Introduction
Diabetes mellitus is a well-known, major independent
risk factor for micro- and macro-vascular diseases and subsequent
complications (1,2). Micro-vascular complications are
comprised of retinopathy, nephropathy and neuropathy, while
macro-vascular complications involve atherosclerosis-related
diseases, including atherosclerotic cardiovascular, cerebrovascular
and peripheral vascular diseases (3). Atherosclerosis accelerated by diabetes
is a process that has a complex pathophysiology, where
dyslipidemia, hormonal abnormalities, oxidative stress,
hyperglycemia and a pro-inflammatory state have all been documented
to serve critical roles (4,5). These changes modulate the direct
consequences of hyperglycemia on diabetic atherosclerosis and alter
the pathogenesis of diabetes itself. Oxidative stress and
inflammation have reciprocal interactions, such that oxidative
stress directly induces the production of pro-inflammatory
cytokines and mediators, which in turn promotes the production of
reactive oxygen species (ROS) (5).
Both inflammation and ROS pathways impair pancreatic β cell
activity, insulin secretion and resistance (5). Therefore, prevention of the vascular
inflammatory state and oxidative stress may prove to be a potential
therapeutic strategy for improving the outcomes of diabetic
atherosclerosis.
Dysregulation of 5'adenosine monophosphate-activated
protein kinase (AMPK) activation also contributes to the onset and
progression of diabetic atherosclerosis (6). AMPK activity has been previously
demonstrated to be reduced in response to chronic inflammation in
adipocytes of a type II diabetic mellitus murine model and in human
adipose tissues (7,8). AMPK suppresses the expression of NF-κB
by increasing the expression of sirtuin 1 (SIRT1), thereby
minimizing the inflammatory response (9). SIRT1 is a class III histone
deacetylase that serves an important role in modulating the
pathogenesis of chronic conditions, including diabetes and
cardiovascular disease (10).
Additionally, SIRT1 has been reported to increase cellular ability
to remove ROS by superoxide dismutase (SOD) activation (9,11).
Quercetin is a natural flavonoid that can be found
in abundance in plant-based foods, including red onions, tea,
apples, capers, broccoli, parsley and red grapes (12). It has been previously revealed to
mediate a multitude of physiological functions with a broad
spectrum of pharmacological properties, including
anti-inflammatory, anti-diabetic, lipid metabolism modulation and
anti-oxidative capacities (13).
Furthermore, several studies have demonstrated that quercetin
reduces the plasma concentrations of total cholesterol (Chol) and
triglycerides (TG), and increases the concentration of high-density
lipoprotein (HDL) Chol (14,15).
Additionally, previous studies have reported that quercetin
exhibits protective effects against atherosclerosis in rodents
(16,17) and enhances SIRT1 and AMPK activity
(18,19). Therefore, due to these
aforementioned protective effects of quercetin against metabolic
disorders and its potential to alleviate oxidative-inflammatory
responses in cardiovascular disease, the aim of the present study
was to evaluate the effects of quercetin on the AMPK/SIRT1/NF-κB
signaling pathway and inflammatory/oxidative stress responses in
diabetes-induced atherosclerosis in rat carotid arteries.
Materials and methods
Animal maintenance and drugs
A total of 30 male Wistar rats (age, 7-8 weeks;
weight, 250±20 g) were maintained and housed in stainless steel,
wire-bottomed cages in a room at 12-h light/dark cycles, an ambient
temperature of 23±2˚C and at 60% humidity. Water and food were
provided to all animals ad libitum. All experiments were
approved by the Ethics Committee of the Second Affiliated Hospital
of Xi'an Medical University (Xian, China; approval no. 2019-1213)
and performed in accordance with the Guidelines of the National
Institutes of Health (publication no. 85-23; 1996) (20). Quercetin and compound-C (CC), an
AMPK inhibitor, were purchased from Sigma-Aldrich, Merck KGaA.
Animal grouping
Following feeding with a normal diet and adaptation
for 2 weeks, the animals were subsequently randomly allocated into
five groups (n=6 rats/group): i) Control; ii) control-quercetin
(control-Q); iii) high-fat diabetic (HFD); iv) HFD-quercetin
(HFD-Q); and v) HFD-quercetin-CC C (HFD-Q-CC).
The control group was fed a normal diet for 8 weeks.
The control-Q group was fed a normal diet for 8 weeks and received
quercetin (30 mg/kg) orally each day for 2 weeks prior to tissue
sampling. Quercetin was dissolved in 2% DMSO and was administered
once daily at a volume of ~10 ml/kg body weight using a 16-gauge
feeding tube. HFD was the atherosclerotic model group and was fed
the high-fat and Chol diet for 8 weeks. By contrast, rats in the
HFD-Q group were fed the high-fat and Chol diet for 8 weeks and
received quercetin (30 mg/kg) orally in the final 2 weeks of the
diet, similar to the control-Q group. The HFD-Q-CC group was fed
the high-fat and Chol diet for 8 weeks and received quercetin (30
mg/kg) orally and CC intravenously (0.2 mg/kg) for 5 days within
the period of quercetin gavage (once every 3 days), starting
alongside quercetin treatment (21,22).
Each rat in the HFD-Q-CC group was injected with CC 5 times in
total. The food, including both high-fat and Chol diet, and normal
pellets, was adjusted to 120 g. The control rats received equal
amounts of saline containing 2% DMSO to minimize the effects of the
procedures on the experimental results.
High-fat diet induction of diabetes
development
A high-fat diet and a low-dose streptozotocin
protocol was used to induce type II diabetes mellitus and the
development of atherosclerosis in the rats. After 2 weeks of
acclimatization, rats were fed on a high-fat Chol-saturated diet
containing standard pellets supplemented with 1% Chol, 8% lard and
0.05% cholate (w/w; 62% calories from fat). Streptozotocin (35
mg/kg) dissolved in citrate buffer at pH 4.5 was then injected
intraperitoneally (i.p) at the beginning of week 4. After 72 h, one
drop of tail vein blood was obtained through a small scratch with
lancet and fasting blood glucose (FBS) levels in the animals were
measured using a glucometer (Convergent Technologies; GmbH &
Co. KG), where rats with FBS >250 mg/dl were assigned into the
HFD diabetic group (23). The total
diabetic period was 8 weeks.
Measurement of lipid profile levels
and atherogenic index (AI)
Following fasting for 12 h, animals were
anesthetized with an i.p administration of sodium pentobarbital (40
mg/kg) before blood samples (~3 ml from portal veins following
laparotomy prior to carotid artery tissue sampling) were collected
and centrifuged at 1,400 x g for 10 min at 4˚C to obtain the
plasma. Subsequently, plasma levels of TG (cat. no. MBS164762;
MyBioSource, Inc.), Chol (cat. no. MBS775433; MyBioSource, Inc.)
and HDL (cat. no. 79970; Crystal Chem, Inc.) and low-density
lipoprotein (LDL; cat. no. 79960; Crystal Chem, Inc.) were measured
using specific assay kits, according to the manufacturer's
protocols. The AI for each rat was calculated using the following
formula: AI=Chol-HDL/HDL.
Oxidative stress marker measurements
in carotid arteries
Following blood sampling, the animals underwent a
surgical procedure to obtain the carotid artery samples under
anesthesia. Followings tissue sampling, animals were euthanized by
an overdose of sodium pentobarbital (200 mg/kg; i.p). Levels of
oxidative stress in the carotid artery samples were evaluated by
measuring malondialdehyde (MDA) content and the activities of
glutathione peroxidase (GPX), SOD and catalase (CAT). Briefly, 100
mg carotid artery tissues were cut into small sections (~2
mm2) and mixed with a 10X volume of pre-cooled saline
before the mixture was homogenized at 4˚C. The homogenates were
centrifuged at 1,400 x g for 10 min at 4˚C. The supernatants were
assessed using specific assay kits or reagents (Randox
Laboratories, Ltd.; GPX, cat. no. RS504; SOD, cat. no. SD125). MDA
and CAT were measured according to methods designed by Aebi et
al (24) and Ohkawa et
al (25), respectively. The
protein MDA levels were expressed as nmol/mg protein, whereas the
GPX, SOD and CAT activities were expressed as U/mg protein.
Measurement of pro-inflammatory and
anti-inflammatory mediators
NF-κB (cat. no. MBS265868), IL-1β (cat. no.
MBS702717) and IL-10 (cat. no. MBS2700945) levels in the carotid
arteries were measured using specific ELISA kits, according to the
manufacturer's protocol (MyBioSource, Inc.). A coating plate was
then placed in the ELISA reader to read the absorbance of each
sample at 450 nm. All optical density values were converted to the
final concentration based on the amount of protein (mg) of each
sample and presented as pg/mg total protein.
Histological examination of
atherosclerotic carotid arteries
Initially, the adipose tissues were removed from the
carotid arteries of the rats prior to being fixed in 10% formalin
for 24 h at room temperature. Following tissue dehydration with an
ascending series of ethanol and washing with xylene, the paraffin
wax-embedded carotid samples were cut (thickness, 4-µm)
transversally and stained with hematoxylin for 20 min and eosin for
8 min at room temperature. Finally, an optical light microscope
(magnification, x40) was used to examine atherosclerotic changes in
the artery.
Western blot analysis
The artery tissue samples were homogenized in RIPA
lysis buffer (cat. no. 9806S; Cell Signaling Technology, Inc.)
containing protease inhibitors (cat. no. 5871S; Cell Signaling
Technology, Inc.). Following centrifugation at 9,800 x g for 10 min
at 4˚C, equal amounts of protein (20 µg) from carotid tissue
supernatants were separated on 12.5% SDS-PAGE and transferred to
PVDF membranes (EMD Millipore). Following blocking with 5% skimmed
milk for 1 h at room temperature, the membranes were incubated with
primary antibodies against SIRT1 (cat. no. 8649S; 1:1,500; Cell
Signaling Technology, Inc.) and β-actin (cat. no. 3700S; 1:5,000;
Cell Signaling Technology, Inc.) overnight at 4˚C. After four 5 min
washes with PBS/0.1% Tween-20, membranes were incubated with
horseradish peroxidase-conjugated goat anti-mouse secondary
antibodies (cat. no. 7076S; 1:2,500; Cell Signaling Technology,
Inc.) for 2 h at room temperature. Following rinsing, the protein
bands were visualized using the enhanced chemiluminescence reagent
(Santa Cruz Biotechnology, Inc.). Protein band intensity was
measured using the ImageJ software (version no. 1.6; National
Institutes of Health) and normalized to β-actin.
Statistical analysis
Data are presented as mean ± SEM. Six rats were
included in each group. One rat was removed from the results for
western blot analysis and histological examination due to
inadequate tissue sampling. Data for the remaining 5 rats were
analyzed for these experiments. Comparisons between groups were
performed with one-way ANOVA with Tukey's post-hoc test using
GraphPad Prism software (version no. 5.0; GraphPad Software, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Plasma lipid levels of rats in the
experimental groups
The levels of Chol, TG, HDL, LDL and FBS in the
control-Q group did not significantly differ compared with the
control group (Table I). However,
the HDL/LDL and HDL/Chol ratios were demonstrated to be
significantly increased in the non-diabetic group receiving
quercetin compared with the control group (P<0.05). Lipid
profiles and FBS in the HFD group were revealed to be significantly
increased compared with rats in the control group (P<0.01).
However, treatment of diabetic rats with quercetin significantly
reduced FBS, Chol, TG and LDL levels (P<0.01), while increasing
the HDL/LDL and HDL/Chol ratios (P<0.05) compared with those in
the HFD group. However, the lipid profile changes as a result of
quercetin treatment were reversed by CC administration compared
with the HFD-Q group (P<0.05 for LDL, HDL/LDL and HDL/Chol;
P<0.01 for Chol and TG; Table
I).
 | Table IBiochemical parameters of the
experimental groups. |
Table I
Biochemical parameters of the
experimental groups.
| Groups | FBS (mg/dl) | Chol (mg/dl) | TG (mg/dl) | LDL (mg/dl) | HDL (mg/dl) | HDL/LDL ratio | HDL/Chol ratio |
|---|
| Control | 98±5 | 49.3±4.0 | 96.6±5.5 | 27.3±2.6 | 14.8±2.7 | 0.50±0.03 | 0.32±0.03 |
| Control-Q | 95±7 | 40.4±3.6 | 81.3±5.3 | 26.4±2.2 | 19.8±1.3 |
0.73±0.08a |
0.47±0.06a |
| HFD | 415±26b |
395.3±26.5b |
236.5±21.0b |
261.0±16.3b |
46.7±5.5b |
0.18±0.04b |
0.12±0.02b |
| HFD-Q | 236±15d |
215.7±13.4d |
114.3±11.9d |
202.5±14.7c | 54.5±5.9 |
0.29±0.06c |
0.26±0.04c |
| HFD-Q-CC | 316±19e |
373.8±29.1f |
226.7±30.3f |
256.3±20.6e | 40.2±5.0 |
0.14±0.05e |
0.10±0.04e |
Carotid artery levels of NF-κB, IL-1β
and IL-10
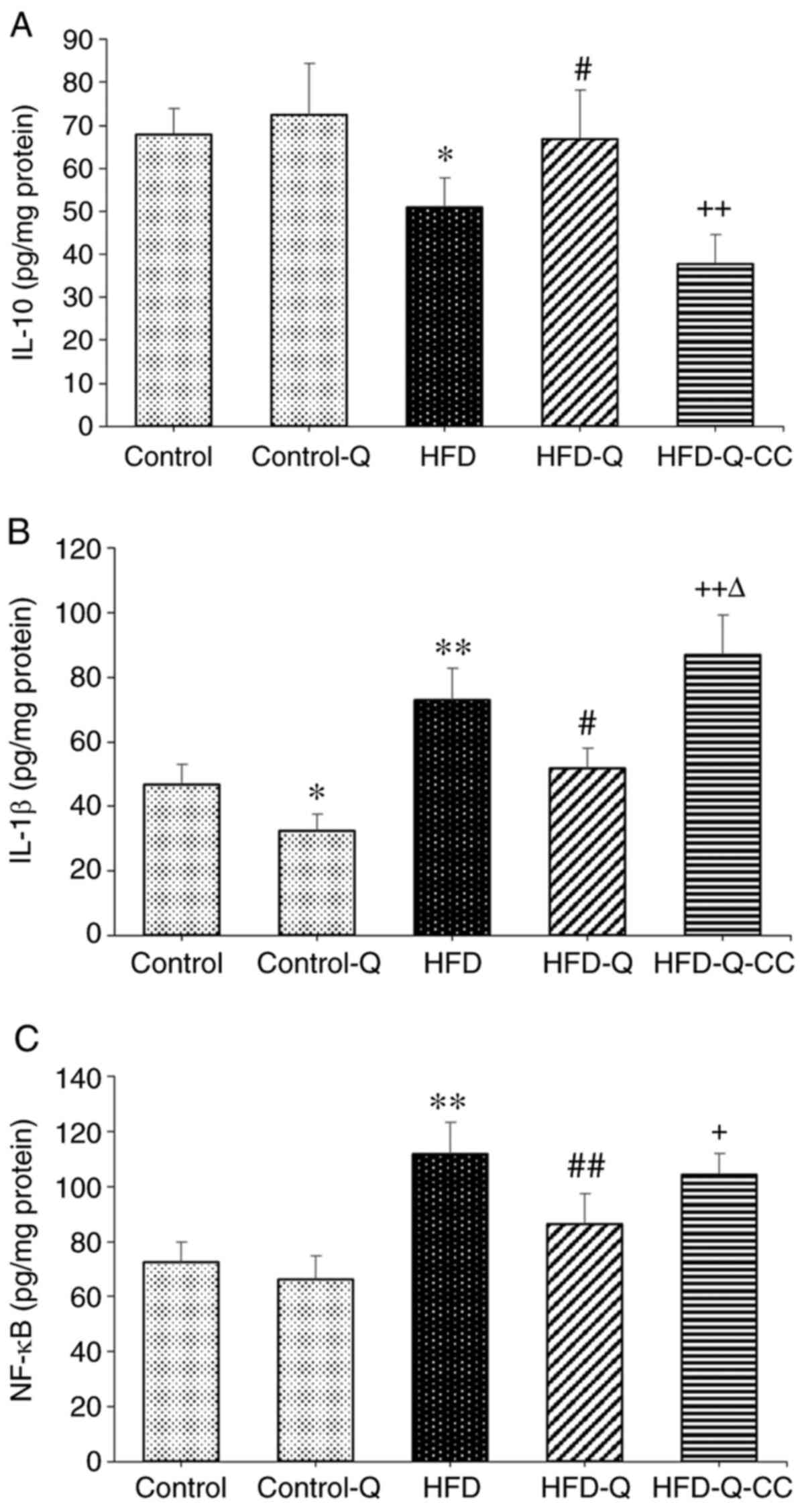
Rats fed on HFD exhibited significantly higher NF-κB
and IL-1β levels (P<0.01) and significantly lower IL-10 levels
(P<0.05) compared with the control animals (Fig. 1). However, quercetin treatment
significantly suppressed the tissue levels of NF-κB and IL-1β
(P<0.01) and increased IL-10 levels (P<0.05) compared with
rats in the HFD group (Fig. 1).
Inhibition of AMPK by CC significantly reversed the protective
effects of quercetin on all three of these parameters (P<0.05;
Fig. 1). Additionally, the levels
of IL-1β in the HFD-Q-CC group was significantly higher than those
in the HFD group (P<0.05; Fig.
1B).
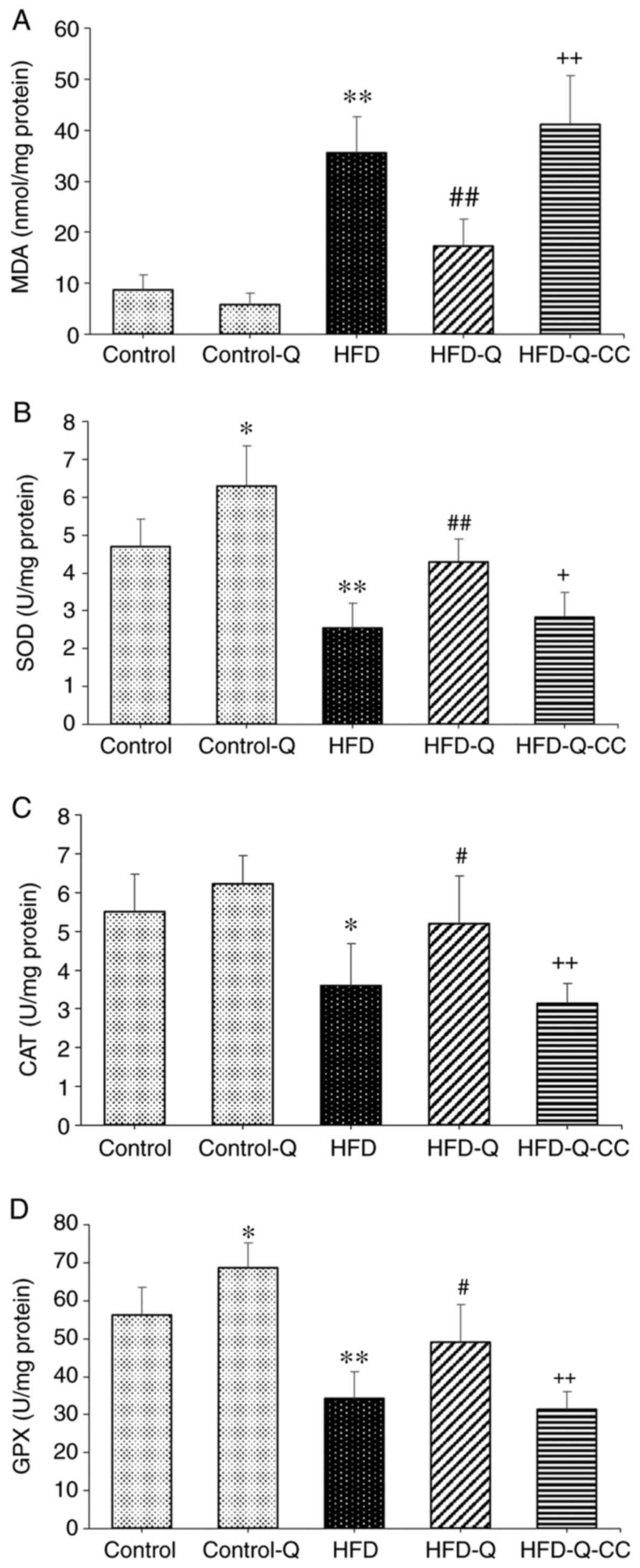
Measurement of oxidative stress
markers
The levels of MDA were reported to be significantly
higher in the HFD group compared with the control group
(P<0.01), which was significantly reversed by quercetin
treatment (P<0.01; Fig. 2).
Additionally, following treatment with the AMPK inhibitor CC in the
HFD-Q group, the MDA level was demonstrated to be significantly
increased (P<0.01; Fig. 2).
Furthermore, HFD was revealed to significantly attenuate the
activities of GPX (P<0.01), SOD (P<0.01) and CAT (P<0.05)
compared with the control group (Fig.
2), all of which were significantly reversed by quercetin
treatment (P<0.05). Administration of the AMPK inhibitor CC to
the HFD-Q group significantly abrogated the effects of quercetin
(P<0.05 for SOD; P<0.01 for GPX and CAT; Fig. 2).
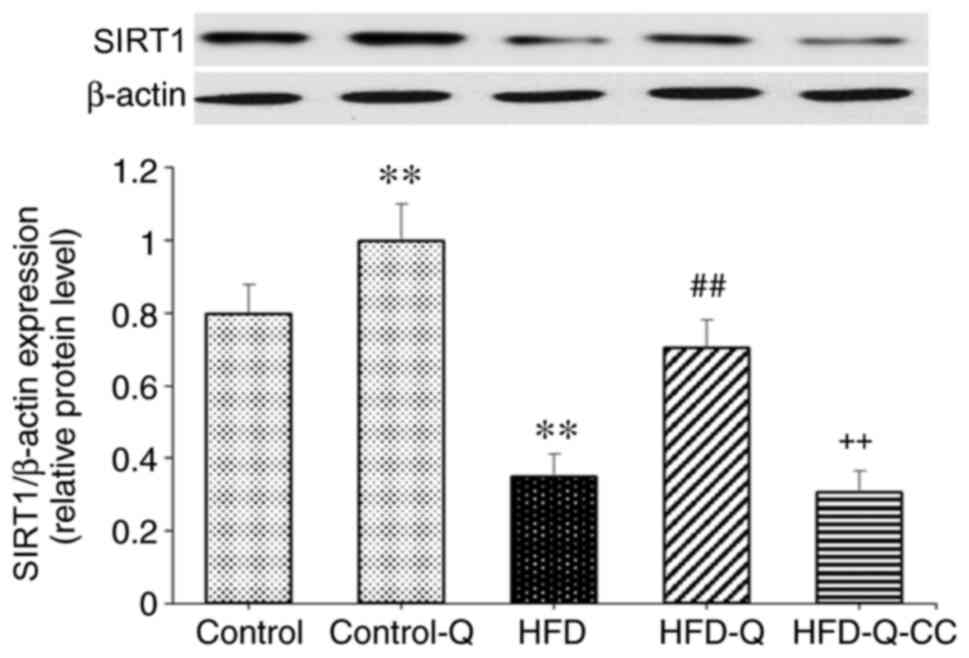
Expression of SIRT1 protein
Quercetin significantly increased the protein
expression of SIRT1 compared with the control group (P<0.05;
Fig. 3). The expression level of
SIRT1 was significantly decreased in the HFD group compared with
the control group (P<0.01). Additionally, quercetin
administration in rats fed on HFD resulted in a significant
increase in SIRT1 protein expression (P<0.01). However,
inhibition of AMPK by CC abolished the effects of quercetin on
SIRT1 expression in HFD rats (P<0.01; Fig. 3).
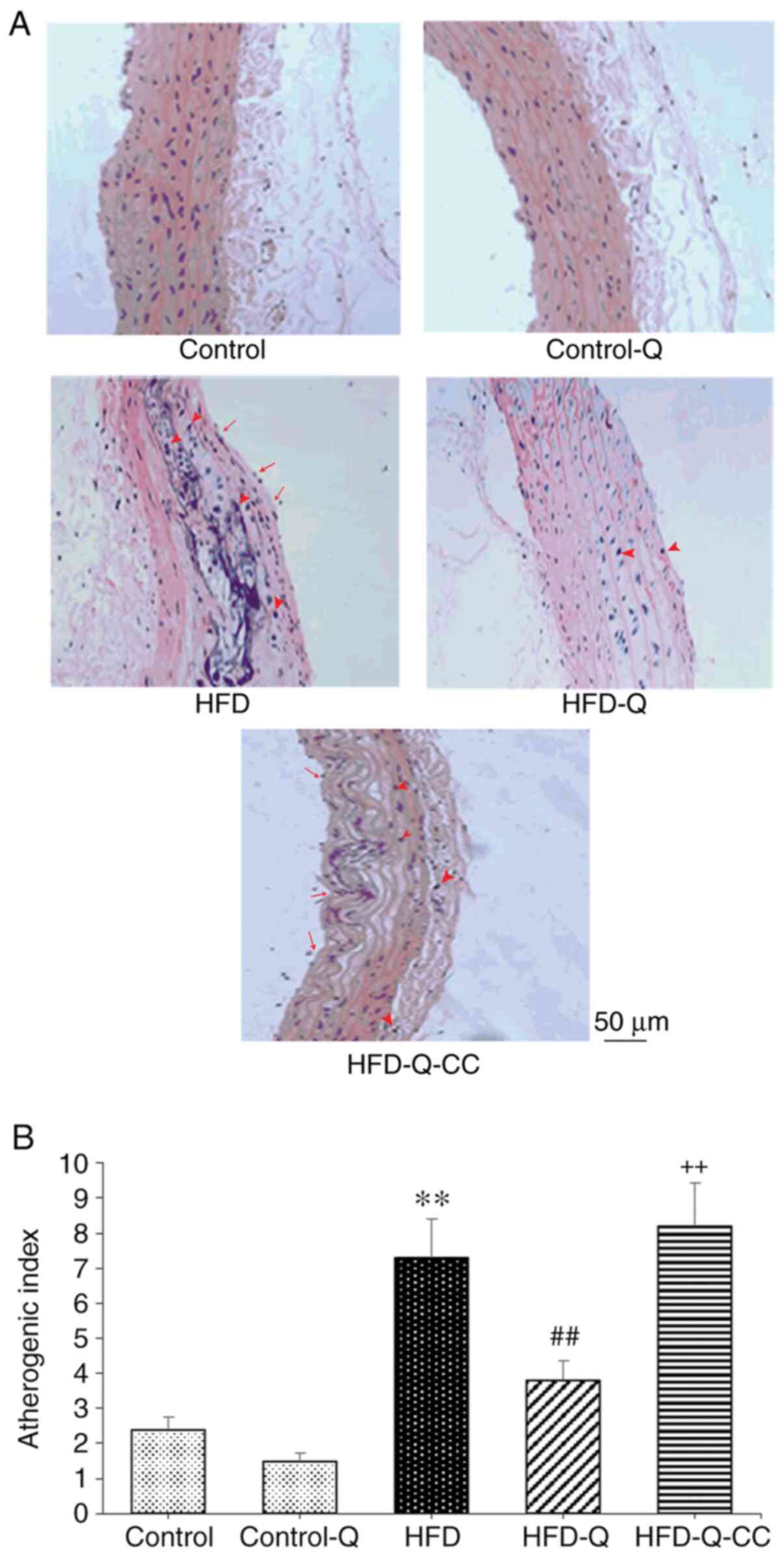
Histological analysis and AI
Rats in the HFD group exhibited superficial erosion
in the carotid artery accompanied by fat deposition and foamy
macrophage accumulation, as indicated by the arrows in Fig. 4A, and a significantly increased AI
was observed compared with the control rats (P<0.01; Fig. 4). Administration of quercetin to the
rats in the HFD group suppressed the formation of atheromatous
plaques and foam cells, in addition to significantly reducing the
AI compared with rats in the HFD group (P<0.01). However, CC
treatment markedly increased the atherosclerotic changes, whilst
significantly increasing the AI to suppress the protective effects
of quercetin (P<0.01; Fig.
4).
Discussion
The present study demonstrated that quercetin
treatment ameliorated the levels of hyperlipidemia, inflammatory
cytokines and oxidative stress in the carotid arteries of diabetic
rats fed on HFD. These protective effects of quercetin were
mediated by the modulation of the AMPK/SIRT1/NF-κB signaling
pathway.
Oxidative stress resulting from an imbalance in the
redox equilibrium may induce vascular dysfunction and contribute to
atherosclerotic plaque formation (5). The importance of oxidative stress in
the progression of vascular complications have been previously
documented in diabetes, particularly in type II diabetes (26,27).
Previous reports have demonstrated that an elevation in the
production of SOD, CAT and GPX antioxidants lead to a reduction in
ROS levels in diabetes (5,27,28).
Any reverse alterations in the levels of these enzymes trigger
oxidative stress in tissues and, consequently, results in diabetic
complications (28). In the present
study, in addition to the elevation in MDA levels, the activity
levels of SOD, CAT and GPX were demonstrated to be decreased in
rats in the HFD diabetic group compared with the control group.
Changes in these oxidative stress indices were revealed to be
associated with increased LDL, Chol, TG, AI and increased formation
of atherosclerotic plaques. Additionally, ROS has been reported to
induce inflammation by increasing the levels of the
pro-inflammatory cytokines IL-1β, IL-6, TNF-α and NF-κB and by
upregulating the expression of adhesion molecules (29,30).
Inflammatory reactions contribute to insulin resistance,
hyperglycemia and, consequently, the progression of diabetic
complications, followed by imbalances in redox signaling in tissues
and further aggravation of diabetic atherosclerosis (31). Consistent with this notion,
HFD-induced diabetes in the present study resulted in the
upregulation of NF-κB and IL-1β and downregulation of IL-10.
Quercetin is a plant polyphenol with a variety of
previously reported pharmacological actions (32). Administration of quercetin to
HFD-receiving rats was demonstrated to markedly protect the carotid
artery against diabetic atherosclerosis in the present study. The
anti-atherosclerotic effects of quercetin have been associated with
its suppressive effects on oxidative stress and inflammatory
responses (12,17). Quercetin reversed HFD-induced
alterations in all inflammatory and oxidative stress parameters in
atherosclerotic rats and significantly increased the HDL/LDL
ratios, SOD and GPX levels, and reduced IL-1β levels in the carotid
arteries of rats in the control group receiving the normal diet.
These findings indicated that quercetin mediated significant
anti-oxidative and anti-inflammatory effects under both normal and
atherosclerotic conditions. Yao et al (33) previously deduced that quercetin
intake was inversely correlated with the prevalence of diabetes in
patients with type II diabetes. Additionally, quercetin was
observed to control disease outcomes in a high-fructose diet model
of diabetes by enhancing the PI3K/AKT signaling pathway, whilst
inhibiting ROS (34). In
particular, a previous in vitro and in vivo study in
rats documented the antioxidant properties of quercetin, which were
reported to be triggered by elevating mitochondrial activity along
with the suppression of adipogenic factors (35).
AMPK serves as a major intracellular energy sensor,
where dysregulations in its activity has been reported to
contribute to the pathogenesis and progression of diabetic
atherosclerosis (6). AMPK inhibited
inflammatory responses by regulating numerous downstream signaling
pathways (5,8,9). In
addition, AMPK has been found to suppress TNF-α-dependent adhesion
of monocytes to endothelial cells in the human aorta (36). Furthermore, it has been demonstrated
that activation of AMPK reduces endoplasmic reticulum stress
induced by oxidized LDL, thereby improving endothelial dysfunction
and atherosclerosis in an in vivo model (37). This is consistent with the present
study, which reported that the beneficial effects of quercetin on
carotid atherosclerosis were abrogated following the inhibition of
AMPK activity. This indicated that the anti-oxidative and
anti-inflammatory effects of quercetin in the carotid artery of HFD
rats are, at least in part, AMPK-dependent. A previous study
reported that quercetin increased the phosphorylation of AMPK in
cultured smooth muscle cells and aortic arteries (38), which also exhibited increased levels
of acetyl CoA carboxylase, a downstream protein of AMPK,
implicating the increased activity of AMPK following quercetin
administration (38). By contrast,
it was observed that the HFD regimen led to the downregulation of
SIRT1 protein expression, which was reversed by the administration
of quercetin. Subsequently, inhibition of AMPK using CC
significantly reversed the effects of quercetin on SIRT1 expression
in HFD rats. Therefore, this observation indicated that quercetin
upregulated AMPK activity to increase SIRT1 protein expression.
SIRT1 serves an important intermediary role in the
anti-atherosclerotic effects of quercetin, the expression of which
is high in human endothelial and vascular cells to regulate
endothelial and vascular function (39). Upregulation of SIRT1 expression or
increased SIRT1 activity has been previously revealed to inhibit
endothelial dysfunction induced by oxidative stress (40). Additionally, AMPK has been
demonstrated to upregulate the expression of SIRT1 and repress the
expression of NF-κB, IL-1β and TNF-α (5,9).
Furthermore, AMPK elevated the expression levels of IL-10(40). Therefore, according to the results
of the present study, modulation of the AMPK/SIRT1/NF-κB signaling
pathway by quercetin was most likely responsible for its
anti-hyperlipidemic, anti-inflammatory and anti-oxidative
properties against atherosclerosis. However, the effects of
quercetin on other candidates for the onset and progression of
atherosclerosis, including nitric oxide, adhesion molecules,
protein kinase C, intracellular Ca2+ signaling,
autophagy and mitochondrial dysfunction, warrant further
investigation (5). These
investigations are required to further the understanding of the
pathophysiology of HFD and diabetes-accelerated atherosclerosis,
and the development of novel preventive and therapeutic
measures.
As a limitation, quercetin was used following the
confirmation of diabetes. Due to a previous report on the
beneficial effects of quercetin against metabolic dysfunction and
inflammatory disease (41), it was
hypothesized that quercetin may have the potential to prevent or
alleviate complications associated with diabetes in patients with
obesity. However, further studies are required to clarify this
important issue. Additionally, one of the most important end
effectors of AMPK/SIRT1 activation in reducing oxidative stress,
tissue inflammation and apoptosis is by promoting mitochondrial
biogenesis and by altering mitochondrial physiology (42). This aspect was not addressed in the
present study and requires further investigation.
In conclusion, the results of the present study
demonstrated that quercetin exerted beneficial effects against
atherosclerosis in the carotid artery of HFD rats. This was
mediated by reducing hyperlipidemia and inflammatory and oxidative
stress. These promising effects of quercetin were demonstrated to
be associated with the SIRT1/NF-κB signaling pathway, where AMPK is
a critical regulator.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Second
Affiliated Hospital of Xi'an Medical University, Xi'an, China
(grant no. 2019-1213).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FZ, JF and DQ designed and performed the
experiments. JZ and DQ aided with hypothesis conceptualization,
analyzed data and wrote the first draft of the manuscript. FZ, JZ
and XK assisted with experimental design and collaborated to
interpret the results. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All experiments were approved by the Ethics
Committee of the Second Affiliated Hospital of Xi'an Medical
University (Xian, China; approval no. 2019-1213).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhu Y, Ye P, Chen SL and Zhang DM:
Functional regulation of large conductance Ca2+-activated K+
channels in vascular diseases. Metabolism. 83:75–80.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chawla A, Chawla R and Jaggi S:
Microvasular and macrovascular complications in diabetes mellitus:
Distinct or continuum? Indian J Endocrinol Metab. 20:546–551.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kitada M, Zhang Z, Mima A and King GL:
Molecular mechanisms of diabetic vascular complications. J Diabetes
Investig. 1:77–89. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Saeid F, Aniseh J, Reza B and Manouchehr
VS: Signaling mediators modulated by cardioprotective interventions
in healthy and diabetic myocardium with ischaemia-reperfusion
injury. Eur J Prev Cardiol. 25:1463–1481. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yuan T, Yang T, Chen H, Fu D, Hu Y, Wang
J, Yuan Q, Yu H, Xu W and Xie X: New insights into oxidative stress
and inflammation during diabetes mellitus-accelerated
atherosclerosis. Redox Biol. 20:247–260. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Almabrouk TA, Ewart MA, Salt IP and
Kennedy S: Perivascular fat, AMP-activated protein kinase and
vascular diseases. Br J Pharmacol. 171:595–617. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mottillo EP, Desjardins EM, Crane JD,
Smith BK, Green AE, Ducommun S, Henriksen TI, Rebalka IA, Razi A,
Sakamoto K, et al: Lack of adipocyte AMPK exacerbates insulin
resistance and hepatic steatosis through brown and beige adipose
tissue function. Cell Metab. 24:118–129. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gauthier MS, O'Brien EL, Bigornia S, Mott
M, Cacicedo JM, Xu XJ, Gokce N, Apovian C and Ruderman N: Decreased
AMP-activated protein kinase activity is associated with increased
inflammation in visceral adipose tissue and with whole-body insulin
resistance in morbidly obese humans. Biochem Biophys Res Commun.
404:382–387. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chen B, Li J and Zhu H: AMP-activated
protein kinase attenuates oxLDL uptake in macrophages through
PP2A/NF-κB/LOX-1 pathway. Vascul Pharmacol. 85:1–10.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Matsuzaki T, Matsushita T, Takayama K,
Matsumoto T, Nishida K, Kuroda R and Kurosaka M: Disruption of
Sirt1 in chondrocytes causes accelerated progression of
osteoarthritis under mechanical stress and during ageing in mice.
Ann Rheum Dis. 73:1397–1404. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kume S, Uzu T, Kashiwagi A and Koya D:
SIRT1, a calorie restriction mimetic, in a new therapeutic approach
for type 2 diabetes mellitus and diabetic vascular complications.
Endocr Metab Immune Disord Drug Targets. 10:16–24. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Xiao L, Liu L, Guo X, Zhang S, Wang J,
Zhou F, Liu L, Tang Y and Yao P: Quercetin attenuates high fat
diet-induced atherosclerosis in apolipoprotein E knockout mice: A
critical role of NADPH oxidase. Food Chem Toxicol. 105:22–33.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kobori M, Takahashi Y, Sakurai M, Akimoto
Y, Tsushida T, Oike H and Ippoushi K: Quercetin suppresses immune
cell accumulation and improves mitochondrial gene expression in
adipose tissue of diet-induced obese mice. Mol Nutr Food Res.
60:300–312. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Egert S, Bosy-Westphal A, Seiberl J,
Kürbitz C, Settler U, Plachta-Danielzik S, Wagner AE, Frank J,
Schrezenmeir J, Rimbach G, et al: Quercetin reduces systolic blood
pressure and plasma oxidised low-density lipoprotein concentrations
in overweight subjects with a high-cardiovascular disease risk
phenotype: A double-blinded, placebo-controlled cross-over study.
Br J Nutr. 102:1065–1074. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pfeuffer M, Auinger A, Bley U,
Kraus-Stojanowic I, Laue C, Winkler P, Rüfer C, Frank J,
Bösch-Saadatmandi C, Rimbach G and Schrezenmeir J: Effect of
quercetin on traits of the metabolic syndrome, endothelial function
and inflammation in men with different APOE isoforms. Nutr Metab
Cardiovasc Dis. 23:403–409. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cao H, Jia Q, Shen D, Yan L, Chen C and
Xing S: Quercetin has a protective effect on atherosclerosis via
enhancement of autophagy in ApoE-/-mice. Exp Ther Med.
18:2451–2458. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Omar HM, Almaeen AH, Elghaffar SKA, Ragab
SM, El-Metwally TH and Ahmed EA: Atherosclerotic rat model after a
high-fat, high-sucrose diet: Protective role of quercetin,
O-coumaric, and berberine. Anal Quant Cytol Histol. 40:76–84.
2018.
|
|
18
|
Feng K, Chen Z, Pengcheng L, Zhang S and
Wang X: Quercetin attenuates oxidative stress-induced apoptosis via
SIRT1/AMPK-mediated inhibition of ER stress in rat chondrocytes and
prevents the progression of osteoarthritis in a rat model. J Cell
Physiol. 234:18192–18205. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Peng J, Li Q, Li K, Zhu L, Lin X, Lin X,
Shen Q, Li G and Xie X: Quercetin improves glucose and lipid
metabolism of diabetic rats: Involvement of Akt signaling and
SIRT1. J Diabetes Res. 2017(3417306)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
National Research Council (US) Institute
for Laboratory Animal Research: Guide for the Care and Use of
Laboratory Animals. National Academies Press (US), Washington, DC,
1996.
|
|
21
|
Kim YM, Kim MY, Kim HJ, Roh GS, Ko GH, Seo
HG, Lee JH and Chang KC: Compound C independent of AMPK inhibits
ICAM-1 and VCAM-1 expression in inflammatory stimulants-activated
endothelial cells in vitro and in vivo. Atherosclerosis. 219:57–64.
2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hasanvand A, Amini-Khoei H, Hadian MR,
Abdollahi A, Tavangar SM, Dehpour AR, Semiei E and Mehr SE:
Anti-inflammatory effect of AMPK signaling pathway in rat model of
diabetic neuropathy. Inflammopharmacology. 24:207–219.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bayrami G, Karimi P, Agha-Hosseini F,
Feyzizadeh S and Badalzadeh R: Effect of ischemic postconditioning
on myocardial function and infarct size following reperfusion
injury in diabetic rats pretreated with vildagliptin. J Cardiovasc
Pharmacol Ther. 23:174–183. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Aebi H: Catalase in vitro. Methods
Enzymol. 105:121–126. 1984.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ohkawa H, Ohishi N and Yagi K: Assay for
lipid peroxides in animal tissues by thiobarbituric acid reaction.
Anal Biochem. 95:351–358. 1979.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Pham-Huy LA, He H and Pham-Huy C: Free
radicals, antioxidants in disease and health. Int J Biomed Sci.
4:89–96. 2008.PubMed/NCBI
|
|
27
|
Bigagli E and Lodovici M: Circulating
oxidative stress biomarkers in clinical studies on type 2 diabetes
and its complications. Oxid Med Cell Longev.
2019(5953685)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Asmat U, Abad K and Ismail K: Diabetes
mellitus and oxidative stress-A concise review. Saudi Pharm J.
24:547–553. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Oguntibeju O: Type 2 diabetes mellitus,
oxidative stress and inflammation: Examining the links. Int J
Physiol Pathophysiol Pharmacol. 11:45–63. 2019.PubMed/NCBI
|
|
30
|
Najafi M, Noroozi E, Javadi A and
Badalzadeh R: Anti-arrhythmogenic and anti-inflammatory effects of
troxerutin in ischemia/reperfusion injury of diabetic myocardium.
Biomed Pharmacother. 102:385–391. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Becatti M, Mannucci A, Taddei N and
Fiorillo C: Oxidative stress and inflammation: New molecular
targets for cardiovascular diseases. Intern Emerg Med. 13:647–649.
2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bule M, Abdurahman A, Nikfar S, Abdollahi
M and Amini M: Antidiabetic effect of quercetin: A systematic
review and meta-analysis of animal studies. Food Chem Toxicol.
125:494–502. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yao Z, Gu Y, Zhang Q, Liu L, Meng G, Wu H,
Xia Y, Bao X, Shi H, Sun S, et al: Estimated daily quercetin intake
and association with the prevalence of type 2 diabetes mellitus in
Chinese adults. Eur J Nutr. 58:819–830. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Xu D, Hu MJ, Wang YQ and Cui YL:
Antioxidant activities of quercetin and its complexes for medicinal
application. Molecules. 24(1123)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Funakoshi T, Kanzaki N, Otsuka Y, Izumo T,
Shibata H and Machida S: Quercetin inhibits adipogenesis of muscle
progenitor cells in vitro. Biochem Biophys Rep. 13:39–44.
2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ewart MA, Kohlhaas CF and Salt IP:
Inhibition of tumor necrosis factor alpha-stimulated monocyte
adhesion to human aortic endothelial cells by AMP-activated protein
kinase. Arterioscler Thromb Vasc Biol. 28:2255–2257.
2008.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Dong Y, Zhang M, Wang S, Liang B, Zhao Z,
Liu C, Wu M, Choi HC, Lyons TJ and Zou MH: Activation of
AMP-activated protein kinase inhibits oxidized LDL-triggered
endoplasmic reticulum stress in vivo. Diabetes. 59:1386–1396.
2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kim SG, Kim JR and Choi HC:
Quercetin-induced AMP-activated protein kinase activation
attenuates vasoconstriction through LKB1-AMPK signaling pathway. J
Med Food. 21:146–153. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Mattagajasingh I, Kim CS, Naqvi A,
Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K and Irani K:
SIRT1 promotes endothelium-dependent vascular relaxation by
activating endothelial nitric oxide synthase. Proc Natl Acad Sci
USA. 104:14855–14860. 2007.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Guo H, Chen Y, Liao L and Wu W:
Resveratrol protects HUVECs from oxidized-LDL induced oxidative
damage by autophagy upregulation via the AMPK/SIRT1 pathway.
Cardiovasc Drugs Ther. 27:189–198. 2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Chen S, Jiang H, Wu X and Fang J:
Therapeutic effects of quercetin on inflammation, obesity, and type
2 diabetes. Mediators Inflamm. 2016(9340637)2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Herzig S and Shaw RJ: AMPK: Guardian of
metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol.
19:121–135. 2018.PubMed/NCBI View Article : Google Scholar
|