Introduction
Air pollution can lead to severe respiratory health
problems, especially in the elderly population (1-3).
Studies have demonstrated that environmental exposure to
particulate matter ≤2.5 µm (PM2.5) threatens the human respiratory
system, and is currently a worldwide concern (4-6).
PM2.5 can be directly inhaled and deeply penetrate into the lung
alveoli, which further leads to severe lung dysfunction, including
chronic cough, bronchitis, asthma and lung cancer (7-9).
DNA damage, cell apoptosis, cell necrosis, autophagy and cell
abnormalities have been identified to occur during PM2.5-induced
cytotoxicity (10-12).
Although a number of reports have attempted to understand
PM2.5-induced lung injury, the underlying processes and signaling
mechanisms governing the effect of PM2.5 on the lungs have not yet
been clearly elucidated (13-16).
A number of signaling pathways have been reported to
be associated with PM2.5-induced biological cell processes
(17-19).
The toxicological effects of PM2.5 can lead to oxidative damage
and/or cytokine secretion in human bronchial epithelial cells
(20). A previous study indicated
that PM2.5 induced apoptosis in L132 cells by changing the
transcription rates of p53, β-cell lymphoma-2 and bax genes
(9). PM2.5 exposure has also been
indicated to induce autophagy via long non-coding RNA loc146880,
which was identified to promote the migration and invasion of lung
cancer cells (11). In addition,
PM2.5 has been demonstrated to induce lung inflammation in mice via
the LPS/MyD88 pathway (21).
Furthermore, PM2.5-induced oxidative stress increased adhesion
molecule expression in human endothelial cells via the
ERK/AKT/NF-κB-dependent pathway (22). Furthermore, PM2.5-induced oxidative
stress increases intercellular adhesion molecule-1 expression in
lung epithelial cells through the interleukin
(IL)-6/AKT/STAT3/NF-κB-dependent pathway (23). However, to the best of our
knowledge, the toxicological mechanisms of PM2.5-induced cell
apoptosis have still not been fully determined.
In the current study, the effects of PM2.5 on
inflammation and apoptosis of mouse bronchial epithelium cells
(MBECs) was assessed. The possible mechanism mediated by PM2.5 was
analyzed in MBECs. The in vivo study was also performed to
investigate the role of PM2.5 in experimental mice.
Materials and methods
Animals
A total of 20 eight week old male C57BL/6 (weight,
20-23 g) mice were purchased from Tianjin Medical University. All
mice were housed at 23±1˚C, 50±5% humidity with a 12 h light/dark
cycle and access to food and water ad libitum. A method of
PM2.5 intratracheal instillation was performed on mice, and was
performed as previously reported (24). In brief, mice were randomly divided
into two groups (n=10 in each group) and lived in PM2.5 and a
normal survival (Control) environment. Mice were housed in their
respective environments for a total of 21 days. The mice were
euthanized using cervical decapitation on day 22.
Lung function evaluation
On day 22, all experimental mice were anesthetized
using 40 mg/kg of sodium pentobarbital. Animals were intubated with
a custom-made laryngoscope blade. Animals were mechanically
ventilated with a rodent ventilator. The pulmonary functions,
including lung capacity, residual volume, tidal volume and airway
resistance were analyzed using the FlexiVent system (SCIREQ)
according to manufacturer's protocol.
ELISA
On day 22, blood was collected from each group and
the plasma was obtained using centrifugation at 10,000 x g for 10
min at 4˚C. An ELISA kit was to measure the levels of IL-1β (cat.
no. MLB00C; Bio-Rad Laboratories, Inc.) and IL-6 (cat. no. M6000B;
Bio-Rad Laboratories, Inc.).
Histopathological examination
The lung tissues were fixed with 4% formaldehyde
overnight at room temperature and embedded in paraffin, and cut
into 4 µm sections. Sections underwent hematoxylin and eosin
staining for 30 min at room temperature. Sections were then washed
with PBS three times and observed under a light microscope at a
magnification of x100 (Olympus Corporation).
Cell culture and treatment
MBECs were purchased from Shanghai Sixin
Biotechnology Co., Ltd. MBECs were cultured in endothelial culture
medium (cat. no. 1001; ScienCell Research Laboratories, Inc.)
containing 10% FBS (cat. no. 0025, ScienCell Research Laboratories,
Inc.), 1% endothelial cell growth supplement (cat. no. 1052,
ScienCell Research Laboratories, Inc.) and 1%
penicillin/streptomycin (Sigma-Aldrich; Merck KGaA) in 5%
CO2 at 37˚C. MBECs (1x105/ml) were then
seeded onto six-well plates and placed in air containing PM2.5,
PM2.5 + PI3K inhibitor (PI3KIR), PI3K inhibitor (PI3KIR; cat. no.
526559; Sigma-Aldrich; Merck KGaA) or 95% air and 5% CO2
at 37˚C for 24 h.
Analysis of reactive oxygen species
(ROS) production
A 2',7'-dicholorofluorescein-diacetate (DCFH-DA)
probe was used to evaluate the level of ROS production in
PM2.5-treated MBECs in six-well plates, as described previously
(25,26). DCFH-DA (10 µM) was added into MBECs
and cells were cultured for 30 min at 37˚C in the dark. MBECs were
then washed three times using ice-cold PBS. ROS production in MBECs
was examined at a magnification of x50 using a fluorescence
microscope (Olympus Corporation). The fluorescence intensity was
calculated using analysis LS 5.0 soft image solution (Olympus
Imaging America Inc.).
Western blot analysis
MBECs (5x106) were lysed in RIPA buffer
(M-PER reagent for the cells and T-PER reagent for the tissues;
Thermo Fisher Scientific, Inc.). Protein concentration was measured
using a BCA protein assay kit (Thermo Fisher Scientific, Inc.).
Protein (20 µg) was electrophoresed on a 15% SDS-PAGE gel and
transferred to a PVDF membrane (EMD Millipore). The membranes were
blocked using 5% BSA at 4˚C overnight. The primary rabbit anti-rat
antibodies used in the immunoblotting assays were as follows: PI3K
(1:1,000; cat. no. ab76315; Abcam), phosphorylated PI3K (1:500;
cat. no. ab182651; Abcam), AKT (1:500; cat. no. ab185633; Abcam),
phosphorylated AKT (1:1,000; cat. no. ab133458; Abcam) and β-actin
(1:2,000; cat. no. ab8226; Abcam). After incubation, the membrane
was washed three times in PBST and incubated with HRP-conjugated
goat anti-rabbit IgG mAb (1:2,000; cat. no. PV-6001; OriGene
Technologies, Inc.) for 1 h at 37˚C. After washing three times with
PBST, the membrane was developed using a chemiluminescence assay
system (EMD Millipore). Densitometric quantification of the
immunoblot data was performed using Quantity-One 1.2 software
(Bio-Rad Laboratories, Inc.).
TUNEL assay
TUNEL analysis was conducted using an In Situ
Cell Death Detection kit (DeadEnd™ Colorimetric Tunel System;
Promega Corporation). For lung tissue, tissue sections (4 µm) were
deparaffinized using xylene, rehydrated in graded ethanol, and
rehydrated for 3 min. The sections were then incubated with TUNEL
(DeadEnd™ Colorimetric Tunel System; Promega Corporation ) for 2 h
at 37˚C according to the manufacturer's protocol. For MBECs, cells
were treated with 4% paraformaldehyde for 15 min at room
temperature. Cells were then washed with PBST three times at room
temperature and incubated with TUNEL (DeadEnd™ Colorimetric Tunel
System, Promega) for 1 h at 37˚C according to the manufacturer's
protocol. Cells were washed with PBS three times at room
temperature and then incubated with 5% DAPI (Sigma-Aldrich; Merck
KGaA) under Antifade mounting medium (cat. no. P0126; Beyotime
Institute of Biotechnology) for 30 min at 37˚C. Finally, images of
sections and cells were captured in six fields of view with a ZEISS
LSM 510 confocal microscope at 488 nm at a magnification of x100.
The apoptosis rate was measured using Developer XD 1.2 software
(Definiens AG).
Autophagy assay
MBECs in culture dishes were fixed with 4%
paraformaldehyde for 15 min at room temperature, and blocked using
0.5% BSA for 30 min at 37˚C. MBECs were washed with PBST three
times at room temperature and incubated with anti-LC3B (1:1,000;
cat. no. ab48394; Abcam) for 12 h at 4˚C. Cells were incubated with
Alexa Fluor 488-labeled goat anti-rabbit secondary antibodies
(1:2,000; Beyotime Institute of Biotechnology) for 12 h at 4˚C.
Images were captured using a confocal microscope (ZEISS GmbH;
LSM510 META; magnification, x100) and analyzed using AxioVision
Rel. 4.6 software (Zeiss GmbH).
Statistical analysis
All data are expressed as means ± SEM and analyzed
using SPSS software 17.0 (SPSS Inc.). Data were analyzed using a
Student's t-test and multiple groups were analyzed using a one-way
ANOVA followed by Tukey's test. P<0.05 was considered to
indicate a statistically significant difference.
Results
PM2.5 damages lung function in
experimental mice
To explore the effects of PM2.5 on lung function,
experimental mice were exposed to PM2.5 or clean air as a control.
The results indicated that PM2.5 significantly decreased lung
function, including total lung capacity, residual volume, vital
capacity and airway resistance in experimental mice (Fig. 1A-D). Body weight of mice was
significantly decreased by PM2.5 compared with the control
(Fig. 1E). H&E staining
demonstrated that PM2.5 induced lung injury (Fig. 1F).
PM2.5 increases inflammatory cytokines
in an experimental animal model
The current study explored the effects of PM2.5 on
inflammatory cytokines in an experimental animal model. PM2.5
increased IL-1β and IL-6 expression levels in the serum of the
experimental animal model compared with the control (Fig. 2A). IL-1β and IL-6 expression was
also markedly increased by PM2.5 in MBECs compared with the control
(Fig. 2B). ROS production was
indicated to be upregulated in MBECs in PM2.5-treated mice compared
with the control (Fig. 2C), which
may induce oxidative injury in lung cells.
PM2.5 induces apoptosis of lung tissue
in experimental mice and MBECs
Apoptosis of lung tissue and MBECs was analyzed
in vivo and in vitro. As indicated in Fig. 3A, PM2.5 increased lung cell
apoptosis in lung tissue compared with the control. The in
vitro assay demonstrated that PM2.5 induced the apoptosis of
MBECs compared with the control (Fig.
3B). These results indicated that PM2.5 can induce apoptosis of
MBECs and in the lung tissue of experimental mice.
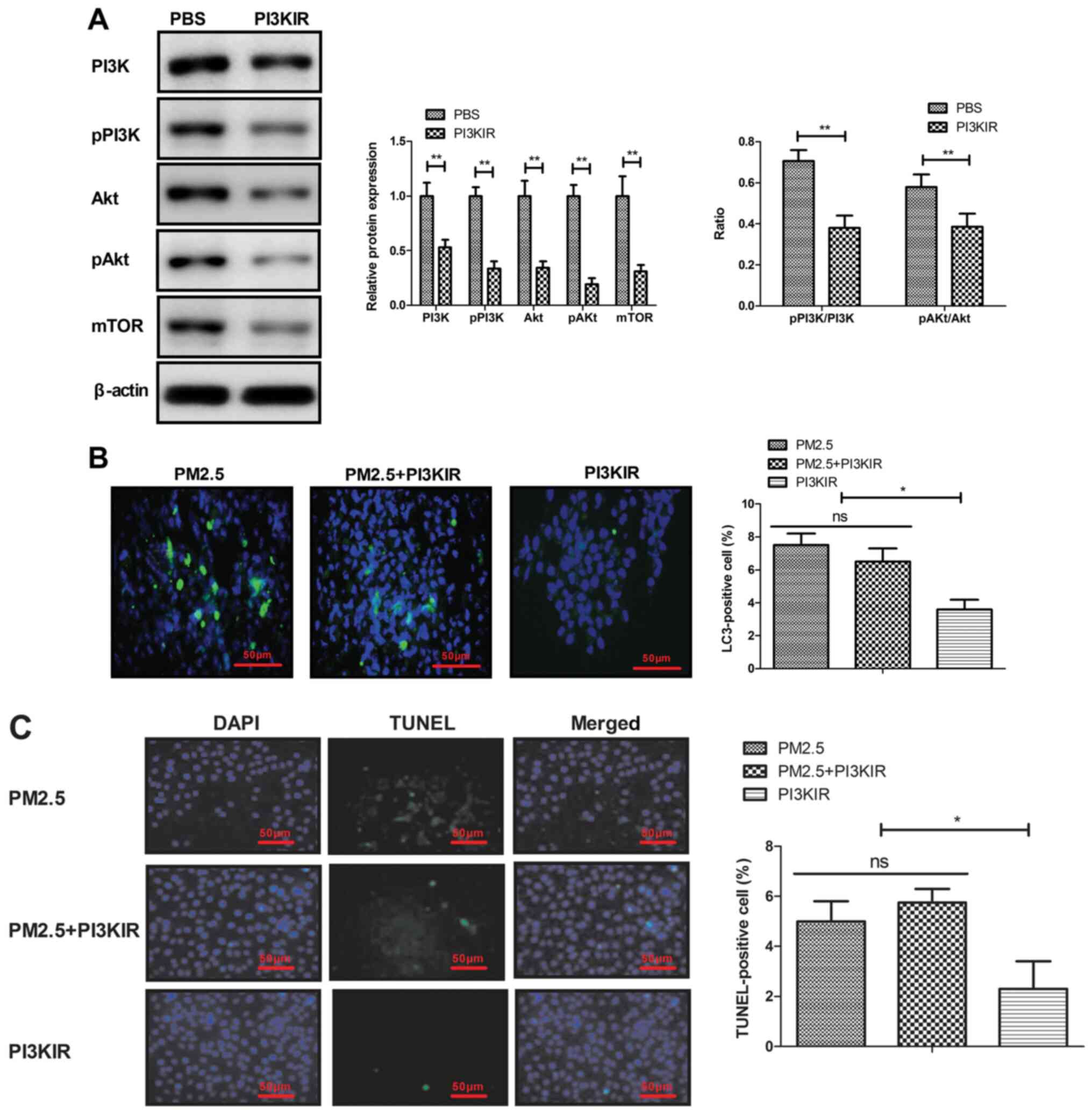
PM2.5 upregulates PI3K, AKT and mTOR
expression, and induces autophagy in MBECs
The PI3K, AKT and mTOR expression in MBECs was
evaluated in MBECs in vitro. The results demonstrated that
PM2.5 increased PI3K, AKT and mTOR expression in MBECs. A
statistical difference was identified in the PI3K and AKT
phosphorylation level between PM2.5 and the control group (Fig. 4A). PM2.5 was also demonstrated to
induced autophagy of MBECs in vitro (Fig. 4B).
PM2.5 induces apoptosis of MBECs via
the PI3K/AKT/mTOR signaling pathway
The current study also explored the potential
mechanism mediated by PM2.5 in MBECs. The results demonstrated that
PI3KIR significantly decreased PI3K, AKT and mTOR expression in
MBECs (Fig. 5A). PI3K inhibitor
also blocked PM2.5-induced autophagy and apoptosis of MBECs
(Fig. 5B-C). These results
indicated that disruption of PI3K/AKT/mTOR signaling can
effectively block autophagy and apoptosis of MBECs induced by
PM2.5.
Discussion
PM2.5 has been demonstrated to rapidly induce
inflammatory responses, oxidative injury and cell apoptosis or
death in human bronchial epithelium cells (21,26,27). A
previous study indicated that PM2.5-induced oxidative stress
increased adhesion molecule expression in human endothelial cells
via the ERK/AKT/NF-κB pathway (22). In the current study, the effects of
PM2.5 on lung function in experimental mice and on the apoptosis of
MBECs was explored in vitro and in vivo. The results
indicated that PM2.5 decreased the overall lung function of
experimental mice and specifically induced autophagy of MBECs to
increase apoptosis. Disruption of PI3K/AKT/mTOR pathway effectively
reduced autophagy level and apoptosis of MBECs. These results
indicated that the mechanism of autophagy-mediated MBECs apoptosis
induced by PM2.5 is mediated by the PI3K/AKT/mTOR pathway, and
provided a potential strategy for the treatment of lung diseases
induced by PM2.5.
Inflammatory cytokine levels serve a crucial role in
the progression of lung disease (28). PM2.5 has been indicated to lead to
increasing IgE, intracellular adhesion molecule-1, vascular cell
adhesion molecule-1, EOS, interferon-γ, IL-4, IL-5, IL-33 and
thymic stromal lymphopoietin in a rat with PM2.5-induced allergic
rhinitis (29). In addition, PM2.5
was indicated to induce ROS formation and NADPH oxidase expression
in 16HBE cells and bone marrow stromal cells, which suggested that
PM2.5 increased inflammatory activation mediated by ROS induction
in the respiratory tract (30).
Furthermore, PM2.5 promoted pro-inflammatory cytokine IL-6 and
IL-1β signaling activation (31).
The current study demonstrated that PM2.5 increased IL-1β and IL-6
expression in the serum of an experimental animal model and
upregulated IL-1β and IL-6 expression in MBECs. PM2.5 has been
revealed to induce respiratory damage, and a mechanistic basis for
preventing outcomes in polluted environments has been identified
previously (32). The results of
the current study demonstrated that ROS production in MBECs were
upregulated in MBECs in PM2.5-treated mice, which further resulted
in oxidative injury of experimental mice.
A previous study indicated that PM2.5 activated a
number of apoptosis pathways in human epithelial lung cells (L132)
in culture (9). PM2.5 also induced
autophagy-mediated cell death via NOS2 signaling in human bronchial
epithelium cells (33). The direct
toxic effect of PM2.5 on human umbilical vein endothelial cells
provides a novel insight into the mechanism of cardiovascular
diseases caused by PM2.5 exposure (34). The results of the present study
revealed that PM2.5 upregulated PI3K, AKT and mTOR expression and
induced autophagy in MBECs. To the best of our knowledge, the
results of the current study first reported that PM2.5 induced the
apoptosis and autophagy of MBECs via the PI3K/AKT/mTOR signaling
pathway.
In conclusion, the current study indicated that
PM2.5 led to lung injury, increased tissue inflammatory factors,
increased ROS production, and induced apoptosis and autophagy in
MBECs. The results indicated that PM2.5 induced apoptosis and
autophagy via the PI3K/AKT/mTOR signaling pathway, which may
provide a potential target in alleviating lung injury in
PM2.5-induced lung diseases.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ performed the experiments and analyzed the data.
XH designed the current study and wrote the manuscript. Both
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This retrospective study was approved by the Ethics
Committee of The Second Hospital of Tianjin Medical University
(Tianjin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lee D, Rushworth A and Sahu SK: A Bayesian
localized conditional autoregressive model for estimating the
health effects of air pollution. Biometrics. 70:419–429.
2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fleischer NL, Merialdi M, van Donkelaar A,
Vadillo-Ortega F, Martin RV, Betran AP and Souza JP: Outdoor air
pollution, preterm birth, and low birth weight: Analysis of the
world health organization global survey on maternal and perinatal
health. Environ Health Perspect. 122:425–430. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wong GW: Air pollution and health. Lancet
Respir Med. 2:8–9. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Choi JH, Kim JS, Kim YC, Kim YS, Chung NH
and Cho MH: Comparative study of PM2.5- and PM10-induced oxidative
stress in rat lung epithelial cells. J Vet Sci. 5:11–18.
2004.PubMed/NCBI
|
|
5
|
Nam HY, Choi BH, Lee JY, Lee SG, Kim YH,
Lee KH, Yoon HK, Song JS, Kim HJ and Lim Y: The role of nitric
oxide in the particulate matter (PM2.5)-induced NFkappaB activation
in lung epithelial cells. Toxicol Lett. 148:95–102. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Harder SD, Soukup JM, Ghio AJ, Devlin RB
and Becker S: Inhalation of PM2.5 does not modulate host defense or
immune parameters in blood or lung of normal human subjects.
Environ Health Perspect. 109 (Suppl 4):599–604. 2001.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Deng F, Guo X, Liu H, Fang X, Yang M and
Chen W: Effects of dust storm PM2.5 on cell proliferation and cell
cycle in human lung fibroblasts. Toxicol In Vitro. 21:632–638.
2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dagher Z, Garcon G, Billet S, Verdin A,
Ledoux F, Courcot D, Aboukais A and Shirali P: Role of nuclear
factor-kappa B activation in the adverse effects induced by air
pollution particulate matter (PM2.5) in human epithelial lung cells
(L132) in culture. J Appl Toxicol. 27:284–290. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Dagher Z, Garcon G, Billet S, Gosset P,
Ledoux F, Courcot D, Aboukais A and Shirali P: Activation of
different pathways of apoptosis by air pollution particulate matter
(PM2.5) in human epithelial lung cells (L132) in culture.
Toxicology. 225:12–24. 2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gu LZ, Sun H and Chen JH: Histone
deacetylases 3 deletion restrains PM2.5-induced mice lung injury by
regulating NF-κB and TGF-β/Smad2/3 signaling pathways. Biomed
Pharmacother. 85:756–762. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Deng X, Feng N, Zheng M, Ye X, Lin H, Yu
X, Gan Z, Fang Z, Zhang H, Gao M, et al: PM2.5
exposure-induced autophagy is mediated by lncRNA loc146880 which
also promotes the migration and invasion of lung cancer cells.
Biochim Biophys Acta Gen Subj. 1861:112–125. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Badyda AJ, Grellier J and Dabrowiecki P:
Ambient PM2.5 exposure and mortality due to lung cancer and
cardiopulmonary diseases in polish cities. Adv Exp Med Biol.
944:9–17. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zwozdziak A, Sowka I, Willak-Janc E,
Zwozdziak J, Kwiecinska K and Balinska-Miskiewicz W: Influence of
PM1 and PM2.5 on lung function parameters in
healthy schoolchildren-a panel study. Environ Sci Pollut Res Int.
23:23892–23901. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ferraz ER, Rainho CR, Fernandes AS and
Felzenszwalb I: Differential toxicity of an organic PM2.5 extract
to human lung cells cultured in three dimensions (3D) and
monolayers. J Toxicol Environ Health A. 79:221–231. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Abbas I, Verdin A, Escande F,
Saint-Georges F, Cazier F, Mulliez P, Courcot D, Shirali P, Gosset
P and Garçon G: In vitro short-term exposure to air pollution
PM2.5-0.3 induced cell cycle alterations and genetic instability in
a human lung cell coculture model. Environ Res. 147:146–158.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fu J, Jiang D, Lin G, Liu K and Wang Q: An
ecological analysis of PM2.5 concentrations and lung cancer
mortality rates in China. BMJ Open. 5(e009452)2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rumelhard M, Ramgolam K, Hamel R, Marano F
and Baeza-Squiban A: Expression and role of EGFR ligands induced in
airway cells by PM2.5 and its components. Eur Respir J.
30:1064–1073. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Billet S, Garcon G, Dagher Z, Verdin A,
Ledoux F, Cazier F, Courcot D, Aboukais A and Shirali P: Ambient
particulate matter (PM2.5): Physicochemical characterization and
metabolic activation of the organic fraction in human lung
epithelial cells (A549). Environ Res. 105:212–223. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Meng Z and Zhang Q: Damage effects of dust
storm PM2.5 on DNA in alveolar macrophages and lung cells of rats.
Food Chem Toxicol. 45:1368–1374. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Dergham M, Lepers C, Verdin A, Cazier F,
Billet S, Courcot D, Shirali P and Garçon G: Temporal-spatial
variations of the physicochemical characteristics of air pollution
Particulate Matter (PM2.5-0.3) and toxicological effects in human
bronchial epithelial cells (BEAS-2B). Environ Res. 137:256–267.
2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
He M, Ichinose T, Yoshida S, Ito T, He C,
Yoshida Y, Arashidani K, Takano H, Sun G and Shibamoto T: :
PM2.5-induced lung inflammation in mice: Differences of
inflammatory response in macrophages and type II alveolar cells. J
Appl Toxicol. 37:1203–1218. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Rui W, Guan L, Zhang F, Zhang W and Ding
W: : PM2.5-induced oxidative stress increases adhesion molecules
expression in human endothelial cells through the
ERK/AKT/NF-κB-dependent pathway. J Appl Toxicol. 36:48–59.
2016.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Liu CW, Lee TL, Chen YC, Liang CJ, Wang
SH, Lue JH, Tsai JS, Lee SW, Chen SH, Yang YF, et al:
PM2.5-induced oxidative stress increases intercellular
adhesion molecule-1 expression in lung epithelial cells through the
IL-6/AKT/STAT3/NF-κB-dependent pathway. Part Fibre Toxicol.
15(4)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang Y, Li Y, Shi Z, Wu J, Yang X, Feng
L, Ren L, Duan J and Sun Z: Metabolic impact induced by total,
water soluble and insoluble components of PM2.5 acute
exposure in mice. Chemosphere. 207:337–346. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ye JZ, Su YB, Lin XM, Lai SS, Li WX, Ali
F, Zheng J and Peng B: Alanine enhances aminoglycosides-induced ROS
production as revealed by proteomic analysis. Front Microbiol.
9(29)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yang D, Ma M, Zhou W, Yang B and Xiao C:
Inhibition of miR-32 activity promoted EMT induced by PM2.5
exposure through the modulation of the Smad1-mediated signaling
pathways in lung cancer cells. Chemosphere. 184:289–298.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhao H, Yang B, Xu J, Chen DM and Xiao CL:
: PM2.5-induced alterations of cell cycle associated gene
expression in lung cancer cells and rat lung tissues. Environ
Toxicol Pharmacol. 52:77–82. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Gauger PC, Vincent AL, Loving CL,
Henningson JN, Lager KM, Janke BH, Kehrli ME Jr and Roth JA:
Kinetics of lung lesion development and pro-inflammatory cytokine
response in pigs with vaccine-associated enhanced respiratory
disease induced by challenge with pandemic (2009) A/H1N1 influenza
virus. Vet Pathol. 49:900–912. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang YL, Gao W, Li Y and Wang YF:
Concentration-dependent effects of PM2.5 mass on
expressions of adhesion molecules and inflammatory cytokines in
nasal mucosa of rats with allergic rhinitis. Eur Arch
Otorhinolaryngol. 274:3221–3229. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jin X, Su R, Li R, Cheng L and Li Z:
Crucial role of pro-inflammatory cytokines from respiratory tract
upon PM2.5 exposure in causing the BMSCs differentiation in cells
and animals. Oncotarget. 9:1745–1759. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Roper C, Chubb LG, Cambal L, Tunno B,
Clougherty JE, Fattman C and Mischler SE: Association of IL-6 with
PM2.5 components: Importance of characterizing
filter-based PM2.5 following extraction. Water Air Soil Pollut.
228(pii: 43)2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jin X, Xue B, Zhou Q, Su R and Li Z:
Mitochondrial damage mediated by ROS incurs bronchial epithelial
cell apoptosis upon ambient PM2.5 exposure. J Toxicol
Sci. 43:101–111. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhu XM, Wang Q, Xing WW, Long MH, Fu WL,
Xia WR, Jin C, Guo N, Xu DQ and Xu DG: : PM2.5 induces
autophagy-mediated cell death via NOS2 signaling in human bronchial
epithelium cells. Int J Biol Sci. 14:557–564. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhou Z, Shao T, Qin M, Miao X, Chang Y,
Sheng W, Wu F and Yu Y: The effects of autophagy on vascular
endothelial cells induced by airborne PM2.5. J Environ Sci (China).
66:182–187. 2018.PubMed/NCBI View Article : Google Scholar
|