Capital Medical
University, and were conducted according to the American
Association for Accreditation of Laboratory Animal Care and the
IACUC guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kasten P, Vogel J, Geiger F, Niemeyer P,
Luginbühl R and Szalay K: The effect of platelet-rich plasma on
healing in critical-size long-bone defects. Biomaterials.
29:3983–3992. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Nooeaid P, Salih V, Beier JP and
Boccaccini AR: Osteochondral tissue engineering: Scaffolds, stem
cells and applications. J Cell Mol Med. 16:2247–2270.
2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cheng X, Wan Q and Pei X: Graphene Family
Materials in Bone Tissue Regeneration: Perspectives and Challenges.
Nanoscale Res Lett. 13(289)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Liao HT, Tsai MJ, Brahmayya M and Chen JP:
Bone Regeneration Using Adipose-Derived Stem Cells in Injectable
Thermo-Gelling Hydrogel Scaffold Containing Platelet-Rich Plasma
and Biphasic Calcium Phosphate. Int J Mol Sci.
19(2537)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wen Y, Gu W, Cui J, Yu M, Zhang Y, Tang C,
Yang P and Xu X: Platelet-rich plasma enhanced umbilical cord
mesenchymal stem cells-based bone tissue regeneration. Arch Oral
Biol. 59:1146–1154. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ferrari M, Zia S, Valbonesi M, Henriquet
F, Venere G, Spagnolo S, Grasso MA and Panzani I: A new technique
for hemodilution, preparation of autologous platelet-rich plasma
and intraoperative blood salvage in cardiac surgery. Int J Artif
Organs. 10:47–50. 1987.PubMed/NCBI
|
|
7
|
Marx RE, Carlson ER, Eichstaedt RM,
Schimmele SR, Strauss JE and Georgeff KR: Platelet-rich plasma:
Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral
Pathol Oral Radiol Endod. 85:638–646. 1998.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Xie X, Wang Y, Zhao C, Guo S, Liu S, Jia
W, Tuan RS and Zhang C: Comparative evaluation of MSCs from bone
marrow and adipose tissue seeded in PRP-derived scaffold for
cartilage regeneration. Biomaterials. 33:7008–7018. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Son SR, Sarkar SK, Nguyen-Thuy BL,
Padalhin AR, Kim BR, Jung HI and Lee BT: Platelet-rich plasma
encapsulation in hyaluronic acid/gelatin-BCP hydrogel for growth
factor delivery in BCP sponge scaffold for bone regeneration. J
Biomater Appl. 29:988–1002. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zou J, Yuan C, Wu C, Cao C and Yang H: The
effects of platelet-rich plasma on the osteogenic induction of bone
marrow mesenchymal stem cells. Connect Tissue Res. 55:304–309.
2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chahla J, Cinque ME, Piuzzi NS, Mannava S,
Geeslin AG, Murray IR, Dornan GJ, Muschler GF and LaPrade RF: A
Call for Standardization in Platelet-Rich Plasma Preparation
Protocols and Composition Reporting: A Systematic Review of the
Clinical Orthopaedic Literature. J Bone Joint Surg Am.
99:1769–1779. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Oryan A, Alidadi S and Moshiri A:
Platelet-rich plasma for bone healing and regeneration. Expert Opin
Biol Ther. 16:213–232. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Landesberg R, Burke A, Pinsky D, Katz R,
Vo J, Eisig SB and Lu HH: Activation of platelet-rich plasma using
thrombin receptor agonist peptide. J Maxillofac Surg. 63:529–535.
2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Oryan A, Alidadi S, Moshiri A and Maffulli
N: Bone regenerative medicine: Classic options, novel strategies,
and future directions. J Orthop Surg Res. 9(18)2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Duan R, Barbieri D, de Groot F, de Bruijn
JD and Yuan H: Modulating Bone Regeneration in Rabbit Condyle
Defects with Three Surface-Structured Tricalcium Phosphate
Ceramics. ACS Biomater Sci Eng. 4:3347–3355. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang XT, Zhou CL, Yan JL, Yan X, Xie HX
and Sun CL: The fate of donor osteocytes in fine particulate bone
powders during repair of bone defects in experimental rats. Acta
Histochem. 114:192–198. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xie H, Ji Y, Tian Q, Wang X, Zhang N,
Zhang Y, Xu J, Wang N and Yan J: Autogenous bone particle/titanium
fiber composites for bone regeneration in a rabbit radius
critical-size defect model. Connect Tissue Res. 58:553–561.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sun YX, Sun CL, Tian Y, Xu WX, Zhou CL, Xi
CY, Yan JL and Wang XT: A comparison of osteocyte bioactivity in
fine particulate bone powder grafts vs larger bone grafts in a rat
bone repair model. Acta Histochem. 116:1015–1021. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sun C, Tian Y, Xu W, Zhou C, Xie H and
Wang X: Development and performance analysis of Si-CaP/fine
particulate bone powder combined grafts for bone regeneration.
Biomed Eng Online. 14(47)2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Graham N and Qian BZ: Mesenchymal Stromal
Cells: Emerging Roles in Bone Metastasis. Int J Mol Sci.
19(1121)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tajima S, Tobita M, Orbay H, Hyakusoku H
and Mizuno H: Direct and indirect effects of a combination of
adipose-derived stem cells and platelet-rich plasma on bone
regeneration. Tissue Eng Part A. 21:895–905. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tian Y, Cui L-H, Xiang S-Y, Xu WX, Chen
DC, Fu R, Zhou CL, Liu XQ, Wang YF and Wang XT: Osteoblast-oriented
differentiation of BMSCs by co-culturing with composite scaffolds
constructed using silicon-substituted calcium phosphate, autogenous
fine particulate bone powder and alginate in vitro. Oncotarget.
8:88308–88319. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang D, Huang D, Huang Y, Liu Y, Lin B,
Yu C, Mou Y, Wu W, Zhang H and Lin H: Efficacy of combined therapy
of periosteum and bone allograft in a critical-sized defect model
in New Zealand white rabbits. Med Sci Monit. 20:2394–2403.
2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Liu F, Chen K, Hou L, Li K, Wang D, Zhang
B and Wang X: Determining the critical size of a rabbit rib
segmental bone defect model. Regen Biomater. 3:323–328.
2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Błaszczyk B, Kaspera W, Ficek K, Kajor M,
Binkowski M, Stodolak-Zych E, Grajoszek A, Stojko J, Bursig H and
Ładziński P: Effects of Polylactide Copolymer Implants and
Platelet-Rich Plasma on Bone Regeneration within a Large Calvarial
Defect in Sheep. BioMed Res Int. 2018(4120471)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Qi Y, Niu L, Zhao T, Shi Z, Di T, Feng G,
Li J and Huang Z: Combining mesenchymal stem cell sheets with
platelet-rich plasma gel/calcium phosphate particles: A novel
strategy to promote bone regeneration. Stem Cell Res Ther.
6(256)2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Guerra I, Morais Branco F, Vasconcelos M,
Afonso A, Figueiral H and Zita R: Evaluation of implant
osseointegration with different regeneration techniques in the
treatment of bone defects around implants: An experimental study in
a rabbit model. Clin Oral Implants Res. 22:314–322. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Broggini N, Hofstetter W, Hunziker E,
Bosshardt DD, Bornstein MM, Seto I, Weibrich G and Buser D: The
influence of PRP on early bone formation in membrane protected
defects. A histological and histomorphometric study in the rabbit
calvaria. Clin Implant Dent Relat Res. 13:1–12. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Fernandes G and Yang S: Application of
platelet-rich plasma with stem cells in bone and periodontal tissue
engineering. Bone Res. 4(16036)2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Niemeyer P, Fechner K, Milz S, Richter W,
Suedkamp NP, Mehlhorn AT, Pearce S and Kasten P: Comparison of
mesenchymal stem cells from bone marrow and adipose tissue for bone
regeneration in a critical size defect of the sheep tibia and the
influence of platelet-rich plasma. Biomaterials. 31:3572–3579.
2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lai F, Kakudo N, Morimoto N, Taketani S,
Hara T, Ogawa T and Kusumoto K: Platelet-rich plasma enhances the
proliferation of human adipose stem cells through multiple
signaling pathways. Stem Cell Res Ther. 9(107)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Eda T, Takahashi K, Kanao S, et al:
Comparison study between plasma rich in growth factors and
platelet-rich plasma for osteoconduction in rat calvaria. J Oral
Maxillofac Surg. 29:563–569. 2017.
|
|
33
|
Wang D, Weng Y, Guo S, Zhang Y, Zhou T,
Zhang M, Wang L and Ma J: Platelet-rich plasma inhibits
RANKL-induced osteoclast differentiation through activation of Wnt
pathway during bone remodeling. Int J Mol Med. 41:729–738.
2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Qian Y, Han Q, Chen W, Song J, Zhao X,
Ouyang Y, Yuan W and Fan C: Platelet-Rich Plasma Derived Growth
Factors Contribute to Stem Cell Differentiation in Musculoskeletal
Regeneration. Front Chem. 5(89)2017.PubMed/NCBI View Article : Google Scholar
|