Introduction
Missed abortions are a common complication in early
pregnancy, affecting ~15% of clinically recognized pregnancies
(1). The incidence of missed
abortions in China is 13.4% and has been rapidly increasing
annually (2). The majority of
missed abortion cases are asymptomatic, which are discovered during
routine ultrasound examination and are currently treated by
dilation and curettage, which causes physiological and
psychological harm to women and their families (3). Previous studies have indicated that
the risk factors for a missed abortion include chromosomal
abnormalities, infections, maternal systemic diseases, endocrine
disorders and anatomical defects (4-6).
However, the cause of ~30% of missed abortions is still
unclear.
Long noncoding RNAs (lncRNAs) are a subtype of
noncoding RNAs with transcripts >200 nucleotides that do not
encode proteins. Recent studies have indicated that three lncRNAs,
Metastasis Associated Lung Adenocarcinoma Transcript 1
(MALAT1), HOX transcript antisense RNA (HOTAIR) and
Maternally expressed gene 3 (MEG3), serve a crucial role in
regulating the migratory and invasive ability of tumor cells
(7-10).
Trophoblasts share a number of common features with tumor cells,
such as high proliferative capacity, invasive nature, secretion of
growth factors and hormones and immunosuppressive activities
(11). Trophoblast dysfunction has
also been associated with adverse pregnancy outcomes, including
missed abortion (12). Insufficient
trophoblast invasion may result in inadequate vascular remodeling
and placental perfusion, which can lead to a variety of pregnancy
complications, such as miscarriage, preeclampsia and intrauterine
growth restriction (13).
Over-invasion of trophoblasts in the first trimester may induce
immune response at the maternal-fetal interface, leading to failure
in the induction of immune tolerance and miscarriage (14). Therefore, the precise regulation of
trophoblast invasion is essential for maintaining a successful
pregnancy (15). The MALAT1, HOTAIR
and MEG3 lncRNAs are thought to be involved in the mobility of
trophoblast cells and embryo implantation (12). However, the expression profile and
role of the three lncRNAs in patients with missed abortion remain
unclear.
In the present study, the expression levels of the
MALAT1, HOTAIR and MEG3 lncRNAs were examined in decidual and
chorionic tissue from women who had a missed abortion and in women
with normal pregnancies. Additionally, serum TNF-α, IL-1β, IL-6 and
IL-10 levels in the two groups were detected. The correlation
between lncRNA expression and the levels of cytokines, estradiol
and progesterone in patients with a missed abortion were
evaluated.
Patients and methods
Clinical samples
A total of 52 patients who underwent dilation and
curettage and a median age of 31 years (age range, 20-45 years)
were enrolled into the present study. Decidual and villous tissue
as well as serum (1.5 ml per patient) were obtained during or
before the dilatation and curettage procedure in the Department of
Obstetrics and Gynecology, Beijing Luhe Hospital, Capital Medical
University (Beijing, China), between October 2017 and May 2018. The
patients exhibiting a missed abortion (n=26) and control
individuals (n=26) were diagnosed via ultrasound examination 6-12
weeks of gestation. The inclusion criteria for missed abortion
include: i) Individuals harboring a fetus <5 mm with either no
growth or heartbeat; ii) a fetus of ≥5 mm without heartbeat; or
iii) an empty gestational sac of ≥20 mm. Women with obvious risk
factors for missed abortion, including chromosomal abnormalities,
autoimmune disorders, infections, endocrine diseases, twin
pregnancy, anatomical abnormalities, those currently on medication
within 1 month, those with insufficient amount of villous samples
and poor quality RNA were excluded. Written informed consent was
obtained from all participants, and the present study was approved
by the ethics committee of the Medical Faculty, Beijing Luhe
Hospital, Capital Medical University.
RNA Extraction and
reverse-transcription quantitative (RT-q) PCR
Total RNA was isolated from decidual and villous
tissues using TRIzol® reagent (Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Reverse
transcription was performed with 1 µg of total RNA, using the
temperature protocol of incubation at 55˚C for 15 min and 85˚C for
5 sec using RevertAid First Strand complementary (c)DNA synthesis
kits (Thermo Fisher Scientific, Inc.). cDNA was quantified using
SYBR Green PCR Master Mix (Applied Biosystems; Thermo Fisher
Scientific, Inc.) according to the following conditions:
Pre-denaturation at 95˚C for 10 min followed by 40 cycles of
amplification at 95˚C for 15 sec and 60˚C for 1 min. Each sample
was examined in triplicate. The primer sequences (GENEWIZ) are
listed in Table I. Data were
analyzed using the 2-ΔΔCq method (16). All samples were normalized to GAPDH,
which served as an endogenous control.
 | Table IThe primer sequences used for
reverse-transcription quantitative-PCR. |
Table I
The primer sequences used for
reverse-transcription quantitative-PCR.
| Gene | Primer sequences
(5'-3') |
|---|
| MALAT1 | |
|
F |
AAAGCAAGGTCTCCCCACAAG |
|
R |
GGTCTGTGCTAGATCAAAAGGCA |
| HOTAIR | |
|
F |
GGTAGAAAAAGCAACCACGAAGC |
|
R |
ACATAAACCTCTGTCTGTGAGTGCC |
| MEG3 | |
|
F |
GCATTAAGCCCTGACCTTTG |
|
R |
TCCAGTTTGCTAGCAGGTGA |
| GAPDH | |
|
F |
CCTGGTATGACAACGAATTTG |
|
R |
CAGTGAGGGTCTCTCTCTTCC |
Measurement of serum tumor necrosis
factor (TNF)-α, interleukin (IL)-1β, IL-6 and IL-10 levels
Blood samples were obtained before vacuum
aspiration. The samples were placed in 4˚C for blood clotting, and
serum was acquired after centrifugation at 1,620 x g for 15 min at
4˚C. Serum was then collected and frozen at -80˚C for cytokine
analysis. The levels of serum TNF-α, IL-1β, IL-6 and IL-10 were
measured with respective human TNF-α (cat. no. ml064303), IL-1β
(cat. no. ml058059), IL-6 (cat. no. ml028583) and IL-10 (cat. no.
ml064299) ELISA kits (Shanghai Enzyme-linked Biotechnology Co.,
Ltd.) according to the manufacturer's protocols. Experiments were
repeated in triplicate.
Measurement of serum estradiol and
progesterone levels
Serum was obtained via centrifugation as
aforementioned and serum estradiol and progesterone levels were
measured via ECL immunoassays using the Roche Cobas E601 Analyzer.
The dilution for estradiol and progesterone is 1:10 (cat. nos.
06656021190 and 07092539190; Roche Diagnostic GmbH) according to
the manufacturer's protocol.
Statistical analysis
Data are presented as the mean ± SD. Expression
differences between groups were analyzed by the Mann-Whitney U
test. Correlations were measured using Spearman's correlation test.
P<0.05 was considered to indicate a statistically significant
difference. GraphPad Prism 5.0 software (GraphPad Software, Inc.)
was used to perform data analysis.
Results
Patients' general and clinical
information
Table II summarizes
the characteristics of missed abortion patients and healthy
controls. No significant differences were indicated in age, BMI,
gestational age, gravidity, parity, number of previous pregnancies,
number of live births or number of previous miscarriages between
the two groups.
 | Table IICharacteristics of patients with
missed abortion and healthy controls. |
Table II
Characteristics of patients with
missed abortion and healthy controls.
| Characteristic | Healthy controls (n
=26) | Patients with
missed abortion (n =26) | P-value |
|---|
| Maternal age
(years) | 29.81±1.30 | 32.73±1.22 | 0.14 |
| BMI
(kg/m2) | 21.97±0.67 | 24.39±1.73 | 0.44 |
| Gestational age
(days) | 51.65±1.80 | 51.23±1.44 | 0.89 |
| Gravidity | 2.46±0.27 | 2.81±0.31 | 0.53 |
| Parity | 0.65±0.11 | 0.69±0.12 | 0.87 |
| Number of previous
pregnancies (%) | | | 0.98 |
|
0 | 1 (3.8) | 0 (0.0) | |
|
1 | 7 (26.9) | 6 (23.1) | |
|
2 | 6 (23.1) | 6 (23.1) | |
|
3 | 5 (19.2) | 7 (26.9) | |
|
4 | 5 (19.2) | 4 (15.4) | |
|
≥5 | 2 (7.7) | 3 (11.5) | |
| Number of previous
miscarriages (%) | | | 0.05 |
|
0 | 26 (100.0) | 21 (80.8) | |
|
≥1 | 0 (0.0) | 5 (19.2) | |
| Number of live
births (%) | | | P>0.99 |
|
0 | 10 (38.5) | 10 (38.5) | |
|
1 | 15 (57.7) | 14 (55.8) | |
|
2 | 1 (3.8) | 2 (7.7) | |
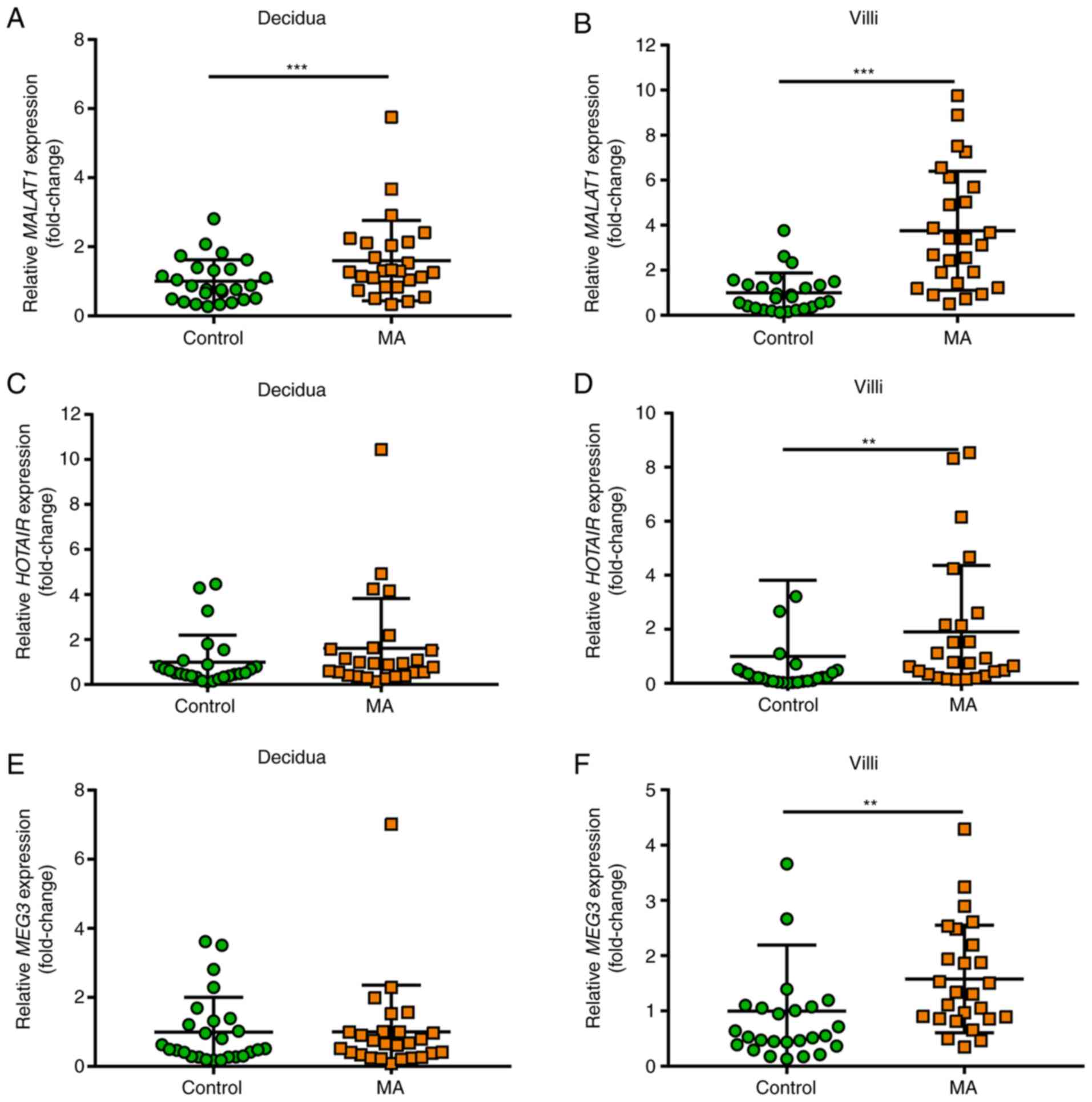
MALAT1, HOTAIR and MEG3 expression is
upregulated in villous tissue from patients with missed
abortion
To explore the role of MALAT1, HOTAIR and MEG3
lncRNAs in the pathogenesis of missed abortion, the expression of
these three lncRNAs were evaluated using RT-qPCR in first trimester
decidua and villous tissue. MALAT1 expression was significantly
upregulated in decidua tissue of patients with missed abortion
(P=0.025; Fig. 1A), while HOTAIR
and MEG3 expression indicated no significant differences between
the missed abortion and healthy control groups (Fig. 1C and E). As presented in Fig. 1B, D
and F, the results of villous
samples showed that MALAT1 (P<0.001), HOTAIR (P=0.001) and MEG3
(P=0.002) expression were significantly upregulated in patients
with missed abortion compared with healthy controls.
Expression of IL-10 in serum of
patients with missed abortion is downregulated
The serum levels of the proinflammatory factors
TNF-α, IL-1β and IL-6 and the anti-inflammatory cytokine IL-10 was
detected in two groups using ELISA. The results indicated that the
levels of TNF-α, IL-1β and IL-6 slightly increased in patients with
a missed abortion, but there were no significant differences
(Fig. 2A-C). Serum IL-10 levels
were significantly lower in the missed abortion group compared with
the control group (P<0.05, Fig.
2D). The correlation of serum IL-10 levels with the expression
of three lncRNAs was further analyzed and the results indicated
that there was no significant correlation of serum IL-10 with
MALAT1 expression in decidua and villous tissue or with HOTAIR and
MEG3 expression in villous tissue (Fig.
3). The expression of HOTAIR and MEG3 in decidual tissues
showed no significant differences between the two groups, therefore
the correlation of serum IL-10 levels with the HOTAIR and MEG3
expression in decidual tissues was not analyzed.
MALAT1 expression in villous tissues
inversely correlates with serum progesterone levels
The levels of the serum hormones progesterone and
estradiol were subsequently detected and the results indicated that
serum progesterone (P<0.001) and estradiol (P<0.001) levels
in patients exhibiting a missed abortion were significantly lower
compared with the healthy controls (Fig. 4). Additionally, the current study
determined whether the expression of the three lncRNAs was
association with the levels of serum progesterone and estradiol. No
statistically significant correlation was indicated between HOTAIR
or MEG3 expression in villous and serum progesterone and estradiol
levels (Fig. 5D-H). However, MALAT1
expression in villous tissues was significantly inversely
associated with serum progesterone levels (Fig. 5C), but there was no significant
correlation between serum progesterone and estradiol levels and
MALAT1 expression in decidua (Fig.
5A and B).
Discussion
lncRNAs serve an important role in normal cell and
tissue development and differentiation, including in embryo
implantation (17). Increasing
evidence has demonstrated that multiple lncRNAs are dysregulated in
pregnancy complications such as miscarriage (18-20),
preeclampsia (21,22) and intrauterine growth restriction
(23). A growing body of evidence
has indicated that MALAT1, HOTAIR and MEG3 serve a
key role in regulating the mobility of trophoblast cells. Knockdown
of MALAT1 and MEG3 in HTR-8/SVneo cells was revealed
to result in suppressed cell invasion, decreased cell viability and
increased cell apoptosis, while overexpression of MALAT1 and
MEG3 had the opposite effect on trophoblast cells (24,25).
Zhang et al (26) reported
that overexpression of HOTAIR in HTR-8/SVneo trophoblast
cells, which was measured using Matrigel cell invasion assays and
ex vivo explant culture models, leads to increased invasive
properties (26). Abnormal
expression of these three lncRNAs have been associated with the
pathogenesis of adverse pregnancy outcomes. In preeclamptic
placenta, MALAT1 and MEG3 were demonstrated to be
downregulated, which suppressed the migratory and invasive
capabilities of trophoblast cells and resulted in preeclampsia
(27). Conversely, MALAT1 is
overexpressed in placenta previa increta/percreta and has been
strongly associated with the high invasion ability of trophoblast
cells (28). Overexpression of
MALAT1 may lead to over-invasion of trophoblast cells, which
contributes to failure of immune tolerance, leading to pathological
pregnancy outcomes (29).
HOTAIR was reported to exhibit significantly higher
expression in placenta from patients with preeclampsia (30). Additionally, the expression of
HOTAIR in the human placenta was reported to be negatively
correlated with the expression of vascular endothelial growth
factor A (VEGFA), and HOTAIR was reports to suppress the
angiogenesis of the human placenta by inhibiting VEGFA expression
(31). Defective angiogenesis in
the first trimester contributes to a majority of early missed
abortions.
In the present study, MALAT1, MEG3 and
HOTAIR lncRNAs were demonstrated to be significantly
overexpressed in villous tissues from patients exhibiting a missed
abortion, which implies that aberrant expression of these three
lncRNAs may be associated with the pathology of missed abortions by
regulating the function of trophoblast cells in the first
trimester. Furthermore, the results of the current study
demonstrated that MALAT1 was expressed in decidual tissue
from healthy pregnancies and was significantly upregulated in
patients exhibiting missed abortion. Although the biological
significance of MALAT1 expression in decidual tissues
remains unclear, it should be noted that MALAT1 may be
associated with decidualization. The current study also detected
serum progesterone and estradiol levels in the two groups and
revealed that serum progesterone and estradiol levels were
significantly lower in the missed abortion group compared with the
healthy control group. The correlation between MALAT1
expression and serum progesterone was also examined, and the
results demonstrated that increased MALAT1 expression was
inversely correlated with serum progesterone levels. Progesterone
is an important hormone in regulating endometrial cell
decidualization, which is an essential step in the establishment
and maintenance of pregnancy (32).
The results of the current study indicated that MALAT1
exerts biological roles in missed abortion by regulating the
behavior of trophoblast cells and also by mediating
decidualization.
An appropriate balance between cytokines produced by
Th1 and Th2 cells is essential for a successful pregnancy (33). It is widely believed that the first
trimester of pregnancy is dominated by proinflammatory Th1 cells to
ensure successful implantation (34). A number of studies have demonstrated
that the skewed balance of Th1/Th2 cells may result in adverse
pregnancy outcomes (35,36), such as early pregnancy loss and
preeclampsia (37). As an important
cytokine in the Th2-dependent immune responses, IL-10 exerts a
regulatory effect on Th1/Th2 balance. IL-10 may induce the
expression of HLA-G in trophoblasts and monocytes at the
maternal-fetal interface and reduce the expression of MHC-I and
MHC-II antigens on the surface of monocytes (38). Additionally, IL-10 can also inhibit
Th1 cytokines secreted by Th1 cells (39). Healthy pregnant women have
significantly higher production of IL-10 and other Th2 cytokines
(40). Previous studies have
indicated that the reduced production of IL-10 may contribute to
recurrent miscarriages (41).
Patients with recurrent pregnancy loss exhibit a lower proportion
of IL-10+ CD19+ B cells, and reduced levels of IL-10 in serum and
supernatant of the stimulated B cell culture medium (42). The results of the current study are
consistent with this, indicating that serum IL-10 was significantly
lower in the missed abortion group. Furthermore, a number of
studies have demonstrated that lncRNAs are associated with the
regulation of Th1/Th2 balance and inflammation at the
maternal-fetal interface (43,44).
Ou et al (45) recently
reported that MALAT1 promotes the production of
proinflammatory cytokines via activation of the NF-kB pathway, and
exacerbates hypertension symptoms in the RUPP-induced rat model. In
trophoblasts, downregulation of MALAT1 inhibits inflammation
by reducing the secretion of inflammatory factors TNF-α, IL-6 and
TGF-β (18). However, correlations
were not revealed between the expression of MALAT1 and serum IL-10,
which may be partially explained by the small sample size. Future
studies should investigate these issues.
In conclusion, the current study demonstrated that
MALAT1 is upregulated in decidual and villous tissue in
patients exhibiting a missed abortion, and higher expression of
MALAT1 is inversely associated with serum progesterone
levels. The results also revealed that HOTAIR and
MEG3 expression are increased in villous samples from
patients with missed abortions. These data indicated that
MALAT1 may be involved in the pathogenesis of missed
abortion. Further studies should aim to elucidate the molecular
mechanisms underlying adverse pregnancy outcomes.
Acknowledgements
Not applicable.
Funding
The current study was supported by the Science and
Technology Commission of Tongzhou District Foundation (grant no.
KJ2019CX014-13) and Health Research and Development of Special Fund
of Tongzhou District (grant no. TFZXPT-20180101).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
ML designed and executed the study, analyzed the
data and prepared the manuscript. HX, LW and JZ collected the
clinical data. JG and WM conceived the study and edited the
manuscript. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The current study was approved by the Ethics
Committee of the Medical Faculty, Beijing Luhe Hospital, Capital
Medical University. Written informed consent was obtained from all
individual participants included in the study prior to collection
of tissue and blood samples.
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests.
References
|
1
|
Rai R and Regan L: Recurrent miscarriage.
Lancet. 368:601–611. 2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhang X, Li J, Gu Y, Zhao Y, Wang Z and
Jia G: A pilot study on environmental and behavioral factors
related to missed abortion. Environ Health Prev Med. 16:273–278.
2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Griebel CP, Halvorsen J, Golemon TB and
Day AA: Management of spontaneous abortion. Am Fam Physician.
72:1243–1250. 2005.PubMed/NCBI
|
|
4
|
Fang J, Xie B, Chen B, Qiao C, Zheng B,
Luan X, Liu J, Yan Y, Zheng Q, Wang M, et al: Biochemical clinical
factors associated with missed abortion independent of maternal
age: A retrospective study of 795 cases with missed abortion and
694 cases with normal pregnancy. Medicine (Baltimore).
97(e13573)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fu M, Mu S, Wen C, Jiang S, Li L, Meng Y
and Peng H: Wholeexome sequencing analysis of products of
conception identifies novel mutations associated with missed
abortion. Mol Med Rep. 18:2027–2032. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hwang JH, Kim JW, Hwang JY, Lee KM, Shim
HM, Bae YK, Paik SS and Park H: Coxsackievirus B infection is
highly related with missed abortion in Korea. Yonsei Med J.
55:1562–1567. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang Y, Zhang Y, Hu K, Qiu J, Hu Y, Zhou M
and Zhang S: Elevated long noncoding RNA MALAT-1 expression is
predictive of poor prognosis in patients with breast cancer: A
meta-analysis. Biosci Rep. 40(BSR20200215)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liao K, Lin Y, Gao W, Xiao Z, Medina R,
Dmitriev P, Cui J, Zhuang Z, Zhao X, Qiu Y, et al: Blocking lncRNA
MALAT1/miR-199a/ZHX1 axis inhibits glioblastoma proliferation and
progression. Mol Ther Nucleic Acids. 18:388–399. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Shen F, Zheng H, Zhou L, Li W and Xu X:
Overexpression of MALAT1 contributes to cervical cancer progression
by acting as a sponge of miR-429. J Cell Physiol. 234:11219–11226.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gordon MA, Babbs B, Cochrane DR, Bitler BG
and Richer JK: The long non-coding RNA MALAT1 promotes ovarian
cancer progression by regulating RBFOX2-mediated alternative
splicing. Mol Carcinog. 58:196–205. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Costanzo V, Bardelli A, Siena S and
Abrignani S: Exploring the links between cancer and placenta
development. Open Biol. 8(180081)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Huppertz B: Traditional and new routes of
trophoblast invasion and their implications for pregnancy diseases.
Int J Mol Sci. 21(289)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Brosens I, Puttemans P and Benagiano G:
Placental bed research: I. The placental bed: from spiral arteries
remodeling to the great obstetrical syndromes. Am J Obstet Gynecol.
221:437–456. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Geldenhuys J, Rossouw TM, Lombaard HA,
Ehlers MM and Kock MM: Disruption in the regulation of immune
responses in the placental subtype of preeclampsia. Front Immunol.
9(1659)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
McAninch D, Roberts CT and Bianco-Miotto
T: Mechanistic Insight into Long Noncoding RNAs and the Placenta.
Int J Mol Sci. 18(1371)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bouckenheimer J, Assou S, Riquier S, Hou
C, Philippe N, Sansac C, Lavabre-Bertrand T, Commes T, Lemaître JM,
Boureux A and De Vos J: Long non-coding RNAs in human early
embryonic development and their potential in ART. Hum Reprod
Update. 23:19–40. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fang Z, Yang Y, Xu Y, Mai H, Zheng W, Pi
L, Fu L, Zhou H, Tan Y, Che D and Gu X: LncRNA HULC polymorphism is
associated with recurrent spontaneous abortion susceptibility in
the southern chinese population. Front Genet.
10(918)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sheng F, Sun N, Ji Y, Ma Y, Ding H, Zhang
Q, Yang F and Li W: Aberrant expression of imprinted lncRNA MEG8
causes trophoblast dysfunction and abortion. J Cell Biochem.
120:17378–17390. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tian FJ, He XY, Wang J, Li X, Ma XL, Wu F,
Zhang J, Liu XR, Qin XL, Zhang Y, et al: Elevated tristetraprolin
impairs trophoblast invasion in women with recurrent miscarriage by
destabilization of HOTAIR. Mol Ther Nucleic Acids. 12:600–609.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen Q, Jiang S, Liu H, Gao Y, Yang X, Ren
Z, Gao Y, Xiao L, Hu H, Yu Y, et al: Association of lncRNA
SH3PXD2A-AS1 with preeclampsia and its function in invasion and
migration of placental trophoblast cells. Cell Death Dis.
11(583)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang Y, He XY, Qin S, Mo HQ, Li X, Wu F,
Zhang J, Li X, Mao L, Peng YQ, et al: Upregulation of PUM1
expression in preeclampsia impairs trophoblast invasion by
negatively regulating the expression of the lncRNA HOTAIR. Mol The.
28:631–641. 2020.PubMed/NCBI
|
|
23
|
Gremlich S, Damnon F, Reymondin D,
Braissant O, Schittny JC, Baud D, Gerber S and Roth-Kleiner M: The
long non-coding RNA NEAT1 is increased in IUGR placentas, leading
to potential new hypotheses of IUGR origin/development. Placenta.
35:44–49. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang R and Zou L: Downregulation of
LncRNA-MEG3 promotes HTR8/SVneo cells apoptosis and attenuates its
migration by repressing Notch1 signal in preeclampsia.
Reproduction. 160:21–29. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang Y, Qu L, Ni H, Wang Y, Li L, Yang X,
Wang X and Hou Y: Expression and function of lncRNA MALAT1 in
gestational diabetes mellitus. Adv Clin Exp Med. 29:903–910.
2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang Y, Jin F, Li XC, Shen FJ, Ma XL, Wu
F, Zhang SM, Zeng WH, Liu XR, Fan JX, et al: The YY1-HOTAIR-MMP2
signaling axis controls trophoblast invasion at the maternal-fetal
interface. Mol Ther. 25:2394–2403. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chen H, Meng T, Liu X, Sun M, Tong C, Liu
J, Wang H and Du J: Long non-coding RNA MALAT-1 is downregulated in
preeclampsia and regulates proliferation, apoptosis, migration and
invasion of JEG-3 trophoblast cells. Int J Clin Exp Pathol.
8:12718–12727. 2015.PubMed/NCBI
|
|
28
|
Tseng JJ, Hsieh YT, Hsu SL and Chou MM:
Metastasis associated lung adenocarcinoma transcript 1 is
up-regulated in placenta previa increta/percreta and strongly
associated with trophoblast-like cell invasion in vitro. Mol Hum
Reprod. 15:725–731. 2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chen J, Ao L and Yang J: Long non-coding
RNAs in diseases related to inflammation and immunity. Ann Transl
Med. 7(494)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zou YF and Sun LZ: Long noncoding RNA
HOTAIR modulates the function of trophoblast cells in
pre-eclampsia. Sichuan Da Xue Xue Bao Yi Xue Ban. 46:113–122.
2015.PubMed/NCBI(In Chinese).
|
|
31
|
Wu K, Liu F, Wu W, Chen Y, Wu H and Zhang
W: Long non-coding RNA HOX transcript antisense RNA (HOTAIR)
suppresses the angiogenesis of human placentation by inhibiting
vascular endothelial growth factor A expression. Reprod Fertil Dev.
31:377–385. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
32
|
Okada H, Tsuzuki T and Murata H:
Decidualization of the human endometrium. Reprod Med Biol.
17:220–227. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Saito S, Nakashima A, Shima T and Ito M:
Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J
Reprod Immunol. 63:601–610. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chaouat G, Zourbas S, Ostojic S,
Lappree-Delage G, Dubanchet S, Ledee N and Martal J: A brief review
of recent data on some cytokine expressions at the materno-foetal
interface which might challenge the classical Th1/Th2 dichotomy. J
Reprod Immunol. 53:241–256. 2002.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang W, Lin Y, Zeng S and Li DJ:
Improvement of fertility with adoptive CD25+ natural killer cell
transfer in subfertile non-obese diabetic mice. Reprod Biomed
Online. 18:95–103. 2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lin Y, Zhong Y, Saito S, Chen Y, Shen W,
Di J and Zeng S: Characterization of natural killer cells in
nonobese diabetic/severely compromised immunodeficient mice during
pregnancy. Fertil Steril. 91:2676–2686. 2009.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lin Y, Wang H, Wang W, Zeng S, Zhong Y and
Li DJ: Prevention of embryo loss in non-obese diabetic mice using
adoptive ITGA2(+)ISG20(+) natural killer-cell transfer.
Reproduction. 137:943–955. 2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Persson G, Bork JBS, Isgaard C, Larsen TG,
Bordoy AM, Bengtsson MS and Hviid TVF: Cytokine stimulation of the
choriocarcinoma cell line JEG-3 leads to alterations in the HLA-G
expression profile. Cell Immunol. 352(104110)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Trinchieri G: Interleukin-10 production by
effector T cells: Th1 cells show self control. J Exp Med.
204:239–243. 2007.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Thaxton JE and Sharma S: Interleukin-10: A
multi-faceted agent of pregnancy. Am J Reprod Immunol. 63:482–491.
2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Thaker R, Oza H, Verma V, Gor M and Kumar
S: The association of circulatory cytokines (IL-6 and IL-10) level
with spontaneous abortion-a preliminary observation. Reprod Sci:
Aug 12, 2020 (Epub ahead of print).
|
|
42
|
Danaii S, Ghorbani F, Ahmadi M, Abbaszadeh
H, Koushaeian L, Soltani-Zangbar MS, Mehdizadeh A, Hojjat-Farsangi
M, Kafil HS, Aghebati-Maleki L and Yousefi M: IL-10-producing B
cells play important role in the pathogenesis of recurrent
pregnancy loss. Int Immunopharmacol. 87(106806)2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Chitnis NS, Shieh M and Monos D:
Regulatory noncoding RNAs and the major histocompatibility complex.
Hum Immunol, 2020 (Epub ahead of print).
|
|
44
|
Huang Z, Du G, Huang X, Han L, Han X, Xu
B, Zhang Y, Yu M, Qin Y, Xia Y, et al: The enhancer RNA
lnc-SLC4A1-1 epigenetically regulates unexplained recurrent
pregnancy loss (URPL) by activating CXCL8 and NF-kB pathway.
EBioMedicine. 38:162–170. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ou M, Zhao H, Ji G, Zhao X and Zhang Q:
Long noncoding RNA MALAT1 contributes to pregnancy-induced
hypertension development by enhancing oxidative stress and
inflammation through the regulation of the miR-150-5p/ET-1 axis.
FASEB J. 34:6070–6085. 2020.PubMed/NCBI View Article : Google Scholar
|